Abstract
Elastic fibers are major components of the extracellular matrix (ECM) in the aorta and support a life-long cycling of stretch and recoil. Elastic fibers are formed from mid-gestation throughout early postnatal development and the synthesis is regulated at multiple steps, including coacervation, deposition, cross-linking, and assembly of insoluble elastin onto microfibril scaffolds. To date, more than 30 molecules have been shown to associate with elastic fibers and some of them play a critical role in the formation and maintenance of elastic fibers in vivo. Because the aorta is subjected to high pressure from the left ventricle, elasticity of the aorta provides the Windkessel effect and maintains stable blood flow to distal organs throughout the cardiac cycle. Disruption of elastic fibers due to congenital defects, inflammation, or aging dramatically reduces aortic elasticity and affects overall vessel mechanics. Another important component in the aorta are the vascular smooth muscle cells (SMCs). Elastic fibers and SMCs alternate to create a highly organized medial layer within the aortic wall. The physical connections between elastic fibers and SMCs form the elastin-contractile units and maintain cytoskeletal organization and proper responses of SMCs to mechanical strain. In this review, we revisit the components of elastic fibers and their roles in elastogenesis and how a loss of each component affects biomechanics of the aorta. Finally, we discuss the significance of elastin-contractile units in the maintenance of SMC function based on knowledge obtained from mouse models of human disease.
1. Introduction
Elastic fibers are required for expansion and recoil of the aortic wall and alteration in quantity or quality of elastic fibers exhibits profound effects on the integrity and biomechanics of the aorta. The aorta is the first segment of the arterial tree and receives blood from the heart. In order to maintain a stable supply of blood to peripheral organs throughout the cardiac cycle, elasticity of the proximal ascending aorta plays a critical role in storing a portion of blood during systole and sending blood out during diastole, exerting the Windkessel effect [1]. The aorta and large elastic arteries are unique in that they contain well-defined elastic lamellar units, in which elastic lamella composed of elastic fibers alternate with vascular smooth muscle cells (SMCs) throughout the medial region. Within each lamellar unit, elastic fibers and SMCs maintain close contacts through connecting filaments that are formed by elastin extensions, thereby keeping SMCs and elastic lamella in a circumferential orientation [2]. These connections allow transmission of mechanical forces generated by the cyclic pumping of blood to the SMCs (outside-in signals), as well as transmission of tension generated by actomyosin within the SMCs to be delivered to the elastic lamella (inside-out signals), thereby forming “elastin-contractile units”. Hence, elastic fibers serve as a mediator of dynamic mechanical signals in the blood vessel wall.
The molecular mechanism of elastic fiber formation has begun to be elucidated through the identification of molecules associated with elastic fibers and their loss-of-function analyses in mice with distinct elastic fiber defects [3–5]. Elastic fiber formation occurs via multiple steps in a tightly regulated manner which actively takes place from mid-embryogenesis throughout early postnatal life [6, 7]. Elastic fibers formed during this period last one’s lifespan. Once elastic fibers are degraded by aging or lost by injuries, they do not regenerate spontaneously, thus compromising the biomechanical properties of the aortic wall. Here, in attempt to provide knowledge on the detailed molecular mechanism of elastic fiber assembly and its role on vessel mechanics in vivo, we first review the components of elastic fibers and provide an updated view of elastic fiber formation, and then we discuss the impact of the loss of major elastic fiber components on the biomechanics of the thoracic aorta. Lastly, we discuss the importance of elastin-contractile units and their association with aortic diseases.
2. Molecular players in elastic fiber assembly
Elastic fibers are comprised of an elastin core and surrounding microfibril scaffold. Elastin is encoded by a single ELN gene and is translated to a ~70 kDa protein secreted as the monomeric form called tropoelastin from elastogenic cells in the skin, lung and vasculature [8]. Elastin contains alternating hydrophobic and cross-linking domains that are responsible for self-aggregation and formation of cross-links, respectively [9]. Coacervation of tropoelastin, which induces self-aggregation and phase separation, occurs spontaneously under physiologic conditions and is important for alignment and cross-linking of tropoelastin [10, 11].However, coacervation alone is not sufficient for polymerization of elastic fibers, which requires the action of cross-linking enzymes. Lysyl oxidase (LOX) and lysyl oxidase-like 1 (LOXL1) belong to a multigene family consisting of five members (LOX and LOXL1 to 4) and are copper- and lysyl-tyrosyl-quinone (LTQ)-dependent secreted enzymes [12, 13]. LOX and LOXL1 are the most well-studied enzymes of the family that contain an N-terminal propeptide, which is cleaved during proteolytic activation, and a conserved catalytic domain. LOX and LOXL1 mediate cross-linking of elastin by catalyzing the oxidative deamination of peptidyl lysine residues, subsequently forming the covalent elastin-specific cross-links desmosine and isodesmosine. LOX also catalyzes cross-linking of collagens. Recent crystal structure analysis of human LOXL2 revealed a conserved catalytic domain among the LOX family and showed generation of LTQ upon copper-mediated activation of the enzyme [14]. It has been shown in vitro that LOX-mediated cross-linking of tropoelastin occurs simultaneously with elastin deposition onto microfibrils [15].
Fibulin-4 (encoded by Efemp2) and fibulin-5 (Fbln5) are secreted glycoproteins with a modular structure. They are expressed in the aorta and are essential for elastic fiber formation in vivo [3–5]. The fibulin family is comprised of seven members and fibulin-4 through fibulin-7 belong to the short fibulins (reviewed in [16, 17]). Among short fibulins, fibulin-4 and fibulin-5 are known as elastogenic fibulins. Fibulin-5 was first reported as an elastin binding protein and its deletion in vivo caused systemic elastic fiber defects in mice [3, 4]. Subsequently, it was shown that fibulin-5 potentiates coacervation of tropoelastin as well as limits the size of coacervated elastin [18, 19]. Consistently, electron microscopy analysis showed that Fbln5-null (Fbln5−/−) skin contains large aggregates formed by insoluble elastin [20]. Fibulin-4 shares high homology with fibulin-5 and also binds tropoelastin in vitro [21]. Deletion of fibulin-4 in mouse (Fbln4−/−) causes a more severe phenotype than the Fbln5−/− mouse, exhibiting aortic rupture, diaphragm herniation and perinatal lethality, which resembles Lox-null (Lox−/−) pups [5, 22–24]. Both fibulins have distinct roles in elastogenesis. Biochemical analyses showed that the N-terminal region of fibulin-4 binds a propeptide of LOX and facilitates tropoelastin binding to LOX [18], whereas fibulin-5 binds LOXL1 via the C-terminal domain and facilitates tethering of LOXL1 onto elastic fibers [25] and/or proteolytic activation of preproLOXL-1 to mature LOXL1 [20]. Taken together, fibulin-4 and fibulin-5 play a critical role in optimizing the conditions for tropoelastin cross-linking through regulation of coacervation and interactions with LOX and LOXL1, respectively.
Visible at the periphery of the elastin core are microfibrils that act as a pre-existing scaffold for elastin polymer deposition as well as regulation of growth factors and cytokines. Microfibrils are formed by polymerization of large ECM proteins, mainly fibrillin-1 (Fbn1) and fibrillin-2 (Fbn2) [26–28]. Heparan sulfate and a fibronectin network are essential for formation of the microfibril scaffold [29–31]. Fibronectin is also shown to be important for microfibril homeostasis in early passage-human skin fibroblasts [32]. A complete deletion of fibrillin-1 in mice causes neonatal lethality due to aortic aneurysm rupture and disruption of elastic fibers [33]. Further deletion of Fbn2 in the Fbn1-null (Fbn1−/−) background in mice worsens the phenotype and induces embryonic lethality with poorly organized elastic fibers, indicating that microfibril scaffold formation is indeed a prerequisite for proper deposition of polymerized elastin [33].
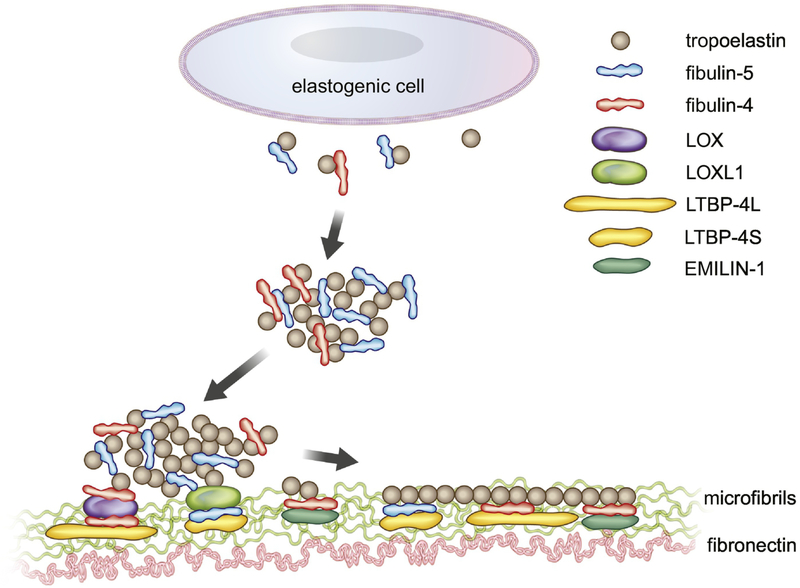
Fibrillins’ role as a scaffold for elastic fiber assembly is not only through homophilic interaction-based long fibril structures, but also by providing interactions with numerous binding proteins [34–36]. Recently, more complex interactions among elastin- and microfibril- associated proteins have been shown to play an important role in navigating assembled elastin onto microfibrils. EMILIN-1 (elastin microfibril interface-located protein), which is abundantly expressed in the aorta and bone, binds and co-localizes with fibulin-4 and is required for the deposition of fibulin-4 onto the ECM in osteoblasts [37]. Latent TGFβ binding proteins (LTBPs) belong to the fibrillin/LTBP family and are comprised of four members LTBP-1 through −4, including splice variants LTBP4S (short form) and LTBP-4L (long form) [38]. LTBP-1, −2, and −4 directly bind to fibrillin-1 whereas LTBP-3 does not bind to fibrillin-1 in vitro. However, LTBP-3 co-localizes with microfibrils, indicating that LTBP-3 interacts with other microfibril-associated proteins [34, 39, 40]. LTBP-1 uses a fibronectin network for its deposition onto microfibrils and LTBP-3 and −4 associate with the fibrillin-1 network [40]. LTBP-1, −3, −4 also bind to the prodomain of TGFβ and to mature TGFβ dimer and regulate bioavailability of TGFβ in vivo [41, 42]. A knockout study in vivo showed that LTBP-4 plays a crucial role in elastic fiber formation [43, 44] and suggested that LTBP-4 aids in deposition of elastin onto microfibrils by interacting with fibulin-5 [45]. By using a combination of binding assays and phenotypic analyses of Ltbp4s-null (Ltbp4s−/−) and Ltbp4-null (Ltbp4−/−) mice, it was recently shown that LTBP-4L and LTBP-4S favor the interaction with fibulin-4 and fibulin-5, respectively, and the absence of both forms of LTBP-4 leads to markedly reduced expression and impaired localization of fibulin-4 and fibulin-5 on the microfibrils, resulting in elastic fiber defects with dysmorphic elastin aggregates [46, 47]. Together with preferential binding between fibulin-4 and LOX and fibulin-5 and LOXL1, it is conceivable that down-regulation or absence of one of the molecules in the fibulin-4-LOX-LTBP-4L or fibulin-5-LOXL1-LTB-P4S complex affects overall assembly efficiency and the process of elastic fiber formation (Figure 1).
Figure 1. Proposed model of elastogenesis.
Tropoelastin is secreted by elastogenic cells, binds to fibulins (predominantly fibulin-5), and undergoes self-aggregation (coacervation). Fibulin-5 binds to LOXL1 and fibulin-4 binds to LOX and the complexes are deposited onto microfibrils (green) with the aid of LTBP-4S and LTBP-4L, respectively. Cross-linking and polymerization of elastic fibers proceed.
3. Elastic fibers and biomechanics of the thoracic aorta
3.1. Scope and definition of mechanical terms
Elastic fibers provide reversible elasticity to the aorta, while collagen fibers provide strength and limit distension at high pressures. Loss of critical elastic fiber components alters the mechanical behavior of the aortic wall. Genetically-modified mice have been instrumental in determining mechanical contributions of different elastic fiber components. In this section, we summarize studies that quantify mechanical behavior of the thoracic aorta in mouse models with elastic fiber defects. In many cases, we compare structural stiffness (depends on material properties and aortic geometry) and material stiffness (depends only on material properties) between groups under physiologic loading conditions. Structural stiffness is often determined from in vitro pressure-diameter tests with the aorta held at the in vivo axial stretch ratio and is the inverse of compliance or distensibility. We present pressure-diameter data for a range of mouse models and discuss the implications of elastic fiber defects on structural stiffness under different loading conditions. Calculation of material stiffness requires information about the unloaded dimensions of the aortic wall, in addition to pressure-diameter data, and hence is not always reported.
Material stiffness depends on multiaxial deformation of the aortic wall and should be calculated by fitting a nonlinear strain energy function to biaxial mechanical testing data. However, an estimate of the circumferential material stiffness called the incremental elastic modulus can be obtained by determining the linear slope of the stress-strain curve under physiologic loading conditions. The incremental elastic modulus of the aorta for a range of animal species from shark to toad to mouse to human is around 0.4 MPa and it has been suggested that this value represents a “universal elastic modulus” that provides optimal mechanical function in a pulsatile cardiovascular system [48]. We highlight mouse models that are able to maintain the universal elastic modulus through remodeling of the aortic wall and those that cannot and are often associated with aneurysmal disease.
Additional mechanical properties compared between mouse models include residual strain (strain remaining in the aortic wall in the unloaded state due to growth and remodeling of the wall components), stored strain energy (the total energy stored due to multiaxial, physiologic deformation of the aortic wall and available to do work on the blood in vivo), and energy dissipation (energy loss due to viscous components in the aortic wall), which is the difference between the stored strain energy with loading and returned strain energy with unloading.
3.2. Elastin
Elastin null (Eln−/−) mice die at birth with obstructive aortic occlusion [49]. The ascending aorta from newborn Eln−/− mice has increased structural stiffness compared to Eln+/+ [50]. There is a large increase in energy dissipation in Eln−/− aorta, which is consistent with the mechanical role of elastin in the large arteries [51]. The aorta from elastin heterozygous (Eln+/−) mice has increased structural stiffness compared to Eln+/+ mice [52] and compensatory remodeling of the axial and circumferential residual strains [53]. Eln+/− mice live a normal lifespan [54]. During postnatal maturation, the increase in aortic structural stiffness precedes and may cause hypertension in Eln+/− mice [55]. Results from constitutive modeling of elastin and collagen contributions to aortic mechanics are consistent with reduced elastin amounts and predict reorganization of collagen fiber alignment and nonlinearity in Eln+/− aorta to maintain the material stiffness near wild-type levels [56]. Eln−/− mice that have been rescued by expression of human elastin through a bacterial artificial chromosome (hBACmNull) have about 30% of the normal elastin levels and even more severe increases in aortic structural stiffness and blood pressure than Eln+/− mice [57]. hBACmNull mice exhibit aortic narrowing similar to humans with supravalvular aortic stenosis (SVAS) that is caused by elastin mutations leading to reduced levels of functional elastin [58]. Aortic narrowing in hBACmNull mice may be caused by deficient circumferential growth [59].
3.3. Fibulin-5
Fbln5−/− mice live a normal lifespan, but have fragmented elastic fibers in elastin rich tissues [3, 4]. Fbln5−/− mice have a longer aorta [4] with increased structural stiffness [3]. Biaxial mechanical testing confirms that Fbln5−/− aorta has increased structural stiffness, but similar material stiffness to wild-type aorta, as well as decreased stored strain energy and increased energy dissipation [60]. The material stiffness is maintained at similar values in Fbln5−/− and wild-type aorta throughout postnatal maturation [61], suggesting a universal elastic modulus for the aorta that is maintained even with genetic mutations that alter the available amounts and structure of elastic fiber components in the wall [48]. Fbln5−/− aorta has decreased active contractility [62], which may be related to altered coupling between SMCs and elastic fibers due to the absence of fibulin-5.
3.4. Fibulin-4
Fbln4−/− mice die at birth with severe vascular and lung defects [5]. Newborn Fbln4−/− mice have ascending aortic aneurysms and increased structural stiffness, but similar material stiffness to wild-type aorta [63] consistent with the hypothesized ability of the aorta to maintain material stiffness despite elastic fiber protein deficiencies. Similar to newborn Eln−/− aorta, newborn Fbln4−/− aorta shows a large increase in energy dissipation with cyclic loading [51] demonstrating that correctly assembled elastic fibers, and not just the elastin protein, are necessary for reversible elasticity of the aorta. Mutations in fibulin-4 cause cutis laxa which is often accompanied by aortic aneurysms. A mouse model with a fibulin-4 mutation (Fbln4E57K/E57K) identified in human patients with cutis laxa develops ascending aortic aneurysms in approximately half of the mice homozygous for the mutation [64]. Fbln4E57K/E57K aorta has increased structural stiffness compared to wild-type, even when an aneurysm is not present [65].
Mice with SMC specific loss of fibulin-4 (Fbln4SMKO) were developed to further investigate the role of fibulin-4 in vascular biology. Fbln4SMKO mice develop ascending aortic aneurysms [24]. Aneurysms are detectable by 2 weeks of age in Fbln4SMKO mice, but increases in structural stiffness are detectable by 1 week of age [66], indicating that increases in structural stiffness precede and may play a causal role in aneurysm formation. Biaxial mechanical testing showed an increase in aortic material stiffness of Fbln4SMKO mice and other mouse models of aneurysmal disease [67] suggesting that aneurysms may manifest in adult animals when the aorta is not able to maintain the universal elastic modulus. In support of this hypothesis, crossing Fbln4SMKO mice with thrombospondin-null (Thbs1−/−) mice rescues the aneurysm phenotype along with the material stiffness [68]. A growth and remodeling model of aortic development was used to understand how changes in elastin, collagen, and SMC amounts and behavior could lead to the resulting mechanical behavior of Fbln4SMKO aortas. Reductions in elastin stress contribution, increases in collagen stress contribution, and altered SMC response to developmental changes in hemodynamic forces were required for the model to reproduce Fbln4SMKO aorta mechanical behavior [69]. The changes in SMC response are consistent with changes in SMC phenotype observed in Fbln4SMKO mice [24, 66].
Lysyl oxidase
Lox−/− mice die at birth with ruptured arterial aneurysms and diaphragm [22, 70]. Newborn Lox−/− mice have ascending aortic aneurysms, increased structural stiffness, and increased material stiffness compared to wild-type aortas [71]. The increased material stiffness in Lox−/− aortas contrasts with the normal material stiffness in Fbln4−/− aortas [63]. Since lysyl oxidase crosslinks both elastin and collagen, it is possible that the compensatory changes in collagen arrangement and nonlinearity hypothesized to maintain the universal elastic modulus in other mouse models of elastic fiber defects [i.e. Eln+/− [56] and Fbln5−/− [61]] cannot occur in Lox−/− aortas. Like newborn Eln−/− and Fbln4−/− aortas, newborn Lox−/− aortas show a large increase in energy dissipation confirming that effective elastic fiber crosslinking is necessary for reversible elasticity in the aorta [51]. Mutations in lysyl oxidase have been identified in human patients with thoracic aortic aneurysms [72]. A mouse model with a mutation identified in humans (LOX p.M298R) shows perinatal lethality and large aneurysms in mice homozygous for the mutation. Mice heterozygous for the mutation live a normal lifespan, but have increased structural stiffness in the aorta [73]. Chemical inhibition of LOX with BAPN induces thoracic aortic aneurysm and dissection in mice [74]. SMCs in BAPN treated aorta are characterized by mechanical stress induced apoptosis, indicating that changes in mechanical behavior of the aorta due to reduced elastic fiber crosslinking may alter SMC mechanobiology and contribute to aneurysm formation and dissection [75].
3.5. Fibrillin-1
Homozygous mice with deletion of a large portion of the fibrillin-1 gene and insertion of a neomycin resistance cassette (Fbn1mgΔ/ mgΔ) die before three weeks of age from cardiovascular complications [76]. Mice that underexpress fibrillin-1 due to insertion of a neomycin resistance cassette, without deletion of any fibrillin-1 gene segments (Fbn1mgR/mgR) die at about 4 months of age with large aortic aneurysms [77]. Fbn1mgR/mgR ascending aorta has increased structural and material stiffnesses, even when an aneurysm is not present [78], consistent with the hypothesis that aneurysms may manifest when the adult mouse aorta is not able to maintain the universal elastic modulus. Similar to Fbln5−/− aorta, Fbn1mgR/mgR aorta has reduced stored strain energy and increased energy dissipation [78].
3.6. Comparison of aortic biomechanics and elastin/collagen contributions in different mouse models
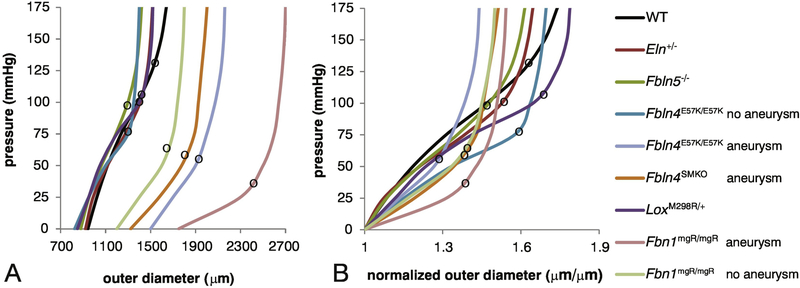
Pressure-diameter tests at the in vivo axial stretch were performed in many of the mouse models discussed above and are summarized in Figure 2. The structural stiffness (or local slope of the curve) is often affected by elastic fiber defects, but is highly dependent on the applied pressure. Due to the pressure dependence, an aorta from a hypertensive mouse (such as Eln+/−) will have increased structural stiffness in vivo, even with no changes in the geometry and material properties of the aortic wall [79]. The applied pressure at which the curve transitions between low and high structural stiffness is also affected by elastic fiber defects. The structural stiffness at low pressure is associated with elastin resistance to deformation, while the structural stiffness at high pressure is associated with collagen resistance to deformation [80]. For many of the mouse models, there are not large differences in the structural stiffness at low pressure where elastin dominates, implying that many genetic modifications (including Eln+/− and Fbln5−/−) have little effect on the low-pressure mechanical behavior attributed to elastin. The more severe mouse models that show highly fragmented elastic fibers and develop aortic aneurysms (including Fbln4SMKO and Fbn1mgR/mgR) have decreased stiffness at low pressure, consistent with reduced mechanical contributions of elastin.
Figure 2. Pressure-diameter curves for ascending aorta from adult mice with elastic fiber defects.
Absolute values of the outer diameter at each pressure (A) and normalized values with respect to the starting outer diameter at 0 mmHg (B) are shown. The local slope of the curve represents the structural stiffness. The black circles on each curve mark the approximate transition from low stiffness (elastin dominated) to high stiffness (collagen dominated) behavior. Aortas from mice with elastic fiber defects transition to high stiffness at lower pressures than wild-type, with aneurysmal models having the lowest transition pressure. Data was approximated from [55] for Eln+/−, [61] for Fbln5−/−, [65] for Fbln4E57K/E57K, [66] for Fbln4SMKO, [73] for Lox+/M298R, and [78] for Fbn1mgR/mgR. The wild-type (WT) curve was estimated from the average behavior of three different studies[55, 61, 66].
Interestingly, all of the mouse models with elastic fiber defects have increased stiffness in the high-pressure region attributed to collagen. They also transition to the stiffer region at lower pressures than wild-type aorta. Some models, such as Fbln4SMKO [81], have collagen defects in addition to elastin defects that may explain the changes in mechanical behavior at high pressure. Others, such as Eln+/− [52, 56], do not have measurable changes in collagen amounts or organization. Experiments with elastase degradation and results from constitutive modeling of aortic wall components suggest that elastic fibers hold collagen fibers in compression, resulting in wavy collagen fibers, and allowing them to deform significantly before contributing to mechanical resistance [82, 83]. The loss of functional elastic fibers may decrease collagen fiber waviness, so the collagen fibers are straighter and contribute to the mechanical resistance at a lower point in the pressure-diameter curves. The loss of functional elastic fibers may also reduce the variation in collagen fiber waviness so that most collagen fibers are recruited at the same deformation, leading to a sharp rather than gradual increase in structural stiffness. These observations imply that changes to elastin and collagen (and SMCs) must be considered together to fully understand the mechanical implications of elastic fiber defects.
4. Biological significance of elastin contractile units
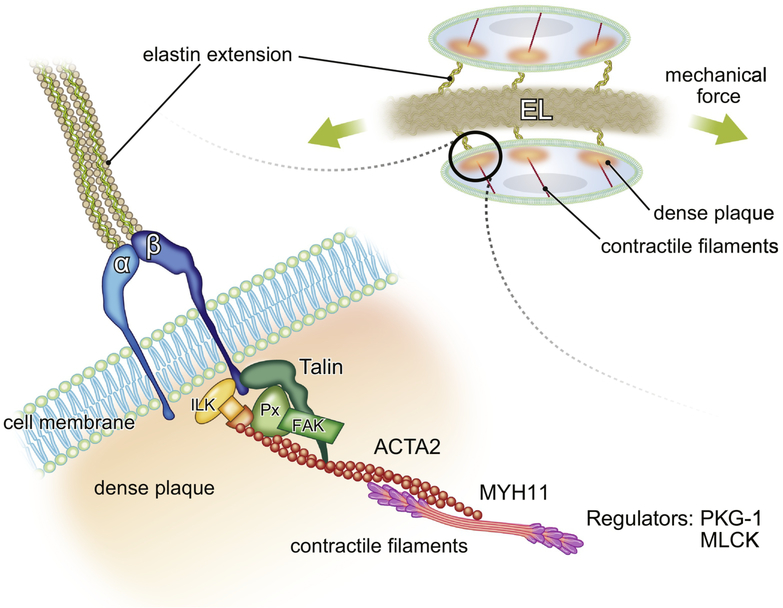
SMCs possess an intrinsic contractile machinery to respond to mechanical loads. The contractile machinery is indirectly connected to elastic fibers to form an elastin-contractile unit, which is the physical and functional unit from elastic fibers to actin contractile filaments that allows the coordinated response of aortic wall components to mechanical stress [2](Figure 3). SMCs do not form sarcomeres like cardiac or skeletal muscle cells and contraction is regulated mainly by a ligand-induced, receptor-mediated signaling pathway involving the mobilization of intracellular calcium and contractile filaments (reviewed in [84]). SMCs organize contractile filaments comprised of smooth muscle (SM) actin and SM myosin in a diagonal direction and they connect to the dense plaque (DP), where elastic fibers attach to integrins from outside of the cell and form focal adhesions (FAs) underneath the cell membrane [85]. Actin isoforms that form contractile filaments in large arteries are mainly α SM actin [86], whereas non-muscle γ actin forms cytoskeleton near the cortex of SMCs [87]. Actin molecules in non-contractile filaments are more dynamic compared to contractile filaments, but both contractile filaments and the cortically-distributed actin cytoskeleton generate tension in SMCs [88].
Figure 3. Schematic presentation of the elastin-contractile unit in SMCs.
Elastic fibers bind to α- and β- heteromeric integrins through elastin extensions and form dense plaques (orange), where focal adhesion proteins such as Talin, paxillin (Px), focal adhesion kinase (FAK) and integrin linked kinase (ILK) bind and regulate contractile filaments (red, major isoform is ACTA2) as well as activate various downstream signaling. Cellular tension is generated by the contraction of actin and myosin (pink, major isoform is MYH11). The regulators of muscle contraction such as PKG-1 and MLCK also play a crucial role in response to mechanical stimuli. The defects in elastin-contractile units result in the formation of thoracic aortic aneurysms. EL: elastic lamina.
Recently, it has been shown that genetic mutations in the components of elastin-contractile units are responsible for thoracic aortic aneurysms (TAAs) (reviewed in [89]). The mutations are largely divided into two groups; the first group includes genes encoding for extracellular proteins that constitute or are associated with microfibrils such as FBN1 [90], LOX [72, 73], EFEMP2 [91], microfibril associated protein 5 (MFAP5) [92], elastin microfibril interface 1 (EMILIN1) [93] and LTBP3 [94]. The bundles of microfibrils are initially linked to SMCs, then elastin progressively infiltrates the microfibrils to form elastin extensions [2]. Fbn1 interacts with integrin α5β1 and αvβ3 via its RGD domain [95] and downregulation of Fbn1 in mouse (Fbn1mgR/mgR) markedly reduces phosphorylation of focal adhesion kinase (FAK) in cardiac myocytes and leads to dilated cardiomyopathy [96]. In addition, compound heterozygous mice for Fbn1 and β1 integrin (Fbn1+/−; Itgb1+/−) recapitulate the phenotype of Fbn1mgR/mgR mice, suggesting that Fbn1 may be crucial to signal activation in SMCs mediated by elastin extensions and integrins. Interestingly, mutations in Eln [58] or Fbln5 [97] that form the elastin core do not cause aortic aneurysms; rather, they develop supravalvular aortic stenosis (SVAS) and tortuous elongated aorta, respectively, exhibiting morphogenetic defects due to impaired elastic fibers in the aorta.
Focal adhesions were described as electron-dense regions in SMCs where elastin extensions attach to the outside of the cell and actin filaments anchor obliquely at the cytoplasmic side underneath the cell membrane [98]. Although mutations in the components of focal adhesions have not been reported in aortic aneurysms, studies in mice have implicated their role in aortic homeostasis. Mechanical force in the aorta is transmitted to focal adhesions via heterodimeric α and β integrins and regulates actin filaments. Several key molecules have been identified within focal adhesions that are involved in mechanotransduction (reviewed by [99, 100]). Integrin linked kinase (ILK) is a scaffold protein that associates with the cytoplasmic tail of β integrin and forms the IPP complex with cysteine-rich protein (PINCH) and parvin and links to actin filaments [101]. Deletion of Ilk in SMCs results in aneurysmal dilatation of the aorta [102] and deletion in neural crest cells shows reduced phosphorylation of pSmad3 and downregulation of SMC markers with aneurysm development [103]. Talin serves as a mechanosensitive molecular hub transducing mechanical force to actin filaments and converting it into biochemical signals (reviewed in [104]). Activation of talin, which binds the β integrin tail, leads to conformational change of the integrin into the active form, and tension generated by actin filaments can stabilize integrins together with talin in its extended open form [105]. Recently, downregulation of talin has been reported in SMCs harvested from patients with aortic dissection, along with increased proliferation and migration [106].
The second group for which elastin-contractile unit mutations are reported in TAAs includes genes encoding for the components of actomyosin contractile filaments and their regulators in SMCs (reviewed in [107]). Mutations in SM-myosin heavy chain (encoded by MYH11), which causes deletion in the C-terminal coiled-coil domain, is predicted to exert a dominant negative effect on myosin motor activity [108]. In addition, 3 missense variants (L1264P, R1275L, R712Q) and 1 rare variant (R247) in MYH11 have been identified and suggested to damage coiled-coil domain (L1264P), affect the ATPase region (R712Q) and disrupt the myosin motor activity (R247C) [109]. More than 40 mutations in ACTA2 have been identified in familial TAA and dissection [110] and R179H and R258C mutant actin have been shown to cause polymerization defects and slower movement compared with wild-type actin filaments in in vitro motility assays [111, 112]. Mutations in regulators of SMC contraction have also been reported, including the MLCK gene encoding for myosin light chain kinase that induces muscle contraction [113] and gain-of-function mutation of PKG1 gene encoding for type I cyclic guanosine monophosphate (cGMP)-dependent protein kinase that controls muscle relaxation [114]. Interestingly, these mutations lead to reduced SMC contractility, presumably affecting the coordinated response of the aortic wall to mechanical forces. It is of note that SMCs isolated from postnatal day 7 Fbn1mgR/mgR aortas have increased SMC markers, reduced elastin expression and decreased proliferation, showing premature SMC differentiation [115]. Marfan iPS-derived SMCs originated from the neural crest recapitulate the Marfan vascular phenotype, exhibiting reduced FBN1 deposition, increased TGFβ, and upregulation of SMC contractile proteins, but decreased carbachol-induced cell contractility and intracellular calcium retention [116]. Fbln4 mutant SMCs also show reduced SMC contractile markers [24]. Taken together, these observations underscore the importance of the elastin-contractile unit for proper maintenance of SMC differentiation and coordinated contraction in the aortic wall.
5. Concluding remarks and future directions
Studies in knockout mice have been instrumental in defining critical players in elastic fiber assembly and identifying molecules that work together to regulate elastin coacervation, cross-linking, deposition onto microfibrils, and interactions with SMCs. Additional in vivo and in vitro studies are needed to fully understand the role(s) of the over 30 different molecules implicated in elastic fiber synthesis so that we may someday be able to encourage elastic fiber repair or de novo synthesis in the case of defects or damage caused by disease or aging. It is important to remember that elastic fiber synthesis occurs in a dynamic mechanical environment, with the aortic wall distending and recoiling with each heartbeat, so that inside out and outside in mechanical signaling likely contribute to the regulation of elastic fiber synthesis in development and dysfunction with disease.
The mechanical data on different mouse models of elastic fiber defects show that increased aortic structural stiffness is a common consequence of reduced elastin levels or misassembled elastic fibers, but that increased aortic material stiffness generally manifests in extreme cases that are associated with aneurysmal disease. The results suggest that failure of the aortic wall to maintain the universal elastic modulus has downstream effects on SMC phenotype that may be caused by the microenvironment within the stiffened ECM. Future work to quantify the material behavior of the aorta under physiologic loading conditions and the resulting SMC phenotype in different mouse models with elastic fiber and other ECM defects is needed to further investigate this hypothesis and understand the limits and consequences of maintaining the universal elastic modulus. When reported, stored strain energy is reduced and energy dissipation is increased in aortas with elastic fiber defects. These energetic alterations increase the work required by the heart to circulate blood and may contribute to secondary cardiovascular diseases associated with elastic fiber defects.
Elasticity is an essential biomechanical property of the aorta and is provided by elastic fibers and enhanced by elastin-contractile units. Whereas insoluble polymerized elastin confers dynamic elasticity and recoil to the aortic wall, elastin extensions aid in connecting elastic lamella to integrins and transmit mechanical forces through focal adhesions, thereby converting the physical stimuli into biochemical signaling and regulating actin contractile filaments. We hypothesize that a homeostatic stress state, determined by external forces from ECM connections and internal forces from contractile filaments, is necessary for maintenance of the SMC phenotype. In the case of genetic defects to elastic fiber proteins, microfibrils may still be in place, but elastin extensions are disrupted, interfering directly with the SMC homeostatic stress state or with continuity of the force sensing apparatus. We also have preliminary data indicating that force promotes micro-remodeling of the elastin contractile unit. Therefore, defects in the mechanical environment (such as changes in the universal elastic modulus) or the cellular interpretation of the mechanical environment (such as defects in the elastin-contractile unit) lead to maladaptive remodeling associated with disease. Disruption of the physical and functional connecting elastin-contractile units weakens the aortic wall and exacerbates pathological conditions due to alterations in SMC phenotype. Ineffective regeneration of elastic fibers hampers the control of these conditions and urges us to have a more comprehensive understanding of the regulation of elastic fibers and organization of elastin-contractile units in vivo.
Highlights.
We revisit the components of elastic fibers and their roles in elastogenesis.
We provide the data and discuss how a loss of each component affects biomechanics of the aorta.
We discuss the significance of elastin-contractile units in the maintenance of smooth muscle cell function based on knowledge obtained from mouse models of human disease.
Acknowledgment
The work in Yanagisawa laboratory and Wagenseil Laboratory were supported in part by grants from the National Institutes of Health (R01HL106305 to HY and R01HL115560 and R01HL105314 to JW), the MEXT KAKENHI (grant No. JP 17H04289 to HY), and the American Heart Association (12EIA8190000 to HY). We thank Mayumi Mori for assistance with graphics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Safar ME, Levy BI, Struijker-Boudier H (2003). Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation 107, 2864–2869. [DOI] [PubMed] [Google Scholar]
- [2].Davis EC (1993). Smooth muscle cell to elastic lamina connections in developing mouse aorta. Role in aortic medial organization. Lab Invest 68, 89–99. [PubMed] [Google Scholar]
- [3].Nakamura T, Lozano PR, Ikeda Y, Iwanaga Y, Hinek A, Minamisawa S, Cheng CF, Kobuke K, Dalton N, Takada Y, Tashiro K, Ross J Jr., Honjo T, Chien KR (2002). Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature 415, 171–175. [DOI] [PubMed] [Google Scholar]
- [4].Yanagisawa H, Davis EC, Starcher BC, Ouchi T, Yanagisawa M, Richardson JA, Olson EN (2002). Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature 415, 168–171. [DOI] [PubMed] [Google Scholar]
- [5].McLaughlin PJ, Chen Q, Horiguchi M, Starcher BC, Stanton JB, Broekelmann TJ, Marmorstein AD, McKay B, Mecham R, Nakamura T, Marmorstein LY (2006). Targeted disruption of fibulin-4 abolishes elastogenesis and causes perinatal lethality in mice. Mol Cell Biol 26, 1700–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wagenseil JE, Mecham RP (2007). New insights into elastic fiber assembly. Birth Defects Res C Embryo Today 81, 229–240. [DOI] [PubMed] [Google Scholar]
- [7].Yanagisawa H, Davis EC (2010). Unraveling the mechanism of elastic fiber assembly: The roles of short fibulins. Int J Biochem Cell Biol 42, 1084–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mecham RP (2018). Elastin in lung development and disease pathogenesis. Matrix Biol 73, 6–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mithieux SM, Weiss AS (2005). Elastin. Adv Protein Chem 70, 437–461. [DOI] [PubMed] [Google Scholar]
- [10].Vrhovski B, Jensen S, Weiss AS (1997). Coacervation characteristics of recombinant human tropoelastin. Eur J Biochem 250, 92–98. [DOI] [PubMed] [Google Scholar]
- [11].Keeley FW, Bellingham CM, Woodhouse KA (2002). Elastin as a self-organizing biomaterial: use of recombinantly expressed human elastin polypeptides as a model for investigations of structure and self-assembly of elastin. Philos Trans R Soc Lond B Biol Sci 357, 185–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lucero HA, Kagan HM (2006). Lysyl oxidase: an oxidative enzyme and effector of cell function. Cell Mol Life Sci 63, 2304–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Barker HE, Cox TR, Erler JT (2012). The rationale for targeting the LOX family in cancer. Nat Rev Cancer 12, 540–552. [DOI] [PubMed] [Google Scholar]
- [14].Zhang X, Wang Q, Wu J, Wang J, Shi Y, Liu M (2018). Crystal structure of human lysyl oxidase-like 2 (hLOXL2) in a precursor state. Proc Natl Acad Sci U S A 115, 3828–3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sato F, Seino-Sudo R, Okada M, Sakai H, Yumoto T, Wachi H (2017). Lysyl Oxidase Enhances the Deposition of Tropoelastin through the Catalysis of Tropoelastin Molecules on the Cell Surface. Biol Pharm Bull 40, 1646–1653. [DOI] [PubMed] [Google Scholar]
- [16].Papke CL, Yanagisawa H (2014). Fibulin-4 and fibulin-5 in elastogenesis and beyond: Insights from mouse and human studies. Matrix Biol 37, 142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Nakamura T (2018). Roles of short fibulins, a family of matricellular proteins, in lung matrix assembly and disease. Matrix Biol 73, 21–33. [DOI] [PubMed] [Google Scholar]
- [18].Hirai M, Ohbayashi T, Horiguchi M, Okawa K, Hagiwara A, Chien KR, Kita T, Nakamura T (2007). Fibulin-5/DANCE has an elastogenic organizer activity that is abrogated by proteolytic cleavage in vivo. J Cell Biol 176, 1061–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cirulis JT, Bellingham CM, Davis EC, Hubmacher D, Reinhardt DP, Mecham RP, Keeley FW (2008). Fibrillins, fibulins, and matrix-associated glycoprotein modulate the kinetics and morphology of in vitro self-assembly of a recombinant elastin-like polypeptide. Biochemistry 47, 12601–12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Choi J, Bergdahl A, Zheng Q, Starcher B, Yanagisawa H, Davis EC (2009). Analysis of dermal elastic fibers in the absence of fibulin-5 reveals potential roles for fibulin-5 in elastic fiber assembly. Matrix Biol 28, 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kobayashi N, Kostka G, Garbe JH, Keene DR, Bachinger HP, Hanisch FG, Markova D, Tsuda T, Timpl R, Chu ML, Sasaki T (2007). A comparative analysis of the fibulin protein family. Biochemical characterization, binding interactions, and tissue localization. J Biol Chem 282, 11805–11816. [DOI] [PubMed] [Google Scholar]
- [22].Hornstra IK, Birge S, Starcher B, Bailey AJ, Mecham RP, Shapiro SD (2003). Lysyl oxidase is required for vascular and diaphragmatic development in mice. J Biol Chem 278, 14387–14393. [DOI] [PubMed] [Google Scholar]
- [23].Horiguchi M, Inoue T, Ohbayashi T, Hirai M, Noda K, Marmorstein LY, Yabe D, Takagi K, Akama TO, Kita T, Kimura T, Nakamura T (2009). Fibulin-4 conducts proper elastogenesis via interaction with cross-linking enzyme lysyl oxidase. Proc Natl Acad Sci U S A 106, 19029–19034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Huang J, Davis EC, Chapman SL, Budatha M, Marmorstein LY, Word RA, Yanagisawa H (2010). Fibulin-4 deficiency results in ascending aortic aneurysms: a potential link between abnormal smooth muscle cell phenotype and aneurysm progression. Circ Res 106, 583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Liu X, Zhao Y, Gao J, Pawlyk B, Starcher B, Spencer JA, Yanagisawa H, Zuo J, Li T (2004). Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat Genet 36, 178–182. [DOI] [PubMed] [Google Scholar]
- [26].Sakai LY, Keene DR, Engvall E (1986). Fibrillin, a new 350-kD glycoprotein, is a component of extracellular microfibrils. J Cell Biol 103, 2499–2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhang H, Apfelroth SD, Hu W, Davis EC, Sanguineti C, Bonadio J, Mecham RP, Ramirez F (1994). Structure and expression of fibrillin-2, a novel microfibrillar component preferentially located in elastic matrices. J Cell Biol 124, 855–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hubmacher D, Tiedemann K, Reinhardt DP (2006). Fibrillins: from biogenesis of microfibrils to signaling functions. Curr Top Dev Biol 75, 93–123. [DOI] [PubMed] [Google Scholar]
- [29].Kinsey R, Williamson MR, Chaudhry S, Mellody KT, McGovern A, Takahashi S, Shuttleworth CA, Kielty CM (2008). Fibrillin-1 microfibril deposition is dependent on fibronectin assembly. J Cell Sci 121, 2696–2704. [DOI] [PubMed] [Google Scholar]
- [30].Sabatier L, Chen D, Fagotto-Kaufmann C, Hubmacher D, McKee MD, Annis DS, Mosher DF, Reinhardt DP (2009). Fibrillin assembly requires fibronectin. Mol Biol Cell 20, 846–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sabatier L, Djokic J, Hubmacher D, Dzafik D, Nelea V, Reinhardt DP (2014). Heparin/heparan sulfate controls fibrillin-1, −2 and −3 self-interactions in microfibril assembly. FEBS Lett 588, 2890–2897. [DOI] [PubMed] [Google Scholar]
- [32].Sabatier L, Djokic J, Fagotto-Kaufmann C, Chen M, Annis DS, Mosher DF, Reinhardt DP (2013). Complex contributions of fibronectin to initiation and maturation of microfibrils. Biochem J 456, 283–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Carta L, Pereira L, Arteaga-Solis E, Lee-Arteaga SY, Lenart B, Starcher B, Merkel CA, Sukoyan M, Kerkis A, Hazeki N, Keene DR, Sakai LY, Ramirez F (2006). Fibrillins 1 and 2 perform partially overlapping functions during aortic development. J Biol Chem 281, 8016–8023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Isogai Z, Ono RN, Ushiro S, Keene DR, Chen Y, Mazzieri R, Charbonneau NL, Reinhardt DP, Rifkin DB, Sakai LY (2003). Latent transforming growth factor beta-binding protein 1 interacts with fibrillin and is a microfibril-associated protein. J Biol Chem 278, 2750–2757. [DOI] [PubMed] [Google Scholar]
- [35].Ono RN, Sengle G, Charbonneau NL, Carlberg V, Bachinger HP, Sasaki T, Lee-Arteaga S, Zilberberg L, Rifkin DB, Ramirez F, Chu ML, Sakai LY (2009). Latent transforming growth factor beta-binding proteins and fibulins compete for fibrillin-1 and exhibit exquisite specificities in binding sites. J Biol Chem 284, 16872–16881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Jensen SA, Handford PA (2016). New insights into the structure, assembly and biological roles of 10–12 nm connective tissue microfibrils from fibrillin-1 studies. Biochem J 473, 827–838. [DOI] [PubMed] [Google Scholar]
- [37].Schiavinato A, Keene DR, Imhof T, Doliana R, Sasaki T, Sengle G (2017). Fibulin-4 deposition requires EMILIN-1 in the extracellular matrix of osteoblasts. Sci Rep 7, 5526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Robertson IB, Horiguchi M, Zilberberg L, Dabovic B, Hadjiolova K, Rifkin DB (2015). Latent TGF-beta-binding proteins. Matrix Biol 47, 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hirani R, Hanssen E, Gibson MA (2007). LTBP-2 specifically interacts with the amino-terminal region of fibrillin-1 and competes with LTBP-1 for binding to this microfibrillar protein. Matrix Biol 26, 213–223. [DOI] [PubMed] [Google Scholar]
- [40].Zilberberg L, Todorovic V, Dabovic B, Horiguchi M, Courousse T, Sakai LY, Rifkin DB (2012). Specificity of latent TGF-beta binding protein (LTBP) incorporation into matrix: role of fibrillins and fibronectin. J Cell Physiol 227, 3828–3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gleizes PE, Beavis RC, Mazzieri R, Shen B, Rifkin DB (1996). Identification and characterization of an eight-cysteine repeat of the latent transforming growth factor-beta binding protein-1 that mediates bonding to the latent transforming growth factor-beta1. J Biol Chem 271, 29891–29896. [DOI] [PubMed] [Google Scholar]
- [42].Saharinen J, Taipale J, Keski-Oja J (1996). Association of the small latent transforming growth factor-beta with an eight cysteine repeat of its binding protein LTBP-1. EMBO J 15, 245–253. [PMC free article] [PubMed] [Google Scholar]
- [43].Sterner-Kock A, Thorey IS, Koli K, Wempe F, Otte J, Bangsow T, Kuhlmeier K, Kirchner T, Jin S, Keski-Oja J, von Melchner H (2002). Disruption of the gene encoding the latent transforming growth factor-beta binding protein 4 (LTBP-4) causes abnormal lung development, cardiomyopathy, and colorectal cancer. Genes Dev 16, 2264–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Dabovic B, Chen Y, Choi J, Vassallo M, Dietz HC, Ramirez F, von Melchner H, Davis EC, Rifkin DB (2009). Dual functions for LTBP in lung development: LTBP-4 independently modulates elastogenesis and TGF-beta activity. J Cell Physiol 219, 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Noda K, Dabovic B, Takagi K, Inoue T, Horiguchi M, Hirai M, Fujikawa Y, Akama TO, Kusumoto K, Zilberberg L, Sakai LY, Koli K, Naitoh M, von Melchner H, Suzuki S, Rifkin DB, Nakamura T (2013). Latent TGF-beta binding protein 4 promotes elastic fiber assembly by interacting with fibulin-5. Proc Natl Acad Sci U S A 110, 2852–2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Bultmann-Mellin I, Conradi A, Maul AC, Dinger K, Wempe F, Wohl AP, Imhof T, Wunderlich FT, Bunck AC, Nakamura T, Koli K, Bloch W, Ghanem A, Heinz A, von Melchner H, Sengle G, Sterner-Kock A (2015). Modeling autosomal recessive cutis laxa type 1C in mice reveals distinct functions for Ltbp-4 isoforms. Dis Model Mech 8, 403–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Bultmann-Mellin I, Essers J, van Heijingen PM, von Melchner H, Sengle G, Sterner-Kock A (2016). Function of Ltbp-4L and fibulin-4 in survival and elastogenesis in mice. Dis Model Mech 9, 1367–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wagenseil JE, Mecham RP (2009). Vascular extracellular matrix and arterial mechanics. Physiol Rev 89, 957–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Li DY, Brooke B, Davis EC, Mecham RP, Sorensen LK, Boak BB, Eichwald E, Keating MT (1998). Elastin is an essential determinant of arterial morphogenesis. Nature 393, 276–280. [DOI] [PubMed] [Google Scholar]
- [50].Wagenseil JE, Ciliberto CH, Knutsen RH, Levy MA, Kovacs A, Mecham RP (2009). Reduced vessel elasticity alters cardiovascular structure and function in newborn mice. Circ Res 104, 1217–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kim J, Staiculescu MC, Cocciolone AJ, Yanagisawa H, Mecham RP, Wagenseil JE (2017). Crosslinked elastic fibers are necessary for low energy loss in the ascending aorta. J Biomech 61, 199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Faury G, Pezet M, Knutsen RH, Boyle WA, Heximer SP, McLean SE, Minkes RK, Blumer KJ, Kovacs A, Kelly DP, Li DY, Starcher B, Mecham RP (2003). Developmental adaptation of the mouse cardiovascular system to elastin haploinsufficiency. J Clin Invest 112, 1419–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Wagenseil JE, Nerurkar NL, Knutsen RH, Okamoto RJ, Li DY, Mecham RP (2005). Effects of elastin haploinsufficiency on the mechanical behavior of mouse arteries. Am J Physiol Heart Circ Physiol 289, H1209–1217. [DOI] [PubMed] [Google Scholar]
- [54].Li DY, Faury G, Taylor DG, Davis EC, Boyle WA, Mecham RP, Stenzel P, Boak B, Keating MT (1998). Novel arterial pathology in mice and humans hemizygous for elastin. J Clin Invest 102, 1783–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Le VP, Knutsen RH, Mecham RP, Wagenseil JE (2011). Decreased aortic diameter and compliance precedes blood pressure increases in postnatal development of elastin-insufficient mice. Am J Physiol Heart Circ Physiol 301, H221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Cheng JK, Stoilov I, Mecham RP, Wagenseil JE (2013). A fiber-based constitutive model predicts changes in amount and organization of matrix proteins with development and disease in the mouse aorta. Biomech Model Mechanobiol 12, 497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hirano E, Knutsen RH, Sugitani H, Ciliberto CH, Mecham RP (2007). Functional rescue of elastin insufficiency in mice by the human elastin gene: implications for mouse models of human disease. Circ Res 101, 523–531. [DOI] [PubMed] [Google Scholar]
- [58].Li DY, Toland AE, Boak BB, Atkinson DL, Ensing GJ, Morris CA, Keating MT (1997). Elastin point mutations cause an obstructive vascular disease, supravalvular aortic stenosis. Hum Mol Genet 6, 1021–1028. [DOI] [PubMed] [Google Scholar]
- [59].Jiao Y, Li G, Korneva A, Caulk AW, Qin L, Bersi MR, Li Q, Li W, Mecham RP, Humphrey JD, Tellides G (2017). Deficient Circumferential Growth Is the Primary Determinant of Aortic Obstruction Attributable to Partial Elastin Deficiency. Arterioscler Thromb Vasc Biol 37, 930–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ferruzzi J, Bersi MR, Uman S, Yanagisawa H, Humphrey JD (2015). Decreased elastic energy storage, not increased material stiffness, characterizes central artery dysfunction in fibulin-5 deficiency independent of sex. J Biomech Eng 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Le VP, Cheng JK, Kim J, Staiculescu MC, Ficker SW, Sheth SC, Bhayani SA, Mecham RP, Yanagisawa H, Wagenseil JE (2015). Mechanical factors direct mouse aortic remodelling during early maturation. J R Soc Interface 12, 20141350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Murtada SI, Ferruzzi J, Yanagisawa H, Humphrey JD (2016). Reduced Biaxial Contractility in the Descending Thoracic Aorta of Fibulin-5 Deficient Mice. J Biomech Eng 138, 051008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kim J, Procknow JD, Yanagisawa H, Wagenseil JE (2015). Differences in genetic signaling, and not mechanical properties of the wall, are linked to ascending aortic aneurysms in fibulin-4 knockout mice. Am J Physiol Heart Circ Physiol 309, H103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Igoucheva O, Alexeev V, Halabi CM, Adams SM, Stoilov I, Sasaki T, Arita M, Donahue A, Mecham RP, Birk DE, Chu ML (2015). Fibulin-4 E57K Knock-in Mice Recapitulate Cutaneous, Vascular and Skeletal Defects of Recessive Cutis Laxa 1B with both Elastic Fiber and Collagen Fibril Abnormalities. J Biol Chem 290, 21443–21459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Halabi CM, Broekelmann TJ, Lin M, Lee VS, Chu ML, Mecham RP (2017). Fibulin-4 is essential for maintaining arterial wall integrity in conduit but not muscular arteries. Sci Adv 3, e1602532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Yamashiro Y, Papke CL, Kim J, Ringuette LJ, Zhang QJ, Liu ZP, Mirzaei H, Wagenseil JE, Davis EC, Yanagisawa H (2015). Abnormal mechanosensing and cofilin activation promote the progression of ascending aortic aneurysms in mice. Sci Signal 8, ra105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Bellini C, Bersi MR, Caulk AW, Ferruzzi J, Milewicz DM, Ramirez F, Rifkin DB, Tellides G, Yanagisawa H, Humphrey JD (2017). Comparison of 10 murine models reveals a distinct biomechanical phenotype in thoracic aortic aneurysms. J R Soc Interface 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Yamashiro Y, Thang BQ, Shin SJ, Lino CA, Nakamura T, Kim J, Sugiyama K, Tokunaga C, Sakamoto H, Osaka M, Davis EC, Wagenseil JE, Hiramatsu Y, Yanagisawa H (2018). Role of Thrombospondin-1 in Mechanotransduction and Development of Thoracic Aortic Aneurysm in Mouse and Humans. Circ Res 123, 660–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Le VP, Yamashiro Y, Yanagisawa H, Wagenseil JE (2014). Measuring, reversing, and modeling the mechanical changes due to the absence of Fibulin-4 in mouse arteries. Biomech Model Mechanobiol 13, 1081–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Maki JM, Rasanen J, Tikkanen H, Sormunen R, Makikallio K, Kivirikko KI, Soininen R (2002). Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation 106, 2503–2509. [DOI] [PubMed] [Google Scholar]
- [71].Staiculescu MC, Kim J, Mecham RP, Wagenseil JE (2017). Mechanical behavior and matrisome gene expression in the aneurysm-prone thoracic aorta of newborn lysyl oxidase knockout mice. Am J Physiol Heart Circ Physiol 313, H446–H456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Guo DC, Regalado ES, Gong L, Duan X, Santos-Cortez RL, Arnaud P, Ren Z, Cai B, Hostetler EM, Moran R, Liang D, Estrera A, Safi HJ, University of Washington Center for Mendelian, G., Leal SM, Bamshad MJ, Shendure J, Nickerson DA, Jondeau G, Boileau C, Milewicz DM (2016). LOX Mutations Predispose to Thoracic Aortic Aneurysms and Dissections. Circ Res 118, 928–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Lee VS, Halabi CM, Hoffman EP, Carmichael N, Leshchiner I, Lian CG, Bierhals AJ, Vuzman D, Brigham Genomic M, Mecham RP, Frank NY, Stitziel NO (2016). Loss of function mutation in LOX causes thoracic aortic aneurysm and dissection in humans. Proc Natl Acad Sci U S A 113, 8759–8764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Ren W, Liu Y, Wang X, Jia L, Piao C, Lan F, Du J (2016). Beta-Aminopropionitrile monofumarate induces thoracic aortic dissection in C57BL/6 mice. Sci Rep 6, 28149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Jia LX, Zhang WM, Zhang HJ, Li TT, Wang YL, Qin YW, Gu H, Du J (2015). Mechanical stretch-induced endoplasmic reticulum stress, apoptosis and inflammation contribute to thoracic aortic aneurysm and dissection. J Pathol 236, 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Pereira L, Andrikopoulos K, Tian J, Lee SY, Keene DR, Ono R, Reinhardt DP, Sakai LY, Biery NJ, Bunton T, Dietz HC, Ramirez F (1997). Targetting of the gene encoding fibrillin-1 recapitulates the vascular aspect of Marfan syndrome. Nat Genet 17, 218–222. [DOI] [PubMed] [Google Scholar]
- [77].Pereira L, Lee SY, Gayraud B, Andrikopoulos K, Shapiro SD, Bunton T, Biery NJ, Dietz HC, Sakai LY, Ramirez F (1999). Pathogenetic sequence for aneurysm revealed in mice underexpressing fibrillin-1. Proc Natl Acad Sci U S A 96, 3819–3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Bellini C, Korneva A, Zilberberg L, Ramirez F, Rifkin DB, Humphrey JD (2016). Differential ascending and descending aortic mechanics parallel aneurysmal propensity in a mouse model of Marfan syndrome. J Biomech 49, 2383–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Halabi CM, Broekelmann TJ, Knutsen RH, Ye L, Mecham RP, Kozel BA (2015). Chronic antihypertensive treatment improves pulse pressure but not large artery mechanics in a mouse model of congenital vascular stiffness. Am J Physiol Heart Circ Physiol 309, H1008–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Roach MR, Burton AC (1957). The reason for the shape of the distensibility curves of arteries. Can J Biochem Physiol 35, 681–690. [PubMed] [Google Scholar]
- [81].Papke CL, Tsunezumi J, Ringuette LJ, Nagaoka H, Terajima M, Yamashiro Y, Urquhart G, Yamauchi M, Davis EC, Yanagisawa H (2015). Loss of fibulin-4 disrupts collagen synthesis and maturation: implications for pathology resulting from EFEMP2 mutations. Hum Mol Genet 24, 5867–5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Chow MJ, Turcotte R, Lin CP, Zhang Y (2014). Arterial extracellular matrix: a mechanobiological study of the contributions and interactions of elastin and collagen. Biophys J 106, 2684–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Fonck E, Prod’hom G, Roy S, Augsburger L, Rufenacht DA, Stergiopulos N (2007). Effect of elastin degradation on carotid wall mechanics as assessed by a constituent-based biomechanical model. Am J Physiol Heart Circ Physiol 292, H2754–2763. [DOI] [PubMed] [Google Scholar]
- [84].Kamm KE, Stull JT (1989). Regulation of smooth muscle contractile elements by second messengers. Annu Rev Physiol 51, 299–313. [DOI] [PubMed] [Google Scholar]
- [85].Yamin R, Morgan KG (2012). Deciphering actin cytoskeletal function in the contractile vascular smooth muscle cell. J Physiol 590, 4145–4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Fatigati V, Murphy RA (1984). Actin and tropomyosin variants in smooth muscles. Dependence on tissue type. J Biol Chem 259, 14383–14388. [PubMed] [Google Scholar]
- [87].Gallant C, Appel S, Graceffa P, Leavis P, Lin JJ, Gunning PW, Schevzov G, Chaponnier C, DeGnore J, Lehman W, Morgan KG (2011). Tropomyosin variants describe distinct functional subcellular domains in differentiated vascular smooth muscle cells. Am J Physiol Cell Physiol 300, C1356–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Kim HR, Gallant C, Leavis PC, Gunst SJ, Morgan KG (2008). Cytoskeletal remodeling in differentiated vascular smooth muscle is actin isoform dependent and stimulus dependent. Am J Physiol Cell Physiol 295, C768–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Karimi A, Milewicz DM (2016). Structure of the Elastin-Contractile Units in the Thoracic Aorta and How Genes That Cause Thoracic Aortic Aneurysms and Dissections Disrupt This Structure. Can J Cardiol 32, 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Dietz HC, Cutting GR, Pyeritz RE, Maslen CL, Sakai LY, Corson GM, Puffenberger EG, Hamosh A, Nanthakumar EJ, Curristin SM, et al. (1991). Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature 352, 337–339. [DOI] [PubMed] [Google Scholar]
- [91].Dasouki M, Markova D, Garola R, Sasaki T, Charbonneau NL, Sakai LY, Chu ML (2007). Compound heterozygous mutations in fibulin-4 causing neonatal lethal pulmonary artery occlusion, aortic aneurysm, arachnodactyly, and mild cutis laxa. Am J Med Genet A 143A, 2635–2641. [DOI] [PubMed] [Google Scholar]
- [92].Barbier M, Gross MS, Aubart M, Hanna N, Kessler K, Guo DC, Tosolini L, Ho-Tin-Noe B, Regalado E, Varret M, Abifadel M, Milleron O, Odent S, Dupuis-Girod S, Faivre L, Edouard T, Dulac Y, Busa T, Gouya L, Milewicz DM, Jondeau G, Boileau C (2014). MFAP5 loss-of-function mutations underscore the involvement of matrix alteration in the pathogenesis of familial thoracic aortic aneurysms and dissections. Am J Hum Genet 95, 736–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Capuano A, Bucciotti F, Farwell KD, Tippin Davis B, Mroske C, Hulick PJ, Weissman SM, Gao Q, Spessotto P, Colombatti A, Doliana R (2016). Diagnostic Exome Sequencing Identifies a Novel Gene, EMILIN1, Associated with Autosomal-Dominant Hereditary Connective Tissue Disease. Hum Mutat 37, 84–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Guo DC, Regalado ES, Pinard A, Chen J, Lee K, Rigelsky C, Zilberberg L, Hostetler EM, Aldred M, Wallace SE, Prakash SK, University of Washington Center for Mendelian, G., Leal SM, Bamshad MJ, Nickerson DA, Natowicz M, Rifkin DB, Milewicz DM (2018). LTBP3 Pathogenic Variants Predispose Individuals to Thoracic Aortic Aneurysms and Dissections. Am J Hum Genet 102, 706–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Bax DV, Bernard SE, Lomas A, Morgan A, Humphries J, Shuttleworth CA, Humphries MJ, Kielty CM (2003). Cell adhesion to fibrillin-1 molecules and microfibrils is mediated by alpha 5 beta 1 and alpha v beta 3 integrins. J Biol Chem 278, 34605–34616. [DOI] [PubMed] [Google Scholar]
- [96].Cook JR, Carta L, Benard L, Chemaly ER, Chiu E, Rao SK, Hampton TG, Yurchenco P, Gen TACRC, Costa KD, Hajjar RJ, Ramirez F (2014). Abnormal muscle mechanosignaling triggers cardiomyopathy in mice with Marfan syndrome. J Clin Invest 124, 1329–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Loeys B, Van Maldergem L, Mortier G, Coucke P, Gerniers S, Naeyaert JM, De Paepe A (2002). Homozygosity for a missense mutation in fibulin-5 (FBLN5) results in a severe form of cutis laxa. Hum Mol Genet 11, 2113–2118. [DOI] [PubMed] [Google Scholar]
- [98].Abercrombie M, Heaysman JE, Pegrum SM (1971). The locomotion of fibroblasts in culture. IV. Electron microscopy of the leading lamella. Exp Cell Res 67, 359–367. [DOI] [PubMed] [Google Scholar]
- [99].Humphrey JD, Dufresne ER, Schwartz MA (2014). Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol 15, 802–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Martino F, Perestrelo AR, Vinarsky V, Pagliari S, Forte G (2018). Cellular Mechanotransduction: From Tension to Function. Front Physiol 9, 824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Vaynberg J, Fukuda K, Lu F, Bialkowska K, Chen Y, Plow EF, Qin J (2018). Non-catalytic signaling by pseudokinase ILK for regulating cell adhesion. Nat Commun 9, 4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Shen D, Li J, Lepore JJ, Anderson TJ, Sinha S, Lin AY, Cheng L, Cohen ED, Roberts JD Jr., Dedhar S, Parmacek MS, Gerszten RE (2011). Aortic aneurysm generation in mice with targeted deletion of integrin-linked kinase in vascular smooth muscle cells. Circ Res 109, 616–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Arnold TD, Zang K, Vallejo-Illarramendi A (2013). Deletion of integrin-linked kinase from neural crest cells in mice results in aortic aneurysms and embryonic lethality. Dis Model Mech 6, 1205–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Goult BT, Yan J, Schwartz MA (2018). Talin as a mechanosensitive signaling hub. J Cell Biol 217, 3776–3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Sun Z, Costell M, Fassler R (2019). Integrin activation by talin, kindlin and mechanical forces. Nat Cell Biol 21, 25–31. [DOI] [PubMed] [Google Scholar]
- [106].Wei X, Sun Y, Wu Y, Zhu J, Gao B, Yan H, Zhao Z, Zhou J, Jing Z (2017). Downregulation of Talin-1 expression associates with increased proliferation and migration of vascular smooth muscle cells in aortic dissection. BMC Cardiovasc Disord 17, 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Milewicz DM, Trybus KM, Guo DC, Sweeney HL, Regalado E, Kamm K, Stull JT (2017). Altered Smooth Muscle Cell Force Generation as a Driver of Thoracic Aortic Aneurysms and Dissections. Arterioscler Thromb Vasc Biol 37, 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Zhu L, Vranckx R, Khau Van Kien P, Lalande A, Boisset N, Mathieu F, Wegman M, Glancy L, Gasc JM, Brunotte F, Bruneval P, Wolf JE, Michel JB, Jeunemaitre X (2006). Mutations in myosin heavy chain 11 cause a syndrome associating thoracic aortic aneurysm/aortic dissection and patent ductus arteriosus. Nat Genet 38, 343–349. [DOI] [PubMed] [Google Scholar]
- [109].Pannu H, Tran-Fadulu V, Papke CL, Scherer S, Liu Y, Presley C, Guo D, Estrera AL, Safi HJ, Brasier AR, Vick GW, Marian AJ, Raman CS, Buja LM, Milewicz DM (2007). MYH11 mutations result in a distinct vascular pathology driven by insulin-like growth factor 1 and angiotensin II. Hum Mol Genet 16, 2453–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Guo DC, Pannu H, Tran-Fadulu V, Papke CL, Yu RK, Avidan N, Bourgeois S, Estrera AL, Safi HJ, Sparks E, Amor D, Ades L, McConnell V, Willoughby CE, Abuelo D, Willing M, Lewis RA, Kim DH, Scherer S, Tung PP, Ahn C, Buja LM, Raman CS, Shete SS, Milewicz DM (2007). Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet 39, 1488–1493. [DOI] [PubMed] [Google Scholar]
- [111].Lu H, Fagnant PM, Bookwalter CS, Joel P, Trybus KM (2015). Vascular disease-causing mutation R258C in ACTA2 disrupts actin dynamics and interaction with myosin. Proc Natl Acad Sci U S A 112, E4168–4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Lu H, Fagnant PM, Krementsova EB, Trybus KM (2016). Severe Molecular Defects Exhibited by the R179H Mutation in Human Vascular Smooth Muscle alpha-Actin. J Biol Chem 291, 21729–21739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Wang L, Guo DC, Cao J, Gong L, Kamm KE, Regalado E, Li L, Shete S, He WQ, Zhu MS, Offermanns S, Gilchrist D, Elefteriades J, Stull JT, Milewicz DM (2010). Mutations in myosin light chain kinase cause familial aortic dissections. Am J Hum Genet 87, 701–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Guo DC, Regalado E, Casteel DE, Santos-Cortez RL, Gong L, Kim JJ, Dyack S, Horne SG, Chang G, Jondeau G, Boileau C, Coselli JS, Li Z, Leal SM, Shendure J, Rieder MJ, Bamshad MJ, Nickerson DA, Gen TACRC, National Heart, L., Blood Institute Grand Opportunity Exome Sequencing, P., Kim C, Milewicz DM (2013). Recurrent gain-of-function mutation in PRKG1 causes thoracic aortic aneurysms and acute aortic dissections. Am J Hum Genet 93, 398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Dale M, Fitzgerald MP, Liu Z, Meisinger T, Karpisek A, Purcell LN, Carson JS, Harding P, Lang H, Koutakis P, Batra R, Mietus CJ, Casale G, Pipinos I, Baxter BT, Xiong W (2017). Premature aortic smooth muscle cell differentiation contributes to matrix dysregulation in Marfan Syndrome. PLoS One 12, e0186603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Granata A, Serrano F, Bernard WG, McNamara M, Low L, Sastry P, Sinha S (2017). An iPSC-derived vascular model of Marfan syndrome identifies key mediators of smooth muscle cell death. Nat Genet 49, 97–109. [DOI] [PubMed] [Google Scholar]