Abstract
Background:
The increased mutational burden for rare structural genomic variants in schizophrenia and other neurodevelopmental disorders has thus far not yielded therapies targeting the biological effects of specific mutations. We identified two carriers (mother and son) of a triplication of the gene encoding glycine decarboxylase, GLDC, presumably resulting in reduced availability of the NMDA receptor (NMDAR) co-agonists glycine and D-serine and NMDAR hypofunction. Both carriers had a diagnosis of a psychotic disorder.
Methods:
We carried out two double-blind placebo-controlled clinical trials of NMDAR augmentation of psychotropic drug treatment in these two individuals. Glycine was used in the first clinical trial. D-cycloserine was used in the second one.
Results:
Glycine or D-cycloserine augmentation of psychotropic drug treatment each improved psychotic and mood symptoms in placebo-controlled trials.
Conclusions:
These results provide two independent proof-of-principle demonstrations of symptom relief by targeting a specific genotype and explicitly link an individual mutation to the pathophysiology of psychosis and treatment response.
The trials were registered on the ClinicalTrials.gov (https://www.clinicaltrials.gov) website ( and ).
Keywords: NMDA receptor hypofunction, schizophrenia, bipolar disorder, copy number variant, genetics, glycine decarboxylase
Introduction
Individually rare structural variants (SVs) of relatively recent evolutionary origin such as copy number variants (CNVs) collectively account for an increased mutational burden for schizophrenia and other neurodevelopmental disorders (e.g., autism spectrum disorders, intellectual disability, epilepsy, and to a lesser extent, bipolar disorder). The most recurrent include microdeletions and microduplications with odds ratios for phenotypic expression ranging from 2 to greater than 60 and have effect sizes much larger than those associated with common genetic risk variants. (1-21) SVs and CNVs can be large, involve many genes, and their underlying structure can be complex. (22) Since pleiotropic clinical effects are the norm, discoveries based on any single mutation are potentially relevant to other individuals who carry the same mutation or mutations that impact the same biological pathways, even if the clinical phenotype differs. The fact that shared molecular mechanisms are implicated in a range of neurodevelopmental disorders (23-26) suggests that a “genotype-first” approach (27-31) may be more instructive about pathophysiology and potential treatments than a disease-oriented approach. Copy number variant loci continue to be linked to cognitive phenotypes. (32, 33) The challenge is to link mutations in specific genes to the underlying disease biology, (34) which can, in turn, be translated into targeted treatment interventions with positive therapeutic effects in appropriately selected patients. (35-39)
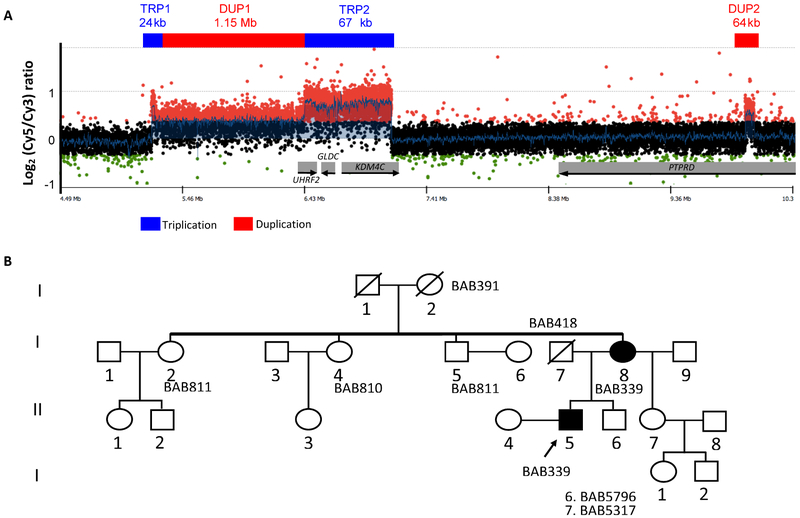
We identified several CNVs spanning 9p24.1 in a proband and his mother, who presented with DSM-IV (40) diagnoses of schizoaffective disorder and bipolar disorder with psychotic features, respectively (Figure 1A); this structural rearrangement seems to segregate with psychosis in this family (Figure 1B). The rearrangement was confirmed as a de novo event in the mother (Figure S1). (41) The complete architecture of this complex rearrangement and the proposed DNA replicative/repair mechanism underlying its formation are described in Grochowski et al. 2018. (42) See supplemental material for details about the CNV.
Figure 1.
Structure of the 9p24.1 duplication-triplication (A) and pedigree (B).
Although several of the genes in the CNV may be potentially relevant to the development of neuropsychiatric disorders (see supplemental material), GLDC is particularly compelling, because it codes for the enzyme that catabolizes glycine, a precursor of D-serine, both of which are co-agonists at the NMDA receptor (NMDAR). (43) Triplication of GLDC would be expected to increase glycine catabolism, resulting in low levels of brain glycine and D-serine. Reduced availability of these two NMDAR co-agonists would result in NMDAR hypofunction, which is associated with psychotic disorders. (44-47) The GLDC triplication seemed fortuitously amenable to a treatment intervention tailored to normalizing the biology hypothesized to be affected by the mutation. Therefore, we undertook a proof-of-principle clinical trial to determine whether augmentation of usual psychotropic drug treatment with glycine, a full agonist at the NMDAR glycine modulatory site (GMS), reduced psychotic and mood symptoms in the two carriers of the GLDC triplication. Due to the encouraging results of the glycine augmentation trial, we undertook a clinical trial with D-cycloserine (DCS), a relatively selective partial agonist at the GMS at low doses. (48, 49)
Methods and Materials
Subjects
Two carriers of the 9p24.1 CNV participated in the clinical trials: the proband (subject 3363) and his mother (subject 5459). Demographic information and details of the study designs and methods are included below and in the supplemental material.
Study Design
Both studies were approved by institutional review boards at McLean Hospital and Partners Healthcare. Subjects provided written informed consent.
Glycine Augmentation Clinical Trial Methods
Procedures.
Symptom severity and treatment side effects were monitored at least weekly; formal clinical ratings were carried out every two weeks blind to drug condition using the following primary instruments: Brief Psychiatric Rating Scale (BPRS), Positive and Negative Syndrome Scale (PANSS), Young Mania Rating Scale, Hamilton Depression Scale, Columbia–Suicide Severity Rating Scale and the Clinical Global Impression (CGI) Scale. (50-55) Motor abnormalities were assessed at baseline and at the end of each treatment arm blind to condition. (56, 57) Plasma concentrations of small and large amino acids, kynurenine (KYN), kynurenic acid (KYNA), quinolinic acid, and homocysteine were obtained at baseline and during week 6 of each arm of the acute glycine trial (see supplemental material). All baseline procedures were carried out in person; some clinical assessments and movement disorder exams were carried out using a secure form of video conferencing, because the subjects were not local.
Short-term Glycine Augmentation Trial.
Both subjects were maintained on stable doses of psychotropic medications during the acute trial (see supplemental material). Design. Double-blind random-order glycine-placebo crossover followed by open-label glycine. Each of these three arms lasted six weeks, separated by two weeks to wash out treatment effects from the previous arm. (58) During each arm, each subject received pharmaceutical grade glycine (Ajinomoto) or placebo as determined by the research pharmacist’s randomization. See supplemental material for details of dose preparation. Starting with a dose of 6 gm, the daily dose of glycine or placebo was titrated upward by 3 gm/d (TID dosing) until the target dose was reached or gastrointestinal (GI) side effects occurred. The target glycine dose was 0.8 gm/kg/d, based on reports that this dose yielded optimal therapeutic effects with minimal side effects. (59, 60) Subject 5459 received glycine during arm 1 and placebo during arm 2; subject 3363 received placebo during arm 1 and glycine during arm 2. See supplemental material for details regarding dosing, titration schedules, side effect management and de facto sustainable dosing due to GI side effects.
Chronic Glycine Augmentation Clinical Trial.
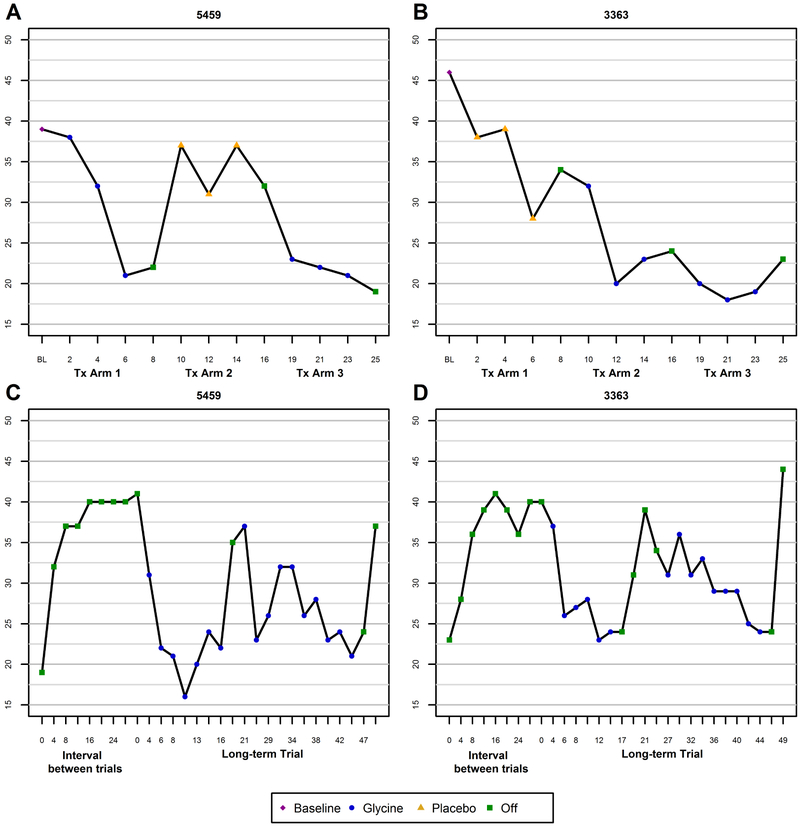
Following completion of the acute glycine study, both subjects experienced an exacerbation of clinical symptoms (Figure 2, C to D). An eight-month period elapsed between the end of the acute trial and the start of the chronic trial. The chronic glycine trial lasted 47 weeks (see supplemental material and tables). Although both subjects showed an initial reduction in total BPRS score (Figure 2, C to D), the chronic trial was temporarily suspended at 16 weeks due to the intolerability of GI side effects with chronic TID dosing. Both subjects asked to end the trial during week 47, having suffered from chronic GI side effects even at reduced doses. See supplemental material for details. The maximum sustainably tolerable doses were ~18.8-27.5% of the target doses.
Figure 2.
Changes in total Brief Psychiatric Rating Scale (BPRS) score as a function of treatment with glycine or placebo in subject 5459 (A) and subject 3363 (B) during the short-term trials. Changes in total BPRS score during the interval between the short-term and long-term glycine trials and during long-term glycine treatment in subject 5459 (C) and subject 3363 (D).
DCS Augmentation Trial
The same subjects participated in the DCS study. Age and medication information are contained in supplemental material.
The first arm was an 8-week open-label trial due to reports of positive symptom exacerbation in two schizophrenia patients treated with conventional neuroleptics (61) and of a significant mean worsening of negative symptoms in patients treated with clozapine (62) during exposure to 50 mg of DCS. It therefore seemed prudent to ascertain that DCS was not negatively affecting symptom severity and that DCS plasma levels were not unusually high [see (61)] before embarking on a double-blind trial. Following a one week washout period after the open-label trial, sufficient for the 7-15 hour half-life of DCS, (63) the double-blind placebo-controlled trial started; each arm lasted 6 weeks with a washout week between arms. The dose of DCS was 50 mg (qAM), a dose at which DCS is a partial agonist of the NMDAR (48, 49, 64-66), which has been shown to reduce negative symptoms of schizophrenia in non-clozapine treated patients (61, 67) and to augment cognitive behavioral therapy for delusions. (68) The double-blind phase was followed by 24 weeks of open-label exposure. Clinical ratings (every two weeks) and movement disorder exams (at the end of each arm) were carried out blind to condition. Due to the unexpected death of an immediate family member at the end of the washout between phase 1 and phase 2 of the double-blind (week 7), the washout period was extended for an additional six weeks until the subjects' clinical states had stabilized sufficiently for the trial to resume.
Statistical Analyses.
We fit linear models with fixed effects for treatment and subject for the primary outcome, total BPRS score, from 1) the post-baseline double-blind arms; and 2) the open-label periods, which included the open-label arm of the short-term trial, the interval between the short- and long-term trials and the long-term trial. We also fit a model that included a treatment-by-subject interaction. Due to the small sample size, p-values were estimated using permutation tests and standard errors were estimated using the bootstrap method. (69) A two-tailed p-value of 0.05 was considered statistically significant.
Results
Glycine Augmentation Clinical Trial
Short-term Glycine Trial.
Both subjects showed improvement in clinical symptoms during administration of glycine. Figures 2A-B present total BPRS scores during the acute trials in each subject. During the two arms of the double-blind trial (treatment arms 1-2), the mean (SD) total BPRS score for 5459 was 30.3 (8.6) while on glycine and 35.0 (3.5) while off glycine; for 3363, the mean (SD) total BPRS score was 25.0 (6.2) while on glycine and 35.0 (6.1) while off glycine (Table 1). See Figures S2 and S3 and supplemental material for data from other salient PANSS symptom domains.
Table 1.
Changes in mean (SD) BPRS as a function of glycine or placebo.
| Condition | ||||
|---|---|---|---|---|
| Trial | Subject | Off Glycine | On Glycine | %Δ |
| Double-Blinda | 5459 | 35.0 (3.5) | 30.3 (8.6) | 13.4 |
| 3363 | 35.0 (6.1) | 25.0 (6.2) | 28.6 | |
| Open Labelb | 5459 | 35.2 (7.0) | 24.7 (5.0) | 29.8 |
| 3363 | 34.5 (6.9) | 27.2 (5.4) | 21.2 | |
Average decrease: 7.3 (3.0) (20.0%)
Average decrease: 8.8 (1.5) (25.9%)
The estimated magnitude of effect, or mean (SE) decrease in total BPRS score while receiving glycine, was 7.3 (3.0) points (20%) lower than while receiving placebo, however this difference did not reach statistical significance (p = 0.083). The interaction between condition and subject was not statistically significant (p=0.494) (Table 1), indicating that the effect of glycine on total BPRS score did not significantly differ between subjects.
During the subsequent 6 weeks of open-label treatment with glycine, both subjects again showed a substantial reduction of symptoms (Figure 2, A to B, treatment arm 3). Following completion of that arm, both subjects experienced an exacerbation of clinical symptoms during the eight-month interval between the end of the short-term trial and the start of the open label chronic glycine trial (Figure 2, C to D).
Long-term Open-Label Glycine Trial.
The mean (SE) decrease in total BPRS score while receiving glycine in all open-label periods was 8.8 (1.5) (p<0.001), a reduction of 26%. The interaction between condition and subject was not statistically significant (p=0.343) (Table 1).
Plasma Levels.
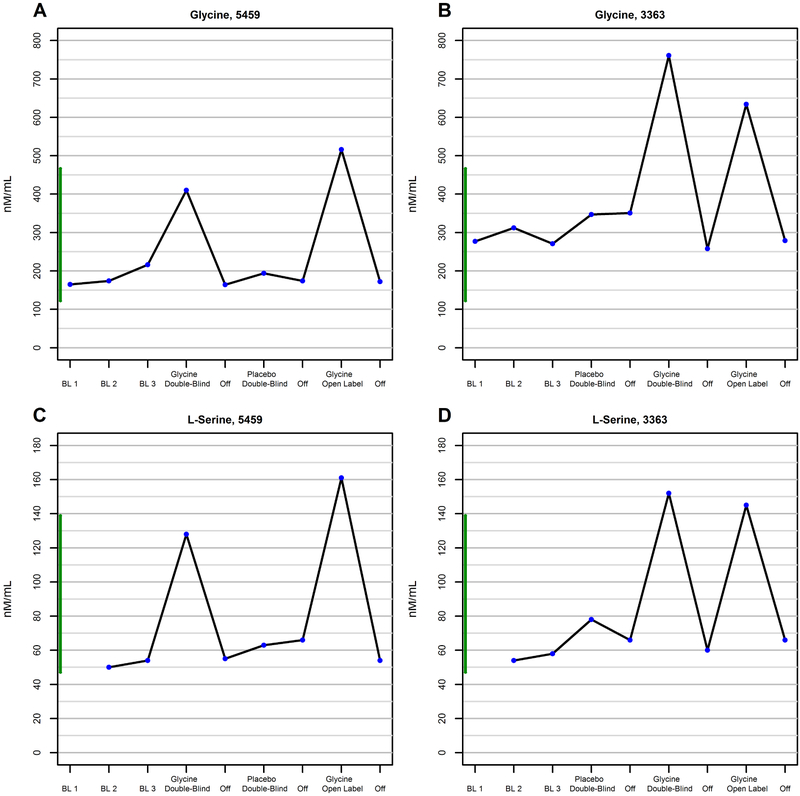
At baseline, plasma glycine levels were within the normal range (Figure 3, A to B). L- serine plasma levels were at the low end of the normal range (Figure 3, C to D). During treatment with glycine, plasma levels of glycine and L-serine increased by 121-179% and 146-210%, respectively (see supplemental material). KYN levels were elevated above the normal range in both subjects independent of glycine-placebo condition and during the chronic glycine trial; the increase was particularly prominent in subject 5459 (Figure S4). KYNA levels were markedly elevated in subject 5459 at baseline, were consistently normalized during treatment with glycine acutely and during short periods when glycine was tolerated chronically; KYNA levels in subject 3363 were in the upper range of normal at baseline, and were also reduced by glycine, but not as consistently as in subject 5459 (Figure S5).
Figure 3.
Changes in plasma glycine level as a function of short-term treatment with glycine or placebo in subject 5459 (A) and subject 3363 (B). Changes in plasma L-serine level as a function of short-term treatment with glycine or placebo in subject 5459 (C) and subject 3363 (D). All data are from the acute trials.
DCS Augmentation Trial
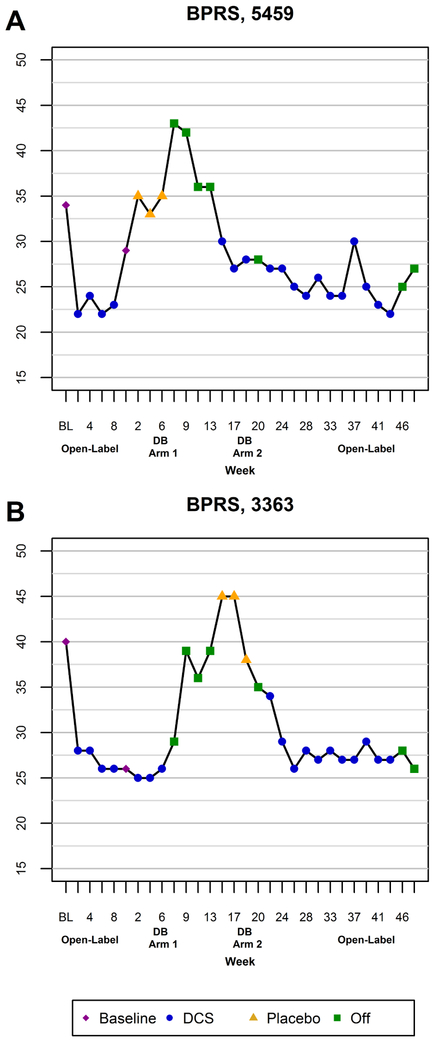
Both subjects showed improvement in clinical symptoms during administration of DCS. Figure 4 presents total BPRS scores during all phases of the DCS trial in each subject. During the two arms of the double-blind trial, the mean (SD) total BPRS score for 5459 was 28.3 (1.5) while on DCS and 34.3 (1.2) while off DCS; for 3363, the mean (SD) total BPRS score was 25.3 (0.6) while on DCS and 42.7 (4.0) while off DCS. Subject 3363 showed improvement in both positive psychotic and negative symptoms. Improvement in subject 5459 was restricted to positive and mood symptoms. The magnitude of the clinical improvement was statistically significant during the open-label and double-blind arms (Table 2). During the double-blind phase, the estimated magnitude of effect, or mean (SE) decrease in total BPRS score while receiving DCS, was 11.7 (1.1) points (30.3%) lower than while receiving placebo (p=0.006). There was a significant interaction between condition and subject (p=0.002). [FN1].
Figure 4.
Changes in total Brief Psychiatric Rating Scale (BPRS) score as a function of treatment with DCS or placebo in subject 5459 (A) and subject 3363 (B).
Table 2.
Changes in mean (SD) BPRS as a function of DCS or placebo.
| Condition | ||||
|---|---|---|---|---|
| Trial | Subject | Off DCS | On DCS | %Δ |
| Double-Blinda | 5459 | 34.3 (1.2) | 28.3 (1.5) | 17.5 |
| 3363 | 42.7 (4.0) | 25.3 (0.6) | 40.7 | |
| Open Labelb | 5459 | 28.7 (4.7) | 24.5 (2.2) | 14.6 |
| 3363 | 313 (7.6) | 27.8 (2.0) | 11.2 | |
Average decrease: 11.7 (1.1) (30.3%)
Average decrease: 3.8 (2.1) (12.7%)
During the acute and chronic open-label periods, the mean (SE) decrease in total BPRS score while receiving DCS in all open-label periods was 3.8 (2.1) (p<0.009), a mean reduction of 12.7%. There was no significant interaction between condition and subject (p=0.834) (Table 2), indicating that the effect of DCS on total BPRS score did not significantly differ between subjects.
Plasma Levels.
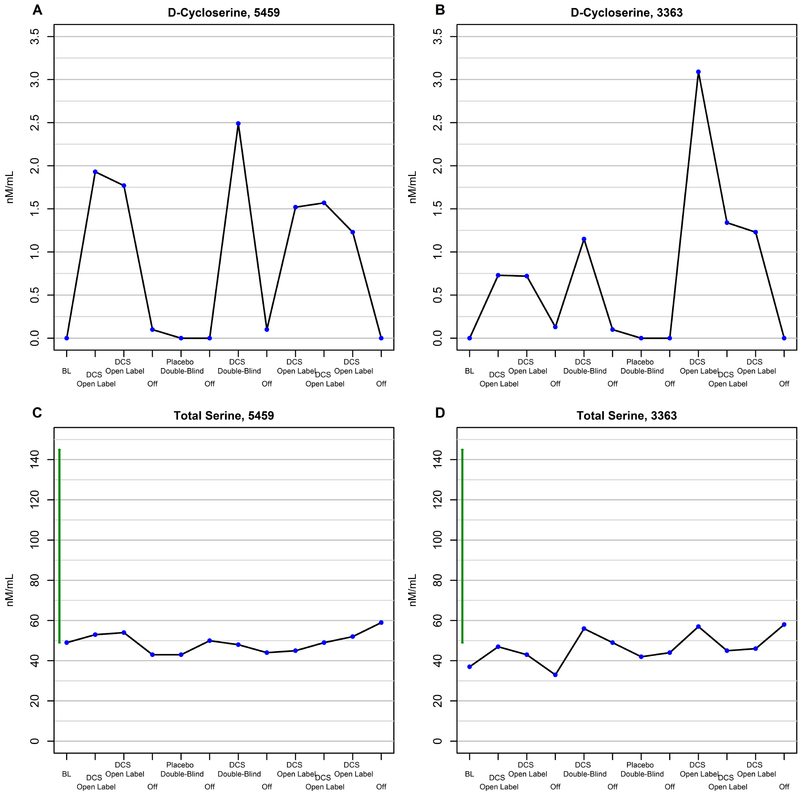
Figures 5A-5B show increases in plasma DCS levels as a function of exposure to DCS.
Figure 5.
Changes in plasma DCS level as a function of treatment with DCS or placebo in subject 5459 (A) and subject 3363 (B). Changes in plasma total serine level as a function of short-term treatment with DCS or placebo in subject 5459 (C) and subject 3363 (D).
In contrast to the dramatic increases in L-serine (96-99% of total serine) observed during exposure to glycine (Figure 3, C to D), total serine plasma levels increased only modestly (10.2-54%) with exposure to DCS but remained below or barely within the normal range (Figure 5, C to D). Plasma glycine level also did not change substantially as a function of treatment with DCS (Figure S6). These findings are consistent with DCS being a weak inhibitor of serine racemase. (70)
KYN levels were consistently elevated in both subjects independent of DCS-placebo condition, especially in subject 5459 (Figure S4). KYNA levels were markedly elevated in subject 5459 at baseline and were more clearly normalized during treatment with DCS than with glycine, but this normalization was not sustained during chronic exposure; KYNA levels in subject 3363 were at the upper end of the normal range at baseline, and were not altered by exposure to DCS (Figure S5).
Neurocognition.
No consistent changes in neurocognition were observed as a function of glycine or DCS exposure (see supplemental material, Figure S7).
Discussion
We report the results of two proof-of-principle clinical trials showing that interventions tailored to a specific genetic mutation reduced symptom severity in two individuals with psychotic disorders. Although both subjects were partially remitted at baseline, the additional 20-26% reduction in symptom severity on glycine and 13%-30% on DCS reflected substantial relief beyond that achieved by their usual psychotropic drug regimen, an effect that is considered clinically meaningful in augmentation treatment studies of schizophrenia. (71) The pleiotropic clinical effects of the GLDC triplication - present in schizoaffective and bipolar disorder - are consistent with the variable expressivity of rare CNVs (see supplemental material). The demonstration of tractable symptom relief by targeting a specific genotype explicitly links an individual mutation to disease biology and pathophysiology and to treatment response. Although we do not know conclusively that the GLDC triplication or any of the other genetic elements in the CNV region is causally implicated in the psychiatric illnesses of the carriers, (72, 73) [FN2] our data show that the severity of their symptoms was reduced by augmentation with glycine or DCS. This result underscores the importance of molecular diagnosis and targeting specific biological processes rather than clinical diagnoses per se. Indeed, it is not unusual for medications to be efficacious in only a subgroup of individuals treated for a particular clinical condition. (74-77) The GLDC gene is among the 15.4% of genes least tolerant of functional variation (see supplemental material), (78) suggesting that gain and loss of function changes in this gene may not be phenotypically neutral. Indeed, triplication of a disease gene may convey a more severe disease phenotype than does duplication of the same locus. (79-81)
The success of this "genotype first" (27) approach underscores the utility of targeting specific biological processes in appropriately selected individuals. NMDAR modulators have shown variable efficacy in schizophrenia patients selected on the basis of refractory negative symptoms, (82, 83) but not for having an identified disturbance in NMDAR function. Inasmuch as rare and common structural and sequence variants converge on specific biological pathways, including but not limited to the NMDAR (e.g., immune function, calcium channel signaling, etc.), (15, 25, 26, 84-89) our results are potentially relevant to a broader group than carriers of increased GLDC copy number per se (e.g., see (90)). Genes involved in glutamate neurotransmission, in particular, are over-represented among the rare variants associated with schizophrenia, autism spectrum disorders (ASD), and non-syndromic intellectual disability. (25, 26) Thus, even though the GLDC triplication is, so far, a private mutation, individuals with mutations in genes impacting glutamatergic and NMDAR signaling may constitute a “molecular subtype” amenable to "pathway defined treatment" (27) who would have a high prior probability of responding to treatments that normalize glutamatergic dysregulation. In keeping with the pleiotropy that is characteristic of CNVs, the pool of potential beneficiaries is likely to transcend diagnostic categories. Notably, multiple lines of evidence implicate genetic variants in KDM4C/JMJD2C, GLDC and other genes in the 9p24.1 region with schizophrenia, ASD, bipolar disorder and neurodevelopmental disorders (summarized in supplemental material). Our findings are consistent with other data illustrating the value of molecular diagnosis in clarifying the significance of newly emerging clinical symptoms (91) or guiding treatment in the context of atypical psychiatric presentations. (92)
At baseline, plasma levels of glycine were within the normal range and those of L-serine were at the low end of the normal range. Plasma levels poorly reflect brain extracellular levels of glycine and L-serine. In the brain, GLDC is expressed exclusively in astrocytes. (93) Astrocytic serine hydroxyl methyl transferase (SHMT-1) converts glycine to L-serine, which is converted to D-serine, the NMDA receptor co-agonist, by neuronal serine racemase. Thus, reduced availability of glycine and L-serine would decrease neuronal synthesis of D-serine. (94) Both glycine and L-serine plasma levels showed marked increases during glycine treatment; these increases were much greater for L-serine than for glycine. Inasmuch as glutamatergic signaling through NMDA receptors requires glycine or D-serine at the GMS, it is possible that exogenous glycine increased the conversion of glycine to L-serine, thereby increasing precursor availability for D-serine synthesis. (94) D-serine is a more potent agonist at the GMS than glycine and is the preferential agonist at forebrain NMDARs. Thus, the mechanism underlying the therapeutic effect of glycine may, at least in part, have been mediated by increased D-serine rather than by glycine per se (Figure S8).
We selected the particular subjects in this study to undergo NMDAR modulation based on the presence of the GLDC triplication, which we hypothesized would result in increased catabolism of glycine and D-serine resulting in NMDAR hypofunction. Unbeknownst to us at the start of the glycine clinical trial, both carriers had elevated baseline KYN and KYNA plasma levels (for reasons that are currently unknown). KYNA is a non-selective competitive glutamate receptor antagonist (95) with a particularly strong affinity for the GMS of the NMDAR. (96) KYNA was normalized by augmentation with glycine (although this effect was not sustained in 3363), suggesting that glycine may have partially counteracted the antagonism of KYNA in vivo, consistent with in vitro data on glycine and D-serine. (97) KYN remained elevated independent of treatment with glycine (or DCS). Conceivably, the magnitude of the glycine effect on symptom severity may have been attenuated by persistent elevations of KYN/KYNA. Thus, elevated KYN and KYNA may have potentiated the deleterious effect of increased glycine and D-serine degradation caused by the GLDC triplication (or vice versa). Either the GLDC triplication or elevated KYNA would have been sufficient to implicate NMDAR hypofunction. The possibility that two processes impairing NMDAR function were present is consistent with evidence of "multiple genetic hits" in neuropsychiatric disorders. (98, 99) Glycine's efficacy in modulating symptom severity may have been an opportunistic effect of different processes that independently contributed to NMDAR dysregulation such that glycine agonism at the GMS or antagonism of KYNA partially normalized NMDAR function, irrespective of whether this triplication or other genes in the rearranged region were causal.
DCS is a partial agonist at the GMS, with only 40-60% of the potency of glycine, with activity ranging from 65%-200% compared with glycine at saturating doses depending on NMDAR subunit composition, (100) but it crosses the blood brain barrier more readily. (63) In addition, it blocks the formation of KYNA in vitro. (101) These dual actions, agonism at the GMS and KYNA inhibition, suggested that DCS might produce an even greater clinical benefit than was achieved with glycine in restoring NMDA receptor function (Figure S8), and guided the rationale for undertaking the second clinical trial. Notably, DCS produced substantially greater normalization of KYNA than glycine (in 5459, although it was not sustained). These dual actions of DCS in normalizing NMDA receptor hypofunction may have contributed to the substantially greater therapeutic benefit of DCS than of glycine.
In addition to its antagonism of the GMS, KYNA is also a potent non-competitive antagonist of the alpha7 nicotinic acetylcholine receptor (α7nAChR) (102-104) (reviewed in (105)). The CHRNA7 gene has been linked to schizophrenia. (106, 107) Both the NMDAR and the α7nAChR have important roles in cognition and synaptic plasticity. (108, 109) Notably, we observed no systematic changes in neurocognitive function in the subjects during exposure to glycine or DCS, possibly related to sustained elevations of KYN and incompletely normalized levels of KYNA. [FN3]
Other Relevant Considerations. Although side-effect-free up until the threshold for GI side effects is reached, glycine is cumbersome to use, especially over the long-term. In our experience, the "optimal"; target dose of 0.8 g/kg not only was much too high to be tolerated without prohibitive side effects, but also was above the dose needed to achieve therapeutic benefit. Indeed, we observed clinical improvement at substantially lower doses. Whether our subjects had an unusual sensitivity to glycine or DCS (related to the GLDC triplication or to elevated KYN/KYNA) is unclear.
Patients taking clozapine (CLZ) are generally excluded from augmentation with NMDAR modulators. [FN4] We hypothesize that the magnitude of CLZ's normalization of NMDAR receptor-dependent neurotransmission will depend on the presence and severity of a glutamatergic deficit. In carriers of mutations that compromise NMDAR-dependent glutamatergic function, CLZ may provide only partial neurochemical remission, leaving room for additional normalization with NMDAR modulators. It thus seems reasonable to propose that patients with mutations in NMDAR and glutamate related genes may benefit from NMDAR modulators even when taking CLZ.
Several limitations should be considered. First, the optimal compound to antagonize an excess of GLDC is a GLDC inhibitor. Since no such compound is available, we used proxies to try to normalize the effects of the GLDC triplication. Ideally, FDA approved compounds found to have GLDC inhibitory activity could be repurposed to more directly target increased degradation. Second, the small sample size and limited number of observations during the double-blind arms reduced power to detect a statistically significant effect of glycine in this short-term trial, and precluded use of more standard analytic techniques (e.g., modeling subject as a fixed, rather than random, effect, modeling trajectory of response or baseline to endpoint change rather than comparing observations across all post-baseline time points). Notably, the magnitude of the reduction in symptom severity in the double-blind phase of the glycine trial was consistent with the statistically significant treatment effect observed during the open-label phases and with the significant reduction in symptom severity in the double-blind and open-label arms of the DCS study. Third, carryover effects from prior glycine exposure may have reduced the estimate of the magnitude of the treatment effect (Figure 2). Given the small sample size, it is not possible to formally evaluate these potential effects. [FN5] Similarly, subject 3363 had a partial response to placebo during the first arm of the double blind in the glycine study, attenuating the magnitude of the difference between placebo and glycine. Fourth, although the blind was not broken until after the short-term glycine study ended, both staff and the subjects correctly guessed when they received glycine on the basis of side effects. Although this recognition may have favorably influenced the subjects' clinical states, they were so much less symptomatic on glycine that they were willing to tolerate the side effects and dosing for extended periods, suggesting that their substantial clinical improvements are unlikely to reflect placebo effects alone. Notably, there were no side effects during exposure to DCS, making it unlikely that the clinical improvements observed reflected placebo effects. Finally, it would have been ideal had the subjects not suffered a personal loss during the DCS trial and had it been feasible to undertake multiple crossovers between drug and placebo conditions in both studies.
In summary, we report two individuals with psychotic disorders in whom identification of a specific genomic variation resulted in improved clinical symptoms during two proof-of-principle trials targeting a similar mechanism implicated by the mutation. This study has important implications for the treatment of other patients with alterations of the same or overlapping biochemical pathways.
Supplementary Material
Key Resource Table
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Other | Plasma Excitatory Amino Acids Assay | Nathan Kline Institute | N/A | |
| Other | Plasma Large Neutral Amino Acids Assay | Nathan Kline Institute | N/A | |
| Other | Plasma d-Serine Assay | Nathan Kline Institute | N/A | |
| Other | Plasma Tryptophan Assay | Nathan Kline Institute | N/A | |
| Other | Plasma GABA Assay | Nathan Kline Institute | N/A | |
| Other | Plasma Citalopram + Metabolites Assay | Nathan Kline Institute | N/A | |
| Other | Plasma Kynurenic Acid Assay | Nathan Kline Institute | N/A | |
| Other | Plasma Quinolinic Acid Assay | Nathan Kline Institute | N/A | |
| Other | Plasma Kynurenine Assay | Nathan Kline Institute | N/A | |
| Other | Plasma Total Homocysteine Assay | Nathan Kline Institute | N/A | |
| Other | Plasma Clozapine & Norclozapine Assay | Nathan Kline Institute | N/A | |
| Other | Plasma Aripiprazole & Dehydroaripraprazole Assay | Nathan Kline Institute | N/A | |
| Other | Plasma Duloxetine Assay | Nathan Kline Institute | N/A | |
| Other | Plasma Gabapentin Assay | Nathan Kline Institute | N/A |
Acknowledgments:
We are grateful to the individuals who participated in this trial. Their investment as collaborators was essential for the successful completion of these studies. The studies were done entirely on an outpatient basis, mostly by long distance, and required very substantial (and at times, onerous) procedural and clinical oversight cooperation from them. We thank Kim Holleran and Mytsie Thevenin for assisting in the pharmacy preparations, and Jerry Crisp, Esther Vance, and Chris Tabor for clinical laboratory support. We gratefully acknowledge the resources provided by the Autism Genetic Resource Exchange (AGRE) Consortium and the participating AGRE families. AGRE is a program of Autism Speaks and is supported in part by grant 1U24MH081810 from the National Institute of Mental Health to C.M. Lajonchere. We also express special appreciation to Dr. Thomas Insel for having had the foresight to support this work. We dedicate this paper to the memory of Dr. Philip S. Holzman, whose vision inspired the Psychology Research Laboratory's focus on family and genetic studies, and to the memories of the loved ones of the subjects and the researchers who passed away during the course of this work.
Funding:
Supported in part by NIH grants R21 MH097470 and R21 MH105732 (DLL), the Fuller Foundation (DLL), the Ellison Foundation (DLL, CMBC, JRL), Anonymous Foundation (DLL, JS), the Carmela and Menachem Abraham Fund (DLL), and Team Daniel (DLL), NIH grant R21 MH104505 (UR), pre-doctoral NHLBI training grant T32HL079888 (AF), NINDS grants R01NS058529 and R35NS105078 (JRL), NIGMS grant R01GM106373 (JRL), and a joint National Human Genome Research Institute/National Heart Blood and Lung Institute (UM1HG006542) grant to the Baylor Hopkins Center for Mendelian Genomics (JRL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
JTC reports consulting relationships with Concert Pharm and BVF Partners. Baylor College of Medicine (BCM) and Miraca Holdings have formed a joint venture with shared ownership and governance of Baylor Genetics (BG), which performs clinical genomics studies including chromosomal microarray analysis and clinical exome sequencing. J.R.L. serves on the Scientific Advisory Board of the BG. J.R.L. has stock ownership in 23andMe, is a paid consultant for Regeneron Pharmaceuticals, has stock options in Lasergen, Inc. and is a co-inventor on multiple United States and European patents related to molecular diagnostics for inherited neuropathies, eye diseases, and bacterial genomic fingerprinting. The other authors report no biomedical financial interests or potential conflicts of interest.
It is likely that the 40.7% difference in severity of symptoms between the DCS-placebo conditions in 3363 exaggerates the clinical worsening associated with the placebo condition, which was confounded by the persisting impact of the unexpected death of his father. Although the study was suspended for six weeks and 3363 had returned to near his original baseline before the study was resumed, further worsening in this context is not surprising. This interpretation is strengthened by the fact that the reduction in severity of clinical symptoms during the initial open-label trial and the first arm of the double-blind, prior to this unexpected life event, was in the range of ~ 32-37%, and was in the 27-35% range for most of the chronic open-label phase, suggesting a notable reduction in symptom severity while on DCS. Similarly, the magnitude of the DCS-placebo difference in 5459 may have been attenuated by the effect of the personal loss prior to exposure to DCS during the second arm of the double-blind where the reduction in total BPRS score ranged from 12-24%, compared to the various open-label conditions when symptom reduction generally ranged from 21-35%.
De novo structural variants have been linked to sporadic psychiatric illness in some studies, (110, 111) but not in others. (41, 112) The extended family of these carriers has a history of psychotic disorders in earlier generations (Figure S9A). If some variant(s) within the 9p24.1 complex rearrangement was causal in the case of our two carriers, different genetic risk factors were likely present in cases in earlier generations.
The elevated baseline KYN and KYNA levels implicate increased activity of indoleamine 2,3-dioxygenase/tryptophan 2,3-dioxygenase and kynurenine aminotransferase (KAT-II). Normalizing KYNA through KAT-II inhibition (113, 114) has been shown to enhance cognition. (115, 116) Such a strategy alone or in combination with partial agonism of the α7nAChR (117) may have had greater benefit on cognition in our subjects.
The reason is that some of CLZ's neurochemical actions affect synaptic glycine levels, either by inhibiting GlyT1 activity (118) or by inhibiting System A mediated transport of glycine and other amino acids.(124) Consistent with these mechanisms for neutralizing the effects of NMDAR modulators, CLZ-induced increase in extracellular D-serine leads to subsequent NMDAR-dependent release of L-glutamate in rats. (119) This is likely the reason that augmentation with NMDAR modulators tends to show clinical efficacy primarily in schizophrenia patients taking antipsychotics other than CLZ, (60, 61, 120, 121) and often not in patients taking CLZ. (82, 122-125) However, no clinical benefit (67) or an equivocal benefit (61) of DCS has also been observed in patients not taking CLZ, small groups of CLZ- and non-CLZ treated patients experienced similar clinical benefit from glycine, (126) and CLZ-resistant patients experienced significant improvement in negative and overall symptom severity with sodium benzoate augmentation of CLZ. (127) Two studies even reported statistically significant (but clinically modest) worsening of negative symptoms with DCS and CLZ (62, 124) compared with CLZ alone. Notably, clinical benefit and worsening with DCS and glycine have been almost entirely limited to negative symptoms (82, 83, 128) (except for enhancing the effect of cognitive behavioral treatment of delusions), (68) whereas the effects in our subjects were primarily on positive symptoms. The most parsimonious explanation for this pattern of findings in non-genotyped individuals is that schizophrenia patients are heterogeneous with respect to NMDAR hypofunction. In the subgroup of patients with NMDAR hypofunction, augmentation is most likely to provide clinical benefit in those patients whose NMDAR function has not been normalized (i.e., non-CLZ antipsychotic medication); in this same subgroup CLZ may generally be sufficient to normalize NMDAR function, resulting in no further benefit from NMDAR modulators. When CLZ is not sufficient to normalize NMDAR function, clinical benefit may occur. In the subgroup of patients who do not have NMDAR hypofunction, augmentation with NMDAR modulators would not be expected to have a clinical benefit.
Two early studies of glycine augmentation in treatment-resistant chronic schizophrenia, one involving seven patients and the other involving nine patients, reported that the significant improvement in negative symptoms observed during six weeks of treatment with glycine was sustained for the next eight weeks (two-week washout and six weeks of placebo). (59, 126) A third study by the same group did not report a significant carryover effect in seven patients. (60) The persisting effects of glycine on positive symptoms that we observed, however, did not last longer than two weeks (Figure 2), but may have reduced the magnitude of the difference in symptom severity between on vs off glycine assessments. Given the half-life of glycine (26-245 minutes depending on dose), (58) a two-week washout would generally be expected to be long enough to eliminate persisting effects of the drug.
References:
- 1.Rees E, Walters JTR, Georgieva L, Isles AR, Chambert KD, Richards AL, et al. (2014b): Analysis of copy number variations at 15 schizophrenia-associated loci. Br J Psychiatry. 204:108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malhotra D, Sebat J (2012): CNVs: Harbinger of a rare variant revolution in psychiatric genetics. Cell. 148:1223–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marshall CR, Howrigan DP, Merico D, Thiruvahindrapuram B, Wu W, Greer DS, et al. (2017): Contribution of copy number variants to schizophrenia from a genome-wide study of 41,321 subjects. Nature genetics. 49:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.International Schizophrenia Consortium (2008): Rare chromosomal deletions and duplications increase risk of schizophrenia. Nature. 455:237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stefansson H, Rujescu D, Cichon S, Pietilainen OPH, Ingason A, Steinberg S, et al. (2008): Large recurrent microdeletions associated with schizophrenia. Nature. 455:232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCarthy S, Makarov V, Kirov G, Addington A, McClellan J, Yoon S, et al. (2009): Microduplications of 16p11.2 are associated with schizophrenia. Nature genetics. 41:1223–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cantor RM, Geschwind DH (2008): Schizophrenia: Genome, interrupted. Neuron. 58:165–167. [DOI] [PubMed] [Google Scholar]
- 8.Weiss LA, Shen Y, Korn JM, Arking DE, Miller DT, Fossdal R, et al. (2008): Association between microdeletion and microduplication at 16p11.2 and autism. N Engl J Med. 358:667–675. [DOI] [PubMed] [Google Scholar]
- 9.Mefford HC, Batshaw ML, Hoffman EP (2012): Genomics, intellectual disability, and autism. N Engl J Med. 366:733–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heinzen EL, Neale BM, Traynelis SF, Allen AS, Goldstein DB (2015): The genetics of neuropsychiatric diseases: Looking in and beyond the exome. Ann Rev Neurosci. 38:47–68. [DOI] [PubMed] [Google Scholar]
- 11.Kirov G, Rees E, Walters JTR, Escott-Price V, Georgieva L, Richards AL, et al. (2014): The penetrance of copy number variations for shizophrenia and developmental delay. Biol Psychiatry. 75:378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirov G (2015): CNVs in neuropsychiatric disorders. Hum Mol Genet. 24:R45–R49. [DOI] [PubMed] [Google Scholar]
- 13.Sullivan PF, Daly MJ, O'Donovan M (2012): Genetic architectures of psychiatric disorders: The emerging picture and its implications. Nat Rev Genet. 13:537–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geschwind DH, Flint J (2015): Genetics and genomics of psychiatric disease. Science. 349:1489–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schizophrenia Working Group of the Psychiatric Genomics Consortium (2014): Biological insights from 108 schizophrenia-associated genetic loci. Nature. 511:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanders SJ, He X, Willsey AJ, Ercan-Sencicek AG, Samocha KE, Cicek AE, et al. (2015): Insights into autism spectrum disorder genomic architecture and biology from 71 risk loci. Neuron. 87:1215–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vacic V, McCarthy S, Malhotra D, Murray F, Chou H-H, Peoples A, et al. (2011): Duplications of the neuropeptide receptor gene VIPR2 confer significant risk for schizophrenia. Nature. 471:499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brunetti-Pierri N, Berg JS, Scaglia F, Belmont J, Bacino CA, Sahoo T, et al. (2008): Recurrent reciprocal 1q21.1 deletions and duplications associated with microcephaly or macrocephaly and developmental and behavioral abnormalities. Nature genetics. 40:1466–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mefford H, Sharp AJ, Baker C, Itsara A, Jiang Z, Buysse K, et al. (2008): Recurrent rearrangements of chromosome 1q21.1 and variable pediatric phenotypes. New England J Med. 359:1685–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levinson DF, Duan J, Oh S, Wang K, Sanders AR, Shi J, et al. (2011): Copy number variants in schizophrenia: Confirmation of five previous findings and new evidence for 3q29 microdeletions and VIPR2 duplications. Am J Psychiatry. 168:302–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shinawi M, Schaaf CP, Bhatt SS, Xia Z, Patel A, Cheung SW, et al. (2009): A small recurrent deletion within 15q13.3 is associated with a range of neurodevelopmental phenotypes. Nature genetics. 41:1269–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carvalho CMB, Lupski JR (2016): Mechanisms underlying structural variant formation in genomic disorders. Nat Rev Genet. 17:224–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The Network and Pathway Analysis Subgroup of the Psychiatric Genomics Consortium (2015): Psychiatric genome-wide association study analyses implicate neuronal, immune and histone pathways. Nat Neurosci. 18:199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parikshak NN, Gandal MJ, Geschwind DH (2015): Systems biology and gene networks in neurodevelopmental and neurodegenerative disorders. Nat Rev Genet. 16:441–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pocklington AJ, Rees E, Walters JTR, Han J, Kavanagh DH, Chambert KD, et al. (2015): Novel findings from CNVs implicate inhibitory and excitatory signaling complexes in schizophrenia. Neuron. 86:1203–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harrison PJ (2015): Recent genetic findings in schizophrenia and their therapeutic relevance. J Psychopharmacol. 29:85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stessman HA, Bernier R, Eichler EE (2014): A genotype-first approach to defining the subtypes of a complex disease. Cell. 156:872–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White J, Beck CR, Harel T, Posey JE, Jhangiani SN, Tang S, et al. (2016): POGZ truncating alleles cause syndromic intellectual disability. Genome Medicine. 8:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Treadwell-Deering DE, Powell MP, Potocki L (2010): Cognitive and behavioral characterization of the Potocki-Lupski syndrome (duplication 17p11.2). J Dev Behav Pediatr 31:137–143. [DOI] [PubMed] [Google Scholar]
- 30.Berg JS, Brunetti-Pierri N, Peters SU, Kang S-HL, Fong C, Salamone J, et al. (2007): Speech delay and autism spectrum behaviors are frequently associated with duplication of the 7q11.23 Williams-Beuren syndrome region. Genet Med 9:427–441. [DOI] [PubMed] [Google Scholar]
- 31.Ben-Shachar S, Lanpher B, German JR, Qasaymeh M, Potocki L, Nagamani SCS, et al. (2009): Microdeletion 15q13.3: A locus with incomplete penetrance for autism, mental retardation, and psychiatric disorders. J Med Genet 46:382–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Männik K, Mägi R, Macé A, Cole B, Guyatt AL, Shihab HA, et al. (2015): Copy number variations and cognitive phenotypes in unselected populations. JAMA. 313:2044–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lupski JR (2016): Clinical genomics: From a truly personal genome viewpoint. Hum Genet. 135:591–601. [DOI] [PubMed] [Google Scholar]
- 34.Scolnick EM (2010): 3rd Annual Report: Executive Summary Version. http://broadharvardedu/files/shared/psych/_Center_Annual_ReportvExecSum_-2009_for_webpdf.
- 35.Bainbridge MN, Wiszniewski W, Murdock DR, Friedman J, Gonzaga-Jauregui C, Newsham I, et al. (2011): Whole-genome sequencing for optimized patient management. Sci Translational Med. 3:87re83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Worthey EA, Mayer AN, Syverson GD, Helbling D, Bonacci BB, Decker B, et al. (2011): Making a definitive diagnosis: Successful clinical application of whole exome sequencing in a child with intractable inflammatory bowel disease. Genetics in Medicine. 13:255–262. [DOI] [PubMed] [Google Scholar]
- 37.Daly AK, Donaldson PT, Bhatnagar P, Shen Y, Pe'er I, Floratos A, et al. (2009): HLA-B*5701 genotype is a major determinant of drug-induced liver injury due to flucloxacillin. Nature genetics. 41:816–819. [DOI] [PubMed] [Google Scholar]
- 38.Loi S, Haibe-Kains B, Majjaj S, Lallemand F, Durbecq V, Larsimont D, et al. (2010): PIK3CA mutations associated with gene signature of low mTORC1 signaling and better outcomes in estrogen receptor-positive breast cancer. Proc Nat'l Acad Sci. 107:10208–10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nathwani AC, Tuddenham EGD, Rangarajan S, Rosales C, McIntosh J, Linch DC, et al. (2011): Adenovirus-associated virus vector– mediated gene transfer in Hemophilia B. N Engl J Med. 365:2357–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.American Psychiatric Association (1994): Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). Washington, DC: American Psychiatric Association. [Google Scholar]
- 41.Malhotra D, McCarthy S, Michaelson JJ, Vacic V, Burdick KE, Yoon S, et al. (2011): High frequencies of de novo CNVs in bipolar disorder and schizophrenia. Neuron. 72:951–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grochowski CM, Gu S, Yuan B, TCW J, Brennand KJ, Sebat J, et al. (2018): Marker chromosome genomic structure and temporal origin implicate a chromoanasynthesis event in a family with pleiotropic psychiatric phenotypes. Human Mutation. 39:939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mothet JP, Le Bail M, Billard JM (2015): Time and space profiling of NMDA receptor co-agonist functions. J Neurochem. 135:210–225. [DOI] [PubMed] [Google Scholar]
- 44.Olney JW, Farber NB (1995): Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry. 52:998–1007. [DOI] [PubMed] [Google Scholar]
- 45.Coyle JT (2006): Glutamate and schizophrenia: Beyond the dopamine hypothesis. Cell Mol Neurobiol. 26:365–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krystal JH, Anand A, Moghaddam B (2002): Effects of NMDA receptor antagonists: Implications for the pathophysiology of schizophrenia. Arch Gen Psychiatry. 59:663–664. [DOI] [PubMed] [Google Scholar]
- 47.Goff DC, Coyle JT (2001): The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psychiatry. 158:1367–1377. [DOI] [PubMed] [Google Scholar]
- 48.Henderson G, Johnson JW, Ascher P (1990): Competitive antagonists and partial agonists at the glycine modulatory site of the mouse N-methyl-D aspartate receptor. J Physiol. 430:189–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watson GB, Bolanowski MA, Baganoff MP, Deppeler CL, Lanthorn TH (1990): D-cycloserine acts as a partial agonist at the glycine modulatory site of the NMDA receptor expressed in Xenopus oocytes. Brain Res. 510:158–160. [DOI] [PubMed] [Google Scholar]
- 50.Overall J, Gorham D (1962): The Brief Psychiatric Rating Scale. Psychol Rep. 10:799–812. [Google Scholar]
- 51.Kay SR, Fiszbein A, Opler LA (1987): The positive and negative syndrome scale (PANSS) for schizophrenia. 13:261–276. [DOI] [PubMed] [Google Scholar]
- 52.Guy W (1976): Clinical global impression (CGI) Scale In: Guy W, editor. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: US Department of Health, Education, and Welfare, pp 125–126. [Google Scholar]
- 53.Hamilton M (1960): A rating scale for depression. J Neurol Neurosurg Psychiatry. 23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Young RC, Biggs JT, Ziegler VE, Meyer DA (1978): A rating scale for mania: Reliability, validity and sensitivity. Br J Psychiatry. 133:429–435. [DOI] [PubMed] [Google Scholar]
- 55.Posner K, Brent D, Lucas C, Gould M, Stanley B, Brown G, et al. (2009): Columbia-Suicide Severity Rating Scale (C-SSRS). New York, New York: The Research Foundation for Mental Hygiene, Inc. [Google Scholar]
- 56.Simpson GM, Angus JW (1970): A rating scale for extrapyramidal side effects. Acta Psychiatr Scand. 212 (Suppl 44): 11–19. [DOI] [PubMed] [Google Scholar]
- 57.Guy W (1976): Abnormal involuntary movement scale (AIMS) In: Guy W, editor. ECDEU assessment Manual for Psychopharmacology: Revised. Rockville, MD: US Department of Health, Education and Welfare, pp 118–119. [Google Scholar]
- 58.Hahn RG (1993): Dose-dependent half-life of glycine. Urol Res. 21:289–291. [DOI] [PubMed] [Google Scholar]
- 59.Heresco-Levy U, Javitt DC, Ermilov M, Mordel C, Silipo G, Lichtenstein M (1999): Efficacy of high-dose glycine in the treatment of enduring negative symptoms of schizophrenia. Arch Gen Psychiatry. 56:29–36. [DOI] [PubMed] [Google Scholar]
- 60.Heresco-Levy U, Ermilov M, Lichtenberg P, Bar G, Javitt DC (2004): High-dose glycine added to olanzapine and risperidone for the treatment of schizophrenia. Biol Psychiatry. 55:165–171. [DOI] [PubMed] [Google Scholar]
- 61.Goff DC, Tsai G, Levitt J, Amico E, Manoach D, Schoenfeld D, et al. (1999b): A placebo-controlled trial of D-cycloserine added to conventional neuroleptics in patients with schizophrenia. Arch Gen Psychiatry. 56:21–27. [DOI] [PubMed] [Google Scholar]
- 62.Goff DC, Tsai G, Manoach DS, Flood J, Darby DG, Coyle JT (1996): D-cycloserine added to clozapine for patients with schizophrenia. Am J Psychiatry. 153:1628–1630. [DOI] [PubMed] [Google Scholar]
- 63.Hanngren H, Hansson E, Ullberg S (1961): An autoradiographic study of the distribution of tritium-labeled cycloserine in mice. Antibiot Chemother. 12:46–54. [PubMed] [Google Scholar]
- 64.Hood WF, Compton RP, Monahan JB (1989): D-cycloserine: a ligand for the N-methyl-D-aspartate coupled glycine receptor has partial agonist characteristics. Neurosci Lett. 98:91–95. [DOI] [PubMed] [Google Scholar]
- 65.Emmett MR, Mick SJ, Cker JA, Rao TS, Iyengar S, Wood PL (1991): Actions of D-cycloserine at the N-methyl-d-aspartate-associated glycine receptor site in vivo. Neuropharmacol. 30:1167–1171. [DOI] [PubMed] [Google Scholar]
- 66.Chessell IP, Procter AW, Francis PT, Bowen DM (1991): D-Cycloserine, a putative cognitive enhancer, facilitates activation of the /V-methyI-D-aspartate receptor-ionophore complex in Alzheimer brain. Brain Res. 565:345–348. [DOI] [PubMed] [Google Scholar]
- 67.Goff DC, Herz L, Posever T, Shih V, Tsai G, Henderson D, et al. (2005): A six-month, placebo-controlled trial of D-cycloserine co-administered with conventional antipsychotics in schizophrenia patients. Psychopharmacol (Berl). 179:144–150. [DOI] [PubMed] [Google Scholar]
- 68.Gottlieb JD, Cather C, Shanahan M, Creedon T, Macklin EA, Goff DC (2011): D-cycloserine facilitation of cognitive behavioral therapy for delusions in schizophrenia. Schiz Res. 131:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Edgington ES, Onghena P (2007): Randomization Tests fourth ed. London: Chapman & Hall. [Google Scholar]
- 70.Cook SP, Galve-Roperh I, Martínez del Pozo A, Rodriguez-Crespo I (2002): Direct calcium binding results in activation of brain serine racemase. J Biol Chem. 277:27782–27792. [DOI] [PubMed] [Google Scholar]
- 71.Freudenreich O, Goff DC (2002): Antipsychotic combination therapy in schizophrenia. A review of efficacy and risks of current combinations. Acta Psychiatr Scand. 106:323–330. [DOI] [PubMed] [Google Scholar]
- 72.Cooper GM, Shendure J (2011): Needles in stacks of needles: Finding disease-causal variants in a wealth of genomic data. Nat Rev Genet. 12:628–640. [DOI] [PubMed] [Google Scholar]
- 73.Buchanan JA, Scherer SW (2008): Contemplating effects of genomic structural variation. Genetics in Medicine. 10:639–647. [DOI] [PubMed] [Google Scholar]
- 74.Perakslis ED, Kohane IS (2016): Treating the enigmatic "exceptional responders" as patients with undiagnosed diseases. Sci Transl Med. 8:340ed348. [DOI] [PubMed] [Google Scholar]
- 75.Goff DC (2014): Bitopertin. The good news and bad news. JAMA Psychiatry. 71:621–622. [DOI] [PubMed] [Google Scholar]
- 76.Chau NG, Lorch JH (2015): Exceptional responders inspire change: Lessons for drug development from the bedside to the bench and back. Oncologist. 20:699–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Iyer G, Hanrahan AJ, Milowsky MI, Al-Ahmadie H, Scott SN, Janakiraman M, et al. (2012): Genome sequencing identifies a basis for everolimus sensitivity. Science. 338:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Petrovski S, Wang Q, Heinzen EL, Allen AS, Goldstein DB (2013): Genic intolerance to functional variation and the interpretation of personal genomes. PLoS Genet. 9:e1003709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu P, Gelowani V, Zhang F, Drory VE, Ben-Shachar S, Roney E, et al. (2014): Mechanism, prevalence, and more severe neuropathy phenotype of the Charcot-Marie-Tooth type 1A triplication. Am J Hum Genet. 94:462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ramocki MB, Peters SU, Tavyev YJ, Zhang F, Carvalho CMB, Schaaf CP, et al. (2009): Autism and other neuropsychiatric symptoms are prevalent in individuals with MECP2 duplication syndrome. Ann Neurol. 66:771–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Carvalho CMB, Ramocki MB, Pehliva D, Franco LM, Gonzaga-Jauregui C, Fang P, et al. (2011): Inverted genomic segments and complex triplication rearrangements are mediated by inverted repeats in the human genome. Nature genetics. 43:1074–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tsai GE, Lin PY (2010): Strategies to enhance N-methyl-D-aspartate receptor-mediated neurotransmission in schizophrenia. A critical review and meta-analysis. Curr Pharm Des. 16:522–537. [DOI] [PubMed] [Google Scholar]
- 83.Singh SP, Singh V (2011): Meta-analysis of the efficacy of adjunctive NMDA receptor modulators in chronic schizophrenia. CNS Drugs. 25:859–885. [DOI] [PubMed] [Google Scholar]
- 84.Kirov G, Pocklington AJ, Holmans P, Ivanov D, Ikeda M, Ruderfer D, et al. (2012): De novo CNV analysis implicates specific abnormalities of postsynaptic signalling complexes in the pathogenesis of schizophrenia. Mol Psychiatry. 17:142–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Raychaudhuri S, Plenge RM, Rossin EJ, Ng ACY, International Schizophrenia Consortium, Purcell SM, et al. (2009): Identifying relationships among genomic disease regions: Predicting genes at pathogenic SNP associations and rare deletions. PLoS Genetics. 5:e1000534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hamdan FF, Gauthier J, Araki Y, Lin D-T, Yoshizawa Y, Higashi K, et al. (2011): Excess of de novo deleterious mutations in genes associated with glutamatergic systems in nonsyndromic intellectual disability. Am J Hum Genet. 88:306–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Elia J, Gai X, Xie HM, Perin JC, Geiger E, Glessner JT, et al. (2010): Rare structural variants found in attention-deficit hyperactivity disorder are preferentially associated with neurodevelopmental genes. Mol Psychiatry. 15:637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yu Y, Lin Y, Takasaki Y, Wang C, Kimura H, Xing J, et al. (2018): Rare loss of function mutations in Nmethyl-D-aspartate glutamate receptors and their contributions to schizophrenia susceptibility. Transl Psychiatry. 8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chang X, Lima LdA, Liu Y, Li J, Li Q, Sleiman PMA, et al. (2018): Common and Rare Genetic Risk Factors Converge in Protein Interaction Networks Underlying Schizophrenia. Frontiers in Genetics. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kathiresan S (2015): Developing medicines that mimic the natural successes of the human genome: lessons from NPC1L1, HMGCR, PCSK9, APOC3, and CETP. Journal of the American College of Cardiology. 65:1562–1566. [DOI] [PubMed] [Google Scholar]
- 91.Farrell M, Lichtenstein M, Crowley JJ, Filmyer DM, Lázaro-Muñoz G, Shaughnessy RA, et al. (2018): Developmental delay, treatment-resistant psychosis, and early-onset dementia in a man with 22q11 deletion syndrome and Huntington’s Disease. Am J Psychiatry. 175:400–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alexandre C, Chaumette B, Martinez G, Christa L, Dupont J-M, Kebir O, et al. (2016): Paradoxical improvement of schizophrenic symptoms by a dopaminergic agonist: An example of personalized psychiatry in a CNV carrying patient. Biol Psychiatry. 80:e21–23. [DOI] [PubMed] [Google Scholar]
- 93.Sakata Y, Owada Y, Sato K, Kojima K, Hisanaga K, Shinka T, et al. (2001): Structure and expression of the glycine cleavage system in rat central nervous system. Brain Res Mol Brain Res. 94:119–130. [DOI] [PubMed] [Google Scholar]
- 94.Wolosker H, Balu DT, Coyle JT (2016): The rise and fall of the D-serine-mediated gliotransmission hypothesis. Trends in Neurosci. 39:712–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kessler M, Terramani T, Lynch G, Baudry M (1989): A glycine site associated with N-methyl-D-aspartic acid receptors: Characterization and identification of a new class of antagonists. J Neurochem. 52:1319–1328. [DOI] [PubMed] [Google Scholar]
- 96.Parsons CG, Danysz W, Quack G, Hartmann S, Lorenz B, Wollenburg C, et al. (1997): Novel systemically active antagonists of the glycine site of the N-methyl-D-aspartate receptor: Electrophysiological, biochemical and behavioral characterization. J Pharmacol Exp Ther. 283:1264–1275. [PubMed] [Google Scholar]
- 97.Birch PJ, Grossman CJ, Hayes AG (1988): Kynurenic acid antagonises responses to NMDA via an action at the strychnine-insensitive glycine receptor. Eur J Pharmacol. 154:85–87. [DOI] [PubMed] [Google Scholar]
- 98.Girirajan S, Brkanac Z, Coe BP, Baker C, Vives L, Vu TH, et al. (2011): Relative burden of large CNVs on a range of neurodevelopmental phenotypes. PLoS Genet. 7:e1002334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.O'Roak BJ, Deriziotis P, Lee C, Vives L, Schwartz JJ, Girirajan S, et al. (2011): Exome sequencing in sporadic autism spectrum disorders identifies severe de novo mutations. Nature genetics. 43:585–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dravid SM, Burger PB, Prakash A, Geballe MT, Yadav R, Le P, et al. (2010): Structural determinants of D-cycloserine efficacy at the NR1/NR2C NMDA receptors. J Neurosci. 30:2741–2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Baran H, Kepplinger B (2014): D-cycloserine lowers kynurenic acid formation - New mechanism of action. Eur Neuropsychopharmacol. 24:639–644. [DOI] [PubMed] [Google Scholar]
- 102.Lopes C, Pereira EF, Wu HQ, Purushottamachar P, Njar V, Schwarcz R, et al. (2007): Competitive antagonism between the nicotinic allosteric potentiating ligand galantamine and kynurenic acid at alpha7* nicotinic receptors. J Pharmacol Exp Ther. 322:48–58. [DOI] [PubMed] [Google Scholar]
- 103.Hilmas C, Pereira EF, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX (2001): The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: Physiopathological implications. J Neurosci. 21:7463–7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wu HQ, Pereira EF, Bruno JP, Pellicciari R, Albuquerque EX, Schwarcz R (2010): The astrocyte-derived alpha7 nicotinic receptor antagonist kynurenic acid controls extracellular glutamate levels in the prefrontal cortex. J Mol Neurosci. 40:204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Albuquerque EX, Schwarcz R (2013): Kynurenic acid as an antagonist of a7 nicotinic acetylcholine receptors in the brain:Facts and challenges. Biochem Pharmacol. 85:1027–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Freedman R, Coon H, Myles-Worsley M, Orr-Urtreger A, Olincy A, Davis A, et al. (1997): Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Natl Acad Sci USA. 94:587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xu J, Pato M, Torre C, Medeiros H, Carvalho C, Basile V, et al. (2001): Evidence of linkage disequilibrium between the alpha 7-nicotinic receptor gene (CHRNA7) locus and schizophrenia in Azorean families. Am J Med Genet. 105:669–674. [DOI] [PubMed] [Google Scholar]
- 108.Albuquerque EX, Pereira EF, Alkondon M, S.W. R (2009): Mammalian nicotinic acetylcholine receptors: From structure to function. Physiol Rev. 89:73–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.MacDonald JF, Jackson MF, Beazely MA (2006): Hippocampal long-term synaptic plasticity and signal amplification of NMDA receptors. Crit Rev Neurobiol. 18:71–84. [DOI] [PubMed] [Google Scholar]
- 110.Xu B, Roos JW, Levy S, van Rensburg EJ, Gogos JA, Karayiorgou M (2008): Strong association of de novo copy number mutations with sporadic schizophrenia. Nature genetics. 40:880–885. [DOI] [PubMed] [Google Scholar]
- 111.Xu B, Woodroffe A, Rodriguez-Murillo L, Roos JL, van Rensburg EJ, Abecasis GR, et al. (2009): Elucidating the genetic architecture of familial schizophrenia using rare copy number variant and linkage scans. Proc Nat'l Acad Sci. 106:16746–16751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Georgieva L, Rees E, Moran JL, Chambert KD, Milanova V, Craddock N, et al. (2014): De novo CNVs in bipolar affective disorder and schizophrenia. Hum Mol Genet. 23:6677–6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schwarcz R, Bruno JP, Muchowski PJ, Wu H-Q (2012): Kynurenines in the mammalian brain: When physiology meets pathology. Nat Rev Neurosci. 13:465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Linderholm KR, Alm MT, Larsson MK, Olsson SK, Goiny M, Hajos M, et al. (2016): Inhibition of kynurenine aminotransferase II reduces activity of midbrain dopamine neurons. Neuropharmacol. 102:42–47. [DOI] [PubMed] [Google Scholar]
- 115.Potter MC, Elmer GI, Bergeron R, Albuquerque EX, Guidetti P, Wu HQ, et al. (2010): Reduction of endogenous kynurenic acid formation enhances extracellular glutamate, hippocampal plasticity, and cognitive behavior. Neuropsychopharmacol. 35:1734–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kozak R, Campbell BM, Strick CA, Horner W, Hoffmann WE, Kiss T, et al. (2014): Reduction of brain kynurenic acid improves cognitive function. J Neurosci. 34:10592–10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Alexander KS, Wu H-Q, Schwarcz R, Bruno JP (2012): Acute elevations of brain kynurenic acid impair cognitive flexibility: normalization by the alpha 7 positive modulator galantamine. Psychopharmcology (Berl). 220:627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Williams JB, Mallorga PJ, Conn PJ, Pettibone DJ, Sur C (2004): Effects of typical and atypical antipsychotics on human glycine transporters. Schiz Res. 71:103–112. [DOI] [PubMed] [Google Scholar]
- 119.Tanahashi S, Yamamura S, Nakagawa M, Motomura E, Okada M (2012): Clozapine, but not haloperidol, enhances glial D-serine and L-glutamate release in rat frontal cortex and primary cultured astrocytes. Br J Pharmacol. 165:1543–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Evins AE, Amico E, Posever TA, Toker R, Goff DC (2002): D-Cycloserine added to risperidone in patients with primary negative symptoms of schizophrenia. Schiz Res. 56:19–23. [DOI] [PubMed] [Google Scholar]
- 121.Heresco-Levy U, Ermilov M, Shimoni J, Shapira B, Silipo G, Javitt DC (2002): Placebo-controlled trial of D-cycloserine added to convential neuroleptics, olanzapine, or risperidone in schizophrenia. Am J Psychiatry. 159:480–482. [DOI] [PubMed] [Google Scholar]
- 122.Potkin SG, Jin Y, Bunney BG, Costa J, Gulasekaram B (1999): Effect of clozapine and adjunctive high-dose glycine in treatment-resistant schizophrenia. Am J Psychiatry. 156:145–147. [DOI] [PubMed] [Google Scholar]
- 123.Evins AE, Fitzgerald SM, Wine L, Rosselli R, Goff DC (2000): Placebo-controlled trial of glycine added to clozapine in schizophrenia. Am J Psychiatry. 157:826–828. [DOI] [PubMed] [Google Scholar]
- 124.Goff DC, Henderson DC, Evins AE, Amico E (1999): A placebo-controlled crossover trial of D-cycloserine added to clozapine in patients with schizophrenia. Biol Psychiatry. 45:512–514. [DOI] [PubMed] [Google Scholar]
- 125.Tsai G, Yang P, Chung LC, Tsai CW, Coyle JT (1999): D-serine added to clozapine for the treatment of schizophrenia. Am J Psychiatry. 156:1822–1825. [DOI] [PubMed] [Google Scholar]
- 126.Heresco-Levy U, Javitt DC, Ermilov M, Mordel C, Horowitz A, Kelly D (1996): Double-blind, placebo-controlled, crossover trial of glycine adjuvant therapy for treatment-resistant schizophrenia. Br J Psychiatry. 169:610–617. [DOI] [PubMed] [Google Scholar]
- 127.Lin C-H, Lin C-H, Chang Y-C, Huang Y-J, Chen P-W, Yang H-T, et al. (2018): Sodium benzoate, a D-amino acid oxidase inhibitor, added to clozapine for the treatment of schizophrenia: A randomized, double-blind, placebo-controlled trial. Biol Psychiatry. 84:422–432. [DOI] [PubMed] [Google Scholar]
- 128.Goff DC (2017): D-cycloserine in schizophrenia: New strategies for improving clinical outcomes by enhancing plasticity. Curr Neuropharmacology. 15:21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.