Introduction
Eosinophilic esophagitis (EoE) is a chronic immune/antigen-mediated disorder defined by eosinophilic-predominant inflammation and esophageal dysfunction.1,2 EoE represents an important etiology of esophageal morbidity and a leading cause of esophagitis.3
Topical corticosteroids (tCS) and food elimination diets (FED) improve the clinical and histologic features of EoE.4 However, appreciable rates of treatment failure exist,3,5 tCS may elicit side effects, and FED may impair quality of life.6 An alternative to monotherapy may be a combined treatment strategy. Few data exist describing combined treatment7 and none examined a combined treatment strategy in adults. This study aims to assess the impact of combined therapy on EoE disease activity in a cohort of adolescents and adults.
Methods
We performed a retrospective cohort study using the University of North Carolina (UNC) EoE Clinicopathologic database between 2002 – 2017. The UNC IRB approved this study.
Included patients received continuous combined treatment with tCS and FED through the follow-up period and had prior non-response to proton pump inhibitors.2 Patients were excluded if additional gastrointestinal tract segments demonstrated eosinophilia, or if they received an alternative EoE therapy.
Most patients (70%) received recommended adult tCS doses on monotherapy, and most (93%) completed a follow-up endoscopy on FED monotherapy.1 Monotherapy was not changed prior to combined treatment.
Demographics, symptoms, endoscopic findings, and outcomes (symptomatic global improvement [yes/no]; endoscopic severity [individual findings]; histologic change [absolute peak eosinophil count]) were extracted. Outcomes were captured before combined treatment, following initial follow-up endoscopy, and following the last endoscopy on combined treatment. Non-response to monotherapy was defined as histologic (≥ 15 eosinophils per high-power field (eos/hpf) on post-treatment assessment) or clinical (i.e. no symptomatic improvement, side effects, or inability to remain compliant). We used bivariate statistics to analyze pre- and post-treatment outcomes. For repeated measures, McNemar’s test and paired t-tests were used.
Results
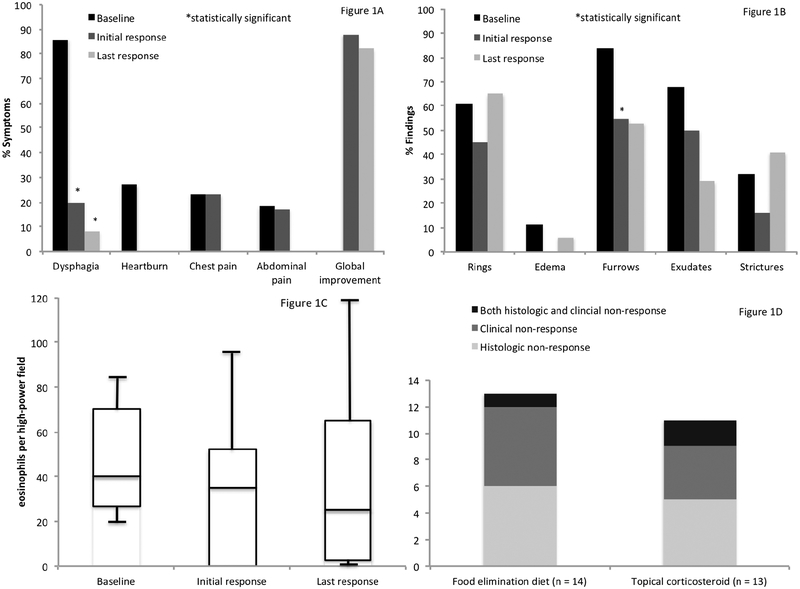
A total of 23 patients met inclusion criteria (mean age 30.5 years, 52% female, 86% white). Dysphagia, heartburn, and chest pain were reported in 86%, 27%, and 23% prior to combined treatment, respectively. Endoscopic findings included rings (61%), furrows (84%), and strictures (32%). The mean peak eosinophil count was 55 ± 36 eos/hpf. There were 21 (91%) patients previously managed with tCS and/or FED monotherapy. A histologic or clinical non-response to monotherapy was documented in 13/14 (93%) treated with a FED, 11/13 (85%) treated with a tCS, and 19/21 (90%) overall (Figure 1).
Figure 1.
A. Proportion of patients reporting clinical symptoms prior to combined treatment, as well as immediately following the initial combined treatment course, and the last available report while on combined treatment. Figure 1B. Proportion of individual endoscopic findings prior to combined treatment, as well as immediately following the initial combined treatment course, and the last available report while on combined treatment. Figure 1C. Box plots depicting eosinophil counts prior to combined treatment, as well as immediately following the initial combined treatment course, and the last available report while on combined treatment. Figure 1D. Clinical and histologic responses to monotherapy with food elimination diet or topical corticosteroids prior to combined therapy. **Note: There were an additional two patients treated with topical corticosteroids reporting treatment-related side effects (e.g. irritability) prompting discontinuation.
The mean initial daily doses of fluticasone and budesonide were 1213 mcg (range: 880 – 2220 mcg) and 2.0 mg (range: 0.5 – 4.0 mg), respectively. Diets removed 1 – 29 foods; 9 patients excluded components of the six-food elimination diet and 14 utilized targeted elimination diets.
The mean time to follow-up after combination therapy was 4 months. Here, 88% reported global symptomatic improvement. Endoscopic findings trended toward or reached statistical significance [rings (61% vs. 45%; p = 0.41), furrows (84% vs. 55%; p = 0.02), and strictures (32% vs. 16%; p = 0.18)]. The mean peak eosinophil count decreased from 54 to 36 eos/hpf (p = 0.12).
At the end of 30.5 months of mean follow-up time, a global symptomatic improvement was documented in 82%. Improvements in rings (61% vs. 65%; p = 0.41) and strictures (32% vs. 41%; p = 0.08) attenuated, though improvements in furrows (84% vs. 55%; p = 0.10) persisted. Mean peak eosinophil counts were comparable to baseline (54 vs. 46 eos/hpf; p = 0.34). FED type and tCS formulation were not associated with outcomes.
Ultimately, 13 (57%) patients remained on combined therapy. Steroids were discontinued in 3, 2, and 5 patients for non-response, patient preference, and histologic response with desire to de-escalate therapy, respectively. Steroid dose was decreased in 5 patients and component(s) of FED re-introduced in 3 patients by end of follow-up.
Discussion
We found that combined treatment improved the histologic, symptomatic, and endoscopic features of EoE in adults and adolescents. This was achieved in a treatment-experienced cohort where 90% had not responded, either histologically or clinically, to previous monotherapy. Patients with active disease, particularly those who failed monotherapy, may represent those who would benefit from this approach. Combined treatment may also be reasonable for patients who respond histologically to monotherapy but suffer side effects or lifestyle burdens.
Limitations include retrospective design, potential misclassification bias, and heterogeneous FEDs, as new evidence suggests that targeted elimination is less effective than empiric.8 Strengths include analysis of a novel treatment approach, homogenous cohort, and real-world applicability.
In summary, combined treatment improved the histologic, symptomatic and endoscopic features of EoE in a cohort largely failing previous monotherapy.
Acknowledgments
Grant Support: This research was funded by NIH Awards T32 DK007634 (CCR, SE), T35 DK007386 (MT), K24 DK100548 (NJS) and R01 DK101856 (ESD)
Abbreviations:
- EoE
Eosinophilic esophagitis
- eos/hpf
eosinophils per high-power field
- FED
food elimination diets
- PPI
proton pump inhibitor
- tCS
topical corticosteroids
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential competing interests: Dr. Dellon is a consultant for Adare, Alivio, Allakos, AstraZeneca, Banner, Calypso, Enumeral, EsoCap, Gossamer Bio, GSK, Receptos/Celegene, Regeneron, Robarts, and Shire, receives research funding from Adare, Allakos, GSK, Meritage, Miraca, Nutricia, Receptos/Celgene, Regeneron, and Shire, and has received an educational grant from Allakos, Banner, and Holoclara. None of the other authors report and potential conflicts of interest with this study.
References
- 1.Dellon ES, Gonsalves N, Hirano I, et al. ACG clinical guideline: Evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol. 2013;108(5):679–92. [DOI] [PubMed] [Google Scholar]
- 2.Lucendo AJ, Molina-Infante J, Arias Á, et al. Guidelines on eosinophilic esophagitis: evidence-based statements and recommendations for diagnosis and management in children and adults. United Eur Gastroenterol J. 2017;5(3):335–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dellon ES, Hirano I. Epidemiology and Natural History of Eosinophilic Esophagitis. Gastroenterology. 2017;154(2):319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reed CC, Fan C, Koutlas NT, et al. Food elimination diets are effective for long-term treatment of adults with eosinophilic oesophagitis. Aliment Pharmacol Ther. 2017;46(9):836–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dellon E Management of refractory eosinophilic oesophagitis. Nat Rev Gastroenterol Hepatol. 2017;14(8):479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang R, Hirano I, Doerfler B, et al. Assessing Adherence and Barriers to Long-Term Elimination Diet Therapy in Adults with Eosinophilic Esophagitis. Dig Dis Sci. 2018;63(7):1756–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reed CC, Safta AM, Qasem S, et al. Combined and Alternating Topical Steroids and Food Elimination Diet for the Treatment of Eosinophilic Esophagitis. Dig Dis Sci. 2018:63(9):2381–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arias Á, González-Cervera J, Tenias JM, Lucendo AJ. Efficacy of dietary interventions for inducing histologic remission in patients with eosinophilic esophagitis: A systematic review and meta-analysis. Gastroenterology. 2014;146(7):1639–1648. [DOI] [PubMed] [Google Scholar]