Abstract
Objectives
Competitive immunoenyzmatic assays for estradiol (E2) and unconjugated estriol (uE3) on UniCel DxI 800 Access immunoassay systems (Beckman Coulter) utilize bovine alkaline phosphatase (ALP) for amplification. In these assays, rare ‘IND’ error flags indicate that a relative light unit (RLU) raw result is past the high or low end of the calibration curve but cannot be differentiated from an instrument error or analytical interference. The present studies were conducted to establish a protocol to identify analytical interference and to characterize its mechanism when present.
Design and methods
Matrix and recovery studies were conducted to establish a protocol for interference identification. Spiking experiments with inactivated calf intestinal ALP were performed to determine whether interference could be blocked. Commercial anti-ALP antibodies (Abs) were spiked into human serum to model assay interference. Three E2 immunoassays which do not include ALP as a reagent component (cobas e602, Roche; Centaur XP, Siemens; ARCHITECT i2000SR, Abbott) were tested for comparative purposes.
Results
1:2 dilution of specimen into Access Sample Diluent A (Beckman) differentiated IND error flags due to true low results (e.g. less than the analytical measurement range; AMR) from those due to assay interference. Interferences were reduced by pre-incubation with inactivated ALP and could be replicated by spiking with commercial anti-ALP Abs.
Conclusions
Patient anti-bovine ALP Abs can cause interference on DxI 800 E2 and uE3 assays. This model can be used to investigate interference risk with other ALP-dependent assays.
Keywords: Immunoassay, Endocrinology, Analytical systems, Interference, Alkaline phosphatase
Abbreviations: ALP, alkaline phosphatase; AMR, analytical measurement range; CLIA, Clinical Laboratory Improvement Amendments; ddH2O, demineralized distilled water; E2, estradiol; IND, indeterminate ‘no value’ error flag; MoM, multiple of the median; PBS, phosphate buffered saline; RLU, relative light unit; uE3, unconjugated estriol
Highlights
-
•
Interferences with estradiol and unconjugated estriol assays were investigated.
-
•
A dilution protocol differentiated interference from low analyte concentrations.
-
•
Endogenous anti-bovine alkaline phosphatase (ALP) antibodies were identified.
1. Introduction
Alkaline phosphatases (ALPs) are ubiquitous enzymes associated with dephosphorylation reactions in bacteria and eukaryotes [1]. ALP can be conjugated to antibodies (Abs) in many immunoassays to amplify signal. ALP-containing immunoassays have also been adapted to use chemiluminescent substrates, allowing for automation of highly sensitive assays that are able to detect analytes in very low concentrations [2].
Immunoassay interference due to increased endogenous ALP activity has previously been described and can cause false positive results in some ALP-containing assays [3,4]. Similarly, exogenous ALP has also been shown to cause immunoassay interference [5]. Abs against reagent ALP is another potential source of immunoassay interference, and while blocking reagents (e.g. inactivated forms of ALP) have previously been developed [6,7], these blocking approaches may not be incorporated into all commercially available clinical immunoassays.
Immunoassays performed on UniCel DxI 800 Access immunoassay systems (Beckman Coulter, Brea, CA) utilize ALP conjugated to reagent components in corresponding chemiluminescent reactions. For two competitive immunoassays on these instruments – estradiol (E2) [8] and unconjugated estriol (uE3) [9] – our laboratory has occasionally observed difficult-to-troubleshoot ‘IND’ error flags on instrument printouts in approximately 0.01–0.04% of specimens analyzed. For competitive immunoassays, the IND error flag designates a relative light unit (RLU) instrument result that is either past the low or high end of the calibration curve [10]. As a competitive immunoassay where RLU results are inversely proportional to analyte concentration, specimen reaction RLUs that are larger than that of the low calibrator could also indicate a quantitative result that is below the analytical measurement range (AMR). Alternatively, IND error flags may also indicate system failure requiring technical support from the vendor [10]. Regardless, no quantitative analyte result is provided, nor is there definitive information as to whether an IND error (with a high RLU) may indicate true low results or assay interferences. This is particularly problematic for panel tests such as maternal quad screening, where uE3 is a required component for fetal defect risk assessment.
Preliminary attempts to characterize this IND error phenomenon for E2 and uE3 in our laboratory demonstrated that it could not be resolved by pre-incubation of specimens with heterophile blocking reagents (Scantibodies, Santee, CA). This suggests that interference was not due to patient Ab against another species Ab in reagent components. Additionally, non-linearity could be observed with dilutions of specimens showing IND error flags, making quantitative evaluation difficult. This suggested the presence of endogenous interference. ALP activity was also not elevated in specimens with IND error flags, arguing against increased ALP activity as a cause of potential interference. Finally, IND error flags were often observed when specimens were re-tested on alternate DxI 800 instruments. This suggested that instrument malfunction was not likely to be a sole cause of IND errors with these specimens. Given this preliminary evidence, we hypothesized that IND errors for these E2 and uE3 assays could be due to both low analyte concentrations as well as endogenous interfering substances in patient specimens. The present studies were therefore conducted to establish a reliable protocol to differentiate these two possibilities for individual specimens and then to explain the mechanism of such interferences when they do occur. Anti-ALP antibodies were identified as the cause of IND error flags when true low results were ruled out.
2. Materials and methods
2.1. General
This project (IRB#00118545) was administratively reviewed by the University of Utah Institutional Review Board, which determined that it did not meet federal definitions of human subject research. Studies were conducted as part of ongoing troubleshooting to identify and characterize endogenous interferences in patient specimens with clinical test orders for E2 and/or uE3 assays. Clinical testing for E2 and uE3 was conducted on DxI 800 immunoanalyzers located in a Clinical Laboratory Improvement Amendments (CLIA)-accredited clinical laboratory setting. Comparative testing (as indicated in the manuscript) was conducted using in-house developed liquid chromatography-tandem mass spectrometry (LC-MS/MS) assays for E2 (quantitative; validated and used for clinical testing by our laboratory) and uE3 (qualitative; validated but not used for clinical testing by our laboratory). Additional comparison studies for E2 using assays which do not include ALP as reagent components were performed on cobas e602 (Roche Diagnostics, Indianapolis, IN), Centaur XP (Siemens Healthineers, Malvern, PA), and ARCHITECT i2000SR (Abbott Diagnostics, Abbott Park, IL) immunoanalyzers using E2 reagent kits from the respective manufacturers [[11], [12], [13]].
2.2. Diluent evaluations
Seven potential diluents were evaluated for matrix effect with E2 and uE3 assays on DxI 800 instruments: demineralized distilled water (ddH2O; General Water Technologies; North Salt Lake, UT), 0.9% saline (Roche), UniCel DxI Access Wash Buffer II (Beckman), Access Sample Diluent A (Beckman), Access Red Blood Cell Folate Lysing Agent (Beckman), Access E2 Zero Calibrator (Beckman), and Access uE3 Zero Calibrator (Beckman). Materials were tested in triplicate on E2 and uE3 immunoassays, while zero calibrator solutions were only tested for their respective assays.
2.3. Recovery studies
Three specimens with measurable E2 and three specimens with measurable uE3 were diluted 1:2, 1:5, and 1:11 into Sample Diluent A and percent recovery was calculated as:
where expected concentration was calculated for each dilution as:
Percent recovery was then averaged across all three patients and presented as mean ± SD.
2.4. Dilution studies
A dilution protocol (100 μl patient specimen + 100 μl Sample Diluent A) was used for evaluation of potential interference in patient specimens that previously were found to show IND error flags with either E2 or uE3 assays. Specimens had previously been stored at −20 °C while the dilution protocol was being developed, and this protocol was used prospectively to evaluate clinical specimens with IND error flags once established.
2.5. Inactivated alkaline phosphatase incubation studies
180 μl patient serum was mixed with either 20 μl of 5 mg/mL Scavenger ALP (Oriental Yeast Co, Osaka, Japan) or 20 μl of phosphate buffered saline (PBS) (Sigma, St. Louis, MO). Mixtures were incubated at room temperature on a rocker for 30 min, centrifuged (30 s at 1000×g; Allegra X-12 Centrifuge; Beckman) and subsequently analyzed for E2 and/or uE3 as indicated.
2.6. Antibody spiking studies
E2 and uE3 (Sigma, St. Louis, MO) were reconstituted in 100% ethanol to make 1 μg/mL stock solutions. In 20 mL of human male serum (Corning, from VWR, Radnor, PA), 34 μl of 1 μg/ml E2 stock and 68 μl of 1 μg/mL uE3 stock were added and mixed for 20 s on a vortex to create a baseline serum pool of measurable E2 and uE3. In 11 mL of this baseline pool, 1 mL of PBS was added and vortexed for 20 s and labeled as baseline pool + PBS. Addition of PBS as described above was performed to match the ratio of volume of Abs against calf intestinal ALP added to baseline pools (Ab mixture) used for serial dilutions. For example, to 550 μl of baseline serum pools, either 50 μl of stock Ab concentration [10 mg/mL sheep anti-calf intestinal ALP Abs (#367321; US Biological Life Sciences, Salem, MA), 50 μl of 3.4 mg/mL mouse anti-calf intestinal ALP Abs (#P4071-15; US Biological Life Sciences), or 50 μl of 2.85 mg/mL mouse anti-calf intestinal ALP Abs (#MIA1703; Invitrogen, Carlsbad, CA)] were added. Serial dilutions were then conducted for each Ab mixture into baseline pool + PBS (for #3677321 Ab mixture, start plus 11 dilutions; for both #P4071-15 and #MIA1703 Ab mixtures, start plus 5 dilutions). More dilutions were conducted for the #3677321 Ab mixture since it started at a higher concentration stock solution. Dilutions were then incubated on a rocker for 30 min at room temperature followed by centrifugation (30 s, 1000×g). Dilutions were then analyzed for E2 and uE3 as indicated.
2.7. Data analysis
Data analysis was performed using Prism (GraphPad Software, San Diego, CA) and Excel 2016 (Microsoft, Redmond, WA). Statistical significance was defined as p < 0.05 as determined by ANOVA (GraphPad). Graphs were generated in SigmaPlot 13 (Systat, San Jose, CA). Results are presented as mean ± SD unless otherwise indicated.
3. Results
3.1. Protocol to identify E2 and uE3 interferences in clinical specimens
To determine which IND error flags were due to endogenous interferents, a dilution protocol was needed to differentiate IND results due to interferences from those due to true low concentration specimens. In preparation for this protocol, seven potential diluents were evaluated for potential matrix effect with E2 and uE3 assays on DxI 800 instruments. As shown in Table 1, matrix effect was observed for several diluents, in particular for the E2 assay (e.g. ddH2O, 0.9% Saline, Wash Buffer II, and Lysing Agent). Sample Diluent A was therefore selected as the protocol diluent for subsequent studies. Given the identification of matrix effect across diluents, percent recovery of E2 and uE3 in normal patient specimens diluted into Sample Diluent A was also investigated. While excellent recovery was observed for uE3 (1:2 dilution, 99.6 ± 3.6%; 1:5 dilution, 97.2 ± 10.0%; 1:11 dilution, 86.3 ± 15.3%), under-recovery was observed with increasing dilutions for E2 (1:2 dilution, 71.2 ± 1.3%; 1:5 dilution, 48.8 ± 2.6%; 1:11 dilution, 36.0 ± 7.2%). A simplified dilution protocol (1:2) was therefore selected to limit the impact of under-recovery for E2 assays.
Table 1.
Matrix Evaluation for uE3 and E2 in Potential Diluents. Testing non-spiked diluents on UniCel DxI 800 Access E2 and uE3 immunoassays.
| uE3 |
E2 |
|
|---|---|---|
| ng/mL Mean ± SD |
pg/mL Mean ± SD |
|
| ddH2O | 0.00 ± 0.00 | 74 ± 15 |
| Saline | 0.01 ± 0.01 | 67 ± 4 |
| Wash Buffer | 0.01 ± 0.00 | 70 ± 14 |
| Sample Diluent A | 0.03 ± 0.01 | 1 ± 2 |
| Lysing Agent | 0.01 ± 0.01 | 74 ± 4 |
| E2 Zero Calibrator | N/A | 0 ± 1 |
| uE3 Zero Calibrator | 0.01 ± 0.01 | N/A |
N/A, Not Applicable, Analyte Not Measured.
Dilutions into Sample Diluent A (1:2) were then made for 13 patient specimens that had previously demonstrated IND flags with original testing (n = 6, IND for E2; n = 7, IND for uE3). As shown in Table 2, dilutions resolved IND error flags in 2 of 6 patient specimens for E2 (e.g. patients C and D), enabling RLU results that would be reported below the assay AMR (i.e. indicative of true low results). In patients B, C, and F, enough residual specimens were available to attempt to confirm the presence of low concentrations of E2 by LC-MS/MS (Table 2). In patients B and F, IND error flags were still observed with 1:2 dilution, suggesting E2 immunoassay interference; E2 was detectable by LC-MS/MS in these specimens. E2 was also detectable by LC-MS/MS for patient C, but at a concentration lower than the AMR of the E2 immunoassay (<20 pg/mL).
Table 2.
IND Error Flags with E2 Results in Six Patients.
| Pt. A | Pt. B | Pt. C | Pt. D | Pt. E | Pt. F | |
|---|---|---|---|---|---|---|
| Age, yrs | 46 | 29 | 42 | 49 | 57 | 55 |
| Ordered Test | E2 | E2 | E2 | E2 | E2 | E2 |
| E2 Undiluted | IND | IND | IND | IND | IND | IND |
| E2 1:2 in Sample DilA | IND | IND | <20 pg/mL | <20 pg/mL | IND | IND |
| Mass Spec Detection of E2 | N/A | 29.6 pg/mL | 17.6 pg/mL | N/A | N/A | 59.2 pg/mL |
| Interference Suspected | Yes | Yes | No | No | Yes | Yes |
N/A, Not Applicable, Insufficient specimen for additional testing.
Results from dilutions of patient specimens showing IND error flags for uE3 are shown in Table 3. Dilutions into Sample Diluent A (1:2) resolved IND error flags in 4 of 7 specimens tested (e.g. patients I, K, L, and M). In one of these specimens (patient I), residual specimen was available to confirm qualitatively the absence of uE3 by LC-MS/MS (i.e. confirmed as a true low concentration specimen, no DxI assay interference). In two specimens (patients G and H), dilutions did not resolve IND error flags on the DxI assay, while uE3 was qualitatively observed on the LC-MS/MS assay (i.e. confirmed as true DxI assay interference). One additional interference (patient J) was observed in a specimen which had no uE3 detectable by LC-MS/MS, but where 1:2 dilution into Sample Diluent A did not resolve the IND error flag. This finding demonstrated that assay interference could be observed even in the absence of measurable analyte.
Table 3.
IND Error Flags with uE3 Results in Seven Patients.
| Pt. G | Pt. H | Pt. I | Pt. J | Pt. K | Pt. L | Pt. M | |
|---|---|---|---|---|---|---|---|
| Age, yrs | 21 | 24 | 34 | 47 | 29 | 18 | 19 |
| Ordered Test | Quad Screen | Quad Screen | Quad Screen | uE3 only |
Quad Screen | Quad Screen | Quad Screen |
| uE3 Undiluted | IND | IND | IND | IND | IND | IND | IND |
| uE3 1:2 in Sample DilA | IND | IND | <0.02 ng/mL | IND | 0.11 ng/mL | <0.02 ng/mL | <0.02 ng/mL |
| Mass Spec Detection of uE3 | Present | Present | Absent | Absent | N/A | N/A | N/A |
| Interference Suspected | Yes | Yes | No | Yes | No | No | No |
N/A, Not Applicable, Insufficient specimen for additional testing.
3.2. Anti-bovine ALP Abs as a mechanism for E2 and uE3 assay interference
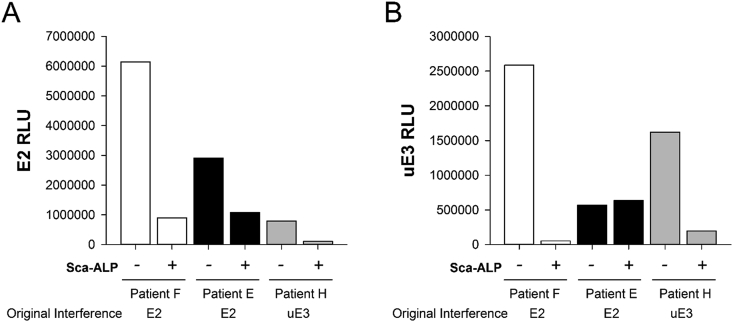
To determine whether E2 and uE3 IND error flag interferences could be blocked by the presence of inactivated ALP, three patient specimens with IND error flags due to suspected interference based on dilution protocol results were incubated with Scavenger ALP (inactivated calf intestine ALP) prior to E2 and uE3 testing. The three patient specimens had IND error flags observed for E2 and uE3 (Pt. F), E2 (Pt. E), or uE3 (Pt. H). As shown in Fig. 1, incubation with Scavenger ALP decreased assay RLUs for E2 (Pt. F, 85.3% decrease; Pt. E, 62.7% decrease; Pt. H, 86.6% decrease) and uE3 (Pt. F, 98.0% decrease; Pt. H, 87.8% decrease). The uE3 RLU for Pt. E increased by 11.9%. As controls, two patient specimens with quantifiable E2 and two specimens with quantifiable uE3 were similarly spiked with Scavenger ALP prior to testing. RLUs in the spiked normal controls showed only minor changes (2.1% average increase for E2; 13.7% average increase for uE3; data not shown).
Fig. 1.
Effect of Scavenger ALP on E2 and uE3 Measurement. Three different patient specimens identified by prior IND error flags for uE3 and/or E2 were tested on UniCel DxI 800 assays for E2 (A) and uE3 (B) with (+) and without (-) incubation with 500 μg/mL Scavenger ALP. Note that as a competitive immunoassay, increased RLUs correlate to decreased apparent analyte concentrations.
To investigate whether assay interferences would be absent in E2 assays which do not incorporate ALP, four specimens previously identified as showing IND error flags for E2 (n = 1; Pt. B) and uE3 (n = 3; Pts. J, H, G) with additional available residual volume were tested across four separate E2 immunoassays on their respective instruments: DxI 800 (ALP-containing), e602 (ALP-absent), Centaur XP (ALP-absent), and ARCHITECT i2000SR (ALP-absent). Additional specimens showing prior IND flags for E2 were depleted in prior experiments and therefore unavailable for further testing. As shown in Table 4, in the specimen from Pt. B (a specimen previously shown to display E2 interference on the DxI), consistent quantifiable results were observable on the other three assays which do not contain ALP as a reagent constituent. The specimen from Pt. H, which previously showed uE3 interference, also showed E2 interference on the DxI 800 (i.e. results markedly discordant with elevated E2 measured on three other platforms and consistent with the patient’s pregnancy status). The specimen from Pt. G did not show clear evidence of E2 interference (all results consistent with pregnancy status), nor was clear interference observed for the specimen from Pt. J. The specimen for Pt. J had also been tested for E2 by a LC-MS/MS method which showed a result of 11 pg/mL, generally consistent with results from the Abbott, Beckman, and Roche assays. Results from Table 4 demonstrate that interference in one competitive assay (e.g. uE3) may or may not correlate with interference with other competitive assays (e.g. E2), and assays which do not include ALP as a reagent constituent are not likely to show ALP-related interferences.
Table 4.
Comparative Testing Across Alternative E2 Assays.
| Prior Characterization | Beckman UniCel DXI |
Roche e602 |
Siemens Centaur XP |
Abbott ARCHITECT i2000SR |
|
|---|---|---|---|---|---|
| E2 (pg/mL) | E2 (pg/mL) | E2 (pg/mL) | E2 (pg/mL) | ||
| Pt. B | E2 interference | INDa | 29.69 | 29 | 39.22 |
| Pt. Jd | uE3 interference | 23b | <5 | 183 | 22.46 |
| Pt. H | uE3 interference | 227b | >3000c | >1000c | >3000c |
| Pt G | uE3 interference | 4061b | >3000c | >1000c | >3000c |
E2 interference identified.
uE3 interference previously identified.
Other assay E2 results consistent with pregnancy status; dilutions not performed due to limited specimen volumes.
Pt. J specimen tested by LC-MS/MS method for E2 (result: 11 pg/mL).
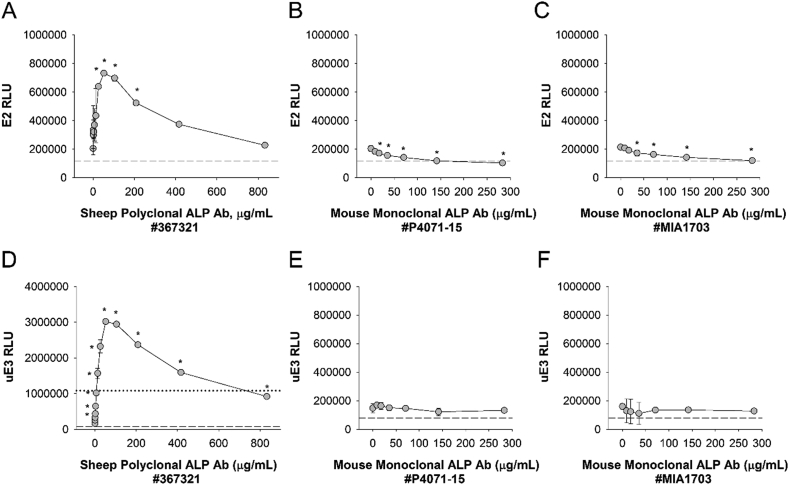
If endogenous patient Abs against reagent (e.g. bovine) ALP interfere with competitive immunoassays for E2 and uE3, then spiking with exogenous anti-bovine ALP Abs should have a similar effect. To determine whether this was the case, normal serum spiked with measurable concentrations of E2 and uE3 was incubated separately with three different commercially available Abs against calf intestinal ALP (sheep polyclonal ALP Ab [#367321], mouse monoclonal ALP Ab [#P4071-15], and mouse monoclonal ALP Ab [#MIA1703]), and serial dilutions were performed prior to testing for E2 and uE3 on DxI 800 instruments. As shown in Fig. 2, sheep polyclonal ALP Ab (#367321) induced strong biphasic effects on assay RLUs for both E2 (Fig. 2A) and uE3 (Fig. 2D) with increasing Ab concentrations. All Ab concentrations quantitatively increased RLUs (i.e. decreased apparent analyte concentration), and in the case of uE3 (Fig. 2D) they increased RLUs to greater than the zero calibrator limit (dotted line) resulting in IND error flags. Smaller magnitude interference of decreasing RLUs (i.e. increased apparent analyte concentration) were observed with mouse monoclonal ALP Ab (#P4071-15) and mouse monoclonal ALP Ab (#MIA1703) for E2 (Fig. 2B,C) but not uE3 (Fig. 2E,F). These results suggest that anti-bovine ALP Ab interference may be epitope dependent, and that antibodies against bovine calf intestinal ALP can interfere with the accurate quantitation of E2 or uE3 immunoassays on the DxI 800 platform.
Fig. 2.
Anti-Bovine ALP Ab Spiking Studies. A, Human serum spiked with 1.7 ng/ml E2 and 3.4 ng/mL uE3 was incubated in the presence or absence of different concentrations of anti-calf intestinal ALP Abs – sheep polyclonal ALP Ab (#367321; A, D), mouse monoclonal ALP Ab (#P4071-15; B, E), and mouse monoclonal ALP Ab (#MIA1703; C, F). Serial dilutions were performed and specimens were tested for E2 (A-C) and uE3 (D-F) on DxI 800 instruments. Dashed lines indicate RLU thresholds for the assay upper calibrator (A-F), whereas dotted lines indicate the RLU threshold for the assay zero calibrator (only visible in D; RLU, 1080727). *, p < 0.05 compared to measurement in the absence of spiked antibody. Note that as a competitive immunoassay, increased RLUs correlate to decreased apparent analyte concentrations.
4. Discussion
The present studies demonstrate that in rare patients, Abs against bovine ALP interfere with the measurement of E2 and uE3 in competitive immunoassays on DxI 800 instruments. Dilution of patient specimens, as well as incubation of specimens with inactivated calf intestine ALP, provide supportive evidence of this interference. Ab spiking experiments demonstrate that both positive and negative interferences are possible with anti-bovine ALP antibodies, a finding which may represent the heterogeneity of possible epitopes available for binding.
IND error flags are particularly problematic in relation to uE3 measurements, which are necessary components of maternal quad screens used in second trimester fetal risk assessments. Quad screens incorporate measurements of human chorionic gonadotropin, alpha-fetoprotein, uE3, and inhibin A. As maternal screening relies upon multiples of the median (MoM) conversions of analyte concentrations prior to risk assessments, specimens with IND errors for uE3 cannot be easily tested using alternative methodologies, as patient medians would not be available. Fortunately, non-biochemical methods for maternal screening (e.g. cell-free DNA) are now available as alternative options for such patients. Identification of true low uE3 concentrations, however, is also critical for the identification of steroid sulfatase deficiencies, Smith-Lemli-Opitz syndrome, and assessment of potential early fetal demise [14]. As such, misinterpretation of an IND assay interference as a true low uE3 measurement could still lead to unnecessary intervention (e.g. amniocentesis) during pregnancy.
Based on the present findings, an algorithm was developed to assist our clinical laboratory in evaluating IND error flags in a manner that allows for the differentiation of true low E2 and uE3 results from assay interferences. If more than one patient has an E2 or uE3 IND interference on a given instrument run, instrument error logs are reviewed and troubleshooting is conducted in accordance with the manufacturer recommendations [10]. If an isolated IND interference for E2 or uE3 is observed, testing is repeated with a 1:2 dilution (100 μl patient specimen + 100 μl Sample Diluent A). If the repeat result has a quantitative value that is below the AMR (with IND error flag cleared), it is reported as less than the AMR for that assay (e.g., a true low result). If the result still shows an IND error flag after dilution, it is reported as a “See Note” with a result comment indicating that the laboratory was unable to quantitate due to interfering substances in the patient sample. When interferences for uE3 are detected as part of a maternal quad screen, alternative cell-free DNA analysis is suggested to the ordering provider. An alternative quantitative LC-MS/MS method is available when E2 interference is observed.
In the absence of an IND error flag raising attention to a potentially problematic specimen, it is difficult to assess whether quantitative interferences may exist. An example of this risk is evident in Pt. H (Table 4), which showed an IND error flag for uE3 but a quantitative negative interference (but no IND error flag) on the Beckman E2 assay, as compared to results from non-ALP-containing alternative E2 assays. It is not possible to definitively determine whether any quantitative interference was present for Pt. G (Table 4), as additional dilutions were not conducted for results that were above assay AMRs. E2 results across assays, however, were consistent with Pt. G’s pregnancy status. Pt J (Table 4). showed E2 results near or below the low end of the AMR for Abbott, Beckman, and Roche assays (analytical sensitivities: Abbott, ≤10 pg/mL; Beckman, 20 pg/mL; Roche, 5 pg/mL). These results were generally consistent with E2 concentrations as previously determined in this specimen by an LC-MS/MS method (11 pg/mL). The E2 result on the Siemens assay (183 pg/mL), however, was notably higher than the assay analytical sensitivity (11.8 pg/mL). Had additional specimen been available, repeat testing on the Siemens instrument may have provided additional supportive evidence in troubleshooting. In the absence of such evidence, the present experiments do not explain the E2 discordance observed for Pt. J with the Siemens assay. Possibilities include low-end calibration differences, endogenous analytical interference due to another mechanism, specimen or reagent aspiration error, and/or analytical error.
The present studies do not provide evidence regarding whether other immunoassays on DxI 800 instruments (competitive and/or non-competitive) are also impacted by anti-bovine ALP Ab interference. Limited specimen volumes and the rarity of IND error flags for E2 and uE3 assays precluded our ability evaluate the potential impact of anti-bovine ALP Ab interferences on other ALP-containing tests. Ab spiking studies, however, provide a potential model that could be used in future investigations.
The prevalence of anti-ALP Abs in healthy and sick individuals is also relatively unknown. Polyreactive human Abs against ALP have previously been reported in an evaluation of patients with bacterial infections [15]. Anti-ALP Abs have also been observed in a subset of patients with anti-acetylcholine receptor positive myasthenia gravis, showing a female preponderance [16,17]. Macro-ALP (e.g. immunoglobulin-ALP complexes) have also been described in several case reports, although such cases specifically refer to human Abs bound to human ALP [[18], [19], [20]].
Without a greater recognition of anti-ALP Ab prevalence, or information on which ALP-containing immunoassays actually contain anti-ALP blocking reagents, it is difficult to assess the risk of assay interference when using ALP-containing reagents. It should be noted that Abs against other reagent constituents such as streptavidin and horseradish peroxidase have also been reported [[21], [22], [23], [24]]. Risks of heterophilic Ab interference are also well-described and are commonly addressed through the addition of blocking reagents in many immunoassay formats [25]. Anti-ALP Abs are an additional mechanism of potential interference that should also be considered in assay design and in clinical laboratory troubleshooting as applicable.
Conflict of interest
JRG, Contract research support to ARUP Laboratories from Fujirebio Diagnostics.
Acknowledgements
The authors would like to thank Beckman Coulter for technical applications assistance during this investigation. Preliminary data were presented at the Academy of Clinical Laboratory Physicians and Scientists (ACLPS) 2018 Annual Meeting in Houston, TX (abstract available at American Journal of Clinical Pathology, Volume 150, Issue suppl_1, 21 September 2018, Pages S163–S164), with additional data presented as a poster (A-241) at the 2019 American Association for Clinical Chemistry (AACC) annual meeting in Anaheim, CA. This work was supported by ARUP Laboratories.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.plabm.2019.e00131.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Millan J.L. Alkaline Phosphatases : structure, substrate specificity and functional relatedness to other members of a large superfamily of enzymes. Purinergic Signal. 2006;2(2):335–341. doi: 10.1007/s11302-005-5435-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schaap A.P., Akhavan H., Romano L.J. Chemiluminescent substrates for alkaline phosphatase: application to ultrasensitive enzyme-linked immunoassays and DNA probes. Clin. Chem. 1989;35(9):1863–1864. [PubMed] [Google Scholar]
- 3.Herman D.S., Ranjitkar P., Yamaguchi D., Grenache D.G., Greene D.N. Endogenous alkaline phosphatase interference in cardiac troponin I and other sensitive chemiluminescence immunoassays that use alkaline phosphatase activity for signal amplification. Clin. Biochem. 2016;49(15):1118–1121. doi: 10.1016/j.clinbiochem.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Marinheiro R., Amador P., Parreira L., Rato Q., Caria R. False positive troponin I rendering two admissions for “recurrent acute myopericarditis”. Open Cardiovasc. Med. J. 2018;12:55–58. doi: 10.2174/1874192401812010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sofronescu A.G., Ross M., Rush E., Goldner W. Spurious testosterone laboratory results in a patient taking synthetic alkaline phosphatase (asfotase alfa) Clin. Biochem. 2018;58:118–121. doi: 10.1016/j.clinbiochem.2018.04.024. [DOI] [PubMed] [Google Scholar]
- 6.Alkaline Phosphatase for the Diagnostics and Life Sciences Industry. Roche Diagnostics; Mannheim, Germany: 2010. http://custombiotech.roche.com/content/dam/internet/dia/custombiotech/custombiotech_com/en_GB/pdf/CustomBiotech_Alkaline_Phosphatase_ProductFlyer.pdf [Google Scholar]
- 7.Scavenger Alkaline Phosphatase (Inactive). Ref 47814900. Oriental Yeast Co, Osaka, Japan.
- 8.Access Estradiol . Beckman Coulter; Brea, CA: July 2018. Instructions for Use. Ref 33540. A56077 N. [Google Scholar]
- 9.Access Unconjugated Estriol . Beckman Coulter; Brea, CA: June 2018. Instructions for Use. Ref 33570. A33458 J. [Google Scholar]
- 10.UniCel DxI - Access Immunoassay System - Instructions for Use. Beckman Coulter; Brea, CA: August 2011. [Google Scholar]
- 11.Estradiol II . Roche Diagnostics; Indianapolis, IN: 2016-09. Package Insert. V 5.0 English. [Google Scholar]
- 12.Enhanced Estradiol (E2) Siemens Healthcare Diagnostics; Tarrytown, NY: 2016-5. Package Insert. 10629847_EN Rev. G. [Google Scholar]
- 13.Architect Estradiol. Package Insert. Ref 7K72. Abbott Laboratories, Abbott Park, IL.
- 14.Schoen E., Norem C., O’Keefe J., Krieger R., Walton D., To T.T. Maternal serum unconjugated estriol as a predictor for Smith-Lemli-Opitz syndrome and other fetal conditions. Obstet. Gynecol. 2003;102(1):167–172. doi: 10.1016/s0029-7844(03)00370-3. [DOI] [PubMed] [Google Scholar]
- 15.Ritter K., Fudickar A., Heine N., Thomssen R. Autoantibodies with a protective function: polyreactive antibodies against alkaline phosphatase in bacterial infections. Med. Microbiol. Immunol. 1997;186(2–3):109–113. doi: 10.1007/s004300050052. [DOI] [PubMed] [Google Scholar]
- 16.Ohta K., Shigemoto K., Kubo S., Maruyama N., Abe Y., Ueda N., Fujinami A., Ohta M. MuSK Ab described in seropositive MG sera found to be Ab to alkaline phosphatase. Neurology. 2005;65(12):1988. doi: 10.1212/01.wnl.0000188881.46043.44. [DOI] [PubMed] [Google Scholar]
- 17.Konishi T., Ohta K., Shigemoto K., Ohta M. Anti-alkaline phosphatase antibody positive myasthenia gravis. J. Neurol. Sci. 2007;263(1–2):89–93. doi: 10.1016/j.jns.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Cervinski M.A., Lee H.K., Martin I.W., Gavrilov D.K. A macro-enzyme cause of an isolated increase of alkaline phosphatase. Clin. Chim. Acta. 2015;440:169–171. doi: 10.1016/j.cca.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 19.McTaggart M.P., Rawson C., Lawrence D., Raney B.S., Jaundrill L., Miller L.A., Murtinho-Braga J., Kearney E.M. Identification of a macro-alkaline phosphatase complex in a patient with inflammatory bowel disease. Ann. Clin. Biochem. 2012;49(Pt 4):405–407. doi: 10.1258/acb.2011.011224. [DOI] [PubMed] [Google Scholar]
- 20.Owen M.C., Pike L.S., George P.M., Barclay M.L., Florkowski C.M. Macro-alkaline phosphatase due to IgG kappa complex: demonstration with polyethylene glycol precipitation and immunofixation. Ann. Clin. Biochem. 2002;39(Pt 5):523–525. doi: 10.1258/000456302320314584. [DOI] [PubMed] [Google Scholar]
- 21.Rulander N.J., Cardamone D., Senior M., Snyder P.J., Master S.R. Interference from anti-streptavidin antibody. Arch. Pathol. Lab Med. 2013;137(8):1141–1146. doi: 10.5858/arpa.2012-0270-CR. [DOI] [PubMed] [Google Scholar]
- 22.Lam L., Bagg W., Smith G., Chiu W.W., Middleditch M.J., Lim J.C., Kyle C.V. Apparent hyperthyroidism caused by biotin-like interference from IgM anti-streptavidin antibodies. Thyroid. 2018;28(8):1063–1067. doi: 10.1089/thy.2017.0673. [DOI] [PubMed] [Google Scholar]
- 23.Berth M., Willaert S., De Ridder C. Anti-streptavidin IgG antibody interference in anti-cyclic citrullinated peptide (CCP) IgG antibody assays is a rare but important cause of false-positive anti-CCP results. Clin. Chem. Lab. Med. 2018;56(8):1263–1268. doi: 10.1515/cclm-2017-1153. [DOI] [PubMed] [Google Scholar]
- 24.Lim Y.Y., Ong L., Loh T.P., Sethi S.K., Sng A.A.J., Loke K.Y., Halsall D.J., Hughes I.A., Lee Y.S. A diagnostic curiosity of isolated androstenedione elevation due to autoantibodies against horseradish peroxidase label of the immunoassay. Clin. Chim. Acta. 2018;476:103–106. doi: 10.1016/j.cca.2017.11.025. [DOI] [PubMed] [Google Scholar]
- 25.Kricka L.J. Human anti-animal antibody interferences in immunological assays. Clin. Chem. 1999;45(7):942–956. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.