Abstract
Objectives:
To compare the risk of resistant hypertension (RHTN) in systemic lupus erythematosus (SLE) patients and controls without SLE; to define factors associated with RHTN in SLE patients.
Methods:
We studied 1,044 SLE patients and 5,241 control subjects using de-identified electronic health records from a tertiary care center. SLE was defined as ≥ 4 ICD codes for SLE and ANA ≥ 1:160. RHTN was defined as uncontrolled blood pressure on three anti-hypertensive medications or requiring four or more anti-hypertensives to attain control. First, we compared the risk of RHTN between groups. Second, we examined the association between RHTN and all-cause mortality in patients with SLE.
Results:
RHTN was nearly twice as prevalent in patients with SLE compared to control subjects (10.2% and 5.3%, respectively), with an incidence rate of 10.2 versus 6.1 cases/1,000 person-years of observation [Hazard Ratio (HR) = 1.72, 95% confidence interval: 1.28–2.30, p<0.001, adjusted for age, sex, race, baseline end-stage renal disease (ESRD), creatinine, and calendar year]. In patients with SLE, we found associations between RHTN and black race, lower renal function, hypercholesterolemia, and increased inflammatory markers. RHTN was associated with a significantly higher mortality risk (HR: 2.91, p=0.0005) after adjustment for age, sex, race, calendar year, creatinine, baseline ESRD, and number of visits.
Conclusions:
Patients with SLE have a higher risk of RHTN compared to frequency-matched controls, independent of multiple covariates. RHTN is an important comorbidity for clinicians to recognize in SLE, as it is associated with a higher risk of mortality.
Patients with systemic lupus erythematosus (SLE) have a five-fold higher prevalence of cardiovascular events compared to age- and sex-matched controls (1). Increasing evidence indicates that traditional cardiovascular risk factors and markers of inflammation do not fully explain the greater risk of coronary artery disease among patients with SLE (2–5).
One novel risk factor—resistant hypertension (RHTN)—carries a 44% increased rate of coronary artery disease, 57% increased rate of stroke, and 30% increased rate of all-cause mortality in the general population (6). RHTN is defined as blood pressure that remains above 140/90 mmHg despite concurrent use of three antihypertensive agents of different classes, or the need for four or more antihypertensive agents (7, 8). RHTN is relatively uncommon in the general population; the incidence rate in newly-diagnosed hypertensive adults is 7 cases per 1,000 person years (9).
Despite the recognition that RHTN increases cardiovascular risk substantially in the general population, there is no information regarding the incidence, prevalence, and associated factors in patients with SLE or its consequences in routine clinical practice. Thus, we set out to complete a retrospective study using electronic health records with the aims to: (1) compare the incidence and prevalence of RHTN in patients with SLE and frequency-matched controls without SLE, (2) define factors associated with RHTN in patients with SLE, and (3) examine the association between RHTN and all-cause mortality in SLE patients.
Patients and Methods
We extracted information from the electronic health records (EHRs) at Vanderbilt University Medical Center (VUMC) between the years 1989 and 2017 via the Synthetic Derivative, a de-identified copy of VUMC inpatient and outpatient records (10). In the Synthetic Derivative, Health Insurance Portability and Accountability Act identifiers are removed by established de-identification software (10). The Synthetic Derivative contains electronic health records from over 2.8 million patients and is a date-shifted (within 1 calendar year) shadow of the EHR, but temporal relationships within each patient’s course are consistent (10). It contains all available information in the EHR but does not contain records outside of VUMC. VUMC is a tertiary referral center, seeing patients throughout the southeastern United States. Both primary care and highly specialized medical care are offered at VUMC. Data on the percentage of patients receiving all care within the VUMC system are not available at this time.
SLE and Control Cohorts:
We identified 1,044 patients with patients with SLE using a validated computerized algorithm developed for EHRs at VUMC. The algorithm requires four or more counts of the ICD-9 code for SLE (710.0) and a positive ANA (titer ≥ 1:160) while excluding codes for dermatomyositis (710.3) and systemic sclerosis (710.1) (11); it has a positive predictive value (PPV) of 89–94% (11). For this project, we randomly selected 50 de-identified charts to review for evidence of a SLE diagnosis by a rheumatologist, nephrologist or dermatologist. This was confirmed in 94%, 4% had probable SLE but incomplete records, and 2% had rheumatoid arthritis. Of patients with confirmed SLE diagnoses, the median number of ACR criteria was 4 [IQR: 3–5].
We established a control cohort that was frequency-matched for age (± 5 years), race, and sex, while excluding subjects who had ICD-9 and ICD-10 codes for autoimmune diseases (Appendix Table 1). Control subjects had to have at least 3 outpatient visits within 5 years of their first visit (12). Thus, controls include both healthy patients seen for primary care as well as patients with a range of medical comorbidities. We identified 5,241 control subjects, yielding an approximately a 5:1 control:SLE patient ratio that allowed close matching while maximizing power.
Follow-up:
For the analysis of resistant hypertension, follow-up started at the time of first ICD-9 code for SLE; for controls, it started at the time of first ICD-9 code. The observation period continued until the first of the following: (1) date of meeting RHTN definition, (2) final ICD-9 code in the medical record, or end of the study (June 2017), or (3) death. The final ICD-9 code in the medical record, for both patients and controls, is the last ICD-9 code of any type. This serves as a proxy for the subject’s last point of follow up at VUMC. For the comparison between patients with SLE with and without RHTN, the observation period spanned the entire medical record.
For the mortality analysis, follow-up started at the time of the fourth ICD-9 for SLE; otherwise, all other procedures remained as above (Supplemental Figure 1).
Resistant hypertension:
Criteria for RHTN were fulfilled by either of two definitions, which used only outpatient blood pressure measurements (Appendix Table 2): Definition 1) At least two measurements of systolic blood pressure (SBP) >140 mmHg or diastolic blood pressure (DBP) >90 mmHg at least one month after being prescribed three anti-hypertensive classes simultaneously, including a thiazide or calcium channel blocker (13). Mean blood pressure had to be SBP >140 mmHg and DBP >90 mmHg during the 6-month period after meeting medication criteria. Definition 2) Simultaneous prescription of ≥ 4 antihypertensives taken concurrently, including a thiazide diuretic or calcium channel blocker (7, 8). The algorithm we used to define RHTN using the EHR had a PPV of 97% (14). The original algorithm excluded patients with nephritis and thyroid disease, but we did not exclude these conditions. We verified performance of this definition in the SLE cohort in a convenience sample of 28 patients, finding a PPV of 100% and a negative predictive value of 92.5%.
Covariates:
We extracted demographic and comorbid variables, including patients’ age, race, ethnicity, sex, height, weight, immunologic markers (antinuclear antibody positivity, anti-double stranded DNA, complement C3, and complement C4), creatinine, inflammatory markers (erythrocyte sedimentation rate and C-reactive protein), cholesterol and triglyceride concentrations, and estimated glomerular filtration rate (eGFR). Data were extracted using computer programming in Structured Query Language (15). The measurement closest to the patient’s first relevant ICD-9 code (i.e., first ICD-9 code for SLE for the SLE cohort and first ICD-9 code of any type for controls) was extracted, as described above in follow-up. We defined end-stage renal disease (ESRD) at baseline by the presence of an ICD-9 code for ESRD (585.6), hemodialysis (39.95), peritoneal dialysis (54.98), or a CPT code for renal dialysis status (V45.11) from the start of observation until 180 days after observation began. A 180-day window was used to ensure baseline ESRD was captured even if it was not coded for on the same day as a subject’s first relevant ICD-9 code in the record.
Blood pressure measures were extracted at outpatient visits (Appendix Table 2). Hypertension (HTN) was defined by two or more HTN ICD-9 or ICD-10 codes on separate dates (Appendix Table 3). Raynaud’s Syndrome was defined by the presence of an ICD-9 code for Raynaud’s Syndrome (443.0) or an ICD-10 code for Raynaud’s Syndrome (I73.0).
Mortality:
We ascertained mortality using a flag defined in the Synthetic Derivative for death through the Social Security Administration’s Death Master File as well as hospital records. Our analysis used the time-shifted date of death.
Statistical Analysis:
Descriptive statistics are presented as number (percent) and median [interquartile range] for categorical and continuous variables, respectively. We used chi-square tests to compare categorical variables and Wilcoxon’s rank sum test to compare continuous variables. Comparisons of blood pressure values over time between groups were performed using a representative sampling of values with Welch’s t-tests.
For the prevalence analysis, we identified all patients who met algorithm criteria for RHTN, regardless of when it occurred respective to study entry (Supplemental Figure 1). Therefore, patients who had RHTN prior to SLE were included as having prevalent RHTN. We defined the prevalence of RHTN for SLE patients and frequency-matched controls, as patients who developed RHTN at any point divided by the total number of patients in the cohort. We assessed the association between prevalence of RHTN and SLE using logistic regression models to adjust for age, sex, race, calendar year, ESRD at baseline, and baseline creatinine. Calendar year was adjusted to control for trends in hypertension (HTN) management over time.
Because our analysis suggested a potential relationship between ESRD at baseline and RHTN, we performed a sensitivity analysis of these logistic regression models, while excluding all patients who had ESRD at baseline. We also compared SLE patients who developed RHTN to those who did not using the baseline demographic, immunologic, lipid and renal characteristics described above.
To analyze the rate of incident RHTN, we excluded patients who developed RHTN earlier than 180 days after study entry, to allow the diagnosis period to capture true incident cases. Inclusions in incident versus prevalent analyses are illustrated in Supplemental Figure 1. We analyzed the cumulative incidence of RHTN for patients with SLE versus control subjects using cumulative incidence curves and Cox proportional hazards models (Figures 1 and 2). Multivariate models were adjusted for age, sex, race, calendar year, ESRD at baseline, and baseline creatinine (Figure 2). We used Kaplan-Meier curves to plot the development of RHTN in patients with SLE over time, stratified by ESRD at baseline, race and sex.
Figure 1. Cumulative Incidence of RHTN in SLE versus Control Cohort.
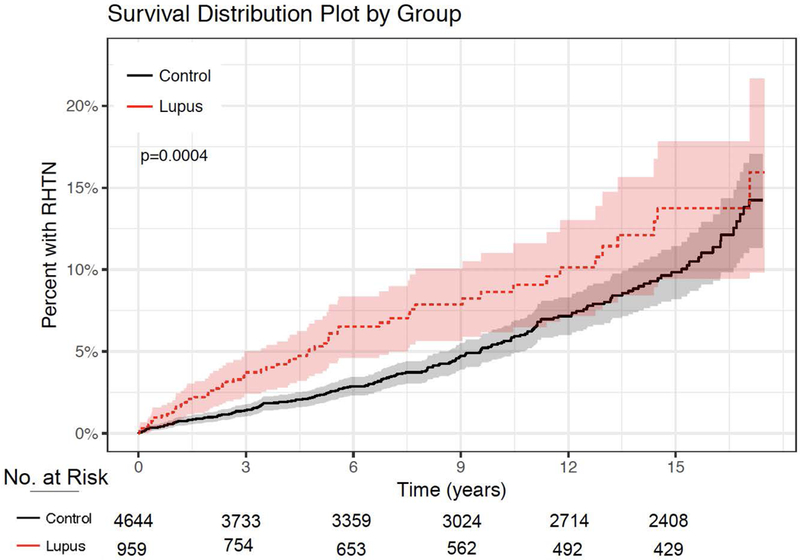
The cumulative incidence of RHTN is plotted for the SLE cohort and Control Cohort, as % of cohort with RHTN over time in years. 95% confidence intervals are shown as highlighted color.
Figure 2. Cox Proportional Hazards and Logistic Regression Models for RHTN Development in Patients with SLE versus Controls.
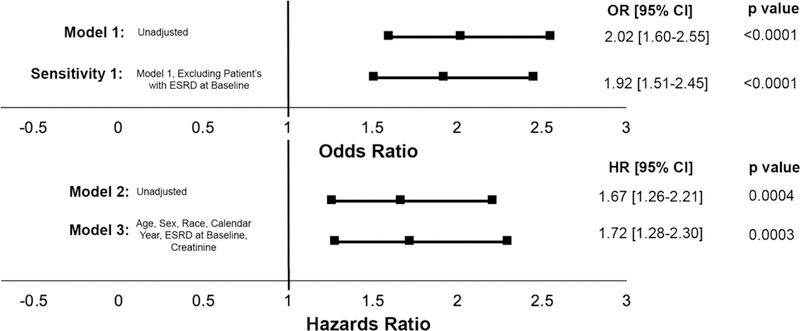
At the top of the figure two logistic regression models are shown (Model 1 and Sensitivity 1) with their adjustments listed beneath each model name. Odds ratio for development of RHTN in patients with SLE versus Controls is shown graphically and in text. Two Cox proportional hazards models are shown (Models 2 and 3), with their adjustments listed beneath each model name. Hazard ratio for risk of development of RHTN in patients with SLE versus Controls is shown graphically and in text.
For the mortality analysis, follow-up started at the time of fourth ICD code in lupus patients in order to allow for a washout period to define incident cases. We used Cox Proportional Hazards modeling to compare the risk of mortality in patients with SLE who developed RHTN compared to SLE patients who did not. These results were adjusted for age, sex, race, calendar year, ESRD at baseline, and creatinine. RHTN was treated as a time-dependent covariate. Only patients from the incident RHTN analyses were included in the mortality analysis. (That is, the first diagnosis of RHTN had to occur after the 4th ICD code for SLE was recorded which also had to be 180 days after a patient’s first ICD code for SLE.) Finally, we used Kaplan-Meier curves to analyze the incidence of mortality for SLE patients with and without RHTN. We conducted analyses using R version 3.4.2 for macOS (16–19); all p-values are two sided; p<0.05 was considered significant.
This study was approved by the Vanderbilt University Medical Center IRB which waived need for informed consent.
Results:
Participant Characteristics:
Baseline characteristics of SLE and control cohorts are shown in Table 1. The cohorts were similar in age, sex, race, and ethnicity due to frequency-matching. They were also similar in total person years of observation time. Patients with SLE had statistically significantly higher creatinine levels, creatinine measures above 1.5 mg/dL, and lower eGFR than controls at study entry. SLE patients were also significantly more likely to have HTN at baseline compared to controls (13.0%, n=136 versus 7.3%, n=385, respectively, p<0.001). Control subjects had significantly higher total cholesterol and LDL-C than patients with SLE, while SLE patients had significantly lower HDL-C and higher triglycerides at time of study entry. Among SLE patients in whom an anti-double-stranded DNA test was performed, 456 of 969 (47.1%) had a positive test. A high number of patients were prescribed immunosuppressants at any point in time: 412 (39.5%) azathioprine, 338 (32.4%) mycophenolate, and 100 (9.6%) cyclophosphamide. A total of 199 patients either presented or developed nephritis over the course of the study (19.1%).
Table 1.
Patient Characteristics at the time of first ICD-9 Code for Patients with SLE and Controls
| N | SLE ^ (N=1044) |
Controls (N=5241) |
p-value* | |
|---|---|---|---|---|
|
| ||||
|
Demographics
Age at first ICD-9 code (years) Female (%) Race White (%) Black (%) Other or Unknown (%) Ethnicity Hispanic (%) BMI (kg/m2) # Person years of observation time |
6285 6285 6285 6285 5466 6285 |
41 [30–53] 941 (90.1) 720 (69.0) 245 (23.5) 79 (7.6) 29 (2.8) 26.7 [23.1–32.2] 5.8 [2.7–10.0] |
42 [31–54] 4728 (90.2) 3678 (70.2) 1167 (22.3) 396 (7.6) 149 (2.8) 27.8 [23.6–33.4] 6.1 [2.3–11.2] |
0.15 0.94 0.69 0.87 <0.001 0.90 |
|
Renal Function Creatinine (mg/d) Creatinine ≥ 1.5 mg/d% eGFR (ml/min/1.73m2) |
5793 5793 5781 |
0.8 [0.7–1.0] 106 (10.2) 85.8 [65.1–107.6] |
0.8 [0.7–0.9] 150 (2.86) 89.6 [74.5–107.5] |
<0.001 <0.001 <0.001 |
|
Cardiovascular Total Cholesterol (mg/dL) HDL-C (mg/dL) LDL-C (mg/dL) Triglycerides (mg/dL) Hypertension% |
2862 2755 2661 2832 6285 |
180.0 [152.0–208.0] 51.0 [39.0–64.0] 99.0 [78.8–124.0] 126.0 [88.0–182.0] 136 (13.0) |
189.0 [161.0–218.0] 53.0 [44.0–67.0] 106.0 [84.0–131.0] 102.0 [69.0–155.0] 385 (7.3) |
<0.001 <0.001 <0.001 <0.001 <0.001 |
| Number of visits | 5722 | 26 [13–51] | 11 [4–27] | <0.001 |
Continuous values are presented as median [interquartile range] and categorical variables are presented as n (%).
Wilcoxon rank-sum test was used to compare continuous variables and Chi-square test was used to compare categorical variables.
Abbreviations are defined as follows: BMI (Body Mass Index), eGFR (estimated glomerular filtration rate), HDL-C (high-density lipoprotein cholesterol), LDL-C (low-density lipoprotein cholesterol)
Creatinine ≥ 1.5 mg/d and Hypertension Flag within the first 180 days of first ICD-9 code.
Prevalence of RHTN and Blood Pressure Values:
The prevalence of RHTN was higher in the SLE cohort (10.2%, n=106) than in control subjects (5.3%, n=278) (OR 2.02 95% CI: 1.60–2.55 p<0.0001, Figure 2, Model 1). In a sensitivity analysis, we excluded patients with ESRD at baseline. The association between SLE and RHTN remained (unadjusted analysis: OR 1.92 95% CI: 1.51–2.45, p<0.0001, Figure 2, Sensitivity Analysis 1).
Median SBP and DBP during study follow-up did not differ between the control and SLE groups. Patients with SLE with prevalent RHTN had significantly higher SBP compared to patients with SLE who did not develop RHTN (median: 127.4 mmHg IQR: [120.0–138.8] versus 114.1 mmHg [105.7–122.9], p<0.001). This finding was also present in controls with and without RHTN (140.4 mmHg [133.5–146.7] versus 123.9 mmHg [115.0–132.4], p<0.001).
Cumulative Incidence of RHTN in Patients with SLE versus Control Subjects:
For this analysis, we excluded 597 control subjects and 85 SLE patients who reached the defined endpoint of RHTN or last study observation within 180 days of the start of follow-up to allow for a washout period to define incident RHTN. After these exclusions, we found 270 incident cases of RHTN—207 in the control group over a follow-up of 33,686 patient-years and 63 in the SLE group during 6,200 patient-years. The incidence rate of RHTN was almost two-fold in the SLE cohort compared to controls—10.1 cases versus 6.2 cases per 1,000 person-years of observation, respectively (HR: 1.67 95% CI: 1.26–2.21, p=0.0004) (Figure 1). This increased risk of RHTN among patients with SLE remained significant after adjustment for age, race, sex, calendar year, ESRD at baseline, and creatinine (HR 1.72 95% CI: 1.28–2.30, p<0.001, Figure 2, Model 3).
The association between RHTN and SLE is robust. In a model adjusted for age, race, sex, calendar year and creatinine and censoring as patients develop ESRD or Raynaud’s the association between RHTN and SLE remained (HR= 1.79 95% CI: 1.32–2.41, p=0.0001). In an analysis restricted to 465 SLE patients and 1,879 control subjects who presented with hypertension yielded similar results (unadjusted: (HR 1.59 95% C.I. 1.18–2.14, p=0.002)) and adjusted for age, sex, race, calendar year, baseline creatinine and Raynaud’s Syndrome at baseline: (HR 1.72 95% CI 1.26–2.35, p=0.0007).
Factors Associated with Prevalent RHTN:
We compared 106 patients with SLE who developed RHTN at any point to 938 patients with SLE who did not (Table 2). SLE patients who developed RHTN were significantly older. They also significantly differed in race: 45.3% of SLE patients who developed RHTN were black versus 21.0% of SLE patients who did not develop RHTN (p<0.001, Table 2). Patients with RHTN were more likely to have taken prednisone or a calcineurin inhibitor than SLE patients without RHTN (prednisone: 83% versus 72%, p=0.02 and calcineurin: 16% versus 7%, p<0.001). This pattern remained when analyzing prednisone and calcineurin inhibitor use within the three months proceeding RHTN or last observation (prednisone: 17% versus 6%, p<0.001 and calcineurin: 3% versus 1%, p=0.04). Higher creatinine and lower eGFR also were associated with RHTN, as was higher total cholesterol and triglycerides, however anti-dsDNA antibodies, C3, and C4 were not. Patients with SLE who developed RHTN had significantly higher inflammatory markers at baseline, with elevation in both C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) (Table 2).
Table 2.
Patient Characteristics at the Time of First ICD-9 Code for Patients with SLE who did and did not Develop RHTN
| N | Patients with SLE with RHTN (N=106) ^*# |
Patients with SLE without RHTN (N=938) |
p value |
|
|---|---|---|---|---|
|
| ||||
|
Demographics
Age at first ICD-9 code (years) Female (%) Race White (%) Black (%) Other or Unknown (%) BMI (kg/m2)% Person years of observation time |
1044 1044 1044 893 1044 |
47 [32–60] 95 (89.6) 52 (49.1) 48 (45.3) 6 (5.7) 27.8 [24.7–32.3] 7.6 [4.3–11.3] |
41 [29–52] 846 (90.2) 668 (71.2) 197 (21.0) 73 (7.8) 26.5 [22.9–32.3] 5.5 [2.6–9.8] |
0.01 0.85 <0.001 0.06 <0.01 |
|
Renal Function Creatinine (mg/d) Creatinine ≥ 1.5 mg/d& eGFR (ml/min/1.73m2) |
1028 1028 979 |
1.1 [0.8–1.8] 34 (32.1) 65.0 [34.6–84.0] |
0.8 [0.7–1.0] 72 (7.7) 88.2 [68.5–108.9] |
<0.001 <0.001 <0.001 |
|
Cardiovascular Cholesterol HDL-C LDL-C Triglycerides Hypertension |
513 466 448 503 1044 |
194.0 [160.5–221.8] 50.0 [35.0–62.0] 103.0 [83.0–128.0] 152.0 [104.0–231.0] 26 (24.5) |
178.0 [151.0–206.0] 51.5 [39.0–64.0] 98.0 [77.5–122.5] 122.0 [86.0–175.8] 110 (11.7) |
0.01 0.24 0.09 <0.001 <0.001 |
|
Medication Use Prednisone ever (%) Calcineurin inhibitor ever (%) |
1035 1035 |
88 (83.0) 17 (16.0) |
671 (72.2) 63 (6.8) |
0.017 <0.001 |
|
Longitudinal Immunologic Markers dsDNA Positive (%)* |
969 |
56 (56.0) |
400 (46.0) |
0.06 |
|
Immunologic Markers at time of SLE Diagnosis ANA (%) ≥ 1:160 dsDNA Positive (%) C3 (mg/dl) C4 (mg/dl) CRP (mg/l)@ ESR (mm/hr)@ |
1044 969 944 857 705 884 |
99 (93.4) 45 (45.0) 106.0 [76.0–129.0] 21.0 [15.0–31.0] 10.2 [2.7–48.7] 40.0 [18.0–80.0] |
913 (97.3) 321 (36.9) 112.0 [86.0–133.0] 21.0 [13.0–28.0] 3.3 [1.0–12.0] 24.0 [9.0–46.0] |
- 0.12 0.25 0.38 <0.001 <0.001 |
Continuous values are presented as median [interquartile range] and categorical variables are presented as n (%).
For percentages with missing values, percentage represents percent of patients with the measurement with a positive value. Missing values may be secondary to SLE diagnosis being made at an outside facility, and therefore labs would not be in the Synthetic Derivative.
Wilcoxon rank-sum test was used to compare continuous variables and Chi-square test was used to compare categorical variables.
Abbreviations are defined as follows: BMI (Body Mass Index), eGFR (estimated glomerular filtration rate), HDL-C (high-density lipoprotein cholesterol), LDL-C (low-density lipoprotein cholesterol), dsDNA (double stranded DNA), ANA (anti-nuclear antibody), C3 (complement 3), C4 (complement 4), CRP (C-reactive protein), ESR (erythrocyte sedimentation rate).
Creatinine ≥ 1.5 mg/d and Hypertension Flag within the first 180 days of first ICD-9 code.
Normal range for CRP is 0.1–1.7mg/l. Normal range for ESR is 2–37 mm/hr.
Factors Associated with Incident RHTN:
In an analysis using Model 3 of the time-to-event analyses (Figure 2, Model 3) black race (HR: 3.43, 95% CI: 2.66–4.42, p<0.0001), age (HR: 1.05, 95% CI: 1.04–1.06, p<0.0001), and creatinine (HR: 1.15, 95% CI: 1.07–1.23, p=0.0001) were associated with RHTN. In a time-to-event analysis, male sex was associated with RHTN in a model adjusted for age, sex and race only (HR: 1.48 95% CI: 1.03–2.12, p=0.03 not pictured) and increasing age and black race were also associated with RHTN as in Model 3. Supplemental Figures 2, 3 and 4 illustrate Kaplan-Meier analysis for cumulative incidence of RHTN stratified by SLE status along with ESRD at baseline, race and gender, respectively.
Mortality and RHTN in SLE patients:
In SLE, 25% of patients with RHTN died compared to 10% of those without RHTN, (Figure 3). Both the unadjusted: (HR: 3.07 95% C.I.=1.85–5.09, p<0.001) and the analysis adjusted for age, sex, race, calendar year, creatinine, ESRD at baseline, and number of visits (HR: 2.91 95% C.I.=1.60–5.29, p<0.001) confirmed the association between RHTN and increased mortality in patients with SLE.
Figure 3. Mortality in SLE Patients with and without RHTN.
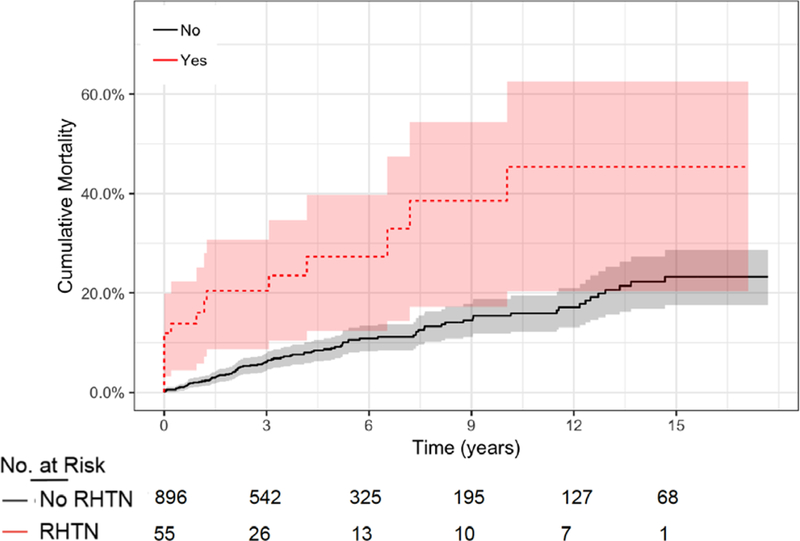
Survival over time (years) is shown for patients with SLE stratified by those who had RHTN versus those who did not have RHTN over the total observation period. Survival is shown as % not surviving over time. 95% confidence intervals are shown as grey highlight.
Discussion:
This study presents several important new findings. First, the incidence and prevalence of RHTN in SLE patients was elevated compared to control subjects without SLE. Second, in patients with SLE, RHTN was associated with older age, black race, male sex, and higher baseline creatinine. Third, in patients with SLE, RHTN was associated with a higher mortality risk.
Our study found that 10.2% of SLE patients had prevalent RHTN, which is similar to that reported in patients with pre-existing HTN (20) in whom the prevalence ranges from 8 to 12% (20–22). Furthermore, we reported that the risk for RHTN among patients with SLE is higher than in the control cohort even when controlling for factors such as age, race, sex, ESRD at baseline, and creatinine. In keeping with the prevalence findings, the incidence rate of RHTN in SLE was twice that in controls, 10.2 versus 6.1 cases per 1,000 person-years of observation time respectively. These findings indicate that RHTN may be an important and previously unrecognized cardiovascular risk factor in SLE patients.
Several demographic and clinical characteristics were associated with RHTN in patients with SLE. Among the demographic characteristics, race and age made the greatest impact. Our finding of an association between RHTN and black race is consistent with previous research in SLE showing that HTN was more common in blacks (23) and with research in the general population showing that RHTN is associated with black race (6, 20, 24). We propose many potential explanations. First, the association may be due to the higher frequency of lupus nephritis and higher progression to ESRD in black patients (25, 26). In addition, black patients have higher rates of obesity, genetic differences such as sodium channel polymorphisms, increased salt sensitivity, and sociological factors such as higher stress levels (27–29). Older age was also associated with RHTN. This is consistent with previous research showing that increasing age is associated with HTN in SLE (30) and with both HTN and RHTN in the general population (8, 20, 31). This study also found an association between male sex and RHTN in patients with SLE. Most commonly, studies have found an association between RHTN and female sex (22, 31–33); however, a few studies have found an association between RHTN and male sex (20). One potential mechanism by which sex could influence risk of RHTN is potentially a more accelerated disease course with increased damage in male patients with SLE (34).
In regard to the association of clinical characteristics, our findings were also consistent with previous research. Similar to studies in the general population, we found an association between renal disease and the risk of RHTN (6, 20–22, 32, 35). There is little information about the pathogenesis of RHTN in SLE, but some mechanisms might be inferred from what is known about the pathogenesis of HTN in this population. A previous study of the Toronto Lupus Cohort found that higher SBP was correlated with SLE disease activity score, suggesting an association between inflammation and HTN (36). Another study in SLE patients found an association between higher SLE damage index and inflammatory markers (i.e., ESR and IL-6) with HTN (30).
An additional possibility is that higher inflammation in the setting of active lupus could increase the use of medications such as corticosteroids and calcineurin inhibitors, which increase the risk of HTN (37). Consistent with that observation, we found that more patients with RHTN had used prednisone or calcineurin inhibitors at any point and within three months of study-end (in this instance, RHTN diagnosis). An additional consideration is the high prevalence of metabolic syndrome in SLE patients, which may contribute to higher risk of RHTN (38). In this cohort, patients with SLE had lower BMI, but higher triglycerides and lower HDL than controls.
Finally, our study found that RHTN was associated with higher risk of mortality in patients with SLE. We postulate that RHTN increases the risk of adverse cardiovascular outcomes in SLE. In general population cohorts, RHTN has been associated with numerous adverse cardiovascular outcomes, including coronary heart disease, stroke, and heart failure (6, 21, 35). Importantly, a large cardiovascular outcomes study with approximately 14,600 patients found that RHTN was associated with increased all-cause mortality (6). This association was seen in a large prospective study of three female cohorts as well (24). Our findings regarding mortality indicate that that RHTN is a marker of clinical risk for SLE patients, meriting specific attention from clinicians.
This study has limitations. Data on the percentage of patients receiving all care within the VUMC system are not available at this time. We were unable to account for medication compliance or white-coat HTN, important causes of pseudo-RHTN (9, 39–42). We acknowledge that previous studies on RHTN that assessed medication adherence eliminated many patients on this basis, and poor adherence to SLE treatment in patients with SLE is common (9, 43). As a result of using an EHR shadow and only including data collected through routine clinical care at a single tertiary care center without access to outside records, a further limitation is missing data. Additionally, as VUMC is a tertiary referral center both the SLE and control cohorts may be sicker than the general population. Moreover, patients with SLE may receive prescriptions for medications with antihypertensive effects due to reasons other than hypertension. For example, angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) are used to treat proteinuria in patients with nephritis, and calcium channel blockers are likewise used for Raynaud’s syndrome (44, 45). However, it is unlikely that a SLE patient would meet criteria for RHTN due to these medications alone without underlying uncontrolled HTN. Another limitation is the lack of ability to fully disentangle the role of lupus nephritis on resistant hypertension, as we do not have complete data on renal biopsy results or the precise date of nephritis onset; the incidence rate for RHTN in patients without a clinical diagnosis of lupus nephritis prior to or during the study period (6.1/1,000 person-years) was similar to the one in control subjects (6.1/1,000 person-years). Finally, mortality data were not available for control subjects at the time of this analysis. Despite these limitations, the association between SLE and RHTN was a robust finding that remained statistically significant even when controlling for confounding factors.
In conclusion, patients with SLE have a higher risk of developing RHTN compared to frequency-matched age, race, and sex controls; this persisted even when controlling for demographics, end-stage renal disease, and creatinine. In patients with SLE, RHTN is associated with black race, lower renal function, hypercholesterolemia, increased inflammatory markers, and increased mortality rates. Our findings support the notion that both primary care and specialty physicians need to recognize RHTN in SLE as a predictor of increased mortality which warrants close monitoring and potentially aggressive management. Future studies including prospective registry-based studies are needed to further address the relationship between nephritis with RHTN in patients with SLE. Future studies are also needed to elucidate if RHTN in SLE patients is associated with higher risk of cardiovascular events as well as to study the pathogenesis of RHTN in SLE.
Supplementary Material
Significance and Innovation:
Resistant hypertension (RHTN) was nearly twice as prevalent in patients with systemic lupus erythematosus (SLE) compared to control subjects and had a higher incidence rate in patients with SLE.
In patients with SLE, there were associations between RHTN and black race, lower renal function, hypercholesterolemia, and increased inflammatory markers.
RHTN was associated with 2.9-fold higher mortality in patients with SLE
RHTN is an important comorbidity for clinicians to recognize in SLE, as it is associated with a higher risk of mortality.
Acknowledgments
Financial /Support: JSG received funding from The Rheumatology Research Foundation, Vanderbilt University School of Medicine Research Immersion Program, Vanderbilt Institute for Clinical and Translational Research funding supported by CTSA award UL1TR000445 from NCATS. MMS received funding from NIH-NIDDK grant DK108444. The datasets used for the analyses described were obtained from Vanderbilt University Medical Center’s Synthetic Derivative which is supported by institutional funding and by the CTSA grant ULTR000445 from NCATS/NIH. AB received funding from NIH/NICHD 5K12HD043483–12 and NIH K08 AR072757–01. PA received funding from NIH/NIGMS T32 GM007569. CMS was supported by the Lupus Research Alliance. CPC was supported by the Rheumatology Research Foundation (K-supplement and R-bridge awards), and K-23 award from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (K23AR064768).
Footnotes
Conflicts of Interest: The authors have no COIs to disclose.
References:
- 1.Manzi S, Meilahn EN, Rairie JE, Conte CG, Medsger TA, Jansen-McWilliams L, et al. Age-specific Incidence Rates of Myocardial Infarction and Angina in Women with Systemic Lupus Erythematosus: Comparison with the Framingham Study. American Journal of Epidemiology. 1997;145(5):408–15. [DOI] [PubMed] [Google Scholar]
- 2.Boulos D, Koelmeyer RL, Morand EF, Hoi AY. Cardiovascular risk profiles in a lupus cohort: what do different calculators tell us? Lupus Sci Med. 2017;4(1):e000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee AB, Godfrey T, Rowley KG, Karschimkus CS, Dragicevic G, Romas E, et al. Traditional risk factor assessment does not capture the extent of cardiovascular risk in systemic lupus erythematosus. Internal medicine journal. 2006;36(4):237–43. [DOI] [PubMed] [Google Scholar]
- 4.Sinicato NA, da Silva Cardoso PA, Appenzeller S. Risk factors in cardiovascular disease in systemic lupus erythematosus. Current Cardiology Reviews. 2013;9(1):15–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gustafsson JT, Svenungsson E. Definitions of and contributions to cardiovascular disease in systemic lupus erythematosus. Autoimmunity. 2014;47(2):67–76. [DOI] [PubMed] [Google Scholar]
- 6.Muntner P, Davis BR, Cushman WC, Bangalore S, Calhoun DA, Pressel SL, et al. Treatment-Resistant Hypertension and the Incidence of Cardiovascular Disease and End-Stage Renal Disease Novelty and Significance. Hypertension. 2014;64(5):1012–21. [DOI] [PubMed] [Google Scholar]
- 7.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Pr. Hypertension (Dallas, Tex: 1979). 2017. [DOI] [PubMed] [Google Scholar]
- 8.Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, et al. Resistant Hypertension: Diagnosis, Evaluation, and Treatment. Circulation. 2008;117(25):e510–e26. [DOI] [PubMed] [Google Scholar]
- 9.Daugherty SL, Powers JD, Magid DJ, Tavel HM, Masoudi FA, Margolis KL, et al. Incidence and Prognosis of Resistant Hypertension in Hypertensive Patients. Circulation. 2012;125(13):1635–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roden DM, Pulley JM, Basford MA, Bernard GR, Clayton EW, Balser JR, et al. Development of a large-scale de-identified DNA biobank to enable personalized medicine. Clinical Pharmacology and Therapeutics. 2008;84(3):362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnado A, Casey C, Carroll RJ, Wheless L, Denny JC, Crofford LJ. Developing Electronic Health Record Algorithms That Accurately Identify Patients With Systemic Lupus Erythematosus. Arthritis Care Res (Hoboken). 2017;69(5):687–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schildcrout JS, Denny JC, Bowton E, Gregg W, Pulley JM, Basford MA, et al. Optimizing drug outcomes through pharmacogenetics: a case for preemptive genotyping. Clin Pharmacol Ther. 2012;92(2):235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shuey MM, Brown NJ, Denny JC. Abstract P147: Differences in the Treatment of Resistant Hypertension in African Americans and European Americans in a Clinical Setting. Hypertension. 2017;70(AP147). [Google Scholar]
- 14.Shuey MM, Gandelman JS, Chung CP, et al. Characteristics and treatment of African-American and EuropeanAmerican patients with resistant hypertension identified using the electronic health record in an academic health centre: a case−control study. BMJ Open 2018;8:e021640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jamison DC. Structured Query Language (SQL) fundamentals. Current protocols in bioinformatics. 2003;Chapter 9:Unit9.2. [DOI] [PubMed] [Google Scholar]
- 16.Team RC. R: A language and environment for statistical computing. In: Computing RFfS, editor. Vienna, Austria; 2017. [Google Scholar]
- 17.Harrell FEJ. Package ‘rms’. Cran.R-Project; 2018. [Google Scholar]
- 18.Harrell FEJ, Dupont, Charles. Hmisc: Harrell Miscellaneous. Cran.R-Project; 2018. [Google Scholar]
- 19.Terry M Therneau TL. survival: Survival Analysis. Cran.R-Project; 2017. [Google Scholar]
- 20.Sarafidis PA, Georgianos P, Bakris GL. Resistant hypertension--its identification and epidemiology. Nature Reviews Nephrology. 2013;9(1):51–8. [DOI] [PubMed] [Google Scholar]
- 21.Persell SD. Prevalence of Resistant Hypertension in the United States, 2003–2008. Hypertension. 2011;57(6):1076–80. [DOI] [PubMed] [Google Scholar]
- 22.McAdam-Marx C, Ye X, Sung JC, Brixner DI, Kahler KH. Results of a retrospective, observational pilot study using electronic medical records to assess the prevalence and characteristics of patients with resistant hypertension in an ambulatory care setting. Clinical Therapeutics. 2009;31(5):1116–23. [DOI] [PubMed] [Google Scholar]
- 23.Chaiamnuay S, Bertoli AM, Roseman JM, McGwin G, Apte M, Durán S, et al. African-American and Hispanic ethnicities, renal involvement and obesity predispose to hypertension in systemic lupus erythematosus: results from LUMINA, a multiethnic cohort (LUMINAXLV). Annals of the Rheumatic Diseases. 2007;66(5):618–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith SM, Huo T, Gong Y, Handberg E, Gulati M, Merz CNB, et al. Mortality Risk Associated With Resistant Hypertension Among Women: Analysis from Three Prospective Cohorts Encompassing the Spectrum of Women’s Heart Disease. Journal of Women’s Health. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burgos PI, Perkins EL, Pons-Estel GJ, Kendrick SA, Liu JM, Kendrick WT, et al. Risk factors and impact of recurrent lupus nephritis in patients with systemic lupus erythematosus undergoing renal transplantation: data from a single US institution. Arthritis and Rheumatism. 2009;60(9):2757–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lea JP. Lupus nephritis in African Americans. The American Journal of the Medical Sciences. 2002;323(2):85–9. [DOI] [PubMed] [Google Scholar]
- 27.Lackland DT. Racial differences in hypertension: implications for high blood pressure management. Am J Med Sci. 2014;348(2):135–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ortega LM, Sedki E, Nayer A. Hypertension in the African American population: A succinct look at its epidemiology, pathogenesis, and therapy. Nefrologia : publicacion oficial de la Sociedad Espanola Nefrologia. 2015;35(2):139–45. [DOI] [PubMed] [Google Scholar]
- 29.Hicken MT, Lee H, Morenoff J, House JS, Williams DR. Racial/ethnic disparities in hypertension prevalence: reconsidering the role of chronic stress. Am J Public Health. 2014;104(1):117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sabio JM, Vargas-Hitos JA, Navarrete-Navarrete N, Mediavilla JD, Jiménez-Jáimez J, Díaz-Chamorro A, et al. Prevalence of and factors associated with hypertension in young and old women with systemic lupus erythematosus. The Journal of Rheumatology. 2011;38(6):1026–32. [DOI] [PubMed] [Google Scholar]
- 31.Holmqvist L, Boström KB, Kahan T, Schiöler L, Hasselström J, Hjerpe P, et al. Cardiovascular outcome in treatment-resistant hypertension: results from the Swedish Primary Care Cardiovascular Database (SPCCD). Journal of Hypertension. 2017. [DOI] [PubMed] [Google Scholar]
- 32.Solini A, Zoppini G, Orsi E, Fondelli C, Trevisan R, Vedovato M, et al. Resistant hypertension in patients with type 2 diabetes: clinical correlates and association with complications. Journal of Hypertension. 2014;32(12):2401–10; discussion 10. [DOI] [PubMed] [Google Scholar]
- 33.Kumbhani DJ, Steg PG, Cannon CP, Eagle KA, Smith SC, Crowley K, et al. Resistant hypertension: a frequent and ominous finding among hypertensive patients with atherothrombosis. European Heart Journal. 2013;34(16):1204–14. [DOI] [PubMed] [Google Scholar]
- 34.Andrade RM, Alarcon GS, Fernandez M, Apte M, Vila LM, Reveille JD, et al. Accelerated damage accrual among men with systemic lupus erythematosus: XLIV. Results from a multiethnic US cohort. Arthritis Rheum. 2007;56(2):622–30. [DOI] [PubMed] [Google Scholar]
- 35.Holmqvist L, Boström KB, Kahan T, Schiöler L, Hasselström J, Hjerpe P, et al. Prevalence of treatment-resistant hypertension and important associated factors-results from the Swedish Primary Care Cardiovascular Database. Journal of the American Society of Hypertension: JASH. 2016. [DOI] [PubMed] [Google Scholar]
- 36.Nikpour M, Urowitz MB, Ibanez D, Harvey PJ, Gladman DD. Importance of cumulative exposure to elevated cholesterol and blood pressure in development of atherosclerotic coronary artery disease in systemic lupus erythematosus: a prospective proof-of-concept cohort study. Arthritis Research & Therapy. 2011;13(5):R156-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaharir SS, Mustafar R, Mohd R, Mohd Said MS, Gafor HA. Persistent hypertension in lupus nephritis and the associated risk factors. Clinical Rheumatology. 2015;34(1):93–7. [DOI] [PubMed] [Google Scholar]
- 38.Chung CP, Avalos I, Oeser A, Gebretsadik T, Shintani A, Raggi P, et al. High prevalence of the metabolic syndrome in patients with systemic lupus erythematosus: association with disease characteristics and cardiovascular risk factors. Annals of the Rheumatic Diseases. 2007;66(2):208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pilla MD, Bruno RM, Taddei S. Resistant Hypertension: An Incurable Disease or Just a Challenge For Our Medical Skill? High Blood Pressure & Cardiovascular Prevention. 2016:1–7. [DOI] [PubMed] [Google Scholar]
- 40.Jung O, Gechter JL, Wunder C, Paulke A, Bartel C, Geiger H, et al. Resistant hypertension? Assessment of adherence by toxicological urine analysis. Journal of Hypertension. 2013;31(4):766–74. [DOI] [PubMed] [Google Scholar]
- 41.Garg JP, Elliott WJ, Folker A, Izhar M, Black HR, Service RUH. Resistant hypertension revisited: a comparison of two university-based cohorts. American Journal of Hypertension. 2005;18(5):619–26. [DOI] [PubMed] [Google Scholar]
- 42.de la Sierra A, Segura J, Banegas JR, Gorostidi M, de la Cruz JJ, Armario P, et al. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension (Dallas, Tex: 1979). 2011;57(5):898–902. [DOI] [PubMed] [Google Scholar]
- 43.Costedoat-Chalumeau N, Houssiau F, Izmirly P, Le Guern V, Navarra S, Jolly M, et al. A Prospective International Study on Adherence to Treatment in 305 Patients With Flaring SLE: Assessment by Drug Levels and Self-Administered Questionnaires. Clin Pharmacol Ther. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levien TL. Advances in the treatment of Raynaud’s phenomenon. Vasc Health Risk Manag. 2010;6:167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bakris GL. Slowing nephropathy progression: focus on proteinuria reduction. Clin J Am Soc Nephrol. 2008;3 Suppl 1:S3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


