Abstract
Objectives
The purpose of this study was to determine the prognostic value of feature tracking global longitudinal strain (GLS) measured during vasodilator stress cardiovascular magnetic resonance (CMR) imaging.
Background
Prior studies have suggested that blunted myocardial strain during dobutamine stress echocardiography maybe associated with adverse prognosis. Recent developments in CMR feature tracking techniques now allow assessment of strain in clinical practice using standard cine images without specialized pulse sequences or complex post-processing. Whether feature tracking GLS measured during vasodilator stress provides independent and incremental prognostic data is unclear.
Methods
Consecutive patients undergoing stress perfusion CMR were prospectively enrolled (n=535). Feature-tracking stress GLS was measured immediately after regadenoson perfusion. Patients were followed for major adverse cardiac events (MACE) - death, non-fatal myocardial infarction, heart failure hospitalization, sustained ventricular tachycardia and late-revascularization. Cox proportional-hazards regression modeling was used to examine the association between stress GLS and MACE. The incremental prognostic value of stress GLS was assessed in nested-models.
Results
Over a median follow-up of 1.5 years, 82 patients experienced MACE. By Kaplan-Meier-analysis, patients with stress GLS≥median (−19%) had significantly reduced event free survival compared to those with stress GLS<median (log-rank p<0.001). Stress GLS was significantly associated with risk-of-MACE after adjustment for clinical and imaging risk factors including ischemia, ejection fraction and LGE (HR=1.267; p<0.001). Addition of stress GLS into a model with clinical and imaging predictors resulted in significant increase in the C-index (from 0.80 to 0.85; p=0.031) and a continuous NRI of 0.898 (95%CI, 0.565-1.124).
Conclusions
Feature tracking stress GLS measured during vasodilator stress CMR is an independent predictor of MACE in patients with known or suspected CAD, incremental to common clinical and imaging risk factors. These findings suggest a role for feature tracking derived stress GLS in identifying patients at highest risk of adverse events following stress CMR.
Keywords: cardiac magnetic resonance imaging, coronary artery disease, global longitudinal strain, prognosis, stress testing
INTRODUCTION
Prior studies have suggested that blunted measures of myocardial strain during dobutamine stress echocardiography maybe associated with adverse prognosis(1). Recent developments in feature tracking techniques now allow assessment of strain with CMR using standard cine images without need for dedicated pulse sequences(2-4). However, stress CMR is most commonly performed by imaging the passage of gadolinium contrast during pharmacologically induced hyperemia and in contrast to dobutamine does not typically induce myocardial ischemia. Nevertheless perfusion abnormalities on vasodilator CMR stress testing are associated with significant reductions in global longitudinal strain (GLS)(5). It has been suggested that this is due to transmural redistribution of blood flow and alterations in the endocardial to epicardial flow gradient, affecting the endocardial longitudinal myocardial fibers(5).
Whether feature tracking strain GLS measured during vasodilator stress provides independent and incremental prognostic data is unclear. Therefore, the aim of this study was to determine the prognostic value of feature tracking GLS measured during vasodilator stress CMR imaging in patients with known or suspected coronary artery disease.
METHODS
Study Population
Consecutive patients undergoing stress perfusion CMR for evaluation of known or suspected coronary artery disease were prospectively enrolled (n=535). Patients were asked to refrain from caffeine for at least 12 hours prior to the test. Twenty-three patients had un-interpretable image quality for GLS assessment or inability to complete the full stress protocol leaving 512 patients. The study was approved by the local institutional review board and all patients provided signed informed consent.
CMR Acquisition
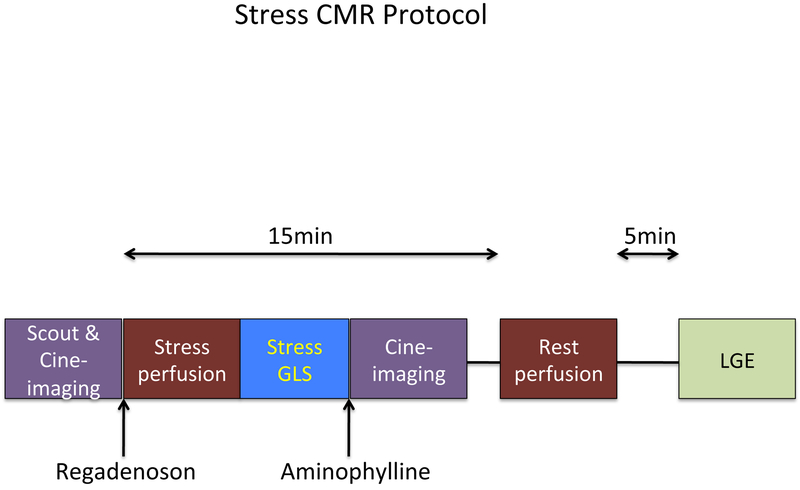
Images were acquired on a 3T scanner (Philips Achieva, Philips Medical Systems, Best, the Netherlands) using a six-element phased-array receiver coil. The stress protocol is shown in Figure 1. Steady-state free-precession cine images were acquired in multiple short-axis and three long-axis views (repetition time, 3.0 ms; echo time, 1.5 ms; flip angle, 40°; slice thickness 6 mm). Short-axis views were obtained every 1 cm to cover the entire left ventricle.
Figure 1. CMR stress protocol.
Image acquisition sequence. Stress GLS was measured immediately after stress perfusion. LGE = late gadolinium enhancement.
The patient table was then partially pulled outside the scanner bore to allow direct observation of the patient and full access. A 0.4 mg bolus of regadenoson (Lexiscan, Astellas Pharma Inc) was infused under continuous electrocardiography. Approximately 1 minute after regadenoson administration, the perfusion sequence was applied and Gadolinium contrast (0.075 mmol/kg) followed by a saline flush (30 ml) was infused (4.5 ml/s) via an antecubital vein. On the console, the perfusion images were observed as they were acquired, with breath-holding starting from the appearance of contrast in the right ventricular cavity. Imaging was completed 10 to 15 s after the gadolinium bolus had transited the left ventricular myocardium. Perfusion images consisted of three to four short-axis slices obtained every heartbeat with a saturation-recovery, gradient-echo sequence (repetition time 2.8 ms; echo time 1.1 ms; flip angle 20°; voxel size 2.5 × 2.5 × 8 mm). A 2-chamber long-axis cine view was obtained immediately after stress perfusion imaging but prior to administration of Aminophylline 100 mg IV. Rest perfusion images were acquired 15 minutes after stress imaging with an additional contrast bolus (0.075 mmol/kg) using identical sequence parameters. Five minutes after rest perfusion, late gadolinium enhancement (LGE) imaging was performed with a 2D segmented gradient echo phase-sensitive inversion-recovery sequence in the same views as cine-CMR. Inversion delay times were typically 280 to 360 ms.
CMR Analysis and GLS Assessment
LGE images were blindly interpreted by standard methods as described previously(6-8). In brief, LGE was scored visually on a 17-segment model with a 5-point scale for each segment (0 = no LGE, 1 = 1% to 25%, 2 = 26% to 50%, 3 = 51% to 75%, 4 = 76% to 100%). LGE extent as a percentage of LV myocardium was calculated by summing the regional scores, each weighted by the LGE range midpoint (i.e., 1 = 13%, 2 = 38%, 3 = 63%, 4 = 88%) and dividing by 17. Stress perfusion CMR images were evaluated according to a 16-segment model (American Heart Association 17-segment model minus the apical segment). The analysis of perfusion images was done visually by applying criteria similar to those used in many prior stress CMR studies (9-11). An ischemic segment was defined as follows: The myocardium appears dark for ≥3 frames after peak myocardial enhancement and is >1 pixel wide and resides in viable (LGE negative) myocardium and conforms to the distribution territory of one or more coronary arteries. The total number of ischemic segments was calculated for each patient.
GLS was measured using cine images acquired before and after regadenoson stress perfusion. Endocardial left ventricular contours were manually traced (by a single physician (S.R.) who was blinded to patient information and outcomes) in the 2-chamber long-axis cine view to derive stress GLS using the Qstrain feature tracking package (Medis Medical Imaging Systems, Leiden, the Netherlands). In 50 randomly selected patients, a second blinded CMR physician measured stress GLS for assessment of inter-observer variability. In another 50 randomly selected patients, the same physician re-measured stress GLS in a blinded fashion for assessment of intra-observer variability.
Follow-up
Patients follow-up was performed retrospectively for the combined primary outcome of major adverse cardiac events (MACE) - death, non-fatal myocardial infarction, hospitalization for heart failure sustained ventricular tachycardia, and late revascularization (>90 days after CMR). Two cardiologists blinded to CMR results performed all standardized follow-up procedures. Clinical follow-up was obtained by review of the electronic medical records. In cases where records were not found in the medical chart, treating physicians and patients were contacted using a standardized questionnaire. Non-fatal myocardial infarction was defined by the presentation of an acute coronary syndrome and elevation of cardiac biomarkers (>99th percentile of the upper limit of normal), temporally consistent with an acute injury. The definition of heart failure hospitalization required the presence of an elevated B-natriuretic peptide (BNP) level in addition to signs and symptoms of heart failure. The Social Security Death Index was used to confirm all cases of death. Time to event was calculated as the period between the CMR study and the first occurrence of a MACE. Patients who did not experience MACE were censored at time of last follow-up.
Statistical Analysis
Normally distributed data were expressed as mean ± SD. Differences in baseline characteristics were compared with the Student’s t-test or Wilcoxon rank-sum test (depending on data normality) for continuous variables and the chi-squared test for dichotomous variables. Kaplan-Meier methods were used to evaluate the relationship between stress GLS and time to the primary outcome of MACE. We used Cox proportional hazards regression modeling to examine the association between stress GLS and MACE. Models were assessed for collinearity and proportional hazards assumption. For the multivariable models, clinical and imaging risk factors which were univariate predictors (at p≤0.10) were considered as covariates. The incremental prognostic value of stress GLS was assessed in nested-models. Model discrimination was compared by calculating the C-index(12). Risk reclassification analyses were conducted with calculation of continuous net reclassification improvement (NRI)(13). A p value of <0.05 was considered statistically significant. Analyses were performed using STATA (StataCorp, TX).
RESULTS
Patient Characteristics
Table 1 summarizes baseline patient characteristics stratified by stress GLS above and below the median (−19%). The mean age of the study population was 58.4(±13.1) years. Forty-five percent of patients were male and 34.6% had diabetes mellitus. The mean ejection fraction was 59.7 ± 11.9% and LGE was present in 16.8% of patients. Primary indications for stress testing were chest pain (71%), dyspnea (18%) and pre-operative evaluation (10%); with about 12% for a mixture of other indications including abnormal ECG, non-sustained ventricular tachycardia, premature ventricular contractions, syncope, and new cardiomyopathy. Some patients had more than one indication for stress testing.
Table 1.
Baseline characteristics of study population stratified by stress GLS above and below the median (−19%).
| CHARACTERISTICS | Total | Stress GLS <median |
Stress GLS ≥median |
P Value |
|---|---|---|---|---|
| CLINICAL VARIABLES | ||||
| Age (±SD), years | 58.4 (±13.1) | 58.1 (±13.2) | 58.7 (±13.1) | 0.604 |
| Male % | 45.3 | 35.0 | 55.4 | <0.001 |
| BMI (±SD), kg/m2 | 30.5 (±6.2) | 30.9 (±6.2) | 30.1 (±6.1) | 0.132 |
| Diabetes % | 34.6 | 28.7 | 40.3 | 0.006 |
| Hyperlipidemia % | 55.1 | 51.6 | 58.5 | 0.114 |
| Hypertension % | 75.6 | 69.7 | 81.4 | 0.002 |
| Smoking % | 17.5 | 14.6 | 20.5 | 0.076 |
| History of MI % | 15.6 | 8.3 | 22.9 | <0.001 |
| CMR VARIABLES | ||||
| Baseline Heart Rate (±SD), beats/min | 65.0 (±12.4) | 63.3 (±11.6) | 66.6 (±13.0) | 0.007 |
| LVEDV index (±SD), ml/m2 | 64.1 (±20.7) | 62.8 (±15.5) | 65.3 (±24.8) | 0.168 |
| LVESV index (±SD), ml/m2 | 24.3 (±17.8) | 20.2 (±11.0) | 28.3 (±21.9) | <0.001 |
| LGE present % | 16.8 | 5.5 | 27.9 | <0.001 |
| LGE size‡ (IQR) | 0 (0,0) | 0 (0,0) | 0 (0,1) | <0.001 |
| Resting GLS (IQR), % | −17(−19,−15) | −19(−20,−18) | −15(−17,−12) | <0.001 |
| Ischemia present % | 10.5 | 2.4 | 18.6 | <0.001 |
| Ischemia extent‡ (IQR) | 0 (0,0) | 0 (0,0) | 0 (0,0) | <0.001 |
| LVEF (±SD), % | 59.7 (±11.9) | 63.1 (±8.4) | 56.4 (±13.8) | <0.001 |
BMI=Body Mass Index, GLS=Global Longitudinal Strain, IQR=Interquartile Range, LGE=Late Gadolinium Enhancement, LVEDV =Left Ventricular End Diastolic Volume Index, LVEF=Left Ventricular Ejection Fraction, LVESV =Left Ventricular End Systolic Volume Index, MI=Myocardial Infarction, SD=standard deviation.
expressed as percent LV.
The median resting GLS was −17.1% (IQR −18.9, −14.6). Median absolute change in GLS was 1.8% (IQR −0.1, 3.2) with a median stress GLS/rest GLS ratio of 1.11 (IQR 0.99, 1.19), which corresponds to a 10.68% increase in GLS (IQR −0.53, 18.55). Stress GLS was −12.5%(IQR −16.4, −8.7) in patients with ischemia vs. −19.4%(IQR −21.7, −16.3) in those without (p<0.001). Stress GLS was −14.8%(IQR −17.2, −10) in patients with LGE vs. −19.7%(IQR −21.9, −16.7) in those without (p<0.001). Amongst patients with history of known CAD, 97% were on antiplatelet medications and 95% were on statins at the time of the CMR study.
Inter and Intra Observer Variability
Bland-Altman analysis of inter-observer variability for stress GLS showed a bias of 0.2%. The 95% limits of agreement were −3.0 to 2.6% (Supplementary Figure 1). Bland-Altman analysis of intra-observer variability for GLS showed a bias of −0.2%. The 95% limits of agreement were −2.6 to 2.1% (Supplementary Figure 1).
Primary Outcome
Of the 512 patients that completed the protocol, 82 (16%) suffered a major adverse event during a median follow-up of 1.5 years (IQR 0.3, 3.4) (death=21, myocardial infarction=21, heart failure hospitalization=13, sustained ventricular tachycardia=2, late revascularization=25).
Stress GLS and Outcomes
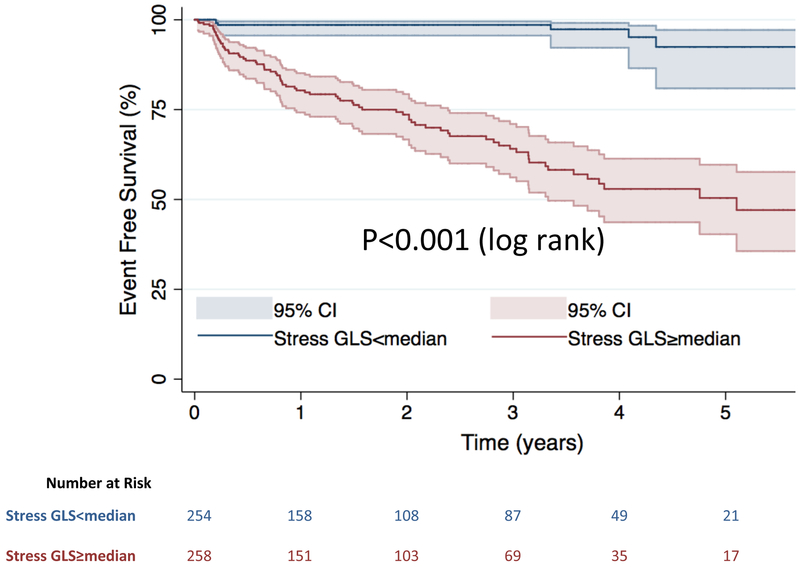
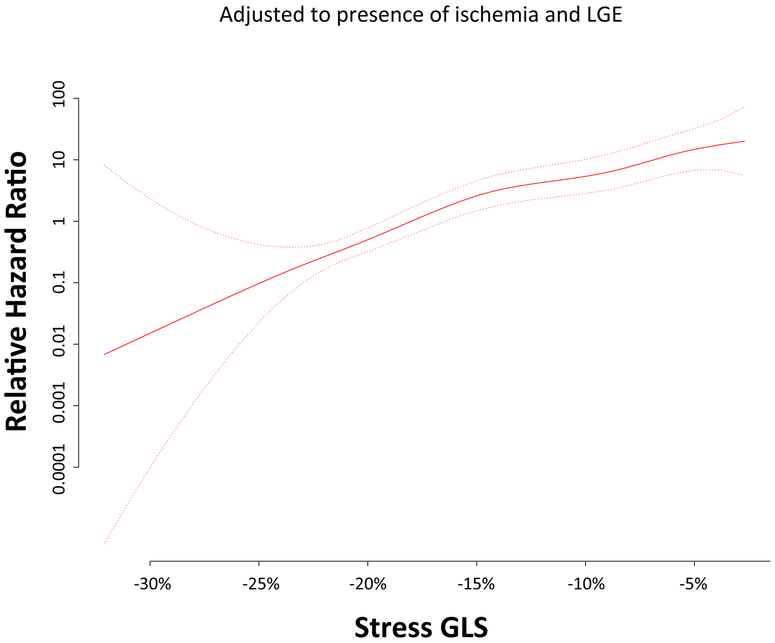
When stratified by the median value of stress GLS (−19%), Kaplan-Meier analysis showed that patients with stress GLS≥median had significantly reduced event free survival compared to those with stress GLS<median (log-rank p<0.001) (Central Illustration). Likewise in patients without myocardial ischemia on first pass perfusion those with stress GLS≥median had significantly reduced event free survival compared to those with stress GLS<median (log-rank p<0.001) (Supplementary figure 2). The continuous relationship between stress GLS and the hazard of MACE (adjusted to the presence of ischemia or LGE) is shown in the cubic spline in Figure 2. Similarly, when using the endpoint of death only, death/MI only, or death/MI/heart failure hospitalization, Kaplan-Meier analyses still showed significantly increased risk in those with stress GLS≥median (log-rank p<0.001 for all) (Supplementary Figures 3-5).
Central Illustration. Kaplan-Meier curves for MACE.
Stratified by stress GLS above and below the median value.
Figure 2. Relationship between stress GLS and hazard of MACE (with 95% confidence intervals) adjusted to presence of ischemia and LGE.
Hazard ratios are relative to those with median stress GLS.
Multivariable Analysis and Incremental Prognostic Value
After adjustment for clinical and imaging risk factors, which were univariate predictors at p≤0.10 (gender, diabetes, hyperlipidemia, history of MI, ischemia, LGE, EF), stress GLS remained a significant independent predictor of MACE (HR=1.267 per % increase; p<0.001) i.e. each % worsening in stress GLS was associated with a 26.7% increase risk of MACE (Table 2). Stress GLS remained a significant independent predictor in the final multivariable model even if LGE size and extent of ischemia were used instead of binary presence/absence of ischemia and LGE (HR=1.278; 1.212-1.348; p<0.001) (Supplementary Table 1).
Table 2.
Final multivariable model of MACE.
| VARIABLES | Final Multivariable Model For MACE |
|
|---|---|---|
| Hazard Ratio (95% CI) |
P Value | |
| Male | 1.667 (1.038-2.678) | 0.034 |
| LVEF‡ | 1.017 (1.001-1.032) | 0.032 |
| Stress GLS* | 1.267 (1.200-1.337) | <0.001 |
| Ischemia | 1.845 (1.070-3.179) | 0.027 |
GLS=Global longitudinal strain, LVEF=Left Ventricular Ejection Fraction,
per % decrease,
per %
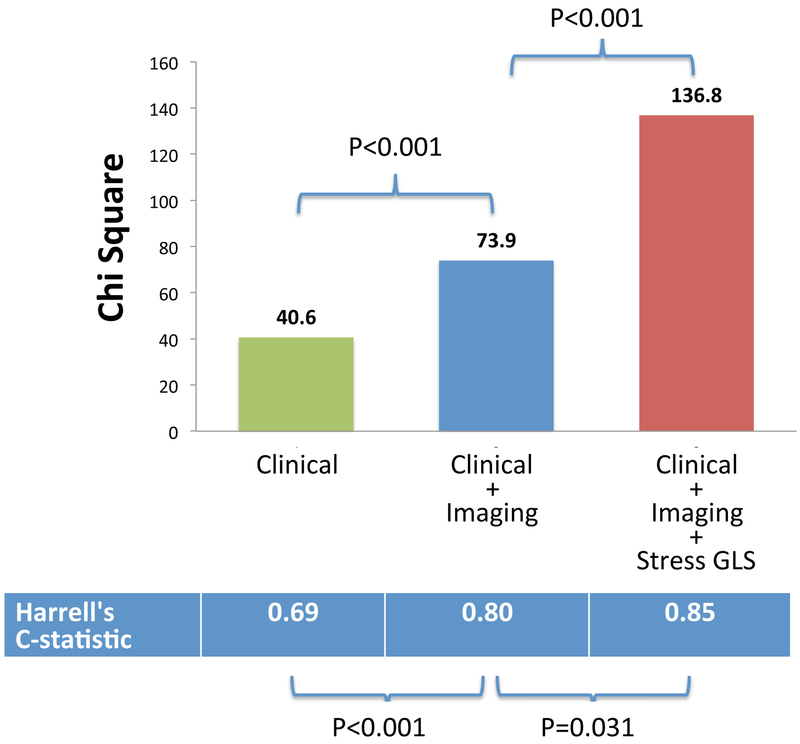
In sequential nested Cox models, a model based on clinical variables alone was significantly improved by addition of imaging variables (EF, LGE, ischemia), and further significantly improved by adding stress GLS (Figure 3). Addition of stress GLS into the model with clinical and imaging predictors resulted in significant increase in the C-index (from 0.80 to 0.85; p=0.031) and a continuous NRI of 0.898 (95% CI, 0.565-1.124).
Figure 3. Sequential nested Cox models for MACE.
A model based on clinical variables alone was significantly improved by addition of imaging variables (ejection fraction, LGE, ischemia), and further significantly improved by adding stress GLS.
In contrast, addition of resting GLS or ratio of stress GLS/rest GLS into the model with basic clinical and imaging predictors resulted in no significant increase in the C-index (p=0.224 and p=0.129 respectively).
DISCUSSION
This study shows that feature tracking GLS measured during vasodilator stress CMR is an independent predictor of MACE in patients with known or suspected CAD. We have demonstrated that stress GLS provides prognostic information incremental to common clinical and imaging risk factors - including ejection fraction, ischemia and late-gadolinium-enhancement. These findings suggest a role for feature tracking derived stress GLS in identifying patients at highest risk of adverse events following stress CMR, even when accounting for ischemia and LGE.
Prior Studies
Echocardiographic studies have shown that blunted measures of longitudinal strain during dobutamine stress can increase the sensitivity of conventional wall motion analysis and may be associated with adverse prognosis(1,14-16). More recently similar observations have been made with dobutamine stress CMR using the technique of strain-encoded magnetic resonance imaging (SENC)(17,18). Korosoglou and colleagues examined the prognostic value of SENC measured during dobutamine stress CMR in 320 patients with known or suspected CAD(18). Over a follow up of 28±9 months, 35 major adverse events occurred (death, non-fatal MI and late revascularization). SENC measures of strain were independently associated with adverse events. However these findings have had limited application to current clinical CMR practice because SENC requires prospective performance of additional specialized pulse sequences during the stress protocol. Moreover, the majority of clinical stress CMR studies are now performed by assessment of vasodilator induced perfusion with adenosine or regadenoson and not dobutamine. Our study therefore differs in two fundamental ways from these prior publications: 1) we measured strain using feature tracking from standard cine images which don’t require specialized pulse sequences; 2) we assessed the prognostic value of GLS during vasodilator stress rather than dobutamine stress. Thus, our findings are likely to be applicable to a much wider group of patients than prior studies of strain measured during stress CMR.
Our findings indicate that feature tracking GLS measured during vasodilator stress CMR is an independent predictor of MACE in patients with known or suspected CAD, incremental to common clinical and imaging risk factors (HR=1.267; p<0.001). Moreover, addition of stress GLS into the model with clinical and imaging predictors resulted in significant increase in the C-index (from 0.80 to 0.85; p=0.031) and a continuous NRI of 0.898 (95% CI, 0.565-1.124). These findings as well as Figure 2 suggest a role for feature tracking derived stress GLS in identifying patients at risk of adverse events following stress CMR, even when accounting for ischemia and LGE.
Potential Mechanisms Linking Stress GLS to Prognosis
The precise mechanism underlying the association between impaired stress GLS and adverse outcomes is unclear. It has been suggested that the subendocardial myocardial fibers (which are more longitudinally aligned) are extremely sensitive to disturbance because of greater compressive forces and higher oxygen consumption(19-23). The subendocardial fibers are therefore more vulnerable to increased wall stress and imbalances between oxygen supply and demand, which may be seen in early stages of many cardiovascular conditions, including atherosclerosis and heart failure. Stress GLS/rest GLS was associated with MACE but did not provide incremental prognostic information beyond common clinical and imaging variables. The reason for this is unclear but highlights the importance of peak hyperemia in demonstrating subendocardial longitudinal dysfunction.
Hyperemic stress does not typically induce myocardial ischemia per se. However, it does lead to a redistribution of myocardial blood flow between the endocardium and epicardium(24). In the resting state there is an endocardial to epicardial blood flow gradient present, reflecting the higher metabolic demands of the endocardial layer(24). Thus in health, pharmacological hyperemia maximizes myocardial blood flow in all layers, leading to a reduction in the epicardial to endocardial gradient(24). Since the subendocardium is primarily composed of longitudinal myocardial fibers(22,23), a blunted GLS response during stress maybe related to these alterations in epicardial to endocardial blood flow gradient. One could speculate that individuals with early damage to the subendocardial layer may be more likely to have a blunted GLS response to vasodilator stress. Future studies using high resolution quantitative stress CMR to measure the epicardial to endocardial gradient in vivo maybe able to assess this more directly(25).
Clinical Implications
Clinically, our findings could be useful in patients without ischemia or scar, who often maybe discharged from follow-up after a “normal” stress CMR. Our results suggest that in the presence of impaired stress GLS such patients are at significantly greater risk of adverse clinical events and may need continued close follow up and perhaps aggressive risk factor modification. Future studies are needed to support such an approach by showing improved clinical outcomes. However, at the present time it is not clear whether and how the finding of impaired stress GLS should direct specific therapy.
Limitations
This is a single center study and may therefore be subject to referral bias and carries all of the inherent limitations of that study design. Moreover, since this is a CMR study, there is further selection bias related to being able to undergo a CMR exam, resulting in exclusion of patients with large body size, severe renal impairment, severe claustrophobia or those with pacemakers and ICDs. Information regarding specific drug therapies triggered by the stress CMR were not assessed.
Similar to some prior studies, we prospectively decided to measure GLS only in the 2-chamber view due to practical time limitations of performing multiple image acquisitions during peak stress within a standard clinical protocol(5). We strongly believe that simple rapid techniques are more likely to be utilized in busy clinical laboratories. Moreover, our results stand on their own and clearly demonstrate that this simple stress GLS measurement is an important independent predictor of adverse events, incremental to standard clinical and imaging variables.
In similar fashion to echo speckle tracking, there are algorithmic differences between various CMR feature tracking software platforms, which may result in differing values(26). Thus the applicability of our findings to other feature tracking vendors requires further investigation. Moreover, Feisst and colleagues recently showed that the correlation of feature tracking measurements to tagging is dependent on reader experience (27). Since the GLS measurements in this study were done by a reader very experienced (>5 years) with feature tracking, it is possible that our findings may differ if performed by less experienced individuals. Observer variability for GLS measurements in this study were approximately similar to that of 2 large prior multicenter studies performed by experienced groups in different patient populations(4,28).
Conclusions
In this prospective single center study, GLS during stress CMR is a significant independent predictor of adverse cardiovascular events in patients with known or suspected CAD – incremental to common clinical and imaging risk factors including EF, ischemia and LGE. Our findings suggest a role for feature tracking derived stress GLS in identifying patients at risk of adverse events following stress CMR - even when accounting for ischemia and LGE. Further work is required to investigate whether and how measurements of stress GLS may direct therapy.
Supplementary Material
CLINICAL PERSPECTIVES.
Competency in Medical Knowledge: Feature tracking stress GLS measured during vasodilator stress CMR is an independent predictor of major adverse cardiovascular events in patients with known or suspected CAD, incremental to common clinical and imaging risk factors Translational Outlook: Additional studies are needed to validate these findings in a multi-center setting and to test whether feature tracking stress GLS can help guide therapies to improve outcomes.
Acknowledgments
SOURCES OF FUNDING:
Dr Shenoy was funded by an NIH grant (K23HL132011-01)
Abbreviations:
- CAD
Coronary Artery Disease
- CMR
Cardiac Magnetic Resonance
- EF
Ejection Fraction
- GLS
Global Longitudinal Strain
- LGE
Late Gadolinium Enhancement
- LV
Left Ventricle
- MACE
Major Adverse Cardiovascular Events
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES: None
REFERENCES
- 1.Bjork Ingul C, Rozis E, Slordahl SA, Marwick TH. Incremental value of strain rate imaging to wall motion analysis for prediction of outcome in patients undergoing dobutamine stress echocardiography. Circulation 2007;115:1252–9. [DOI] [PubMed] [Google Scholar]
- 2.Pedrizzetti G, Claus P, Kilner PJ, Nagel E. Principles of cardiovascular magnetic resonance feature tracking and echocardiographic speckle tracking for informed clinical use. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance 2016; 18:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romano S, Judd RM, Kim RJ et al. Association of Feature-Tracking Cardiac Magnetic Resonance Imaging Left Ventricular Global Longitudinal Strain With All-Cause Mortality in Patients With Reduced Left Ventricular Ejection Fraction. Circulation 2017;135:2313–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romano S, Judd RM, Kim RJ et al. Feature-Tracking Global Longitudinal Strain Predicts Death in a Multicenter Population of Patients With Ischemic and Nonischemic Dilated Cardiomyopathy Incremental to Ejection Fraction and Late Gadolinium Enhancement. JACC Cardiovascular imaging 2018;11:1419–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garg P, Aziz R, Al Musa T et al. Effects of hyperaemia on left ventricular longitudinal strain in patients with suspected coronary artery disease : A first-pass stress perfusion cardiovascular magnetic resonance imaging study. Netherlands heart journal : monthly journal of the Netherlands Society of Cardiology and the Netherlands Heart Foundation 2018;26:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu E, Judd RM, Vargas JD, Klocke FJ, Bonow RO, Kim RJ. Visualisation of presence, location, and transmural extent of healed Q-wave and non-Q-wave myocardial infarction. Lancet 2001;357:21–8. [DOI] [PubMed] [Google Scholar]
- 7.Kim HW, Farzaneh-Far A, Kim RJ. Cardiovascular magnetic resonance in patients with myocardial infarction: current and emerging applications. J Am Coll Cardiol 2009;55:1–16. [DOI] [PubMed] [Google Scholar]
- 8.Romano S, Judd RM, Kim RJ et al. Prognostic Implications of Mitral Annular Plane Systolic Excursion in Patients with Hypertension and a Clinical Indication for Cardiac Magnetic Resonance Imaging: A Multicenter Study. JACC Cardiovascular imaging 2018. doi: 10.1016/j.jcmg.2018.10.003. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah R, Heydari B, Coelho-Filho O et al. Stress cardiac magnetic resonance imaging provides effective cardiac risk reclassification in patients with known or suspected stable coronary artery disease. Circulation 2013;128:605–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenwood JP, Maredia N, Younger JF et al. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet 2012;379:453–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Indorkar R, Kwong RY, Romano S et al. Global Coronary Flow Reserve Measured During Stress Cardiac Magnetic Resonance Imaging Is an Independent Predictor of Adverse Cardiovascular Events. JACC Cardiovascular imaging 2018. doi: 10.1016/j.jcmg.2018.08.018. [Epub ahead of print]. [DOI] [Google Scholar]
- 12.Pencina MJ, D'Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Statistics in medicine 2004;23:2109–23. [DOI] [PubMed] [Google Scholar]
- 13.Pencina MJ, D'Agostino RB Sr., D'Agostino RB Jr., Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Statistics in medicine 2008;27:157–72; discussion 207–12. [DOI] [PubMed] [Google Scholar]
- 14.Voigt JU, Nixdorff U, Bogdan R et al. Comparison of deformation imaging and velocity imaging for detecting regional inducible ischaemia during dobutamine stress echocardiography. European heart journal 2004;25:1517–25. [DOI] [PubMed] [Google Scholar]
- 15.Ingul CB, Stoylen A, Slordahl SA, Wiseth R, Burgess M, Marwick TH. Automated analysis of myocardial deformation at dobutamine stress echocardiography: an angiographic validation. J Am Coll Cardiol 2007;49:1651–9. [DOI] [PubMed] [Google Scholar]
- 16.Ng AC, Sitges M, Pham PN et al. Incremental value of 2-dimensional speckle tracking strain imaging to wall motion analysis for detection of coronary artery disease in patients undergoing dobutamine stress echocardiography. American heart journal 2009;158:836–44. [DOI] [PubMed] [Google Scholar]
- 17.Korosoglou G, Lehrke S, Wochele A et al. Strain-encoded CMR for the detection of inducible ischemia during intermediate stress. JACC Cardiovascular imaging 2010;3:361–71. [DOI] [PubMed] [Google Scholar]
- 18.Korosoglou G, Gitsioudis G, Voss A et al. Strain-encoded cardiac magnetic resonance during high-dose dobutamine stress testing for the estimation of cardiac outcomes: comparison to clinical parameters and conventional wall motion readings. J Am Coll Cardiol 2011;58:1140–9. [DOI] [PubMed] [Google Scholar]
- 19.Henein MY, Gibson DG. Normal long axis function. Heart 1999;81:111–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henein MY, Gibson DG. Long axis function in disease. Heart 1999;81:229–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chilian WM. Microvascular pressures and resistances in the left ventricular subepicardium and subendocardium. Circulation research 1991;69:561–70. [DOI] [PubMed] [Google Scholar]
- 22.Ho SY. Anatomy and myoarchitecture of the left ventricular wall in normal and in disease. European journal of echocardiography : the journal of the Working Group on Echocardiography of the European Society of Cardiology 2009; 10: iii3–7. [DOI] [PubMed] [Google Scholar]
- 23.Greenbaum RA, Ho SY, Gibson DG, Becker AE, Anderson RH. Left ventricular fibre architecture in man. British heart journal 1981;45:248–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klocke FJ, Lee DC. Probing transmural myocardial perfusion with CMR. JACC Cardiovascular imaging 2014;7:23–5. [DOI] [PubMed] [Google Scholar]
- 25.Chiribiri A, Hautvast GL, Lockie T et al. Assessment of coronary artery stenosis severity and location: quantitative analysis of transmural perfusion gradients by high-resolution MRI versus FFR. JACC Cardiovascular imaging 2013;6:600–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Almutairi HM, Boubertakh R, Miquel ME, Petersen SE. Myocardial deformation assessment using cardiovascular magnetic resonance-feature tracking technique. The British journal of radiology 2017;90:20170072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feisst A, Kuetting DLR, Dabir D et al. Influence of observer experience on cardiac magnetic resonance strain measurements using feature tracking and conventional tagging. International journal of cardiology Heart & vasculature 2018;18:46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eitel I, Stiermaier T, Lange T et al. Cardiac Magnetic Resonance Myocardial Feature Tracking for Optimized Prediction of Cardiovascular Events Following Myocardial Infarction. JACC Cardiovascular imaging 2018;11:1433–1444. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.