Abstract
In a minority of relapsed myeloma, patient's disease may spread into extramedullary sites, associated with high degrees of heterogeneity. The breadth of myeloma therapeutic armamentarium allows clinicians to manage its heterogeneous presentation, including intracranial relapses, with fair success resulting in a significant prolongation of survival.
Keywords: bortezomib, extramedullary disease, multiple myeloma, refractoriness
1. INTRODUCTION
Multiple myeloma (MM) accounts for approximately 1% of all new cancer cases, and it is the 2nd most common hematologic malignancy,1, 2, 3 with an age‐adjusted incidence of five cases per 100 000 per year in the western world.4, 5 The disease is characterized by uncontrolled clonal proliferation of malignant plasma cells (PC) with a strong dependence on the bone marrow (BM) microenvironment, leading to lytic bone lesions, severe anemia, hypercalcemia, and renal impairment. MM is extremely heterogeneous, with multiple clones coexisting in the same patient, expressing ranging degrees of disease progression potential and treatment resistance.6
Clonal heterogeneity at diagnosis and at subsequent relapses results in a disease with varying clinical and phenotypic features that requires unique treatment considerations. Deep sequencing of plasma cell DNA at different time points over the course of the disease allows for targeted treatment approaches that could potentially prevent the development of dominant clones over time. Therefore, combination regimens, rather than single agent sequential therapies, could potentially be more effective in eradicating dominant, as well as minor, clones that often emerge during disease relapse.7, 8, 9
The past decade has seen extraordinary advances in the treatment of MM, particularly with the discovery of proteasome inhibitors (such as bortezomib) and immunomodulatory agents (such as lenalidomide), that have become the pillars of frontline treatment regimens.10 Furthermore, with an explosion of novel agents (including carfilzomib, pomalidomide, ixazomib, daratumumab, elotuzumab, and panobinostat), most of them already FDA and EMA approved, for use in the relapsed/refractory (R/R) setting, the overall response rates (ORR), progression‐free survival (PFS) and overall survival (OS) have dramatically improved.11, 12
However, as with other oncologic diseases that have been successfully treated with increasingly effective regimens, marked prolongation of OS has led to previously uncommon clinical presentations. In the case of MM, these include relapses in extramedullary sites, such as visceral organs, lymph nodes, and the central nervous system (mainly as meningeal myelomatosis), and secondary plasma cell leukemia. Even in the era of novel agents, extramedullary disease (EMD) remains a rare condition associated with poor prognosis and drug resistance.13 The reported incidence in newly diagnosed MM varies from 7% to 18%, with 6% to 20% of patients developing EMD later in the course of the disease.14, 15, 16, 17
Here, we will describe an extremely uncommon case of MM with intracranial EMD relapse. This patient received multiple lines of combination therapy, including double ASCT and novel agents that provided responses at every relapse.
2. CASE PRESENTATION
A 54‐year‐old Caucasian man was diagnosed with micromolecular lambda (λ) light chain MM in February 2013 due to severe back pain. His past medical history included a transitory ischemic attack in 2003. There was no other relevant medical, surgical, or family history finding. The diagnosis was based on a bone marrow (BM) biopsy that revealed infiltration of monoclonal plasma cells (35%), while fluorescence in situ hybridization (FISH) analysis identified 13q deletion and translocation t(11;14). The initial diagnostic MM workup demonstrated a presence of micromolecular free light chain (FLC) λ monoclonal protein (MP) (0.24 g/dL), elevated urine FLC 4.28 mg/dL, with an abnormal urine κ/λ ratio (rFLC) of 0.12. Laboratory tests also revealed anemia (Hb 10.7 g/dL), increased level of beta‐2 microglobulin (4.8 mg/L), mild increase of serum creatinine and lactate dehydrogenase (LDH) levels, with normal level of serum calcium. The patient was evaluated first with skeletal X‐ray, and later with both spinal magnetic resonance imaging (MRI) and positron emission tomography/computed tomography (PET/CT) that revealed multiple lytic lesions of the sternum, humerus, scapula, femur, and the skull, along with cervical, dorsal, lumbar, and pelvic lesions. Stage IIIA according to Durie‐Salmon and stage II according to Revised International Staging System (R‐ISS) criteria were confirmed.
The patient was enrolled in the European Intergroup Trial of the European Myeloma Network (EMN02/HOVON 95 MM)18 and, according to the protocol, an induction therapy was started using bortezomib, cyclophosphamide, and dexamethasone (VCD regimen). After three cycles of induction therapy, the patient achieved a stringent complete response (sCR) with negative minimal residual disease (MRD). Subsequently, the patient underwent cyclophosphamide‐based mobilization and collection of peripheral blood progenitor cells. He was then randomized, according to protocol, to the nontransplant arm and treated with bortezomib, melphalan, and prednisone, using an intensified VMP‐regimen. The patient completed four cycles of intensified VMP in December 2013, maintaining stringent CR.
In January 2014, he experienced a sudden onset of neurological symptoms (tongue deviation with swallowing difficulties, headache, diplopia, left trigeminal neuralgia, and paresthesia). An emergency CT was performed, revealing a large mass surrounding the left internal carotid artery, posterior to the pterygoid muscles and lateral to the levator veli palatini, confirmed later by brain MRI (Figure 1). Laboratory tests showed an absence of MP, with negative serum and urine immunofixation (IFE). A needle biopsy of the mass confirmed a nonsecretory EMD relapse of MM. The patient underwent 2nd‐line therapy with lenalidomide and dexamethasone (Rd), followed by a conditioning regimen with high‐dose melphalan (200 mg/m2) and double ASCT (respectively in April and July 2014). Disease re‐evaluation with brain MRI showed a size reduction of the extramedullary disease greater than 50% (Figure 2), along with a significant improvement in clinical and neurological symptoms, confirming a partial response (PR). A BM biopsy and MM laboratory tests were all negative.
Figure 1.
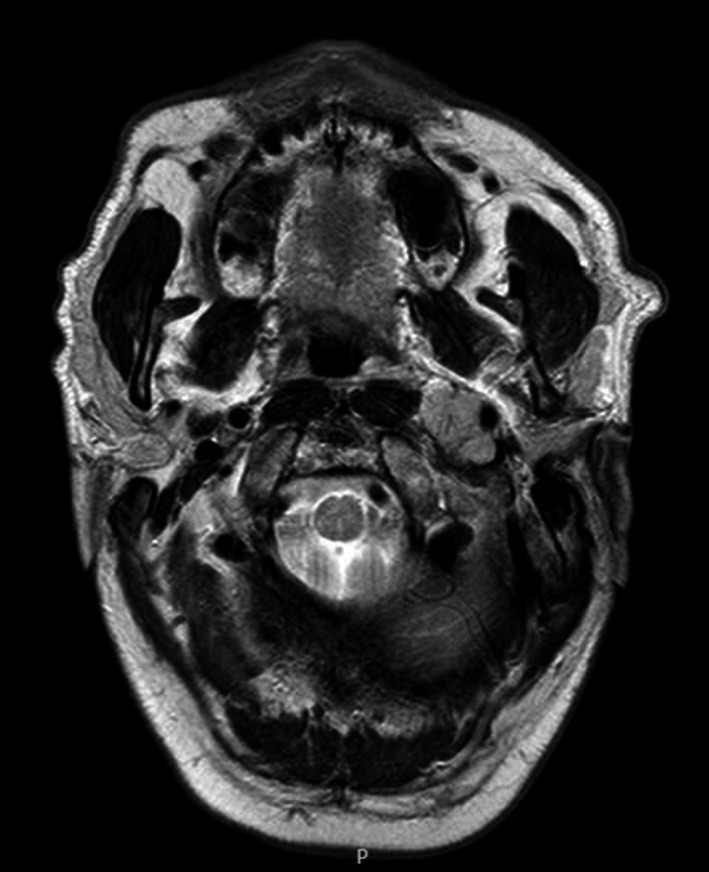
MRI scan of extramedullary disease at onset
Figure 2.
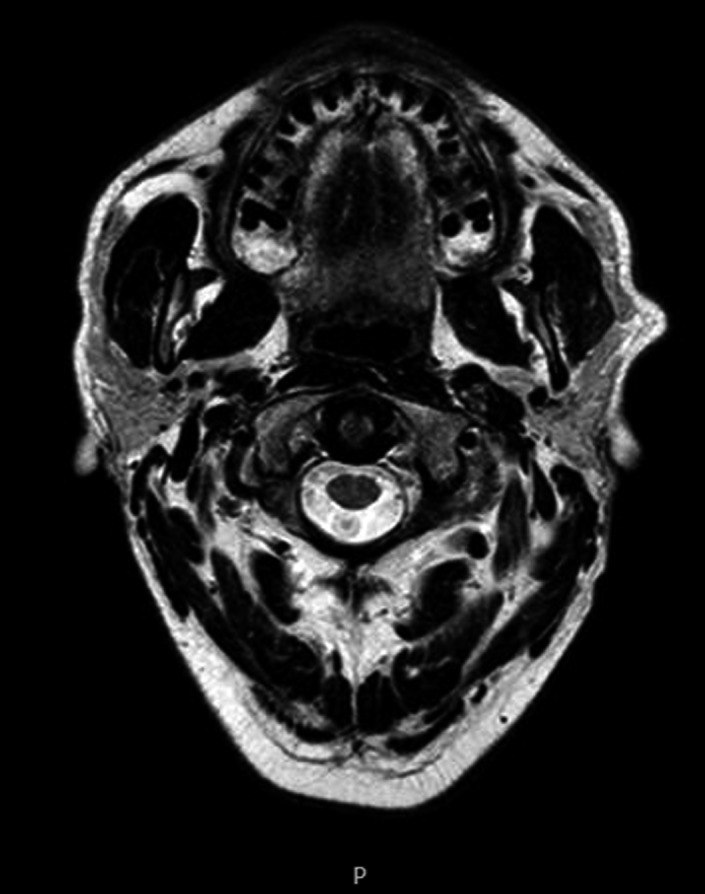
MRI scan of extramedullary disease after double ASCT (autologous stem cell transplant)
A 3rd‐line therapy with lenalidomide alone was started in August 2014 to maintain the response. In October 2014, the patient was enrolled in a preallogeneic stem cell transplant screening program, with a sibling identified as a matching donor. As a bridge to transplant, the patient received a 4th‐line therapy with lenalidomide, doxorubicin, and dexamethasone, for a total of two cycles. Unfortunately, due to a temporary lack of eligibility, the donor withdrew consent and the patient declined to join the search program for Matched Unrelated Donors (MUD).
Next, we decided to use a combination of ifosfamide and lenalidomide as the 5th‐line therapy from December 2014 until April 2015, due to the need to treat the patient with agents that cross the blood brain barrier. During this time, laboratory assessments remained negative, brain MRIs were stable, and total body MRI showed a reduction in both the infiltration and the size of the previously identified osteolytic lesions, confirming maintenance of the partial response.
In April 2015, the patient progressed with numerous lesions in the ribs, vertebrae, scapulae, humeri, right femur, and pelvis. Laboratory tests did not reveal a recurrence of the MP, suggesting a nonsecretory disease relapse. Therefore, a polychemotherapy regimen including cyclophosphamide, etoposide, cisplatin, and dexamethasone (DCEP regimen) was administered for a total of two cycles. Unfortunately, PET/CT scans revealed further progression of the disease, so the patient was switched to the 7th‐line therapy with bendamustine, bortezomib, and dexamethasone, the BVD regimen, from July 2015 until April 2016, for a total of eight cycles. During this period, the patient experienced significant improvement of symptoms, including a marked pain relief. In February 2016, re‐staging with PET/CT revealed the presence of residual disease; however, metabolic activity and size of bone lesions was significantly reduced compared to July 2015. Brain MRI was stable and laboratory examinations remained negative for serological disease. Results indicated that another partial remission was achieved.
In May 2016, taking advantage of the development of novel agents, we decided to start the 8th line of therapy with pomalidomide and dexamethasone. Unfortunately, the therapy was temporarily withheld in June 2016, due to grade IV hematological toxicity and severe diarrhea. The patient was hospitalized and treated with blood product transfusion, fluids, and electrolyte therapy. Subsequently, in July 2016, the patient developed interstitial lung disease, so he was hospitalized and treated with antibiotics and transfusion. The treatment was restarted again in August 2016, but this time without dexamethasone to avoid steroid‐related side effects. While the patient was on pomalidomide therapy, the disease was re‐evaluated by BM biopsy, measurement of MP, and brain and total body MRI scans, which were negative for new bone lesions. The partial response was confirmed.
In June 2017, while still receiving pomalidomide, the patient experienced a sudden onset of burning pain on the left side of the face, which was unresponsive to analgesics. Routine tests were performed, revealing the reappearance of MP (0.06 g/dL), with a positive serum IFE test for IgG κ and a negative urine IFE. A total body MRI scan did not reveal the presence of any new osteolytic lesions, while the intracranial mass remained stable in size. The pain responded well to steroid therapy and was most likely caused by inflammatory neuralgia. Due to disease progression, the patient was started on a 9th line of therapy with carfilzomib, lenalidomide, and dexamethasone, KRd regimen. After two cycles, both the serum and urine IFE results were negative, and the MRI scan remained stable. The patient was in complete remission, with stable EMD. The treatment was well tolerated, and significant pain relief was achieved with analgesic therapy.
In July 2018, after completing thirteen cycles of the KRd regimen, disease started to progress once again, with significant back pain, and the presence of IgG κ MP (0.05 g/dL). Multiple skeletal segments were detected by PET/CT, including the vertebral column (Figure 3), while the BM biopsy remained negative. The patient was switched to the 10th‐line treatment with daratumumab, bortezomib, and dexamethasone, the DaraVd regimen, in August 2018.
Figure 3.
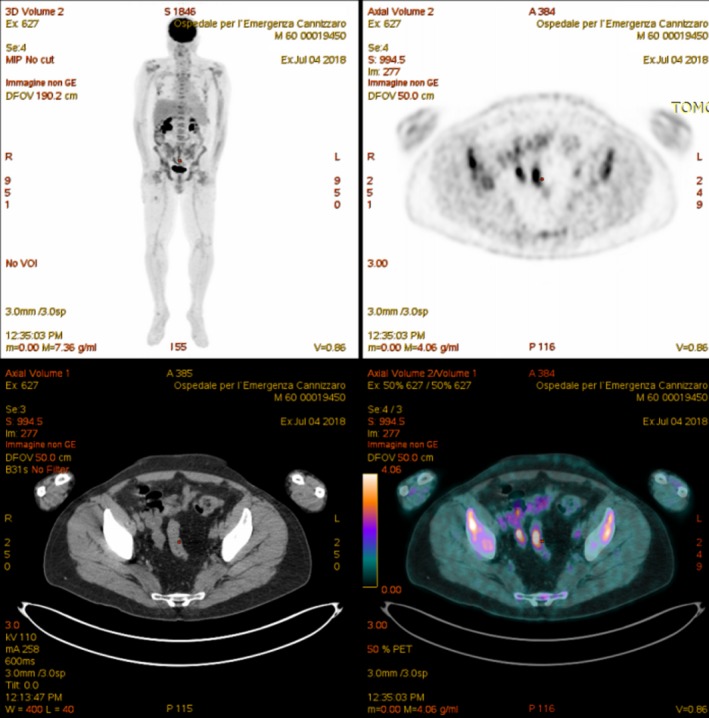
PET/CT scan of multiple myeloma relapse after 9th line of therapy KRd regimen (carfilzomib, lenalidomide, and dexamethasone)
Currently, the patient is doing well, reporting a significant improvement in disease‐related pain. After completing five cycles of DaraVd, the MP levels are still present but reduced, while the EMD remains stable. We did not assess the presence of therapy‐related IgG κ due to technical difficulties (daratumumab being an IgG κ antibody), but serum FLC κ value has decreased since the DaraVd regimen was initiated.
3. DISCUSSION
This rare case of myeloma has several unusual characteristics:
Probable clonal evolution with clinical and phenotypic disease changes at different relapses.
Aggressive nonsecretory EMD relapse, even with concomitant BM response to therapy, currently stable.
Treatment with multiple combination therapies, with interesting results in favor of novel agents, including pomalidomide, carfilzomib, and daratumumab, despite the aggressiveness of the disease (Figure 4).
Figure 4.
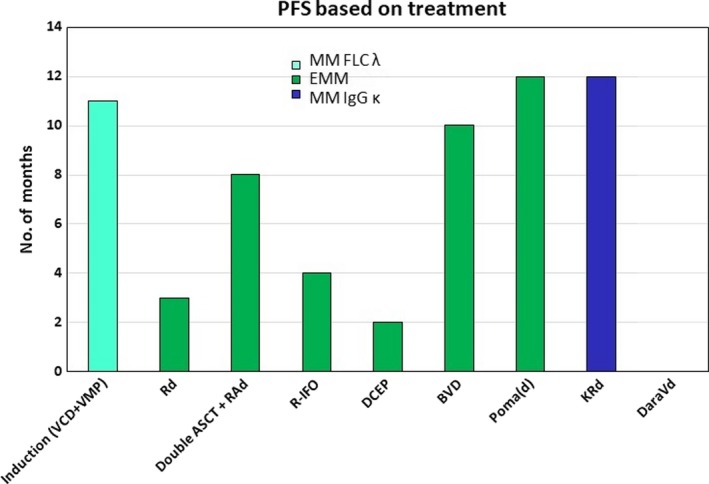
Progression‐free survival (PFS) comparison in different lines of therapy (MM—multiple myeloma; EMM— extramedullary multiple myeloma; ASCT—autologous stem cell transplant; VCD—bortezomib, cyclophosphamide, dexamethasone; VMP—bortezomib, melphalan, prednisone: Rd— lenalidomide, dexamethasone; RAd—lenalidomide, adriamycin, dexamethasone; R‐IFO— lenalidomide, ifosfamide; DCEP—dexamethasone, cyclophosphamide, etoposide, cisplatin; BVD— bendamustine, bortezomib, dexamethasone; PomaD— pomalidomide, dexamethasone; KRd—carfilzomib, lenalidomide, dexamethasone; DaraVd—daratumumab, bortezomib, dexamethasone)
Intraclonal diversity and clonal evolution during the course of MM with different clinical and phenotypic characteristics have been reported and shown to be associated with one or more abnormalities, such as the presence of biclonal disease, a switch in the monoclonal protein subtype (light chain conversion), the presence of numeric, and structural chromosomal aberrations in a subset of MM‐cells, and occasionally the discordant therapeutic response (between extramedullary and bone marrow disease) within the same patient.19 This heterogeneous clonal mixture at relapse and tiding over time supports the Darwinian branching model of tumor evolution. It is characterized by several clonal progenitors, or tumor‐initiating cells, present at diagnosis with therapeutic or ecosystem‐dependent selection pressures driving the alternating dominance of these clones over time.7 Therefore, cytogenetic risk re‐evaluation of MM with FISH after every relapse and/or progression of disease should be considered.
Unfortunately, in the described case, FISH analysis of EMD was not performed because of the difficult disease location in the CNS (around left ICA), accessible for biopsy only by maxillofacial surgery. Furthermore, FISH analyses at each relapse were negative for new alterations other than del 13q and t(11;14) that were present at diagnosis. However, even without additional chromosomal aberrations, we suspect that the disease has evolved, expressing a phenotypic shift from micromolecular FLC λ, to nonsecretory, IFE negative, EMD, and finally IgG κ MM, suggesting the presence of different clones.
An EMD is a rare event at the onset of disease and more frequent at the time of progression or relapse, usually caused by changing interactions between plasma cells and the bone marrow microenvironment.20, 21 It has been associated with an aggressive course of disease, presence of high‐risk genetic abnormalities, resistance to treatment, and poor outcomes.22, 23 The frequency of EMD onset in relapsed/refractory MM is estimated to range from 3% to 14%, with CNS involvement accounting for approximately 3% of cases. An increased EMD incidence seen in the last decade is thought to be associated with prolonged overall survival (related to introduction of novel agents such as IMiDs, PIs, and monoclonal antibodies) and better detection (related to improved imaging techniques).13, 24, 25, 26
Bortezomib‐induced peripheral neuropathy (BIPN) is present in about 75% of treated patients. Variable neurological manifestations associated with BIPN may pose a clinical challenge in differentiating between therapy‐related and disease‐related symptoms, such as the CNS relapse.27
In our case, a sudden onset of intracranial EMD happened during the period of stringent CR, one month after completion of VCD induction and intensified VMP consolidation therapy. The disease further progressed during lenalidomide monotherapy, confirming the lack of response in EMD (particularly in the CNS location) during the earlier bortezomib and lenalidomide combination regimens.
Transplant‐eligible patients with high‐risk MM are good candidates for tandem ASCT, as it may offer improved benefits in terms of PFS and OS, not only in the frontline, but also in the relapsed/refractory setting.28 Our patient underwent double tandem ASCT during the EMD relapse, achieving a partial response with ≥50% reduction in the size of the pathological mass and significant neurological recovery.
Pomalidomide is a 3rd‐generation IMiDs, with well‐established efficacy in the setting of relapsed/refractory MM.29 In a small number of EMD patients, the drug has shown a response rate of approximately 30%.26, 30 Our group has recently submitted a real‐life experience with pomalidomide in 76 patients among which about 10% had an EMD relapse, with a superior PFS and OS compared to the current literature.31
In the case we report here, the patient was treated with pomalidomide, (4 mg) as the 8th line of therapy, two years after double ASCT and different chemotherapy combination regimens. A partial response was achieved and maintained during 13 months of therapy (Figure 5), with a PFS significantly longer compared to the MM‐003 study (PFS 3.9 months; OS 11.9 months).32 During this period, the patient discontinued the therapy twice because of substantial hematologic toxicity (severe anemia and febrile neutropenia with pneumonia), a common therapy‐related side effect.32 The adverse effects of pomalidomide therapy were managed by G‐CSF as secondary prophylaxis, without further drug discontinuation.
Figure 5.
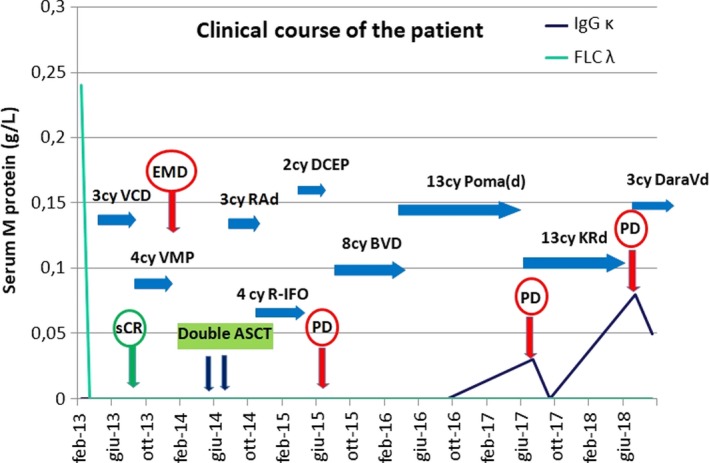
Clinical course of the disease and efficacy of different lines of therapy, compared to correspondent clinical studies (EMD—extramedullary disease; sCR—stringent complete response; PD—progressive disease; PFS—progression‐free survival; OS—overall survival; ASCT—autologous stem cell transplant; VCD—bortezomib, cyclophosphamide, dexamethasone; VMP—bortezomib, melphalan, prednisone: RAd—lenalidomide, adriamycin, dexamethasone; R‐IFO—lenalidomide, ifosfamide; DCEP—dexamethasone, cyclophosphamide, etoposide, cisplatin; BVD—bendamustine, bortezomib, dexamethasone; PomaD—pomalidomide, dexamethasone; KRd—carfilzomib, lenalidomide, dexamethasone; DaraVd—daratumumab, bortezomib, dexamethasone)
Carfilzomib is the 2nd‐generation proteasome inhibitor, investigated as a KRd regimen in the R/R MM setting (mainly as the 2nd or 3rd line of therapy) in the ASPIRE study (PFS 26.3 months; OS 48.3 months) and several other smaller studies.33, 34 There is limited evidence of carfilzomib efficacy in the setting of R/R EMD myeloma, although several abstracts have been published recently or are in preparation, that describe extramedullary disease subgroup from the KRd studies, including the experience from our group.35, 36
Our patient was treated with KRd in the 9th line for a total of 12 months, achieving a CR (stable EMD) after only two cycles, without significant therapy‐related side effects. The PFS was inferior compared to the ASPIRE study, but satisfactory, considering that the therapy was started in a lenalidomide‐refractory patient treated with different multi‐drug regimens in the past (Figure 5).
Daratumumab is an anti‐CD38 IgG κ monoclonal antibody. It is actually used in R/R myeloma patients starting from the 2nd line as a single agent (Sirius study: PFS 3.7 months; OS 18.6 months) or as part of a 3‐drug regimen, with either bortezomib(CASTOR study: PFS 16.7 months; OS not reached) or lenalidomide (POLLUX study: PFS and OS not reached), with superior response, especially in patients after 1st and 2nd relapse of the disease.37, 38 The analysis of daratumumab‐based regimens in extramedullary populations of MM patients is limited, although there are reports of promising results in patients not eligible for high‐dose chemotherapy and double ASCT.34
In our case, after disease progression on KRd, a DaraVd regimen was initiated. Currently, the patient has completed five cycles (Figure 5). The monoclonal protein is still present, although the results are uncertain, because of the same type of light chains of the patient's disease and the monoclonal antibody—IgG κ. The treatment plan is to proceed until progression and re‐evaluate the disease with FLC ratio and PET/CT scan.
As for future treatment options, after possible DaraVd progression of disease, there are several approaches to be considered. Allo‐SCT with myeloablative conditioning has been reported to result in a long‐term PFS, with a plateau in survival curves suggesting possible curative benefit in some patients,39, 40 including a subgroup of EMD patients with del 17p or multi‐organ involvement.41
Our patient is 59 years old at the moment, already heavily treated, and still eligible for transplant, but so far has consistently refused the procedure.
In our case report, FISH analysis was done at diagnosis demonstrating positivity for t(11;14), confirmed at later relapses of the disease. Therefore, a venetoclax (oral BCL‐2 inhibitor)‐based therapy could be an option, as a single agent or in combination regimens with bortezomib, dexamethasone ± daratumumab, even if the therapy outcome would be extremely uncertain.42, 43, 44
MM remains a heterogeneous disorder where every available treatment could be useful at any phase of disease progression. We are far from being able to define which therapeutic options are superior to others in the R/R MM setting, especially in the intracranial EMD, but we can, at least, suggest that in the majority of cases in the era of novel agents, there are viable therapeutic options for every patient.
Clinical trials with novel drugs and monoclonal antibodies should always be considered in R/R myeloma patients.
4. CONCLUSION
MM patients demonstrate clonal heterogeneity with phenotype variations and eventual changes in therapy sensitivity, so a complete re‐evaluation, including cytogenetic analysis with FISH should be done after every relapse and/or progression of disease.
Progressive EMD is an uncommon, more aggressive disease presentation, even in patients with medullar response, and possible salvage therapy solutions could include single or tandem ASCT, which could have a critical impact on reducing a tumor burden. Novel agents, such as pomalidomide, carfilzomib, and daratumumab, have demonstrated a reasonably good efficacy even in the advanced lines of therapy and thus present a valuable therapeutic option in the relapsed setting, with potential to improve outcomes in highly pretreated patients.
We believe that pomalidomide, carfilzomib, and daratumumab could all play an important role in the treatment of an increasing number of patients exposed and/or refractory to lenalidomide after the first‐line and maintenance therapy, and also in the long‐term control of more challenging sites of relapse, such as extramedullary disease. Larger studies and real‐life experiences are needed in this setting to further evaluate the efficacy of these new regiments.
CONFLICT OF INTEREST
None declared.
AUTHOR’S CONTRIBUTION
All authors have made substantial contributions to all of the following: UM: interpreted data, drafted the final article and critically revised it. VC: obtained patient consent, collected initial data, and wrote the first draft of manuscript. UM, CC, VC, EM, RG, MSP, and VDF: involved in the acquisition of data, analysis, and interpretation of data. CC, AR, and FDR: revised the article for important intellectual content and approved the final version for submission.
ACKNOWLEDGMENTS
The authors would like to thank Andrej Pervan for editorial assistance on the manuscript.
Markovic U, Calafiore V, Martino E, et al. A rare case of multiple myeloma with intracranial extramedullary relapse: One or more myeloma clones? Clin Case Rep. 2019;7:1629–1636. 10.1002/ccr3.2292
Contributor Information
Uros Markovic, Email: urosmarkovic09041989@gmail.com.
Concetta Conticello, Email: ettaconticello@gmail.com.
REFERENCES
- 1. Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046‐1060. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller K. Cancer statistics, 2017. CA Cancer J Clin. 2017;65:5‐29. [DOI] [PubMed] [Google Scholar]
- 3. Teras LR, DeSantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR. US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016;66:443‐459. [DOI] [PubMed] [Google Scholar]
- 4. Phekoo KJ, Schey SA, Richards MA, et al. A population study to define the incidence and survival of multiple myeloma in a National Health Service Region in UK. Br J Haematol. 2004;127(3):299‐304. [DOI] [PubMed] [Google Scholar]
- 5. Sant M, Allemani C, Tereanu C, et al. Incidence of hematologic malignancies in Europe by morphologic subtype: Results of the HAEMACARE project. Blood. 2010; 116(19):3724‐3734. [DOI] [PubMed] [Google Scholar]
- 6. Kuehl WM, Bergsagel PL. Molecular pathogenesis of multiple myeloma and its premalignant precursor. J Clin Invest. 2012;122(10):3456‐3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Keats JJ, Chesi M, Egan JB, et al. Clonal competition with alternating dominance in multiple myeloma. Blood. 2012;120(5):1067‐1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Egan JB, Shi C‐X, Tembe W, et al. Whole‐genome sequencing of multiple myeloma from diagnosis to plasma cell leukemia reveals genomic initiating events, evolution, and clonal tides. Blood. 2012;120(5):1060‐1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Walker BA, Wardell CP, Melchor L, et al. Intraclonal heterogeneity and distinct molecular mechanisms characterize the development of t(4;14) and t(11;14) myeloma. Blood. 2012;120(5):1077‐1086. [DOI] [PubMed] [Google Scholar]
- 10. Moreau P, Attal M, Facon T. Frontline therapy of multiple myeloma. Blood. 2015;125(20):3076‐3084. [DOI] [PubMed] [Google Scholar]
- 11. Kumar SK, Rajkumar SV. The current status of minimal residual disease assessment in myeloma. Leukemia. 2014;28(2):239‐240. [DOI] [PubMed] [Google Scholar]
- 12. Nooka AK, Kastritis E, Dimopoulos MA, Lonial S. Treatment options for relapsed and refractory multiple myeloma. Blood. 2015;125(20):3085‐3099. [DOI] [PubMed] [Google Scholar]
- 13. Usmani SZ, Heuck C, Mitchell A, et al. Extramedullary disease portends poor prognosis in multiple myeloma and is over‐represented in high‐risk disease even in the era of novel agents. Haematologica. 2012;97:1761‐1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Varettoni M, Corso A, Pica G, Mangiacavalli S, Pascutto C, Lazzarino M. Incidence, presenting features and outcome of extramedullary disease in multiple myeloma: A longitudinal study on 1003 consecutive patients. Ann Oncol. 2010;21(2):325‐330. [DOI] [PubMed] [Google Scholar]
- 15. Wu P, Davies FE, Boyd K, et al. The impact of extramedullary disease at presentation on the outcome of myeloma. Leuk Lymphoma. 2009;50(2):230‐235. [DOI] [PubMed] [Google Scholar]
- 16. Bladé J, Lust JA, Kyle RA. Immunoglobulin D multiple myeloma: Presenting features, response to therapy, and survival in a series of 53 cases. J Clin Oncol. 1994;12(11):2398‐2404. [DOI] [PubMed] [Google Scholar]
- 17. Bladé J, Kyle RA, Greipp PR. Presenting features and prognosis in 72 patients with multiple myeloma who were younger than 40 years. Br J Haematol. 1996;93(2):345‐351. [DOI] [PubMed] [Google Scholar]
- 18. Cavo M, Petrucci M, Di Raimondo F, et al. Versus double autologous stem cell transplantation for newly diagnosed multiple myeloma: an intergroup, multicenter, phase III study of the European myeloma network (EMN02/HO95 MM Trial). Blood. 2016;128:991. [Google Scholar]
- 19. Bahlis NJ. Darwinian evolution and tiding clones in multiple myeloma. Blood. 2012;120(5):927‐928. [DOI] [PubMed] [Google Scholar]
- 20. Varga C, Xie W, Laubach J, et al. Development of extramedullary myeloma in the era of novel agents: No evidence of increased risk with lenalidomide‐bortezomib combinations. Br J Haematol. 2015;169(6):843‐850. [DOI] [PubMed] [Google Scholar]
- 21. Bladé J, Fernández De Larrea C, Rosiñol L, Cibeira MT, Jiménez R, Powles R. Soft‐tissue plasmacytomas in multiple myeloma: Incidence, mechanisms of extramedullary spread, and treatment approach. J Clin Oncol. 2011;29(28):3805‐3812. [DOI] [PubMed] [Google Scholar]
- 22. Rasmussen T, Kuehl M, Lodahl M, Johnsen HE, Dahl I. Possible roles for activating RAS mutations in the MGUS to MM transition and in the intramedullary to extramedullary transition in some plasma cell tumors. Blood. 2005;105:317‐323. [DOI] [PubMed] [Google Scholar]
- 23. López‐Anglada L, Gutiérrez NC, García JL, Mateos MV, Flores T, San Miguel JF. P53 deletion may drive the clinical evolution and treatment response in multiple myeloma. Eur J Haematol. 2010;84:359‐361. [DOI] [PubMed] [Google Scholar]
- 24. Qu X, Chen L, Qiu H, et al. Extramedullary manifestation in multiple myeloma bears high incidence of poor cytogenetic aberration and novel agents resistance. Biomed Res Int. 2015;2015:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pour L, Sevcikova S, Greslikova H, et al. Soft‐tissue extramedullary multiple myeloma prognosis is significantly worse in comparison to bone‐related extramedullary relapse. Haematologica. 2014;99(2):360‐364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Short KD, Rajkumar SV, Larson D, et al. Incidence of extramedullary disease in patients with multiple myeloma in the era of novel therapy, and the activity of pomalidomide on extramedullary myeloma. Leukemia. 2011;25(6):906‐908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Abid MB, De Mel S, Abid MA, Tan KB, Chng WJ. Bortezomib‐related neuropathy may mask CNS relapse in multiple myeloma: A call for diligence. Cancer Biol Ther. 2016;17(7):723‐726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lonial S, Boise LH, Kaufman J. How I treat high‐risk myeloma. Blood. 2015;126(13):1536‐1543. [DOI] [PubMed] [Google Scholar]
- 29. Dimopoulos MA, Weisel KC, Song KW, et al. Cytogenetics and long‐term survival of patients with refractory or relapsed and refractory multiple myeloma treated with pomalidomide and low‐dose dexamethasone. Haematologica. 2015;100(10):1327‐1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mussetti A, Dalto S, Montefusco V. Effective treatment of pomalidomide in central nervous system myelomatosis. Leuk Lymphoma. 2013;54(4):864‐866. [DOI] [PubMed] [Google Scholar]
- 31. Parisi MS, Calafiore V, Martino E, et al. Long term disease control with pomalidomide and dexamethasone in relapsed/refractory multiple myeloma patients: a real life experience; 2018. [DOI] [PMC free article] [PubMed]
- 32. Miguel JS, Weisel K, Moreau P, et al. Pomalidomide plus low‐dose dexamethasone versus high‐dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM‐003): A randomised, open‐label, phase 3 trial. Lancet Oncol. 2013;14(11):1055‐1066. [DOI] [PubMed] [Google Scholar]
- 33. Siegel DS, Dimopoulos MA, Ludwig H, et al. Improvement in overall survival with carfilzomib, lenalidomide, and dexamethasone in patients with relapsed or refractory multiple myeloma. J Clin Oncol. 2018;36(8):728‐734. [DOI] [PubMed] [Google Scholar]
- 34. Dingli D, Ailawadhi S, Bergsagel PL, et al. Therapy for Relapsed Multiple Myeloma: Guidelines From the Mayo Stratification for Myeloma and Risk‐Adapted Therapy. Mayo Clin Proc. 2017;92(4):578‐598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Conticello C, Romano A, Del Fabro V, et al. Feasibility, Tolerability and Efficacy of Carfilzomib in Combination with Lenalidomide and Dexamethasone in Relapsed Refractory Myeloma Patients: A Retrospective Real-Life Survey of the Sicilian Myeloma Network. J Clin Med. 2019;8(6):877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Barila G, Meneghini V, Bonalumi A, et al. KRD treatment of Relapsed/Refractory Multiple Myeloma: a real life experience. Haematologica. 2017;102(s2):1‐882.28040785 [Google Scholar]
- 37. Usmani SZ, Weiss BM, Plesner T, et al. Clinical efficacy of daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma. Blood. 2016;128(1):37‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Palumbo A, Chanan‐Khan A, Weisel K, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(8):754‐766. [DOI] [PubMed] [Google Scholar]
- 39. Bensinger WI, Buckner CD, Anasetti C, et al. Allogeneic marrow transplantation for multiple myeloma: an analysis of risk factors on outcome. Blood. 1996;88:2787‐2793. [PubMed] [Google Scholar]
- 40. Bjorkstrand P, Svensson H, Hermans J, et al. Allogeneic bone marrow transplantation versus autologous stem cell transplantation in multiple myeloma: a retrospective case‐matched study from the European Group for Blood and Marrow Transplantation. Blood. 1996;88:4711‐4718. [PubMed] [Google Scholar]
- 41. Rasche L, Röllig C, Stuhler G, et al. Allogeneic hematopoietic cell transplantation in multiple myeloma: focus on longitudinal assessment of donor chimerism, extramedullary disease, and high‐risk cytogenetic features. Biol Blood Marrow Transplant. 2016;22(11):1988‐1996. [DOI] [PubMed] [Google Scholar]
- 42. Kumar S, Kaufman JL, Gasparetto C, et al. Efficacy of venetoclax as targeted therapy for relapsed/refractory t(11;14) multiple myeloma. Blood. 2017;130(22):2401‐2409. [DOI] [PubMed] [Google Scholar]
- 43. Rahbari KJ, Nosrati JD, Spektor TM, Berenson JR. Spektor TM, Berenson JR. Venetoclax in Combination With Bortezomib, Dexamethasone, and Daratumumab for Multiple Myeloma. Clin Lymphoma, Myeloma Leuk. 2018;18(9):e339‐e343. [DOI] [PubMed] [Google Scholar]
- 44. Moreau P, Chanan‐Khan A, Roberts AW, et al. Promising efficacy and acceptable safety of venetoclax plus bortezomib and dexamethasone in relapsed/refractory MM. Blood. 2017;130(22):2392‐2400. [DOI] [PubMed] [Google Scholar]


