Abstract
Objectives
We thoroughly explored the demographic and imaging characteristics, as well as all-cause and cause-specific mortality of CAC≥1000 patients in the largest dataset of this population to date.
Background
Coronary artery calcium (CAC) is commonly used to quantify cardiovascular risk. Current guidelines classify CAC>300 or 400 as the highest risk group, yet little is known about the potentially unique imaging characteristics and mortality risk in individuals with CAC≥1000.
Methods
We included 66,636 asymptomatic adults from the CAC Consortium, a large retrospective multicenter clinical cohort. Mean patient follow-up was 12.3 ± 3.9 years for CVD, CHD, cancer, and all-cause mortality. Using multivariable Cox proportional hazards regression models adjusted for age, sex, and traditional risk factors, we assessed the relative mortality hazard of individuals with CAC≥1000 compared first against a reference of CAC=0, and then against CAC 400–999.
Results
There were 2,869 patients with CAC≥1000 (86.3% male, mean age 66.3 ± 9.7 years). Most CAC≥1000 patients had 4-vessel CAC (mean 3.5 ± 0.6 vessels), and had greater total CAC area, higher mean CAC density, and more extra-coronary calcium (79% with TAC, 46% with AVC, 21% with MVC) compared to CAC 400–999. After full adjustment, those with CAC≥1000 had 5.04 (3.92–6.48), 6.79 (4.74–9.73), 1.55 (1.23–1.95), and 2.89-fold (2.53–3.31) risk of CVD, CHD, cancer, and all-cause mortality, respectively, compared to those with CAC=0. The CAC≥1000 group had a 1.71- (1.41–2.08), 1.84- (1.43–2.36), 1.36- (1.07–1.73), and 1.51-fold (1.33–1.70) increased CVD, CHD, cancer, and all-cause mortality compared to CAC 400–999. Graphical analysis of CAC≥1000 revealed continued logarithmic increase in risk, with no clear evidence of a risk plateau.
Conclusions
Patients with extensive CAC (CAC≥1000) represent a unique very high-risk phenotype with mortality outcomes commensurate with high-risk secondary prevention patients. Future guidelines should consider CAC≥1000 a distinct risk group which may benefit from the most aggressive preventive therapy.
Keywords: coronary artery calcium, risk scoring, cardiovascular imaging, high risk, primary prevention
INTRODUCTION
Coronary artery calcium (CAC), acquired using cardiac-gated non-contrast computed tomography, is now routinely used to quantify atherosclerotic burden in the coronary arteries. Higher levels of CAC have been strongly associated with an increased risk of coronary heart disease (CHD) and all-cause mortality.1 In fact, most studies have found CAC to be a more robust predictor of coronary events in the asymptomatic primary prevention population than traditional risk scores such as the Framingham Risk Score (FRS) or the Pooled Cohort Equations (PCE).2–4 CAC, as a measure of cumulative subclinical vascular injury, also appears to be an independent predictor of other important clinical outcomes such as stroke, dementia, cancer, and chronic kidney disease.5–7
Current guidelines classify persons with CAC > 300 or > 400 as the highest risk group for cardiovascular disease (CVD) events, with no further differentiation above this threshold8–11. To date, few studies have explored the commonly encountered extensively calcified plaque phenotype of CAC ≥ 1000. There is little data on the demographic and imaging characteristics of this population, and even less long-term data on the relative risks of cause-specific mortality. For example, it remains unclear if these patients constitute a unique population with extremely high CHD risk, or if the extensively calcified nature of their atherosclerosis puts them at high all-cause mortality risk but no higher CHD risk than those with CAC > 300 or > 400. Prior studies investigating these extensive Agatston scores have been limited by small sample sizes and by studying only all-cause mortality.1,12
Therefore, we sought to comprehensively describe the demographic characteristics, baseline cardiovascular risk factors, and CT imaging features of this unique and clinically important population, as well as to determine the risks for long-term cause-specific long-term mortality. To accomplish this, we used data from the CAC Consortium, which is the largest cohort of patients with measured CAC to date.13
METHODS
Study design and study population
Our analysis involves 66,636 asymptomatic adults (age ≥ 18 years) without known CHD from the multi-center CAC Consortium study, which was designed to study the relationship between clinical CAC scoring and long-term cause-specific mortality. Details on the data collection, preparation, and harmonization are published elsewhere.13 In summary, this study collected data from 1991 through 2010, with follow-up data until June 2014. Four medical centers with ≥ 10 years CAC scanning experience (per CAC Consortium study site inclusion criteria) from three different states (California, Ohio, and Minnesota) contributed patient data to the CAC Consortium. All CAC scans were clinically indicated and physician-referred. All study participants provided informed consent at the time of CAC scanning. Institutional Review Board approval for coordinating center activities was obtained at the Johns Hopkins Hospital.
Computed tomography data
Each individual study site performed routine non-contrast cardiac-gated CT scans for the clinical determination of CAC scores. A common standard protocol was used for each scanner technology and scans were read locally at each center using the Agatston method13. Electron beam tomography (EBT) was used for the CT scans performed by most centers (93%), while two centers (7%) which had more recent CAC data utilized multi-detector CT (MDCT). It has been previously shown that EBT versus MDCT scanners have no clinically meaningful differences in CAC scores14.
In addition, data on total number of vessels with CAC (0–4) was available in 54,678 patients (82%), thoracic aortic calcium scores in 34,024 patients (51%), aortic valve calcium scores in 10,007 patients (15%), mitral valve calcium scores in 10,008 patients (15%), and mean density (CT attenuation) of calcified coronary lesions in 20,052 patients (30%). In patients with CAC density data, a summed area of all CAC lesions (in mm2) was determined by dividing the total Agatston CAC score by the mean density (in Hounsfield units) divided by 100 (to back calculate the mean density weighting factor in the Agatston protocol)15.
Measurement and definition of baseline characteristics and risk factors
Participants had baseline characteristics, risk factors, and laboratory data collected at the time of the CAC scan and/or as part of their routine clinical visit. Data on race was available in only a subset of the study population (42,964 patients, 64%). Hypertension was defined as current treatment with anti-hypertensive medications or prior diagnosis of hypertension. Dyslipidemia was defined as prior diagnosis of dyslipidemia (elevated triglycerides and/or low HDL-C), prior diagnosis of hyperlipidemia, or treatment with lipid-lowering medications. If participants had concomitant laboratory data, dyslipidemia was considered present if HDL-C<50 mg/dL in women and HDL-C<40 mg/dL in men, LDL-C>160 mg/dL, or fasting triglycerides>150 mg/dL. Smoking status was defined as current cigarette smoker or not (yes/no). Diabetes was defined as prior diagnosis of diabetes or treatment with anti-diabetic drugs. At all centers except for the Columbus, OH site, family history of CHD was determined by presence of a first-degree relative with history of CHD. The Columbus, OH site used a more stringent definition of family history of CHD, which was < 55 years old in male relatives and < 65 years old in female relatives. Multiple imputation was conducted in the instance of partially missing risk factor data (28% of cohort had at least one missing data element). The imputation algorithm has been previously validated.13 The Pooled Cohort Equations (PCE) was used to calculate 10-year risk of ASCVD as previously described.13
Outcome ascertainment
Mortality status was determined by linking to the Social Security Administration Death Master File using a previously validated algorithm16. Individual-level cause of death was ascertained through ICD coded death certificates from the National Death Index (NDI). Participants had follow-up data until June 2014. Mean follow-up was 12.3 ± 3.9 years.
Statistical methods
CAC scores were categorized as CAC 0, CAC 1–399, CAC 400–999, and CAC ≥ 1000. Baseline characteristics were stratified by CAC groups, reporting number (percentage) and means (SD) as appropriate.
Mortality rates (per 1000 person years) were calculated for all-cause and cause-specific mortality. For purposes of comparison, the proportion of a particular cause-specific death was calculated (Ncause-specific deaths / Ntotal deaths) for CAC score groups 400–999 and ≥ 1000. Multivariable-adjusted Cox regression models were used to assess the relative hazards of CAC groups for cause-specific and all-cause mortality compared to a reference group of Agatston score 0. Additionally, for the purposes of specific comparison, the same models were used to assess risk of patients with CAC ≥ 1000 compared to a reference group of CAC 400–999 (in the CAC ≥ 400 subset).
For the Cox regression models, we chose to include an unadjusted model (Model 1) and a fully-adjusted model adjusted for age, sex, and traditional cardiovascular risk factors (Model 2). We also included three other models as supplementary analyses (Supplement Tables S1 and S2), which are as follows: 1) Adjusted for age and sex (Model 3); 2) Model 2 additionally adjusted for race in the race subset (Model 4); 3) Model 2 additionally adjusted for study site (Model 5). To graphically study risks around the CAC=1000 threshold, we used cubic splines to study the dose response relationship between CAC score and mortality outcomes in a multivariable model adjusted for age, sex, and traditional risk factors. Knots were placed at CAC=100 (to capture risk acceleration at low CAC scores) and CAC=1000.
A two-sided p-value < 0.05 was considered statistically significant. All analyses were performed using Stata/SE 14.0 (Stata Corporation LP, College Station, TX, USA).
RESULTS
Baseline characteristics
There were 2,869 patients (4.3% of study cohort) with CAC ≥ 1000. These patients tended to be older (66.3 ± 9.7 years), more likely to be men (86.3%), and higher risk (by number of risk factors, FRS, and ASCVD risk score) than those with lower CAC (Table 1). In the CAC ≥ 1000 group, mean age was 66.3 ± 9.7 years and 27.4% were under 60 years of age. In contrast, among those with CAC scores of 400–999, 39.0% were under 60 years of age.
Table 1 -.
Baseline characteristics according to Agatston score group
| Demographic Characteristics | CAC 0 (n = 29,757) | CAC 1–399 (n = 29,601) | CAC 400–999 (n = 4409) | CAC ≥ 1000 (n = 2869) |
|---|---|---|---|---|
| Age, yr, mean (SD) | 49.9 (9.2) | 56.5 (9.8) | 63.0 (9.4) | 66.3 (9.7) |
| < 50 | 50.2% | 24.7% | 6.9% | 3.7% |
| 50–59 | 36.0% | 40.6% | 32.1% | 23.7% |
| 60–69 | 11.6% | 24.8% | 36.4% | 35.5% |
| 70–79 | 2.0% | 8.6% | 20.3% | 29.2% |
| ≥ 80 | 0.2% | 1.2% | 4.3% | 7.9% |
| Race* (n = 42,964) | ||||
| White | 88.7% | 89.4% | 90.7% | 87.6% |
| Asian | 4.2% | 3.5% | 3.2% | 4.0% |
| Black | 2.2% | 2.3% | 1.5% | 3.1% |
| Hispanic | 3.2% | 3.0% | 2.8% | 3.4% |
| Sex (male), % | 55.5% | 74.3% | 82.5% | 86.3% |
| Hypertension, % | 22.8% | 34.5% | 46.5% | 55.4% |
| Smoking, % | 8.9% | 10.1% | 11.0% | 10.2% |
| Diabetes, % | 3.9% | 7.5% | 12.8% | 19.4% |
| Dyslipidemia, % | 48.0% | 57.9% | 65.0% | 67.3% |
| Family history** | 45.6% | 46.3% | 46.5% | 48.5% |
| # of Risk Factors, mean (SD) | 1.3 (0.9) | 1.5 (0.9) | 1.8 (0.9) | 1.9 (0.9) |
| 0 | 21.7% | 14.6% | 10.2% | 7.7% |
| 1 | 39.0% | 34.5% | 28.1% | 23.7% |
| 2 | 29.2% | 33.6% | 36.4% | 36.4% |
| > 2 | 10.2% | 17.3% | 25.4% | 32.2% |
| FRS, % score, mean (SD) | 7.8% (5.9%) | 12.4% (9.0%) | 18.0% (12.3%) | 21.8% (15.0%) |
| < 10 % | 71.8% | 48.5% | 29.8% | 22.2% |
| 10–19 % | 24.3% | 36.4% | 36.5% | 33.8% |
| ≥ 20 % | 3.8% | 15.1% | 33.6% | 44.0% |
| ASCVD*** Risk Score, % score, mean (SD) | 3.8% (4.7%) | 8.6% (8.7%) | 15.1% (12.0%) | 20.2% (14.9%) |
| < 5 % | 76.9% | 43.8% | 16.0% | 8.6% |
| 5–20% | 21.7% | 47.5% | 59.0% | 52.1% |
| ≥ 20% | 1.4% | 8.7% | 25.0% | 39.2% |
Data only available in subset of study population in “Demographic Characteristics 1”. Detailed data on number of participants in each subgroup can be found in Supplement Table S3
Family history of coronary heart disease
Pooled cohort equations ASCVD risk score
Men comprised 55.5% of the CAC 0 group, 74.3% of the CAC 1–399 group, 82.5% of the CAC 400–999 group, and 86.3% of the CAC ≥ 1000 group. Generally, with increasing CAC score, the percentage of participants with traditional cardiovascular risk factors increased. The distribution of specific risk factors is shown in Table 1.
Those with CAC ≥ 1000 had a mean ASCVD Pooled Cohort Equations (PCE) risk score of 20.2 ± 14.9%, in contrast to a mean score of 15.1 ± 12.0% for the CAC 400–999 group. While mean risk scores were high in the CAC ≥ 1000 group, the distribution (shown in Table 1) indicates that many scores were lower in the range where clinical risk may be considered uncertain (ASCVD 5–20%).
Imaging characteristics
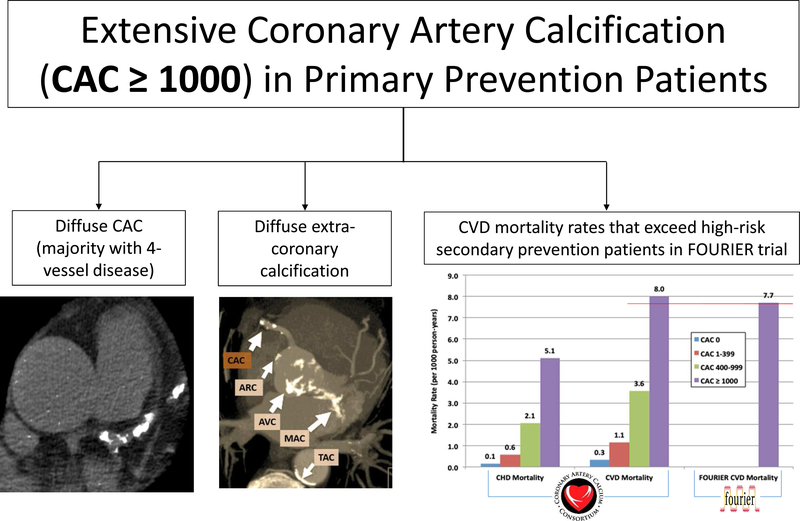
In those with CAC ≥ 1000, the majority had 4-vessel CAC (52.4%) (Table 2). Table 2 shows the distribution of imaging characteristics, including extra-coronary artery calcium, by CAC group. The CAC ≥ 1000 group tended to have not only a higher total mean density, but also a substantially greater total CAC area than lower CAC scores. Additionally, those with extensive CAC ≥ 1000 also tended to have more diffuse systemic vascular disease than lower CAC scorers, with 79.3% having TAC, 45.7% having AVC, and 21.4% having MVC.
Table 2 -.
Imaging characteristics according to Agatston score group
| Imaging Characteristics 1 | CAC 0 (n = 26,531) | CAC 1–399 (n = 22,572) | CAC 400–999 (n = 3329) | CAC ≥ 1000 (n = 2246) |
| # Vessels with CAC, mean (SD) | 0 (0) | 1.9 (0.9) | 3.2 (0.7) | 3.5 (0.6) |
| 0, % | 100.0% | 0.0% | 0.0% | 0.0% |
| 1, % | 0.0% | 42.6% | 0.7% | 0.0% |
| 2, % | 0.0% | 31.6% | 12.1% | 3.7% |
| 3, % | 0.0% | 20.7% | 53.5% | 43.9% |
| 4, % | 0.0% | 5.2% | 33.7% | 52.4% |
| Imaging Characteristics 2 | CAC 0 (n = 16,250) | CAC 1–399 (n = 14,214) | CAC 400–999 (n = 2147) | CAC ≥ 1000 (n = 1413) |
| TAC, % | 11.7% | 39.1% | 67.3% | 79.3% |
| TAC 1–399, % | 10.7% | 30.9% | 39.4% | 33.1% |
| TAC 400–999, % | 0.6% | 4.7% | 12.4% | 15.1% |
| TAC ≥ 1000, % | 0.4% | 3.6% | 15.6% | 31.1% |
| Imaging Characteristics 3 | CAC 0 (n = 4842) | CAC 1–399 (n = 3842) | CAC 400–999 (n = 739) | CAC ≥ 1000 (n = 584) |
| AVC, % | 4.1% | 16.6% | 39.5% | 45.7% |
| AVC 1–399, % | 3.7% | 15.4% | 32.6% | 36.8% |
| AVC 400–999, % | 0.2% | 0.5% | 3.5% | 6.2% |
| AVC ≥ 1000, % | 0.1% | 0.7% | 3.4% | 2.7% |
| Imaging Characteristics 4 | CAC 0 (n = 4842) | CAC 1–399 (n = 3843) | CAC 400–999 (n = 739) | CAC ≥ 1000 (n = 584) |
| MVC, % | 1.4% | 6.6% | 19.4% | 21.4% |
| MVC 1–399, % | 1.2% | 4.9% | 13.7% | 14.4% |
| MVC 400–999, % | 0.2% | 1.0% | 3.2% | 2.4% |
| MVC ≥ 1000, % | 0.0% | 0.7% | 2.4% | 4.6% |
| Imaging Characteristics 5 | CAC 0 (n = 9678) | CAC 1–399 (n = 8575) | CAC 400–999 (n = 1159) | CAC ≥ 1000 (n = 640) |
| Estimated Total Area, mm2, mean (SD) | 0 (0) | 37.5 (41.9) | 254.1 (70.3) | 691.6 (365.8) |
| Total Mean Density, HU, mean (SD) | 0 (0) | 201.5 (45.7) | 251.9 (35.1) | 272.7 (34.8) |
| 130–199, % | 0.0% | 53.0% | 2.9% | 0.2% |
| 200–299, % | 0.0% | 43.7% | 88.1% | 79.5% |
| 300–399, % | 0.0% | 3.3% | 9.0% | 20.2% |
| ≥ 400, % | 0.0% | 0.1% | 0.0% | 0.2% |
All-cause and cause-specific mortality by CAC group
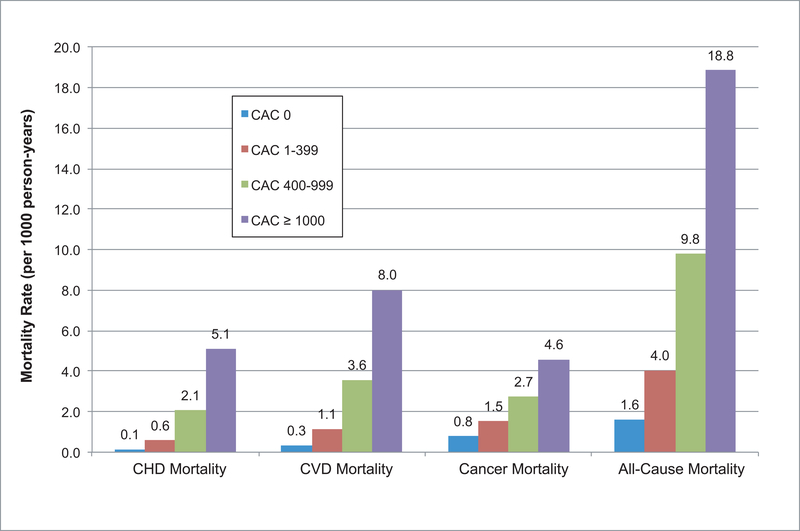
Incidence rates of all outcomes increased across all causes of mortality with increasing CAC score (Figure 1). Individuals with CAC ≥ 1000 had approximately twice the mortality rate of those with CAC 400–999 across all causes of mortality. For CVD mortality, the mortality rate per 1000 person-years was 8.0 for those with CAC ≥ 1000 vs. 3.6 for those with CAC 400–999. Similarly for CHD mortality, the mortality rate of the CAC ≥ 1000 group was more than twice that of the CAC 400–999 group (5.1 vs. 2.1 per 1000 person-years).
Figure 1. Mortality rate per 1000 person-years for CVD, CHD, cancer, and all-cause mortality by CAC score group.
Incidence rates increased for all-cause and cause-specific mortality with increasing CAC score. In particular, those with CAC ≥ 1000 had a 5.1, 8.0, 4.6, and 18.8 mortality rate per 1000 person-years for CHD, CVD, cancer, and all-cause mortality, respectively. In contrast, those with CAC 400–99 had a 2.1, 3.6, 2.7, and 9.8 mortality rate per 1000 person-years for CHD, CVD, cancer, and all-cause mortality, respectively. *A version of this figure including error bars for 95% CI can be found in Supplement Figure S1.
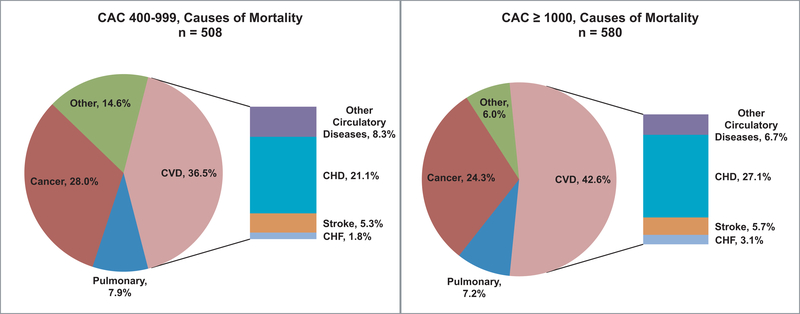
In those with CAC ≥ 1000, the most common cause of death was CVD (42.6%) followed by cancer (24.3%), (Figure 2), while CVD death (36.5%) constituted a smaller portion of all deaths in the CAC 400–999 group, followed by cancer (28.0%).
Figure 2. Causes of mortality for CAC 400–999 and CAC ≥ 1000 groups.
In both CAC groups, the leading cause of death was CVD (CAC 400–999 = 36.5%; CAC ≥ 1000 = 42.6%), followed by cancer (CAC 400–999 = 28.0%; CAC ≥ 1000 = 24.3%). CHD mortality, as a subset of CVD mortality, constituted 21.1% of deaths in the CAC 400–999 group and 27.1% of deaths in the CAC ≥ 1000 group.
Multi-variable adjusted hazard ratios
When adjusting for traditional cardiovascular risk factors, those with CAC ≥ 1000 had a 5.04 (95% CI: 3.92–6.48), 6.79 (95% CI: 4.74–9.73), 1.55 (95% CI: 1.23–1.95), and 2.89-fold (95% CI: 2.53–3.31) risk of CVD, CHD, cancer, and all-cause mortality, respectively (Table 3A), compared to those with CAC=0.
Table 3 -.
Hazard Ratios for all-cause and cause-specific mortality by CAC score group
| A. CAC 0 as reference group | ||||
| Model 1 - Unadjusted HRs | ||||
| Agatston score | Cause of Mortality, HR (95% CI) | |||
| CVD | CHD | Cancer | All-cause | |
| 0 | REF | REF | REF | REF |
| 1–399 | 3.49 (2.85 – 4.27) | 4.14 (3.05 – 5.63) | 1.96 (1.70 – 2.26) | 2.50 (2.28 – 2.75) |
| 400–999 | 10.85 (8.63 – 13.64) | 14.86 (10.64 – 20.74) | 3.52 (2.88 – 4.30) | 6.07 (5.39 – 6.83) |
| ≥ 1000 | 24.23 (19.49 – 30.12) | 36.26 (26.44 – 49.73) | 5.87 (4.80 – 7.18) | 11.64 (10.38 – 13.05) |
| Model 2 - Fully adjusted HRs | ||||
| Agatston score | Cause of Mortality, HR (95% CI) | |||
| CVD | CHD | Cancer | All-cause | |
| 0 | REF | REF | REF | REF |
| 1–399 | 1.77 (1.43 – 2.18) | 1.99 (1.45 – 2.74) | 1.09 (0.94 – 1.27) | 1.37 (1.24 – 1.52) |
| 400–999 | 3.09 (2.41 – 3.97) | 3.90 (2.72 – 5.59) | 1.19 (0.95 – 1.48) | 1.98 (1.73 – 2.25) |
| ≥ 1000 | 5.04 (3.92 – 6.48) | 6.79 (4.74 – 9.73) | 1.55 (1.23 – 1.95) | 2.89 (2.53 – 3.31) |
| B. CAC 400–999 as reference group | ||||
| Model 1 - Unadjusted HRs | ||||
| Agatston score | Cause of Mortality, HR (95% CI) | |||
| CVD | CHD | Cancer | All-cause | |
| 400–999 | REF | REF | REF | REF |
| ≥ 1000 | 2.23 (1.84 – 2.70) | 2.43 (1.90 – 3.11) | 1.65 (1.31 – 2.09) | 1.91 (1.69 – 2.15) |
| ≥ 1000 | 1.71 (1.41 – 2.08) | 1.84 (1.43 – 2.36) | 1.36 (1.07 – 1.73) | 1.51 (1.33 – 1.70) |
| Model 2 - Fully adjusted HRs | ||||
| Agatston score | Cause of Mortality, HR (95% CI) | |||
| CVD | CHD | Cancer | All-cause | |
| 400–999 | REF | REF | REF | REF |
| ≥ 1000 | 1.71 (1.41 – 2.08) | 1.84 (1.43 – 2.36) | 1.36 (1.07 – 1.73) | 1.51 (1.33 – 1.70) |
Adjusted on age, sex, hypertension, dyslipidemia, smoking, diabetes, and family history of CHD
In a similarly adjusted model, those with CAC ≥ 1000 had a 1.71- (95% CI: 1.41–2.08), 1.84- (95% CI: 1.43–2.36), 1.36- (95% CI: 1.07–1.73), and 1.51-fold (95% CI: 1.33–1.70) increase in CVD, CHD, cancer, and all-cause mortality, respectively (Table 3B), compared to those with CAC 400–999.
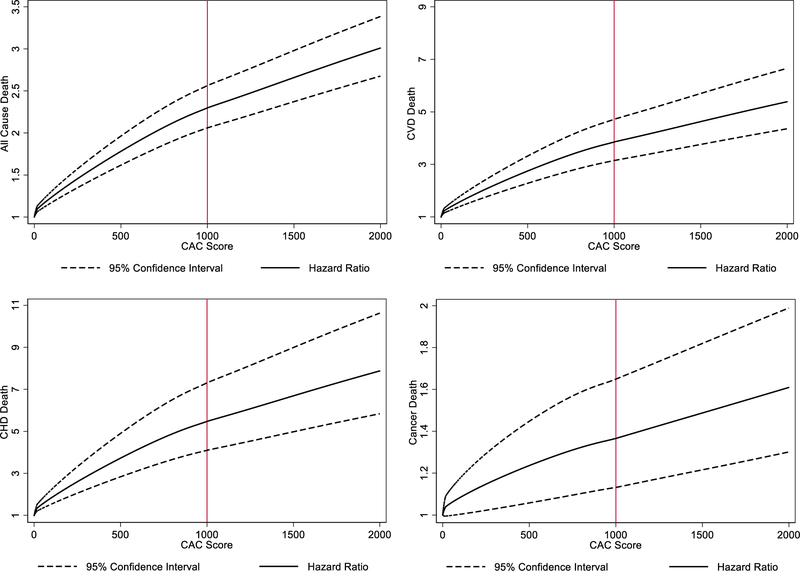
The relationship between CAC score and multivariable adjusted risk of cause-specific and all-cause mortality is displayed graphically in figure 3. Increasing CAC above 1000 led to higher hazard ratios for all causes of mortality (Figure 3). While the hazard ratio increases with a slightly steeper slope when CAC < 1000, the hazard ratio continues to increase when CAC ≥ 1000, with no apparent upper CAC threshold for this increase of both cause-specific and all-cause mortality.
Figure 3. Adjusted hazard ratios and 95% CI for CVD, CHD, cancer, and all-cause mortality by continuous CAC score.
Cubic splines were used to study the relationship between CAC score and mortality outcomes, with hazard ratios adjusted for age, sex, and traditional risk factors. Knots were placed at CAC=100 and CAC=1000. With increasing CAC score, mortality risk continues to increase logarithmically for all-cause and cause-specific mortality, with no apparent plateau in risk.
DISCUSSION
In this study, we provide the most extensive description of individuals with extreme CAC scores (CAC ≥ 1000) to date. We showed that those with CAC ≥ 1000 have both a higher area and density of calcification, a more dispersed pattern of calcification in their coronary artery tree (the majority with 4-vessel disease), with a markedly more diffuse distribution of extra-coronary calcification compared to the other CAC groups. Additionally, we demonstrate that extreme CAC (≥ 1000) is associated with a substantially increased risk of CVD, CHD, cancer, and all-cause mortality, and importantly, those with CAC ≥ 1000 are at an almost 2-fold higher risk of CVD mortality compared to those with CAC 400–999. While the mortality risk levels off slightly after CAC = 1000, risk still increases with no apparent upper CAC threshold for both all-cause and cause-specific mortality.
The few prior smaller studies of CAC ≥ 1000 have explored only all-cause rather than cause-specific mortality or have investigated individual coronary endpoints rather than mortality outcomes1,12. For example, Patel et al showed that among 1593 patients with extensive Agatston scores, increasing CAC led to decreased survival, with continued increased mortality risk past CAC scores > 20001. Other prior studies have suggested that although extensive CAC scores may be associated with higher angina, they are not associated with more hard CHD events12. For example, in the MESA study, Coylewright et al found that in those with extensive CAC scores ≥ 1000 (n = 257 participants), there was no greater risk of CHD death or myocardial infarction compared to those with high CAC scores (n = 420; CAC 400–999)12. This finding has been interpreted as consistent with the notion that a more dense plaque phenotype may be no more risky than lesser CAC scores, and could perhaps be protective.
Indeed, with increasing recognition from other imaging modalities that more dense calcified plaque may be more stable, many have cast doubt on the exceptional risk of extensive CAC scores (CAC ≥ 1000). For example, in a seminal paper Criqui et al found that while higher CAC volume led to increased CHD and CVD risk, higher CAC density was actually significantly protective against CHD and CVD risk when keeping CAC volume constant.17 The protective effect of high density plaque makes sense, because calcified plaque is more stable than low attenuation plaque (predominantly non-calcified) in prior studies utilizing IVUS and CTA.18–21 Since CAC score is a combination of plaque volume and density, some have speculated that many patients who have extensive CAC scores might simply have higher plaque density yet not more plaque burden, which might actually lower CVD risk.17,18,22,23 Similarly, there has been much discussion on endurance athletes, such as marathon runners, whose higher CAC scores may be driven by higher plaque density, which may be relatively protective.24–28
However, in our study, we show that those with CAC ≥ 1000 constitute a distinct population of patients who are at a significantly higher risk of CVD, CHD, cancer, and all-cause mortality than those with CAC 400–999. Furthermore, not only did these patients have markedly higher CAC burden (CAC area), they also had more extra-coronary calcium, such as TAC, AVC, and MVC, than patients with lower CAC. Therefore, it appears that patients with extreme CAC scores have a higher total burden of both coronary and extra-coronary atherosclerosis compared to those with just high CAC scores (CAC 400–999). The most likely reason why our data contradict the prior Coylewright et al study is statistical power. While the Coylewright et al analysis of the MESA study had just 257 patients with CAC ≥ 1000 (too few to show a difference in CHD mortality), our cohort included 2869 patients with CAC ≥ 1000, which is over 10-fold the number in the MESA cohort, with longer follow-up.12
Guidelines from organizations such as the American College of Cardiology (ACC) and American Heart Association (AHA) currently describe the highest risk group for coronary events and mortality as patients with CAC > 300 or CAC > 400.8–11 Based on our data, we argue that those with extensive CAC scores (CAC ≥ 1000) represent a distinct group of patients at the highest risk for all-cause mortality and cardiovascular mortality. Our analyses indicate a potential for future guidelines to recognize asymptomatic patients with extensive Agatston scores (CAC ≥ 1000) as a distinct group where targeted, more aggressive treatment should be considered.
For example, many current recommendations in preventive cardiology include goals for LDL lowering and blood pressure reduction, among other modifiable risk factors.29–32 Specifically, a reduction of approximately 38 mg/dl (1 mmol/L) of LDL-C has been found to reduce the risk of cardiovascular mortality and non-fatal infarctions by 20–25%,33,34 with the newest evidence from the IMPROVE-IT, FOURIER, and ODYSSEY-OUTCOMES clinical trials suggesting that combining statins with non-statins, such as ezetimibe or PCSK9 inhibitors, can significantly improve CVD outcomes even in patients who are on a maximally tolerated intensive statin therapy.35–38
Guidelines from medical societies, particularly the American Association of Clinical Endocrinologists (AACE) have begun recommending very low LDL-C goals (<55 mg/dL) in those at “extreme risk”.35,39 Based on our data, we argue that many patients with CAC ≥ 1000 are at extreme risk and can potentially be considered for the most aggressive therapies, including non-statin lipid lowering therapies such as ezetimibe or PCSK9 inhibitors. For example, in the FOURIER trial, which enrolled stable secondary prevention patients a median of 2.2 years after their last CVD event, the annualized cardiovascular death rate (0.77%/year) in the placebo group was lower than the CVD mortality rate we observed in asymptomatic primary prevention patients with CAC ≥ 1000 (8.0 per 1000 patient-years, or 0.80%/year).36 Such data on those with CAC ≥ 1000 helps to blur the lines between primary and secondary prevention.40 In addition, prior data suggests a high risk of ischemia in these patients,41 arguing for a more thorough history-taking to ensure that they are truly asymptomatic. Patients who are truly asymptomatic should be managed with preventative risk-reducing medications only.
Study Limitations
There are several limitations to this study. First, since the CAC Consortium consists of patients referred for CAC screening, they may not be representative of the general population. However, previous studies indicate that the CAC Consortium contains patients with generally similar characteristics to participants in the Framingham Heart Study and MESA studies.13,42 Second, data on covariates such as diabetes, hypertension, and dyslipidemia relied in part on self-report, and furthermore these were adjusted for in analytical models rather than actual blood pressure and lipid profile. Therefore our models may be subject to some residual confounding. Third, data on race and advanced imaging characteristics was only available in a subset of the study cohort. However, this data was missing at random relative to CAC score and outcomes, and therefore we do expect differential bias in analyses using these data points. Fourth, we do not have creatinine measurements or information on CKD in our population. Thus, we were unable to adjust for kidney function in our analysis. However, none of our patients had ESRD at baseline and via our review of the source populations from which the CAC Consortium was derived, we expect <1% to have advanced CKD (CKD 3B or above). Fifth, CAC scans were not read at a central lab, but rather at four different centers as part of the clinical workflow. However, the site-specific reading of CAC scores adds generalizability to clinical practice, as these scans closely resemble those done routinely in the community. Finally, a key limitation is that we do not have data on the follow-up treatment for patients after receiving their CAC scores. Those with CAC ≥ 1000 were likely treated the most aggressively, however such treatment would bias our results to the null, making our findings even more impactful and powerful.
Conclusion
In conclusion, in the largest sample of patients with CAC ≥ 1000 yet assembled, we show that patients with extensive CAC are unique in their burden of coronary and extra-coronary disease and in their long-term outcomes. Our data argues for consideration of CAC ≥ 1000 as a distinct group with CVD mortality greater than that of contemporary secondary prevention trials like FOURIER.
Supplementary Material
Central Illustration: Understanding Extensive CAC (CAC ≥ 1000) in Primary Prevention Patients.
Primary prevention patients with extensive CAC (CAC ≥ 1000) are unique in their their burden of coronary and extra-coronary disease and in their long-term outcomes. Those with CAC ≥ 1000 can be found on imaging to have a dispersed pattern of calcification in their coronary artery tree (the majority with 4-vessel disease) and diffuse extra-coronary calcification (TAC, AVC, and MVC). In addition, their annualized CVD mortality rates exceed those of high-risk secondary prevention patients from the FOURIER trial (0.80%/year vs. 0.77%/year).
PERSPECTIVES.
Competency in Medical Knowledge
Patients with extensive CAC (CAC ≥ 1000, 4.3% of our population) have distinct imaging characteristics, with higher area and density of CAC, nearly ubiquitous multi-vessel disease, and characteristically diffuse extra-coronary calcification (TAC, MVC, and AVC). Furthermore, they are unique in their high risk of all-cause and cause-specific mortality, with CAC ≥ 1000 patients showing 50% increased risk of CVD mortality compared to those with CAC 400–999, independent from traditional CVD risk factors. Further, risk continues to climb logarithmically with higher CAC above 1000, with no clear evidence of a risk plateau. Our data shows that these patients have CVD mortality greater than that of secondary prevention trials (such as FOURIER), lending them a unique risk status that may inform intensity of preventive therapy.
Translational Outlook
Identification of asymptomatic patients with CAC ≥ 1000 is important in clinical practice given their very high risk of mortality. More studies comparing outcomes in CAC ≥ 1000 patients to routine secondary prevention patients are needed to further inform treatment guidelines. In addition, future randomized controlled trials of aggressive preventative therapies, for example PCSK9-inhibitors and anti-inflammatory drugs, in patients with CAC ≥ 1000, may prove helpful to evaluate the benefits of such treatment in this unique group. Lastly, it may be important to update current guidelines reflecting the best practices in this distinct group of patients with CAC ≥ 1000.
Acknowledgments
Grants and Financial Support: MJB is supported by NIH/NHLBI L30 HL110027. There are no financial disclosures to support.
ABBREVIATIONS
- AVC
aortic valve calcium
- CAC
coronary artery calcium
- CHD
coronary heart disease
- CTA
computed tomography angiography
- CVD
cardiovascular disease
- EBT
electron-beam tomography
- IVUS
intravascular ultrasound
- MDCT
multi-detector computed tomography
- MESA
multi-ethnic study of atherosclerosis
- MVC
mitral valve calcium
- PCE
pooled cohort equations
- TAC
thoracic artery calcium
Footnotes
Disclosures: The authors have no disclosures to support.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Patel J et al. All-cause mortality in asymptomatic persons with extensive Agatston scores above 1000. Journal of Cardiovascular Computed Tomography 8, 26–32 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Hecht HS Coronary Artery Calcium Scanning: Past, Present, and Future. JACC: Cardiovascular Imaging 8, 579–596 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Blaha MJ et al. The Legacy of MESA – Providing Evidence for Subclinical Cardiovascular Disease in Risk Assessment. Glob Heart 11, 275–285 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeboah J et al. Utility of Nontraditional Risk Markers in Atherosclerotic Cardiovascular Disease Risk Assessment. J. Am. Coll. Cardiol 67, 139–147 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gibson AO et al. Coronary artery calcium and incident cerebrovascular events in an asymptomatic cohort. The MESA Study. JACC Cardiovasc Imaging 7, 1108–1115 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Handy CE et al. The Association of Coronary Artery Calcium With Noncardiovascular Disease: The Multi-Ethnic Study of Atherosclerosis. JACC Cardiovasc Imaging 9, 568–576 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuller LH et al. Subclinical Cardiovascular Disease and Death, Dementia, and Coronary Heart Disease in Patients 80+ Years. J. Am. Coll. Cardiol 67, 1013–1022 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neves PO, Andrade J & Monção H Coronary artery calcium score: current status. Radiol Bras 50, 182–189 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnson Y et al. Comparison of the Coronary Artery Calcium Score and Number of Calcified Coronary Plaques for Predicting Patient Mortality Risk. Am. J. Cardiol 120, 2154–2159 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Goff DC et al. 2013. ACC/AHA Guideline on the Assessment of Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology 63, 2935–2959 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Budoff MJ Progression of coronary calcium: not as predictable as 1–2-3. Eur Heart J 35, 2934–2935 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Coylewright M et al. Differentiation of severe coronary artery calcification in the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis 219, 616–622 (2011). [DOI] [PubMed] [Google Scholar]
- 13.Blaha MJ et al. Rationale and design of the coronary artery calcium consortium: A multicenter cohort study. J Cardiovasc Comput Tomogr 11, 54–61 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daniell AL et al. Concordance of coronary artery calcium estimates between MDCT and electron beam tomography. AJR Am J Roentgenol 185, 1542–1545 (2005). [DOI] [PubMed] [Google Scholar]
- 15.Agatston AS et al. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol 15, 827–832 (1990). [DOI] [PubMed] [Google Scholar]
- 16.Al-Mallah MH, Keteyian SJ, Brawner CA, Whelton S & Blaha MJ Rationale and design of the Henry Ford Exercise Testing Project (the FIT project). Clin Cardiol 37, 456–461 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Criqui MH et al. Calcium Density of Coronary Artery Plaque and Risk of Incident Cardiovascular Events. JAMA 311, 271–278 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blaha MJ, Mortensen MB, Kianoush S, Tota-Maharaj R & Cainzos-Achirica M Coronary Artery Calcium Scoring: Is It Time for a Change in Methodology? JACC Cardiovasc Imaging 10, 923–937 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Camici PG, Rimoldi OE, Gaemperli O & Libby P Non-invasive anatomic and functional imaging of vascular inflammation and unstable plaque. Eur Heart J 33, 1309–1317 (2012). [DOI] [PubMed] [Google Scholar]
- 20.Obaid DR et al. Coronary CT angiography features of ruptured and high-risk atherosclerotic plaques: Correlation with intra-vascular ultrasound. J Cardiovasc Comput Tomogr 11, 455–461 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feuchtner G et al. The high-risk criteria low-attenuation plaque <60 HU and the napkin-ring sign are the most powerful predictors of MACE: a long-term follow-up study. Eur Heart J Cardiovasc Imaging 18, 772–779 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Criqui MH et al. Coronary Artery Calcium Volume and Density: Potential Interactions and Overall Predictive Value: The Multi-Ethnic Study of Atherosclerosis. JACC Cardiovasc Imaging 10, 845–854 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Forbang NI et al. Greater Volume But Not Higher Density of Abdominal Aortic Calcium Is Associated With Increased Cardiovascular Disease Risk: The Multi-Ethnic Study of Atherosclerosis (MESA). Circ Cardiovasc Imaging 9, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aengevaeren VL et al. Relationship Between Lifelong Exercise Volume and Coronary Atherosclerosis in Athletes. Circulation 136, 138–148 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Merghani A et al. Prevalence of Subclinical Coronary Artery Disease in Masters Endurance Athletes With a Low Atherosclerotic Risk Profile. Circulation 136, 126–137 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Möhlenkamp S et al. Running: the risk of coronary events : Prevalence and prognostic relevance of coronary atherosclerosis in marathon runners. Eur. Heart J 29, 1903–1910 (2008). [DOI] [PubMed] [Google Scholar]
- 27.Schwartz RS, Kraus SM, Schwartz JG & Wickstrom K Increased coronary artery plaque volume among male marathon runners. Mo. Med 111, 85–90 (2014). [PMC free article] [PubMed] [Google Scholar]
- 28.Braber TL et al. Occult coronary artery disease in middle-aged sportsmen with a low cardiovascular risk score: The Measuring Athlete’s Risk of Cardiovascular Events (MARC) study. Eur J Prev Cardiol 23, 1677–1684 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Hong KN, Fuster V, Rosenson RS, Rosendorff C & Bhatt DL How Low to Go With Glucose, Cholesterol, and Blood Pressure in Primary Prevention of CVD. Journal of the American College of Cardiology 70, 2171–2185 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Yusuf S et al. Blood-Pressure and Cholesterol Lowering in Persons without Cardiovascular Disease. New England Journal of Medicine 374, 2032–2043 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Leening MJG, Berry JD & Allen NB Lifetime Perspectives on Primary Prevention of Atherosclerotic Cardiovascular Disease. JAMA 315, 1449–1450 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Sniderman AD, Toth PP, Thanassoulis G, Pencina MJ & Furberg CD Taking a longer term view of cardiovascular risk: the causal exposure paradigm. BMJ 348, g3047 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Baigent C et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 366, 1267–1278 (2005). [DOI] [PubMed] [Google Scholar]
- 34.Cholesterol Treatment Trialists’ (CTT) Collaboration et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 376, 1670–1681 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Russell C, Sheth S & Jacoby D A Clinical Guide to Combination Lipid-Lowering Therapy. Curr Atheroscler Rep 20, 19 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Sabatine MS et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. New England Journal of Medicine 376, 1713–1722 (2017). [DOI] [PubMed] [Google Scholar]
- 37.Cannon CP et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. New England Journal of Medicine 372, 2387–2397 (2015). [DOI] [PubMed] [Google Scholar]
- 38.Schwartz GG et al. Effect of alirocumab, a monoclonal antibody to PCSK9, on long-term cardiovascular outcomes following acute coronary syndromes: rationale and design of the ODYSSEY outcomes trial. Am. Heart J 168, 682–689 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Adhyaru BB & Jacobson TA Role of Non-Statins, LDL-C Thresholds, and Special Population Considerations: A Look at the Updated 2016 ACC Consensus Committee Recommendations. Curr Atheroscler Rep 19, 29 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Blaha MJ Personalizing Treatment: Between Primary and Secondary Prevention. Am. J. Cardiol 118, 4A–12A (2016). [DOI] [PubMed] [Google Scholar]
- 41.Berman DS et al. Relationship between stress-induced myocardial ischemia and atherosclerosis measured by coronary calcium tomography. J. Am. Coll. Cardiol 44, 923–930 (2004). [DOI] [PubMed] [Google Scholar]
- 42.DeFilippis AP et al. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann. Intern. Med 162, 266–275 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.