Abstract
Objectives
Breast cancer (BC) is the leading cancer among women. Almost 20% of patients develop brain metastases (BM) and die shortly afterward. There is a dearth of data on the survival outcome of BC patients with BM from the Arab world.
Methods
Consecutive women diagnosed with BC who developed radiologically-confirmed BM during their illness were identified through the hospital’s electronic patient’s records. Clinicopathological features and treatment outcomes were recorded. Survival was calculated using the Kaplan-Meier method, and factors affecting survival were studied using log-rank analysis.
Results
Between January 2003 and June 2015, a total of 692 patients were treated for BC at our institute. Forty-eight (6.9%) developed BM. The median age at the diagnosis of BM was 45.2 years. More than half of cohort (54.2%) had HER2 positive disease, while 27.1% had the triple-negative disease. The median time interval between the diagnosis of BC and the development of BM was 21 months, and median survival after development of brain disease was seven months. On univariate analysis, pathological grade, previous systemic treatment, brain as the first site of metastases, brain as the only site of metastases, treatment of BM, systemic treatment after BM, and diagnosis-specific graded prognostic assessment (DS-GPA) score significantly affected survival. On multivariate Cox regression analysis, the brain as the first site of metastases, treatment for brain disease, treatment type, and DS-GPA score significantly affected survival post-BM.
Conclusions
Our data indicate that Omani women are diagnosed with BC at a younger age, develop BM earlier, and carry a poor outcome.
Keywords: Brain Neoplasms, Breast Cancer, Oman, Radiotherapy
Introduction
Breast cancer (BC) is the most common cancer among women worldwide with significant geographical variation in the age-standardized incidence rate (ASR). In 2018, 2.1 million new cases of BC were diagnosed, and it remained the leading cause of cancer-related deaths in most countries.1 The vast majority of women are diagnosed with either localized or locally advanced disease, and around 10–20% of patients develop brain metastases (BM) in the course of their disease.2 The incidence of BM in BC is increasing worldwide, and this is attributed to an aging population, better systemic treatment, longer survival after treatment, and increased surveillance using sophisticated diagnostic imaging facilities.3
Historically, BC patients with BM were considered to have a uniformly poor prognosis with little consideration for identifying patients who might benefit from more aggressive therapy.2-5 However, there is significant heterogeneity not only in BC but also in patients who develop BM.4,6,7 Several risk factors have been identified, including early-onset BC, higher tumor grade, molecular subtype, and treatment type.4-8 Molecular subtyping has identified three distinct histological types of BC: hormone receptor (HR) positive, human epidermal growth factor receptor (HER2/neu) positive, and triple-negative (TN).9 Recognition of the heterogeneity helps to tailor the treatment according to the prognostic and predictive factors.10 More recently, it has been shown that the median survival after the diagnosis of BM is highest among the HR-positive subtype, intermediate in HER2/neu positive, and shortest in the TN subtype.11-15 Reasons for this variation in overall outcome is the availability of targeted treatment for HR and HER2/neu positive BC, which is not the case for the TN form, as well as underlying molecular heterogeneity.12,16,17
The pattern of presentation of BC in Oman and other countries in the region is different from that reported in the literature. For example, the ASR in western countries is about 75/100 000 and 25.5/100 000 in Oman.18 The median age at diagnosis is 47 years, and between 50–60% of patients present with locally advanced or metastatic disease.19-21 Although the outcomes of BC have been reported, there is a paucity of data on the outcomes of BC patients with BM from Oman and other countries in the region.22,23 This study was designed to report the outcomes of patients with the three molecular subtypes of BC described above from a single institution in Oman receiving the standard of care treatment. Secondary aims included studying the prognostic factor responsible for the development of BM.
Methods
All patients with BC who presented with BM or later developed BM and were treated at the Sultan Qaboos University Hospital (SQUH) between January 2003 and June 2015 were included in the study. The diagnosis of BM was established by cerebral computed tomography scan or magnetic resonance imaging (MRI). Electronic medical records were reviewed to study the clinicopathological features and treatment outcomes of BC patients with BM.
Male BC patients, patients with a history of previous, concomitant other primary carcinoma, or the presence of leptomeningeal carcinomatosis were excluded.
Clinicopathological features including age, pathological type and grade, prognostic and predictive markers, stage at the time of diagnosis, presenting symptoms at the time of brain disease, treatment type before and after developing BM, and diagnosis-specific graded prognostic assessment (DS-GPA) score were recorded and analyzed. The time from diagnosis to development of BM was defined as the time from the diagnosis of BC to the evidence of brain parenchymal disease on imaging. Overall survival-1 (OS1) was defined as the time from initial BC diagnosis to death from any cause, while OS2 was the time from the diagnosis of BM to death. Patients who were alive or lost to follow-up without an event (BM) were censored at the closing date of the study analysis (30 April 2017).
The DS-GPA score was used to determine survival prediction post-BM. Patients were assigned a score from 0–4 depending upon the type of BC, performance status at the time of BM and age.24
All data were recorded on a questionnaire and later entered in SPSS Statistics (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.). Descriptive statistics were used to describe the patient’s clinicopathological characteristics. Survival rates were estimated using the Kaplan-Meier method and compared using the log-rank test. Multivariate Cox regression (forward and backward stepwise) analysis was used to identify factors influencing survival after development of BM.
Results
Between January 2003 and June 2015, 692 patients were treated at SQUH with BC; 48 (6.9%) developed BM. Only two patients presented with BM at the time of diagnosis, while others developed it during their illness trajectory. The median age at diagnosis of BC was 43.5 (26–69) years.
Over half of patients (54.2%) were aged less than 40 years at the time of BM development. The majority of women had infiltrating ductal carcinoma (85.4%) with grade III disease in 70.8%. More than half of cohort (54.2%) had HER2 positive disease while 27.1% were diagnosed with the TN subtype. More than one-third (39.6%) of women had stage IV disease at the time of diagnosis. Headache was the most common symptom at presentation with BM (62.5%) and 47.9% of women had more than three lesions on imaging. Almost equal numbers of patients were divided into the three subcategories according to DS-GPA score [Table 1].
Table 1. Patient characteristics and its significance on survival after developing brain metastases (BM).
| Characteristics | n (%) |
|---|---|
| Median age, years | 43.5 |
| Median age at diagnosis of BM, years | 45.2 |
| Age, years | |
| < 44 | 26 (54.2) |
| > 44 | 22 (45.8) |
| Stage at diagnosis of breast cancer | |
| II | 14 (29.2) |
| III | 15 (31.3) |
| IV | 19 (39.6) |
| Histopathology | |
| Infiltrating ductal carcinoma | 41 (85.4) |
| Infiltrating lobular carcinoma | 2 (4.2) |
| Miscellaneous | 4 (8.4) |
| Tumor grade | |
| I | 1 (2.1) |
| II | 11 (22.9) |
| III | 34 (70.8) |
| Missing | 2 (4.2) |
| Prognostic and predictive markers | |
| Hormone receptor-positive, HER2 negative | 9 (18.8) |
| Hormone receptor-positive, HER2 positive | 13 (27.1) |
| Hormone receptor-negative, HER2 positive | 13 (27.1) |
| Triple-negative | 13 (27.1) |
| Symptoms at presentation | |
| Headache (with or without vomiting) | 30 (62.5) |
| Any neurological deficit | 14 (29.2) |
| Asymptomatic | 4 (8.3) |
| Number of brain lesions | |
| 1 | 16 (33.3) |
| 2–3 | 8 (16.7) |
| > 3 | 23 (47.9) |
| Missing | 1 (2.1) |
| DS-GPA | |
| 0–1 | 2 (4.2) |
| 1.5–2 | 15 (31.3) |
| 2.5–3 | 16 (33.3) |
| 3.5–4 | 15 (31.3) |
DS-GPA: diagnosis-specific graded prognostic assessment score; HER2: human epidermal growth factor receptor.
The median time to development of BM after the diagnosis was 21 months (range 0–113 months). The median time for developing BM was 17 months in patients with the TN and HR-negative/HER2 positive subtypes, and 34.5 months for HR-positive/HER2 positive cases, and 33 months for HR positive/HER2 negative BC patients.
All but two patients received some form of chemotherapy (mostly anthracycline and taxane-based regimens) at the time of presentation, while those two were treated with hormonal therapy. All patients with HER2 positive disease were treated with trastuzumab as a first-line treatment and with trastuzumab, lapatinib, or ado-trastuzumab after developing BM [Table 2].
Table 2. Treatment offered before and after developing brain metastases (BM) and its significance on survival.
| Treatment offered | n (%) |
|---|---|
| Before BM | |
| Neo-adjuvant chemotherapy followed by surgery and radiotherapy | 12 (25.0) |
| Surgery followed by adjuvant chemotherapy and radiotherapy | 14 (29.2) |
| Palliative chemotherapy | 20 (41.7) |
| Surgery followed by adjuvant hormonal therapy and radiotherapy | 1 (2.1) |
| Palliative hormonal therapy | 1 (2.1) |
| After BM | |
| Localized treatment for BM | |
| WBRT | 30 (62.5) |
| Surgery + WBRT | 8 (16.7) |
| BSC | 5 (10.4) |
| Observation | 3 (6.3) |
| SRS + WBRT | 1 (2.1) |
| SRS only | 1 (2.1) |
| Systemic treatment after BM | |
| Chemotherapy + anti-HER2 therapy | 16 (33.3) |
| BSC | 14 (29.2) |
| Palliative chemotherapy | 11 (22.9) |
| Anti-HER2 therapy only | 2 (4.2) |
| Hormonal therapy | 2 (4.2) |
| Tyrosine kinase inhibitors | 2 (4.2) |
| Anti-HER2 therapy and hormonal therapy | 1 (2.1) |
WBRT: whole-brain radiotherapy; BSC: best supportive care; SRS: stereotactic radiosurgery. Anti-HER2 therapy included trastuzumab, lapatinib, and ado-trastuzumab.
Two (4.2%) patients had de novo BM, seven (14.6%) patients were on surveillance for early BC and were found to have BM during routine clinical follow-up visits, while all others developed BM while receiving palliative treatment (19 patients with stage IV disease at the time of diagnosis and the remaining developed metastases to other organs before developing BM). A median of two lines (0–6 lines) of chemotherapy was administered to patients before they developed BM. Anthracyclines and taxanes were the most commonly used agents in standard doses, and all cycles were administered three weekly. After developing BM, the majority of patients (62.5%) were treated with whole-brain radiotherapy (WBRT), while eight (16.7%) underwent surgical resection of brain lesions followed by radiotherapy [Table 2]. After local treatment for brain disease, a median of one line (0–4 lines) of systemic therapy (including different chemotherapeutic agents, hormonal treatment, and anti-HER2/neu agents) was administered. Newer anti-HER2/neu agents (i.e., pertuzumab) were not available.
After a median follow-up time of 39 months, the median OS1 was 40 months (5–112 months) for all patients [Figure 1a]. The median time to develop BM was 21 months (0–113) [Figure 1b]. On univariate log-rank analysis, age group (< 44 years), stage at presentation, tumor type, BC type (survival for HR-positive better than TN), treatment type at the time of diagnosis, time to develop distant disease after diagnosis, anthracycline-based chemotherapy, and duration of developing BM (before or one-year post-diagnosis) were significant factors for development of BM (p ≤ 0.050). Using multivariate Cox regression analysis, none of the factors were significant.
Figure 1.
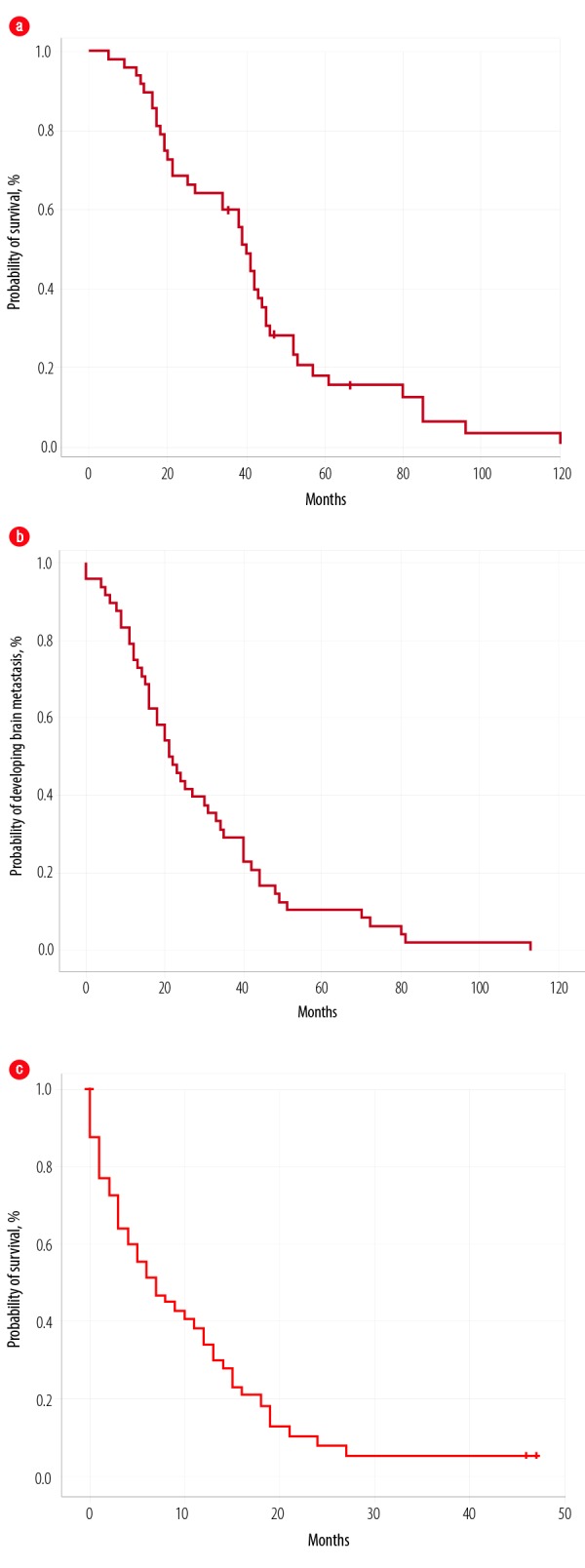
Overall survival of all patients from the (a) time of diagnosis, (b) time to develop metastases, and (c) after developing brain metastases.
Median OS2 after developing BM was seven months (0–47), with a one-year survival rate of 68.0% [Figure 1c]. On univariate log-rank analysis, age range, brain as first site of metastases, brain as only site of metastases, systemic disease along with brain disease, treatment of BM, surgery for BM [Figure 2], administration of systemic treatment after BM, type of systemic therapy, lines of systemic therapy, and DS-GPA score were significant factors affecting survival after development of BM (p ≤ 0.050) [Table 3]. On multivariate Cox regression analysis, the brain as the first site of metastases, treatment type, and DS-GPA score range were significant factors affecting survival post-BM [Table 3].
Figure 2.
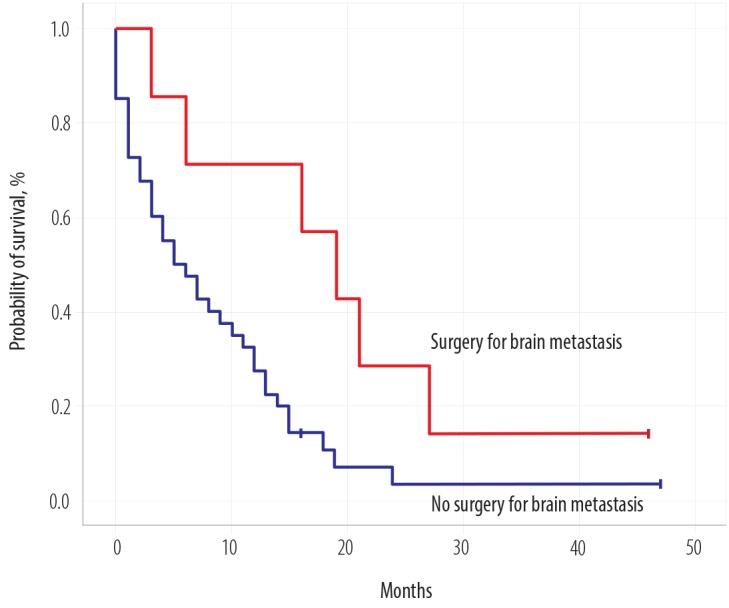
Overall survival of all patients according to tumor histology subtype.
Table 3. Results of univariate and multivariate analysis affecting overall survival after brain metastases (BM) for all patients.
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| 95% CI | p-value | HR (95% CI) | p-value | |
| Age range (< 44 vs. > 44 years) | 1.93–8.06 | 0.008 | 0.42 (0.10–1.81) | 0.240 |
| Brain as first disease site | 10.34–21.65 | 0.001 | 0.30 (0.12–0.75) | 0.010 |
| Brain as only site of metastasis | 7.60–24.40 | 0.030 | 4.15 (0.57–30.02) | 0.150 |
| Local treatment for BM | - | < 0.001 | 0.67 (0.47–0.95) | 0.020 |
| Surgery for brain disease | 11.30–26.69 | 0.020 | 1.85 (0.27–12.65) | 0.520 |
| Type of systemic treatment after BM | 6.02–19.97 | < 0.001 | 1.31 (0.97–1.77) | 0.070 |
| Treatment lines after BM | 9.87–16.13 | 0.001 | 0.45 (0.20–1.02) | 0.050 |
| DS-GPA range | 8.42–23.57 | 0.006 | 0.29 (0.15–0.55) | < 0.001 |
DS-GPA: diagnosis-specific graded prognostic assessment score; CI: confidence interval; HR: hormone receptor.
Discussion
BM is considered a catastrophic event in the care of cancer in general and BC in particular due to its high mortality and morbidity.15,25-27 BC is the second most frequent cancer to cause BM after lung cancer.2,7,28 The incidence of BM in BC ranges between 15–50%.2,28-30 It was 6.9% in our cohort.
Omani women develop BC (median age 43.5 years) and BM (median age 45.2 years) at a younger age compared to women in other parts of the world.3,29,31,32 The median time interval between diagnosis and development of BM in our cohort was 21 months (range 0–113), which is significantly less than reported in other studies.3,28,29,32 Usually, the time to develop BM after the diagnosis of BC is 27 months for the TN subtype and 54 months for HR and HER2 positive BC.28 The time to develop BM in our cohort was remarkably low (17 months for TN and 34.5 months for HR and HER2 positive BC). We suspect that the different genetic makeup of Arab women is responsible for the younger age at the time of BC diagnosis and development of BM in Omani women.
Our results matched the reported literature regarding the higher incidence of BM in women with HER2 positive and TN BC,3,25,29-35 as almost half of our cohort had HER2 positive BC (54.2%). Despite the higher prevalence of developing BM in patients with TN or HER2 positive subtypes, there was no significant difference in the time to develop BM, survival after BM, or OS in comparison with HR positive BC in our cohort. Our results contradict that of another study, which reported better survival for patients with luminal A and B disease type than with HER2 positive and TN subtypes.31 This can be explained by the small sample size of our cohort.
The majority of women (62.5%) presented with headache or vomiting while 29.1% had some neurological deficit, which corresponds to results reported earlier.2,29 Roughly half of women (47.9%) had three or more brain lesions on imaging, but the number of lesions did not affect survival. Treatment of BM affects the overall outcome as patients who could undergo surgery followed by WBRT lived longer (median survival of 19 months). The improved survival post-brain metastasectomy is proven by several studies.2,5,29,30 Two phase III trials showed improved survival after surgery followed by WBRT in patients with a single brain lesion, while another phase III trial showed no improved outcome with the combined approach. Patient selection is paramount as patients with limited or controlled systemic disease and good neurological status fare well with the combined approach. There were significant issues with these trials as patients with different primary histologies were enrolled with small number of patients with primary BC.28 The role of post-operative WBRT versus stereotactic radiosurgery (SRS) was assessed in a phase III trial after a resection of a solitary brain lesion and showed no difference in OS, but better cognition status favoring SRS group and better local control after 12 months favoring the WBRT group.35,36 SRS was also assessed in an adjuvant setting versus observation in a single-center study and showed promising results mostly for patients with tumor cavities up to 2.5 cm.36,37 A meta-analysis favored SRS for patients younger than 50 with one or four BM.28 In our cohort, all patients except two received WBRT. We did not collect the data on cognitive status or quality of life post-WBRT for our patients.
Most patients require systemic treatment of some form after treatment for BM. No systemic agent has been approved so far for the treatment of BC. Varying success rates have been reported for different agents in case series including capecitabine, anthracyclines, and platinum compounds (mainly in the TN subtype or with BRCA 1/2 mutated patients). Generally, all the systemic therapeutic agents approved by regulatory authorities can be used depending upon the BC subtype.28 There is no evidence to support modifying the systemic treatment after the treatment of BM.28 Subset analysis of a phase III study (EMILIA), showed no difference or responses between ado-trastuzumab treatment in comparison to capecitabine and lapatinib in patients who were asymptomatic after BM treatment.28,38 More than two-thirds (70.8%) of women in our cohort continued to receive systemic therapy for their metastatic disease, which included HER2 directed therapies (trastuzumab and lapatinib) as a single agent or in combination with other anti-cytotoxic agents, platinum compounds, or hormonal agents. Administration of systemic treatment after documentation of BM improved the survival (p < 0.001, 95% CI: 9.87–16.13). Ongoing studies are exploring new agents for patients with BM secondary to BC (including CDK4/6 inhibitors and checkpoint inhibitors) with agents that can cross the blood-brain barrier.28
The DS-GPA score was developed to predict the survival of different subtypes of BC after receiving treatment with WBRT, SRS, surgery, or a combination of these treatments.24 This score was proposed by the Radiation Therapy Oncology Group while treating patients with different modalities of radiotherapy and different cancers including BC. This index should not be used to predict outcome after any treatment, but rather is a useful tool for clinical trials and can help oncologists to make decisions on sending a patient to a hospice.28
Multiple attempts have been made to study this entity to prevent and/or minimize morbidity and mortality. Efforts to diagnose BM early by screening patients using brain MRI did not show improvement in OS.39,40 But the data is scant and further prospective studies are required, especially for patients with HER2 positive and TN subtypes who have a high incidence of BM.28 Prophylactic cranial irradiation (PCI) is the standard of care in small cell lung cancer.41 It has been attempted in BC as well, but so far it is not part of the standard care.42,43 There is an ongoing phase III clinical trial trying to prevent BM with PCI in advanced HER2 positive BC.43
Survival after diagnosis of BC associated BM was poor in our cohort with a median survival of seven months (0–35), which is far less than reported internationally.3,29,31 Younger age is usually considered a favorable risk factor for OS,29 but this was not the case for our cohort and despite a median age of 43.5 years at BC diagnosis and 45.2 years at the time of BM, OS was significantly poor. BC subtypes had no significant effect on OS post-BM in our patients, which may be explained by the small sample size, as the literature suggests different survival according to BC subtypes.29,31,43 The exception is a recently reported study from Poland, published as an abstract, which showed almost similar OS in comparison to our cohort.44 The median survival of our cohort after developing BM was only seven months compared to 8.3 months reported in another study, which was almost identical for patients with the TN subtype. Survival after one year was only 36% in another study32 while it was 68% in our cohort, which indicates that the difference in survival may be secondary to differences in the disease due to the differences in ethnic background or genetic makeup of BC in Arab women.
Significant progress has been made on molecular profiling of different cancers including BC. Most studies have looked at the molecular landscape of primary breast tumor, but recently some investigators have shown evolving genetic mutations in the metastatic sites from BC45 though none of the patients had BM from their primary BC.
Our study was limited by its retrospective nature, small sample size, and data collection over a long time. However, it does provide some useful information about the poor outcome in Omani women after the development of BM. It can also be used as a basis for further research into the different genetic makeup of the disease in general and on brain tissue in particular.
Conclusion
BM represents a major complication in BC. Further molecular predictors and predictive models are needed to try and prevent this complication. Treatment of BM continues to improve with a better understanding of the biology of the disease, and sophisticated surgical and advanced treatment approaches.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018. Nov;68(6):394-424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Lin NU, Bellon JR, Winer EP. CNS metastases in breast cancer. J Clin Oncol 2004. Sep;22(17):3608-3617. 10.1200/JCO.2004.01.175 [DOI] [PubMed] [Google Scholar]
- 3.Sanna G, Franceschelli L, Rotmensz N, Botteri E, Adamoli L, Marenghi C, et al. Brain metastases in patients with advanced breast cancer. Anticancer Res 2007. Jul-Aug;27(4C):2865-2869. [PubMed] [Google Scholar]
- 4.Boogerd W, Vos VW, Hart AA, Baris G. Brain metastases in breast cancer; natural history, prognostic factors and outcome. J Neurooncol 1993. Feb;15(2):165-174. 10.1007/BF01053937 [DOI] [PubMed] [Google Scholar]
- 5.Frisk G, Tinge B, Ekberg S, Eloranta S, Bäcklund LM, Lidbrink E, et al. Survival and level of care among breast cancer patients with brain metastases treated with whole brain radiotherapy. Breast Cancer Res Treat 2017. Dec;166(3):887-896. 10.1007/s10549-017-4466-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryberg M, Nielsen D, Osterlind K, Andersen PK, Skovsgaard T, Dombernowsky P. Predictors of central nervous system metastasis in patients with metastatic breast cancer. A competing risk analysis of 579 patients treated with epirubicin-based chemotherapy. Breast Cancer Res Treat 2005. Jun;91(3):217-225. 10.1007/s10549-005-0323-x [DOI] [PubMed] [Google Scholar]
- 7.Kyeong S, Cha YJ, Ahn SG, Suh SH, Son EJ, Ahn SJ. Subtypes of breast cancer show different spatial distributions of brain metastases. PLoS One 2017. Nov;12(11):e0188542. 10.1371/journal.pone.0188542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braccini AL, Azria D, Thezenas S, Romieu G, Ferrero JM, Jacot W. Prognostic factors of brain metastases from breast cancer: impact of targeted therapies. Breast 2013. Oct;22(5):993-998. 10.1016/j.breast.2013.05.011 [DOI] [PubMed] [Google Scholar]
- 9.Weigelt B, Geyer FC, Reis-Filho JS. Histological types of breast cancer: how special are they? Mol Oncol 2010. Jun;4(3):192-208. 10.1016/j.molonc.2010.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, et al. Panel members Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 2013. Sep;24(9):2206-2223. 10.1093/annonc/mdt303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dawood S, Broglio K, Esteva FJ, Ibrahim NK, Kau S-W, Islam R, et al. Defining prognosis for women with breast cancer and CNS metastases by HER2 status. Ann Oncol 2008. Jul;19(7):1242-1248. 10.1093/annonc/mdn036 [DOI] [PubMed] [Google Scholar]
- 12.Dawood S, Broglio K, Esteva FJ, Yang W, Kau S-W, Islam R, et al. Survival among women with triple receptor-negative breast cancer and brain metastases. Ann Oncol 2009. Apr;20(4):621-627. 10.1093/annonc/mdn682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hines SL, Vallow LA, Tan WW, McNeil RB, Perez EA, Jain A. Clinical outcomes after a diagnosis of brain metastases in patients with estrogen- and/or human epidermal growth factor receptor 2-positive versus triple-negative breast cancer. Ann Oncol 2008. Sep;19(9):1561-1565. 10.1093/annonc/mdn283 [DOI] [PubMed] [Google Scholar]
- 14.Jang G, Lee SS, Ahn J-H, Jung KH, Lee H, Gong G, et al. Clinical features and course of brain metastases in triple-negative breast cancer: comparison with human epidermal growth factor receptor 2-positive and other type at single institution in Korea. Breast Cancer Res Treat 2011. Jul;128(1):171-177. 10.1007/s10549-011-1526-y [DOI] [PubMed] [Google Scholar]
- 15.Lee SS, Ahn JH, Kim MK, Sym SJ, Gong G, Ahn SD, et al. Brain metastases in breast cancer: prognostic factors and management. Breast Cancer Res Treat 2008. Oct;111(3):523-530. 10.1007/s10549-007-9806-2 [DOI] [PubMed] [Google Scholar]
- 16.Brenton JD, Carey LA, Ahmed AA, Caldas C. Molecular classification and molecular forecasting of breast cancer: ready for clinical application? J Clin Oncol 2005. Oct;23(29):7350-7360. 10.1200/JCO.2005.03.3845 [DOI] [PubMed] [Google Scholar]
- 17.Dawood S, Broglio K, Esteva FJ, Ibrahim NK, Kau S-W, Islam R, et al. Defining prognosis for women with breast cancer and CNS metastases by HER2 status. Ann Oncol 2008. Jul;19(7):1242-1248. 10.1093/annonc/mdn036 [DOI] [PubMed] [Google Scholar]
- 18.Al-Lawati JA, Al-Lawati NA, Al-Siyabi NH, Al-Ghabri DO. Cancer incidence in Oman. Report of 2012. Muscat: Ministry of Health; 2012 [cited 2016 June 14]. Available from: https://internal.moh.gov.om/documents/272928/1232802/Cancer+Incident+2012+Final.pdf/bf8b0ac3-ad0f-4a1e-8f92-340b2b78c34e. [Google Scholar]
- 19.Al-Moundhri M, Al-Bahrani B, Pervez I, Ganguly SS, Nirmala V, Al-Madhani A, et al. The outcome of treatment of breast cancer in a developing country–Oman. Breast 2004. Apr;13(2):139-145. 10.1016/j.breast.2003.10.001 [DOI] [PubMed] [Google Scholar]
- 20.Kumar S, Burney IA, Al-Ajmi A, Al-Moundhri MS. Changing trends of breast cancer survival in sultanate of oman. J Oncol 2011;2011:316243. 10.1155/2011/316243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Moundhri M. The need for holistic cancer care framework: breast cancer care as an example. Oman Med J 2013. Sep;28(5):300-301. 10.5001/omj.2013.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arslan UY, Oksuzoglu B, Aksoy S, Harputluoglu H, Turker I, Ozisik Y, et al. Breast cancer subtypes and outcomes of central nervous system metastases. Breast 2011. Dec;20(6):562-567. 10.1016/j.breast.2011.07.017 [DOI] [PubMed] [Google Scholar]
- 23.Yap YS, Cornelio GH, Devi BC, Khorprasert C, Kim SB, Kim TY, et al. Brain metastases in Asian HER2-positive breast cancer patients: anti-HER2 treatments and their impact on survival. Br J Cancer 2012. Sep;107(7):1075-1082. 10.1038/bjc.2012.346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 2012. Feb;30(4):419-425. 10.1200/JCO.2011.38.0527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leone JP, Lee AV, Brufsky AM. Prognostic factors and survival of patients with brain metastasis from breast cancer who underwent craniotomy. Cancer Med 2015. Jul;4(7):989-994. 10.1002/cam4.439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogawa K, Yoshii Y, Nishimaki T, Tamaki N, Miyaguni T, Tsuchida Y, et al. Treatment and prognosis of brain metastases from breast cancer. J Neurooncol 2008. Jan;86(2):231-238. 10.1007/s11060-007-9469-1 [DOI] [PubMed] [Google Scholar]
- 27.Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, et al. Effect of tumor subtype on survival and the graded prognostic assessment for patients with breast cancer and brain metastases. Int J Radiat Oncol Biol Phys 2012. Apr;82(5):2111-2117. 10.1016/j.ijrobp.2011.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin N, Gaspar LE, Soffietti R, eds. Breast cancer in the central nervous system: multidisciplinary considerations and management. American Society of Clinical Oncology Educational Book. American Society of Clinical Oncology Meeting; 2017. [DOI] [PubMed] [Google Scholar]
- 29.Rostami R, Mittal S, Rostami P, Tavassoli F, Jabbari B. Brain metastasis in breast cancer: a comprehensive literature review. J Neurooncol 2016. May;127(3):407-414. 10.1007/s11060-016-2075-3 [DOI] [PubMed] [Google Scholar]
- 30.Koo T, Kim IA. Brain metastasis in human epidermal growth factor receptor 2-positive breast cancer: from biology to treatment. Radiat Oncol J 2016. Mar;34(1):1-9. 10.3857/roj.2016.34.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nam BH, Kim SY, Han HS, Kwon Y, Lee KS, Kim TH, et al. Breast cancer subtypes and survival in patients with brain metastases. Breast Cancer Res 2008;10(1):R20. 10.1186/bcr1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eichler AF, Kuter I, Ryan P, Schapira L, Younger J, Henson JW. Survival in patients with brain metastases from breast cancer: the importance of HER-2 status. Cancer 2008. Jun;112(11):2359-2367. 10.1002/cncr.23468 [DOI] [PubMed] [Google Scholar]
- 33.Tham YL, Sexton K, Kramer R, Hilsenbeck S, Elledge R. Primary breast cancer phenotypes associated with propensity for central nervous system metastases. Cancer 2006. Aug;107(4):696-704. 10.1002/cncr.22041 [DOI] [PubMed] [Google Scholar]
- 34.Pestalozzi BC, Zahrieh D, Price KN, Holmberg SB, Lindtner J, Collins J, et al. International Breast Cancer Study Group (IBCSG) Identifying breast cancer patients at risk for Central Nervous System (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG). Ann Oncol 2006. Jun;17(6):935-944. 10.1093/annonc/mdl064 [DOI] [PubMed] [Google Scholar]
- 35.Brown PD, Ballman KV, Cerhan JH, Anderson SK, Carrero XW, Whitton AC, et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol 2017. Aug;18(8):1049-1060. 10.1016/S1470-2045(17)30441-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lo SS, Chang EL, Sahgal A. Radiosurgery for resected brain metastases-a new standard of care? Lancet Oncol 2017. Aug;18(8):985-987. 10.1016/S1470-2045(17)30448-5 [DOI] [PubMed] [Google Scholar]
- 37.Mahajan A, Ahmed S, McAleer MF, Weinberg JS, Li J, Brown P, et al. Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol 2017. Aug;18(8):1040-1048. 10.1016/S1470-2045(17)30414-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krop IE, Lin NU, Blackwell K, Guardino E, Huober J, Lu M, et al. Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: a retrospective, exploratory analysis in EMILIA. Ann Oncol 2015. Jan;26(1):113-119. 10.1093/annonc/mdu486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller KD, Weathers T, Haney LG, Timmerman R, Dickler M, Shen J, et al. Occult central nervous system involvement in patients with metastatic breast cancer: prevalence, predictive factors and impact on overall survival. Ann Oncol 2003. Jul;14(7):1072-1077. 10.1093/annonc/mdg300 [DOI] [PubMed] [Google Scholar]
- 40.Niwińska A, Tacikowska M, Murawska M. The effect of early detection of occult brain metastases in HER2-positive breast cancer patients on survival and cause of death. Int J Radiat Oncol Biol Phys 2010. Jul;77(4):1134-1139. 10.1016/j.ijrobp.2009.06.030 [DOI] [PubMed] [Google Scholar]
- 41.Rudin CM, Ismaila N, Hann CL, Malhotra N, Movsas B, Norris K, et al. Treatment of Small-Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians Guideline. J Clin Oncol 2015. Dec;33(34):4106-4111. 10.1200/JCO.2015.63.7918 [DOI] [PubMed] [Google Scholar]
- 42.Shimada K, Ishikawa T, Yoneyama S, Kita K, Narui K, Sugae S, et al. Early-onset brain metastases in a breast cancer patient after pathological complete response to neoadjuvant chemotherapy. Anticancer Res 2013. Nov;33(11):5119-5121. [PubMed] [Google Scholar]
- 43.Clinicaltrials.gov. Radiation therapy to the head in preventing brain metastases in women receiving trastuzumab and chemotherapy for metastatic or locally advanced breast cancer. 2015 [cited 2018 October]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT00639366.
- 44.Niwinska A, Pogoda K, Rudnicka H, Jagiello-Gruszfeld AI, Rybski S, Nowecki Z. Outcomes from 735 patients with breast cancer brain metastases (BM) according to biological subtype, number of BMs, and systemic treatment after local therapy. J Clin Oncology 2017;35(15_suppl):2078.
- 45.Yates LR, Knappskog S, Wedge D, Farmery JH, Gonzalez S, Martincorena I, et al. Genomic evolution of breast cancer metastasis and relapse. Cancer Cell 2017. Aug;32(2):169-184.e7. 10.1016/j.ccell.2017.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]


