Abstract
Zika and the four serotypes of dengue are closely related flaviviruses that share a high degree of structural and sequence homology and co-circulate in many regions of the world. Here, we review recent studies investigating antigenic cross-reactivity between the two viruses. We discuss the pathogenic and protective roles of cross-reactive anti-viral antibody and T cell responses, respectively, in modulating the outcome of secondary dengue or Zika infection. Based on recent findings and increased incidence of severe disease in seronegative recipients of the first dengue vaccine to be licensed, we propose that the time has come to focus on developing pan-flavivirus vaccines that protect against Zika and four dengue serotypes by eliciting protective cross-reactive T cell responses while concomitantly reducing production of cross-reactive antibodies that can exacerbate disease.
Introduction
Zika virus (a single serotype, ZIKV) and dengue virus (four serotypes, DENV1–4) are mosquito-transmitted members of the Flaviviridae family, Flavivirus genus that includes West Nile, Japanese encephalitis, and yellow fever viruses. Flaviviruses contain a positive-sense single-stranded RNA genome of ~11 kb that encodes three structural proteins (capsid [C], premembrane [prM], and envelope [E]) and seven nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5). ZIKV was first isolated from a sentinel rhesus monkey in Uganda in 1947, but few human infections were recorded until 2013–2014, when a large outbreak occurred in French Polynesia that affected about 28,000 patients [1]. ZIKV then spread to South America during 2014–2016 [2], and it had been reported in at least 84 countries and territories worldwide by 2017 [3]. In comparison, hundreds of millions of cases of DENV infection have been recorded annually worldwide since it was first described in Egypt and Indonesia in 1779 [4]. At present, an estimated 390 million DENV infections occur globally each year, and the virus is endemic in at least 139 countries [5]. DENV thus continues to present a significant global health problem.
While DENV and ZIKV are both spread by mosquito bites, ZIKV can also be transmitted vertically (mother-to-fetus) and by sexual contact [6]. Most ZIKV and DENV infections are asymptomatic, but both viruses can cause mild disease, characterized by fever, rash, and muscle and joint pain [6], as well as more severe life-threatening disease. ZIKV infection during pregnancy is linked to serious fetal defects, termed congenital Zika syndrome [7], and in adults, viral infection is associated with autoimmune disorders such as Guillain–Barré syndrome and immune thrombocytopenic purpura [8]. In the case of DENV infection, the most serious clinical manifestation is “severe dengue” (formerly known as dengue hemorrhagic fever/dengue shock syndrome), the hallmark of which is plasma leakage that may progress to hypovolemic shock and death [9].
Antigenic cross-reactivity between ZIKV and DENV
DENV exists as four serotypes that differ substantially at the protein level and are antigenically distinct. Since immunity to one serotype does not fully protect against infection with a different serotype, the patient remains vulnerable to heterotypic reinfections. Paradoxically, pre-existing DENV immunity is actually the single greatest risk factor for developing severe dengue (see below) [9]. ZIKV, which exists as a single serotype [10], also shares high protein sequence homology with DENV. Thus, the emergence of ZIKV in DENV-endemic regions has raised the critical question of how the interplay between ZIKV and DENV immunity influences the clinical outcomes of sequential infection with heterologous viruses/serotypes. The principal target of the antibody (Ab) response to DENV and ZIKV is the structural E protein, which is 60–75% identical among the four DENV serotypes and 54–59% identical between ZIKV and the four DENV serotypes at the amino acid level [9,11]. In contrast, the T cell response to DENV and ZIKV is targeted mainly to nonstructural proteins, many of which are more conserved than structural proteins among flaviviruses (Table 1). In humans and in mouse models, CD8+ T cell responses to DENV are directed mainly to NS3, NS4B, and NS5 [12–15], whereas the CD4+ T cell response targets NS3, NS5, and the structural C protein [16–21]. In comparison, CD4+ and CD8+ T cell responses to ZIKV appear to target both structural proteins (E, C, and prM) and nonstructural proteins (NS1, NS2A, NS3, NS4B, and NS5 [22–26].
Table 1.
Amino acid homology between DENV and ZIKV proteins
| Identity vs. ZIKVa |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | prM/M | E | NS1 | NS2A | NS2B | NS3 | NS4A | NS4B | NS5 | |
| ZIKV | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% |
| DENV1 | 43% | 45% | 58% | 53% | 18% | 36% | 66% | 42% | 56% | 67% |
| DENV2 | 40% | 42% | 54% | 52% | 29% | 43% | 67% | 47% | 57% | 68% |
| DENV3 | 40% | 43% | 59% | 55% | 29% | 42% | 67% | 40% | 58% | 68% |
| DENV4 | 33% | 48% | 57% | 53% | 18% | 41% | 67% | 39% | 56% | 69% |
The percent identities were determined using BLAST search.
Collectively, these observations have prompted the crucial question: how does pre-existing DENV immunity modulate the immune response to and clinical outcome of a subsequent ZIKV infection, and vice versa? As illustrated by the following discussion, this is currently an area of intense investigation.
Pre-existing cross-reactive Abs to DENV enhance DENV and ZIKV pathogenesis
Epidemiological studies have long established that secondary DENV infection (in children or adults) and waning levels of maternal Abs in infants with primary DENV infection are risk factors for the development of severe dengue disease [27]. Ab-dependent enhancement of infection (ADE) is one of the two major hypotheses proposed to explain these epidemiologic observations. ADE occurs when a patient with pre-existing serotype-cross-reactive nonneutralizing or subneutralizing Abs is reinfected with DENV. Binding of the pre-existing Abs to the virus facilitates its infection of Fcγ receptor-bearing cells, and the increased viral burden then triggers severe dengue disease manifestations [9]. Direct evidence for the ADE hypothesis of DENV pathogenesis was obtained in 2010, almost half a century after it was first proposed, when a mouse model of ADE-mediated severe dengue disease was developed [28,29]. In recent years, several human studies have provided additional compelling evidence in support of the ADE hypothesis. A long-term pediatric cohort study in Nicaragua confirmed that ADE occurred in patients with a low titer of pre-existing anti-DENV Abs (defined by inhibition ELISA), whereas patients with high Ab titers were protected against severe dengue [30]. Similarly, a school-based cohort study in Thailand identified a specific low titer of anti-DENV Abs (defined by hemagglutination inhibition or plaque reduction and neutralization assays) that correlated with severe dengue disease manifestations [31]. Consistent with these recent human studies and earlier epidemiological data, clinical trials of the first approved DENV vaccine Dengvaxia®, which includes DENV structural proteins but yellow fever virus (YFV) nonstructural proteins, revealed a higher incidence of severe dengue among vaccinated DENV-naïve compared with unvaccinated DENV-naïve individuals during subsequent primary DENV infection [32], suggesting that the vaccine primed the naïve individuals for development of severe dengue disease. These recent findings have solidified ADE as a well-accepted hypothesis to explain DENV pathogenesis during primary infection of infants with maternally acquired anti-DENV Abs or secondary infection of older children and adults with heterotypic DENV.
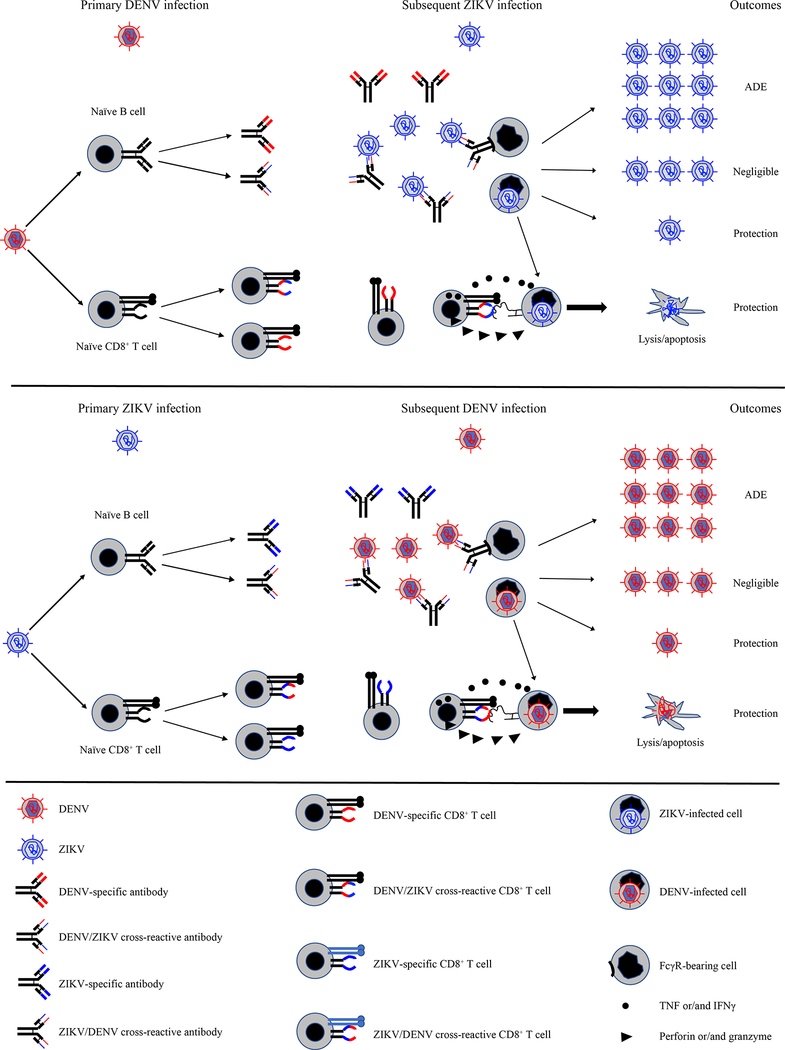
Since the 2014–2016 ZIKV outbreak in DENV-endemic areas of the Americas, many groups have investigated whether ZIKV infection in DENV-immune individuals may exacerbate disease, analogous to the effects of secondary infection with heterotypic DENV. Within a short time-frame, several groups reported that the anti-DENV Ab response cross-reacted with ZIKV, and vice versa [33–37], underscoring the need for studying the consequences of ZIKV emergence in DENV-endemic countries. DENV-elicited cross-reactive Abs were found to both neutralize [36–38] and enhance [35,39,40] ZIKV infection. E-dimer epitope (EDE)-targeting monoclonal Abs, originally isolated from DENV-infected individuals and shown to have potent neutralizing activity against DENV1–4, also effectively controlled ZIKV infection and prevented ZIKV pathogenesis in rhesus macaques [41] and in mice lacking one or more components of the interferon (IFN) system, which are highly susceptible to viral infection [36,42]. However, plasma isolated from convalescent DENV patients was able to either protect against or enhance ZIKV infection and increase disease severity in Stat2−/− mice depending on the Ab concentration [40]. In vitro, monoclonal Abs isolated from DENV-infected patients increased ZIKV infection of human placental macrophages and explants [43]. In contrast to these observations, maternally derived anti-DENV Abs showed little to no protective effect against ZIKV infection in LysMCre+Ifnar1fl/fl mice, which lack the type I IFN receptor on myeloid cells [44]. Similarly, other studies have shown that pre-existing DENV immunity of 1–3 years duration had no effect on ZIKV infection in macaques [45,46] and that the cross-neutralizing capacity of the human anti-DENV Ab response became less potent over time [47,48]. Collectively, the studies in humans and mouse models demonstrate that pre-existing DENV Abs can (i) contribute to cross-protection, (ii) mediate ADE, or (iii) have little to no effect during subsequent ZIKV infection (Figure 1), which is analogous to the scenario during secondary infection with heterotypic DENV. Future epidemiologic studies will be crucial in determining whether pre-existing DENV Abs in humans protect against or enhance ZIKV pathogenesis. If evidence continues to mount that pre-existing DENV Abs increase ZIKV disease severity via ADE, a pan-flavivirus vaccine targeting both DENV and ZIKV will be required. Since there is clear evidence that anti-DENV Abs can cross-protect against ZIKV infection, it seems likely that a universal vaccine could be designed to simultaneously protect against ZIKV and all four DENV serotypes via induction of cross-neutralizing Abs. However, it will first be necessary to understand the precise qualitative and quantitative features of the pre-existing DENV Ab response that dictate whether it has a cross-protective, enhancing, or neutral effect during ZIKV infection.
Figure 1. The proposed roles of the pre-existing cross-reactive immunity to DENV during subsequent ZIKV infection, and vice versa.
Cross-reactive Abs mediate neutralizing, enhancing or negligible effects during subsequent infection with heterologous virus. Cross-reactive CD8+ T cells provide protection against subsequent infection with heterologous virus.
Pre-existing cross-reactive T cell immunity to DENV protects against DENV and ZIKV infection
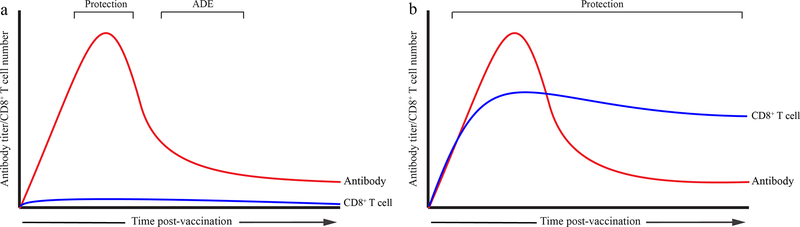
In addition to Abs, T cells may also contribute to the pathogenesis of severe dengue upon secondary infection of older children and adults. Early studies demonstrated that patients with severe dengue could mount only a weak cross-reactive CD8+ T cell response against the infecting DENV heterotype, comprised of inefficient, low-affinity cells that expressed high levels of cytokines such as IFNγ and TNF, low levels of the cytotoxic degranulation marker CD107a, and were prone to apoptosis [49,50]. However, more recent studies with both humans and mouse models suggest that DENV serotype cross-reactive T cells can protect against DENV infection and severe disease [12,13,51–54]. Comprehensive studies in two DENV-endemic countries, Sri Lanka and Nicaragua, have revealed that the magnitude and breadth of the epitope-specific CD8+ T cell response to DENV is HLA-linked and positively correlates with resistance to severe dengue [12,13]. Similarly, the magnitude and functional capacity of epitope-specific cytotoxic CD4+ T cells are also associated with HLA alleles linked to resistance to severe dengue [52]. Studies in Ifnar1−/− mice support these results and provide direct evidence that DENV serotype cross-reactive CD8+ T cells can protect against heterotypic DENV reinfection [55,56]. In fact, in a short-term model, CD8+ T cells could protect against severe dengue even in the presence of subneutralizing levels of cross-reactive Abs [56], suggesting that interplay between the cross-reactive Ab and T cell responses may determine the outcome of subsequent infection. Mouse models in which different vaccination strategies and maternal Ab-mediated ADE were investigated also demonstrated that CD8+ T cells could prevent ADE and reduce DENV pathogenesis [57–59]. Consistent with these findings, the unequal protection and enhancement problems of Dengvaxia® may be due to the lack of DENV-specific CD8 T cell responses, as this vaccine contains YFV but not DENV nonstructural proteins [60,61]. Taken together, these recent studies in humans and mouse models indicate that a DENV vaccine should ideally elicit serotype-cross-protective T cells, particularly CD8+ T cells, to abrogate ADE resulting from suboptimal or waning Ab responses induced by the vaccine (Figure 2).
Figure 2. The characteristics of an antibody-centric DENV or ZIKV vaccine and a pan-flavivirus vaccine that elicits both Ab and CD8+ T cell responses.
(a) Ab-centric DENV or ZIKV vaccines induce strong antibody but weak or negligible CD8+ T cell responses. (b) The proposed pan-flavivirus vaccines induce both Ab and CD8+ T cell responses.
The recent emergence of ZIKV in DENV-endemic regions has prompted investigators to address whether the outcome of secondary infection with ZIKV is also influenced by pre-existing T cell immunity to DENV. ZIKV epitopes, particularly those derived from the conserved nonstructural proteins such as NS3, are recognized by human CD4+ and CD8+ T cells elicited by prior DENV infection or vaccination [62–65]. In fact, ZIKV-cross-reactive T cells from DENV-immune individuals expressed higher levels of activation markers, such as granzyme B and PD-1, and exhibited a more vigorous response compared with T cells from DENV-naïve individuals [62]. In agreement with this observation in humans, DENV-immune HLA transgenic mice displayed a more robust and earlier cross-reactive CD8+ T cell response to ZIKV infection than did naïve transgenic mice [23]. This study is of particular importance because it showed that immunization with DENV epitopes that are relevant to the response in humans could protect against ZIKV infection, suggesting that similar vaccination strategies to elicit cross-protective CD8+ T cell responses might be beneficial in humans. In support of this, mouse studies modeling sequential DENV and ZIKV infection demonstrated CD8+ T cell-mediated short-term cross-protection against ZIKV in both non-pregnant and pregnant mice [66,67]. Taken together, these studies firmly establish that pre-existing DENV-primed CD8+ T cells provide at least transient cross-protection against subsequent infection with either ZIKV or heterotypic DENV (Figure 1). The transient nature of cross-protection mediated by DENV-primed CD8+ T cells against ZIKV is consistent with the short duration of cross-protection, ranging from 1–2 weeks to 3 years, that is observed during secondary DENV infections in humans [9]. Although further studies are needed to determine whether DENV-primed CD8+ T cells also mediate cross-protection against ZIKV and heterotypic DENV in humans, these recent mouse studies suggest that a universal vaccine could be designed to elicit cross-protective CD8+ T cell responses against ZIKV and the four DENV serotypes.
Pre-existing cross-reactive Abs to ZIKV enhance DENV pathogenesis
Several vaccine candidates against ZIKV are currently in development, and their planned deployment in DENV-endemic countries underscores the urgent need to understand how pre-existing immunity to ZIKV affects the outcome of subsequent DENV infection. Several studies have already shed light on the interplay between ZIKV immunity and DENV pathogenesis. In AG129 (Ifnar−/−) mice, injection of monoclonal Abs isolated from ZIKV-infected patients or vaccination with inactivated ZIKV both enhanced disease severity following DENV infection [34]. Similarly, LysMCre+Ifnar1fl/fl pups with maternally acquired anti-ZIKV Abs, but not pups born to naïve mothers developed severe dengue disease [44]. These findings are in line with a report that prior ZIKV infection significantly enhanced peak DENV viremia in macaques [68]. Collectively, these studies indicate that pre-existing ZIKV Abs can enhance DENV pathogenesis (Figure 1). The precise qualities of the pre-existing anti-ZIKV Ab response that induce ADE during subsequent DENV infection should be defined to ensure the design of ZIKV vaccines that avoid ADE-induce severe dengue. One potential strategy is to engineer mutations in the E protein, as suggested by a mouse study in which a ZIKV vaccine containing fusion loop mutations in the E protein reduced cross-reactive Ab production and circumvented ADE-mediated disease development upon DENV infection [69]. Clearly, ongoing and future clinical trials of ZIKV vaccine candidates in DENV-endemic countries should be carefully monitored for vaccine-induced enhancement of dengue disease severity.
Pre-existing cross-reactive T cell immunity to ZIKV protects against DENV infection
In contrast to the multiple studies demonstrating the pathogenic role of pre-existing cross-reactive ZIKV Abs during DENV infection, relatively few studies have examined the role of pre-existing T cell immunity during DENV infections. Two mouse studies reported that ZIKV-elicited CD8+ T cells were cross-reactive with DENV, but in only one of these studies, the investigators evaluated the role of this response, which conferred cross-protection against DENV infection [66,70]. Nevertheless, these findings, in combination with the studies described above, do suggest that CD8+ T cell responses to ZIKV and DENV are mutually cross-protective (Figure 1). Although further research will be necessary to validate these findings in humans and to evaluate the precise features (e.g., magnitude, specificity, phenotype, and location) of the cross-protective CD8+ T cell response in animal models and humans, the studies to date support the feasibility of a pan-flavivirus vaccine to elicit cross-protective CD8+ T cell responses against both viruses.
Concluding remarks and future perspectives
Bearing in mind that DENV and ZIKV co-circulate in a number of countries, the need to understand the protective vs. pathogenic impact of pre-existing DENV immunity on subsequent ZIKV infection, and vice versa, is made more urgent by the fact that a recently introduced DENV vaccine is suboptimal/inefficient and that ZIKV vaccines are under rapid development. Emerging research suggests that DENV- or ZIKV-elicited cross-reactive humoral immunity contributes to the pathogenesis of the reciprocal virus, whereas cross-reactive T cell immunity, particularly that mediated by CD8+ T cells, is mutually protective. Studies are now needed to (i) determine whether cross-reactive CD4+ T cells also contribute to protection and (ii) define the precise attributes of the cross-protective vs. pathogenic immune responses during ZIKV and DENV infections. Studies in mouse models suggest that DENV or ZIKV vaccines that elicit cross-reactive Abs alone may be more dangerous than those inducing both cross-reactive Ab and CD8+ T cell responses. Therefore, the ideal universal vaccine may be one that elicits robust long-lasting protection against both viruses via a combination of Ab and CD8+ T cell responses. This would not only avoid ADE mediated by an inefficient or waning vaccine-induced cross-reactive Ab response but also harness the protective capacity of cross-reactive CD8+ T cell responses. Importantly, a universal vaccine would also be desirable to provide cost-effective protection for resource-poor countries.
Acknowledgments
We would like to thank Anh-Viet Nguyen for making Figure 2. SS is supported by grants from the National Institutes of Health (AI116813 and AI140063). JW is supported by grant from the National Natural Science Foundation of China (31870159).
Footnotes
Conflict of interest statement
Nothing declared
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Control ECfDPa: Rapid risk assessment: Zika virus infection outbreak. EDCD, French Polynesia; 2014. http://ecdc.europa.eu/en/publications/Publications/zika-virus-rapid-risk-assessment-8-february-2016.pdf 2014.
- 2.Campos GS, Bandeira AC, Sardi SI: Zika Virus Outbreak, Bahia, Brazil. Emerg Infect Dis 2015, 21:1885–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO: Zika situation report: Zika virus, microcephaly and Guillain-Barre syndrome World Health Organization 2017:1–5. [Google Scholar]
- 4.Gubler DJ: Dengue and dengue hemorrhagic fever. Clin Microbiol Rev 1998, 11:480–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, et al. : The global distribution and burden of dengue. Nature 2013, 496:504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakkas H, Economou V, Papadopoulou C: Zika virus infection: Past and present of another emerging vector-borne disease. J Vector Borne Dis 2016, 53:305–311. [PubMed] [Google Scholar]
- 7.Ventura CV, Maia M, Bravo-Filho V, Gois AL, Belfort R Jr.: Zika virus in Brazil and macular atrophy in a child with microcephaly. Lancet 2016, 387:228. [DOI] [PubMed] [Google Scholar]
- 8.Monsalve DM, Pacheco Y, Acosta-Ampudia Y, Rodriguez Y, Ramirez-Santana C, Anaya JM: Zika virus and autoimmunity. One-step forward. Autoimmun Rev 2017, 16:1237–1245. [DOI] [PubMed] [Google Scholar]
- 9.Ngono AE, Shresta S: Immune Response to Dengue and Zika. Annu Rev Immunol 2018, 36:279–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dowd KA, DeMaso CR, Pelc RS, Speer SD, Smith ARY, Goo L, Platt DJ, Mascola JR, Graham BS, Mulligan MJ, et al. : Broadly Neutralizing Activity of Zika Virus-Immune Sera Identifies a Single Viral Serotype. Cell Rep 2016, 16:1485–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diamond MS, Ledgerwood JE, Pierson TC: Zika Virus Vaccine Development: Progress in the Face of New Challenges. Annu Rev Med 2018, 70:121–135. [DOI] [PubMed] [Google Scholar]
- 12.Weiskopf D, Angelo MA, de Azeredo EL, Sidney J, Greenbaum JA, Fernando AN, Broadwater A, Kolla RV, De Silva AD, de Silva AM, et al. : Comprehensive analysis of dengue virus-specific responses supports an HLA-linked protective role for CD8+ T cells. Proc Natl Acad Sci U S A 2013, 110:E2046–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Alwis R, Bangs DJ, Angelo MA, Cerpas C, Fernando A, Sidney J, Peters B, Gresh L, Balmaseda A, de Silva AD, et al. : Immunodominant Dengue Virus-Specific CD8+ T Cell Responses Are Associated with a Memory PD-1+ Phenotype. J Virol 2016, 90:4771–4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiskopf D, Yauch LE, Angelo MA, John DV, Greenbaum JA, Sidney J, Kolla RV, De Silva AD, de Silva AM, Grey H, et al. : Insights into HLA-restricted T cell responses in a novel mouse model of dengue virus infection point toward new implications for vaccine design. J Immunol 2011, 187:4268–4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yauch LE, Zellweger RM, Kotturi MF, Qutubuddin A, Sidney J, Peters B, Prestwood TR, Sette A, Shresta S: A protective role for dengue virus-specific CD8+ T cells. J Immunol 2009, 182:4865–4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grifoni A, Angelo MA, Lopez B, O’Rourke PH, Sidney J, Cerpas C, Balmaseda A, Silveira CGT, Maestri A, Costa PR, et al. : Global Assessment of Dengue Virus-Specific CD4(+) T Cell Responses in Dengue-Endemic Areas. Front Immunol 2017, 8:1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weiskopf D, Angelo MA, Grifoni A, O’Rourke PH, Sidney J, Paul S, De Silva AD, Phillips E, Mallal S, Premawansa S, et al. : HLA-DRB1 Alleles Are Associated With Different Magnitudes of Dengue Virus-Specific CD4+ T-Cell Responses. J Infect Dis 2016, 214:1117–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rivino L, Kumaran EA, Jovanovic V, Nadua K, Teo EW, Pang SW, Teo GH, Gan VC, Lye DC, Leo YS, et al. : Differential targeting of viral components by CD4+ versus CD8+ T lymphocytes in dengue virus infection. J Virol 2013, 87:2693–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Angelo MA, Grifoni A, O’Rourke PH, Sidney J, Paul S, Peters B, de Silva AD, Phillips E, Mallal S, Diehl SA, et al. : Human CD4(+) T Cell Responses to an Attenuated Tetravalent Dengue Vaccine Parallel Those Induced by Natural Infection in Magnitude, HLA Restriction, and Antigen Specificity. J Virol 2017, 91:pii: e02147–02116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grifoni A, Angelo M, Sidney J, Paul S, Peters B, de Silva AD, Phillips E, Mallal S, Diehl SA, Botten J, et al. : Patterns of Cellular Immunity Associated with Experimental Infection with rDEN2Delta30 (Tonga/74) Support Its Suitability as a Human Dengue Virus Challenge Strain. J Virol 2017, 91:pii: e02133–02116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yauch LE, Prestwood TR, May MM, Morar MM, Zellweger RM, Peters B, Sette A, Shresta S: CD4+ T cells are not required for the induction of dengue virus-specific CD8+ T cell or antibody responses but contribute to protection after vaccination. J Immunol 2010, 185:5405–5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delgado FG, Torres KI, Castellanos JE, Romero-Sanchez C, Simon-Loriere E, Sakuntabhai A, Roth C: Improved Immune Responses Against Zika Virus After Sequential Dengue and Zika Virus Infection in Humans. Viruses 2018, 10:pii: E480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wen J, Tang WW, Sheets N, Ellison J, Sette A, Kim K, Shresta S: Identification of Zika virus epitopes reveals immunodominant and protective roles for dengue virus cross-reactive CD8+ T cells. Nat Microbiol 2017, 2:17036.• This study revealed that DENV-primed cross-reactive CD8+ T cells dominate the CD8+ T cell response to ZIKV in a sequential model of DENV infection followed by ZIKV challenge in HLA transgenic mice.
- 24.Reynolds CJ, Suleyman OM, Ortega-Prieto AM, Skelton JK, Bonnesoeur P, Blohm A, Carregaro V, Silva JS, James EA, Maillere B, et al. : T cell immunity to Zika virus targets immunodominant epitopes that show cross-reactivity with other Flaviviruses. Sci Rep 2018, 8:672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koblischke M, Stiasny K, Aberle SW, Malafa S, Tschouchnikas G, Schwaiger J, Kundi M, Heinz FX, Aberle JH: Structural Influence on the Dominance of Virus-Specific CD4 T Cell Epitopes in Zika Virus Infection. Front Immunol 2018, 9:1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elong Ngono A, Vizcarra EA, Tang WW, Sheets N, Joo Y, Kim K, Gorman MJ, Diamond MS, Shresta S: Mapping and Role of the CD8+ T Cell Response During Primary Zika Virus Infection in Mice. Cell Host Microbe 2017, 21:35–46.• This study demonstrated that CD8+ T cells protect against ZIKV infection.
- 27.Halstead SB: Dengue. Lancet 2007, 370:1644–1652. [DOI] [PubMed] [Google Scholar]
- 28.Zellweger RM, Prestwood TR, Shresta S: Enhanced infection of liver sinusoidal endothelial cells in a mouse model of antibody-induced severe dengue disease. Cell Host Microbe 2010, 7:128–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balsitis SJ, Williams KL, Lachica R, Flores D, Kyle JL, Mehlhop E, Johnson S, Diamond MS, Beatty PR, Harris E: Lethal antibody enhancement of dengue disease in mice is prevented by Fc modification. PLoS Pathog 2010, 6:e1000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katzelnick LC, Gresh L, Halloran ME, Mercado JC, Kuan G, Gordon A, Balmaseda A, Harris E: Antibody-dependent enhancement of severe dengue disease in humans. Science 2017, 358:929–932.•• This long-term pediatric cohort study identified the precise low and high titers of anti-DENV Abs that are associated with susceptibility to and protection against severe dengue disease, respectively.
- 31.Salje H, Cummings DAT, Rodriguez-Barraquer I, Katzelnick LC, Lessler J, Klungthong C, Thaisomboonsuk B, Nisalak A, Weg A, Ellison D, et al. : Reconstruction of antibody dynamics and infection histories to evaluate dengue risk. Nature 2018, 557:719–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sridhar S, Luedtke A, Langevin E, Zhu M, Bonaparte M, Machabert T, Savarino S, Zambrano B, Moureau A, Khromava A, et al. : Effect of Dengue Serostatus on Dengue Vaccine Safety and Efficacy. N Engl J Med 2018, 379:327–340.•• This report presents analysis of the Sanofi dengue vaccine trial results, providing evidence for a higher risk of severe dengue disease in vaccinated DENV-naïve relative to unvaccinated DENV-naïve individuals.
- 33.Priyamvada L, Quicke KM, Hudson WH, Onlamoon N, Sewatanon J, Edupuganti S, Pattanapanyasat K, Chokephaibulkit K, Mulligan MJ, Wilson PC, et al. : Human antibody responses after dengue virus infection are highly cross-reactive to Zika virus. Proc Natl Acad Sci U S A 2016, 113:7852–7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stettler K, Beltramello M, Espinosa DA, Graham V, Cassotta A, Bianchi S, Vanzetta F, Minola A, Jaconi S, Mele F, et al. : Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science 2016, 353:823–826. [DOI] [PubMed] [Google Scholar]
- 35.Dejnirattisai W, Supasa P, Wongwiwat W, Rouvinski A, Barba-Spaeth G, Duangchinda T, Sakuntabhai A, Cao-Lormeau VM, Malasit P, Rey FA, et al. : Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat Immunol 2016, 17:1102–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swanstrom JA, Plante JA, Plante KS, Young EF, McGowan E, Gallichotte EN, Widman DG, Heise MT, de Silva AM, Baric RS: Dengue Virus Envelope Dimer Epitope Monoclonal Antibodies Isolated from Dengue Patients Are Protective against Zika Virus. MBio 2016, 7:pii: e01123–01116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barba-Spaeth G, Dejnirattisai W, Rouvinski A, Vaney MC, Medits I, Sharma A, Simon-Loriere E, Sakuntabhai A, Cao-Lormeau VM, Haouz A, et al. : Structural basis of potent Zika-dengue virus antibody cross-neutralization. Nature 2016, 536:48–53. [DOI] [PubMed] [Google Scholar]
- 38.Zhang S, Kostyuchenko VA, Ng TS, Lim XN, Ooi JS, Lambert S, Tan TY, Widman DG, Shi J, Baric RS, et al. : Neutralization mechanism of a highly potent antibody against Zika virus. Nat Commun 2016, 7:13679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Charles AS, Christofferson RC: Utility of a Dengue-Derived Monoclonal Antibody to Enhance Zika Infection In Vitro. PLoS Curr 2016, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bardina SV, Bunduc P, Tripathi S, Duehr J, Frere JJ, Brown JA, Nachbagauer R, Foster GA, Krysztof D, Tortorella D, et al. : Enhancement of Zika virus pathogenesis by preexisting antiflavivirus immunity. Science 2017, 356:175–180.• This study demonstrated that DENV-immune plasma isolated from convalescent patients can enhance ZIKV infection and pathogenesis in mice.
- 41.Abbink P, Larocca RA, Dejnirattisai W, Peterson R, Nkolola JP, Borducchi EN, Supasa P, Mongkolsapaya J, Screaton GR, Barouch DH: Therapeutic and protective efficacy of a dengue antibody against Zika infection in rhesus monkeys. Nat Med 2018, 24:721–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernandez E, Dejnirattisai W, Cao B, Scheaffer SM, Supasa P, Wongwiwat W, Esakky P, Drury A, Mongkolsapaya J, Moley KH, et al. : Human antibodies to the dengue virus E-dimer epitope have therapeutic activity against Zika virus infection. Nat Immunol 2017, 18:1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zimmerman MG, Quicke KM, O’Neal JT, Arora N, Machiah D, Priyamvada L, Kauffman RC, Register E, Adekunle O, Swieboda D, et al. : Cross-Reactive Dengue Virus Antibodies Augment Zika Virus Infection of Human Placental Macrophages. Cell Host Microbe 2018, 24:731–742 e736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fowler AM, Tang WW, Young MP, Mamidi A, Viramontes KM, McCauley MD, Carlin AF, Schooley RT, Swanstrom J, Baric RS, et al. : Maternally Acquired Zika Antibodies Enhance Dengue Disease Severity in Mice. Cell Host Microbe 2018, 24:743–750 e745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCracken MK, Gromowski GD, Friberg HL, Lin X, Abbink P, De La Barrera R, Eckles KH, Garver LS, Boyd M, Jetton D, et al. : Impact of prior flavivirus immunity on Zika virus infection in rhesus macaques. PLoS Pathog 2017, 13:e1006487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pantoja P, Perez-Guzman EX, Rodriguez IV, White LJ, Gonzalez O, Serrano C, Giavedoni L, Hodara V, Cruz L, Arana T, et al. : Zika virus pathogenesis in rhesus macaques is unaffected by pre-existing immunity to dengue virus. Nat Commun 2017, 8:15674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Collins MH, McGowan E, Jadi R, Young E, Lopez CA, Baric RS, Lazear HM, de Silva AM: Lack of Durable Cross-Neutralizing Antibodies Against Zika Virus from Dengue Virus Infection. Emerg Infect Dis 2017, 23:773–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Montoya M, Collins M, Dejnirattisai W, Katzelnick LC, Puerta-Guardo H, Jadi R, Schildhauer S, Supasa P, Vasanawathana S, Malasit P, et al. : Longitudinal Analysis of Antibody Cross-Neutralization Following Zika and Dengue Virus Infection in Asia and the Americas. J Infect Dis 2018, 218:536–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mongkolsapaya J, Dejnirattisai W, Xu XN, Vasanawathana S, Tangthawornchaikul N, Chairunsri A, Sawasdivorn S, Duangchinda T, Dong T, Rowland-Jones S, et al. : Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat Med 2003, 9:921–927. [DOI] [PubMed] [Google Scholar]
- 50.Duangchinda T, Dejnirattisai W, Vasanawathana S, Limpitikul W, Tangthawornchaikul N, Malasit P, Mongkolsapaya J, Screaton G: Immunodominant T-cell responses to dengue virus NS3 are associated with DHF. Proc Natl Acad Sci U S A 2010, 107:16922–16927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weiskopf D, Cerpas C, Angelo MA, Bangs DJ, Sidney J, Paul S, Peters B, Sanches FP, Silvera CG, Costa PR, et al. : Human CD8+ T-Cell Responses Against the 4 Dengue Virus Serotypes Are Associated With Distinct Patterns of Protein Targets. J Infect Dis 2015, 212:1743–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weiskopf D, Bangs DJ, Sidney J, Kolla RV, De Silva AD, de Silva AM, Crotty S, Peters B, Sette A: Dengue virus infection elicits highly polarized CX3CR1+ cytotoxic CD4+ T cells associated with protective immunity. Proc Natl Acad Sci U S A 2015, 112:E4256–4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simon-Loriere E, Duong V, Tawfik A, Ung S, Ly S, Casademont I, Prot M, Courtejoie N, Bleakley K, Buchy P, et al. : Increased adaptive immune responses and proper feedback regulation protect against clinical dengue. Sci Transl Med 2017, 9:pii: eaal5088. [DOI] [PubMed] [Google Scholar]
- 54.Rivino L, Kumaran EA, Thein TL, Too CT, Gan VC, Hanson BJ, Wilder-Smith A, Bertoletti A, Gascoigne NR, Lye DC, et al. : Virus-specific T lymphocytes home to the skin during natural dengue infection. Sci Transl Med 2015, 7:278ra235. [DOI] [PubMed] [Google Scholar]
- 55.Elong Ngono A, Chen HW, Tang WW, Joo Y, King K, Weiskopf D, Sidney J, Sette A, Shresta S: Protective Role of Cross-Reactive CD8 T Cells Against Dengue Virus Infection. EBioMedicine 2016, 13:284–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zellweger RM, Tang WW, Eddy WE, King K, Sanchez MC, Shresta S: CD8+ T Cells Can Mediate Short-Term Protection against Heterotypic Dengue Virus Reinfection in Mice. J Virol 2015, 89:6494–6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zellweger RM, Eddy WE, Tang WW, Miller R, Shresta S: CD8+ T cells prevent antigen-induced antibody-dependent enhancement of dengue disease in mice. J Immunol 2014, 193:4117–4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zellweger RM, Miller R, Eddy WE, White LJ, Johnston RE, Shresta S: Role of humoral versus cellular responses induced by a protective dengue vaccine candidate. PLoS Pathog 2013, 9:e1003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lam JH, Chua YL, Lee PX, Martinez Gomez JM, Ooi EE, Alonso S: Dengue vaccine-induced CD8+ T cell immunity confers protection in the context of enhancing, interfering maternal antibodies. JCI Insight 2017, 2:pii: 94500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guy B, Nougarede N, Begue S, Sanchez V, Souag N, Carre M, Chambonneau L, Morrisson DN, Shaw D, Qiao M, et al. : Cell-mediated immunity induced by chimeric tetravalent dengue vaccine in naive or flavivirus-primed subjects. Vaccine 2008, 26:5712–5721. [DOI] [PubMed] [Google Scholar]
- 61.Harenberg A, Begue S, Mamessier A, Gimenez-Fourage S, Ching Seah C, Wei Liang A, Li Ng J, Yun Toh X, Archuleta S, Wilder-Smith A, et al. : Persistence of Th1/Tc1 responses one year after tetravalent dengue vaccination in adults and adolescents in Singapore. Hum Vaccin Immunother 2013, 9:2317–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grifoni A, Pham J, Sidney J, O’Rourke PH, Paul S, Peters B, Martini SR, de Silva AD, Ricciardi MJ, Magnani DM, et al. : Prior Dengue virus exposure shapes T cell immunity to Zika virus in humans. J Virol 2017, 91:e01469–01417.• This study revealed that prior DENV immunity alters the magnitude, quality, and kinetics of T cell response to subsequent ZIKV infection in humans.
- 63.Lim MQ, Kumaran EAP, Tan HC, Lye DC, Leo YS, Ooi EE, MacAry PA, Bertoletti A, Rivino L: Cross-Reactivity and Anti-viral Function of Dengue Capsid and NS3-Specific Memory T Cells Toward Zika Virus. Front Immunol 2018, 9:2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Herrera BB, Tsai WY, Chang CA, Hamel DJ, Wang WK, Lu Y, Mboup S, Kanki PJ: Sustained Specific and Cross-Reactive T Cell Responses to Zika and Dengue Virus NS3 in West Africa. J Virol 2018, 92:pii: e01992–01917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paquin-Proulx D, Leal FE, Terrassani Silveira CG, Maestri A, Brockmeyer C, Kitchen SM, Cabido VD, Kallas EG, Nixon DF: T-cell Responses in Individuals Infected with Zika Virus and in Those Vaccinated Against Dengue Virus. Pathog Immun 2017, 2:274–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wen J, Elong Ngono A, Regla-Nava JA, Kim K, Gorman MJ, Diamond MS, Shresta S: Dengue virus-reactive CD8(+) T cells mediate cross-protection against subsequent Zika virus challenge. Nat Commun 2017, 8:1459.•• This study demonstrated that DENV-elicited CD8+ T cells provide cross-protection against subsequent ZIKV infection.
- 67.Regla-Nava JA, Elong Ngono A, Viramontes KM, Huynh AT, Wang YT, Nguyen AT, Salgado R, Mamidi A, Kim K, Diamond MS, et al. : Cross-reactive Dengue virus-specific CD8+ T cells protect against Zika virus during pregnancy. Nat Commun 2018, 9:3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.George J, Valiant WG, Mattapallil MJ, Walker M, Huang YS, Vanlandingham DL, Misamore J, Greenhouse J, Weiss DE, Verthelyi D, et al. : Prior Exposure to Zika Virus Significantly Enhances Peak Dengue-2 Viremia in Rhesus Macaques. Sci Rep 2017, 7:10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Richner JM, Himansu S, Dowd KA, Butler SL, Salazar V, Fox JM, Julander JG, Tang WW, Shresta S, Pierson TC, et al. : Modified mRNA Vaccines Protect against Zika Virus Infection. Cell 2017, 168:1114–1125 e1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang H, Li S, Zhang Y, Han X, Jia B, Liu H, Liu D, Tan S, Wang Q, Bi Y, et al. : CD8(+) T Cell Immune Response in Immunocompetent Mice during Zika Virus Infection. J Virol 2017, 91:pii: e00900–00917.• This study showed that ZIKV-elicited CD8+ T cells mediate cross-protection against DENV infection.