Abstract
Introduction
Krüppel-like factor 4 (KLF4) is considered one of the Yamanaka factors, and recently, we and others have shown that KLF4 is one of the transcription factors essential for reprogramming non-human corneal epithelial cells (HCECs) into HCECs. Since epithelial to mesenchymal transition (EMT) suppression is vital for homeostasis of HCECs via regulation of transcription factors, in this study, we aimed to investigate whether KLF4 prevents EMT in HCECs and to elucidate the underlying mechanism within the canonical TGF-β signalling pathway, which is involved in corneal epithelial wound healing.
Methods
HCECs were collected from cadaver donors and cultivated. We generated KLF4-knockdown (KD) HCECs using siRNA transfection and analysed morphology, gene or protein expression, and endogenous TGF-β secretion. KLF4 was overexpressed using lentiviral KLF4 expression vectors and underwent protein expression analyses after TGF-β2 treatment.
Results
KLF4-KD HCECs showed a fibroblastic morphology, downregulation of the epithelial markers, keratin 12 and keratin 14, and upregulation of the mesenchymal markers, fibronectin 1, vimentin, N-cadherin, and SLUG. Although E-cadherin expression remained unchanged in KLF4-KD HCECs, immunocytochemical analysis showed that E-cadherin–positive adherens junctions decreased in KLF4-KD HCECs as well as the decreased total protein levels of E-cadherin analysed by immunoblotting. Moreover, within the TGF-β canonical signalling pathway, TGF-β2 secretion by HCECs increased up to 5 folds, and several TGF-β–associated markers (TGFB1, TGFB2, TGFBR1, and TGFBR2) were significantly upregulated up to 6 folds in the KLF4-KD HCECs. SMAD2/3, the main signal transduction molecules of the TGF-β signalling pathway, were found to be localised in the nucleus of KLF4-KD HCECs. When KLF4 was overexpressed, cultivated HCECs showed upregulation of epithelial markers, keratin 14 and E-cadherin, indicating the contributory role of KLF4 in the homeostasis of human corneal epithelium in vivo. In addition, KLF4 overexpression in HCECs resulted in decreased SMAD2 phosphorylation and altered nuclear localisation of SMAD2/3, even after TGF-β2 treatment.
Conclusions
These results show that KLF4 prevents EMT in HCECs and suggest a novel role of KLF4 as an endogenous TGF-β2 suppressor in the human corneal epithelium, thus highlighting the potential of KLF4 to prevent EMT and subsequent corneal fibrotic scar formation by attenuating TGF-β signalling.
Keywords: KLF4, EMT, Corneal epithelium, TGF-β signalling pathway, SMAD2, SMAD3
Abbreviations: KLF4, Krüppel-like factor 4; HCECs, human corneal epithelial cells; EMT, epithelial to mesenchymal transition; TGF-β, transforming growth factor-β
Highlights
-
•
KLF4 inhibited EMT within corneal epithelia.
-
•
TGF-β expression of human corneal epithelial cells is regulated by KLF4.
-
•
KLF4 prevented phosphorylation and nuclear localisation of SMAD2.
-
•
KLF4 may be an important transcription factor in wound healing.
1. Introduction
The corneal epithelium, consisting of several squamous epithelial layers, forms the outermost surface of the cornea. Epithelial to mesenchymal transition (EMT) is the process by which cells lose their epithelial characteristics and gain mesenchymal properties. In human corneal epithelial cells (HCECs), EMT suppression supports a healthy and transparent corneal epithelium, which is vital for homeostasis of HCECs via regulation of transcription factors [1], [2]. Recently, we and others demonstrated that corneal epithelial sheets can be obtained from human induced pluripotent stem cells or non-corneal epithelial cells [1], [3], [4], and EMT suppression is also a vital condition for maintaining such corneal sheets.
Krüppel-like factor 4 (KLF4) is a zinc finger-containing transcription factor that regulates cell growth, proliferation, and differentiation [5], [6], [7], and is considered one of the Yamanaka reprogramming factors [8]. KLF4 has also been shown as one of the transcription factors essential in reprogramming non-HCECs into HCECs and maintaining human corneal epithelium homeostasis [1], [4]. In addition, our previous findings using cap analysis of gene expression (CAGE) revealed that KLF4 co-regulates HCEC-specific genes in conjunction with PAX6, another key transcription factor in HCECs [9].
KLF4 has been shown to regulate EMT and function as an oncogene or a tumour-suppressor gene depending on the cell type [10], [11], [12], [13]. In the murine corneal epithelium, Klf4 is reported to play an important role in cell differentiation and maintenance of the barrier function or epithelial characteristics [2], [14], [15], and one report shows that KLF4 suppresses mesenchymal properties [2]. Whilst EMT is also involved in corneal epithelial wound healing [16], [17], the detailed mechanism underlying the role of KLF4 between this EMT suppression and wound healing has not yet been fully elucidated. Since TGF-β signalling pathway is well known to drive EMT [18], [19], in this study, we investigated the involvement of KLF4 within EMT in HCECs, and the underlying mechanism of the TGF-β signalling pathway. By elucidating the underlying mechanism, KLF4 can be explored as a therapeutic target for corneal wound healing.
2. Materials and methods
2.1. Cell culture
Research-grade corneoscleral rims of human cadaver donors were procured from the eye bank CorneaGen Inc. (Seattle, WA, USA) and were handled in accordance with the tenets of the Declaration of Helsinki. Corneal limbal cells were carefully collected as previously described [9]. Cells were seeded at 1 × 104 cells/cm2 onto plastic tissue culture plates coated with 0.5 μg/cm2 laminin 511 (iMatrix-511, Nippi, Tokyo, Japan). Cells were cultured in corneal epithelium maintenance medium (CEM), as it is reported to be suitable for HCEC maintenance [20], and incubated with 5% CO2 at 37 °C. CEM consists of DMEM:F-12 medium (1:1) containing l-glutamine and 2.438 g/L sodium bicarbonate (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 2% B-27 (Thermo Fisher Scientific), 10 μM of Y-27632 (Wako Pure Chemical Industries, Osaka, Japan), 10–20 ng/mL of human recombinant KGF/FGF-7 (R&D Systems, Minneapolis, MN, USA), and 1% Penicillin-Streptomycin (Thermo Fisher Scientific).
2.2. RNA interference
Small interfering RNAs (siRNAs) against KLF4 (5′-GCAGCUUCACCUAUCCGAUTT-3′) and a negative control siRNA (Silencer® Select Pre-designed (Inventoried) siRNA) were purchased from Thermo Fisher Scientific. When HCECs were confluent, they were treated with TrypLE™ Express (Thermo Fisher Scientific) for 20 min at 37 °C, to create a single-cell suspension and seeded at 2.5 × 104 cells/cm2 onto 0.5 μg/cm2 laminin 511-coated plastic tissue culture plates. Seeded cells were transfected with 30 nM KLF4 siRNA using Lipofectamine™ RNAiMAX Transfection Reagent (Thermo Fisher Scientific) as per the manufacturer's instructions. Immediately after seeding, HCECs were transfected at day 0, the CEM medium was changed, and the HCECs were transfected again on days 2 and 4, to sustain the knockdown. On day 7, the supernatant was collected, and the HCECs were harvested for further analysis.
2.3. Viral transduction
KLF4 was subcloned into the CSIV-CMV-MCS-IRES2-Venus vector, which was kindly provided by RIKEN BioResource Center, as previously described [4]. EmGFP was subcloned into the CSIV-CMV-IRES2-Venus vector as control. Both lentiviral vectors were co-transfected into 293T cells with pCMV-VZV-G-RSV-Rev and pCAG-HIV-gp using X-tremeGENE™ 9 DNA Transfection Reagent (Roche Applied Science, Manheim, Germany). Virus-containing supernatants were ultra-centrifuged in an Optima L-90K Preparative Ultracentrifuge for 1.5 h at 50,000×g at 4 °C. When the HCECs were sub-confluent, they were treated with TrypLE™ Express, seeded at 1 × 104 cells/cm2 onto 0.5 μg/cm2 laminin 511-coated plastic tissue culture plates, and subjected to viral treatment with 6 μg/mL of polybrene (Nacalai Tesque, Kyoto, Japan). HCECs were incubated for 24 h, the medium was changed, and the HCECs were further incubated for 48 h before collection.
2.4. TGF-β treatment
After 72 h of cultivation, KLF4-overexpressing cells and control HCECs were pre-starved for 2 h in DMEM:F12 medium (1:1) supplemented with 0.1% bovine serum albumin (Merck & Co., Kenilworth, NJ, USA) and then treated with TGF-β2 (10 ng/mL). TGF-β2–treated cells were harvested at 30 min and 4 h and analysed for immunocytochemistry or immunoblotting. SB431542, a potent and specific inhibitor of TGF-β type I receptor kinases (Wako Pure Chemical Industries; 10 μM), was used for inhibition of TGF-β signalling.
2.5. Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from the corneal epithelial cells using RNeasy Plus Micro Kit (QIAGEN, Hilden, Germany). cDNA synthesis and quantitative PCR were performed as described previously [4]. TaqMan™ Gene Expression Assay probes (Thermo Fisher Scientific) are provided in Supplementary Fig. 1. GAPDH was used as the reference gene, and the relative percentages of the desired markers were determined.
2.6. Immunoblotting
Total protein was extracted from cultivated HCECs using RIPA Buffer (Nacalai Tesque) or Laemmli sample buffer for detection of phosphorylated SMAD2, and immunoblotting was performed as previously described [21]. Briefly, 10 μg of protein was subjected to SDS-PAGE and then electro-transferred. Immunoblot membranes containing protein samples were treated with antibodies at dilutions detailed in Supplementary Figs. 2 and 3. ACTB was used as reference control and protein expression levels relative to those of ACTB were appropriately calculated using densitometric analyses using Image Lab software (BIO-RAD, Hercules, CA) as a ratio of desired protein to control protein.
2.7. Immunocytochemistry and high-content imaging assay
Immunocytochemistry was performed as previously described [3], using primary antibodies (Supplementary Fig. 2), with Alexa Fluor 488, 568, or 647-conjugated secondary antibodies (Thermo Fisher Scientific) at 1:200 dilution. Images were captured using an inverted microscope (Axio Observer. Z1, D1, Carl Zeiss, Jena, Germany). Cells were also viewed using the Operetta high-content imaging system (PerkinElmer, Waltham, MA, USA) to determine cell numbers, cell size, cell roundness, and cell border fluorescence intensity using the Harmony Software (PerkinElmer). Details of the imaging assay are listed in Table 1.
Table 1.
Details of high-content imaging system assays.
| Purpose | Methods |
|---|---|
| Single cell size | Cell nuclei stained with Hoechst |
| Cytoplasm stained with keratin 14 (KRT14) | |
| Ratio of round cells | Step 1: Analyse relative roundness index: ‘1’ represents a circle and ‘0’ represents a line |
| Step 2: Count the number of round cells with a roundness index >0.9 and width:length ratio >0.5 | |
| Cell border fluorescence intensity | Cell nuclei stained with Hoechst |
| Cytoplasm stained with KRT14 | |
| Trace region of cell border for membrane fluorescence quantification |
2.8. Immunoassays
Cell culture supernatants were analysed to measure total TGF-β1 and TGF-β2 using the Human TGF-β1 and TGF-β2 Quantikine ELISA kits (R&D systems) respectively, according to the manufacturer's instructions. For latent TGF-β release, samples were acidified with HCl and neutralised with NaOH. The minimum detectable dose was 31.3 pg/mL for both TGF-β1 and TGF-β2. Optical densities were measured at 540 nm with correction at 570 nm using a multi-plate reader (2030 Multilabel Reader ARVO™ X4, PerkinElmer).
2.9. Statistical analysis
Data were analysed using JMP version 13.0 (SAS Institute, Cary, NC, USA). One-way analysis of variance or Mann Whitney tests were performed as appropriate. For multiple non-paired comparisons, Steel's tests were performed for each gene as appropriate. P < 0.05 was considered significant.
3. Results
3.1. KLF4 knockdown caused loss of phenotype in corneal epithelial cells
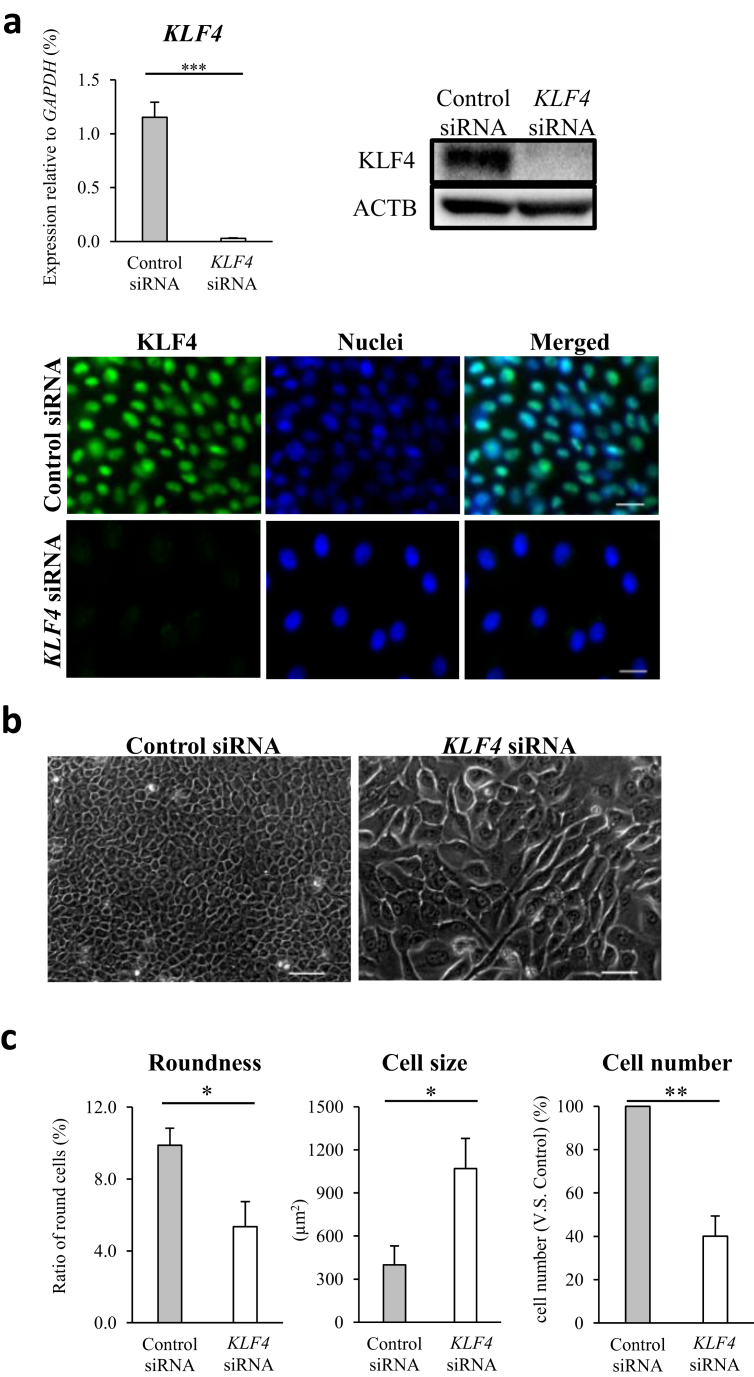
To determine the role of KLF4 in the process of corneal epithelial homeostasis, we generated KLF4-knockdown (KLF4-KD) HCECs using siRNA. KLF4-KD was successful in HCECs up to day 7 in vitro, as confirmed by qPCR, immunoblotting, and immunocytochemistry (Fig. 1a). In addition, cell morphology and gene expression of non-transfected HCECs compared to those of control siRNA transfected HCECs, displayed no major differences (Supplementary Figs. 4 and 5). Therefore, we used control siRNA as a comparative of KLF4 siRNA. On day 7, KLF4-KD HCECs displayed notable morphological changes to elongated and asymmetrical cells, compared to the classical polygonal and rounded control HCECs (Fig. 1b); roundness and cell size were quantified using high-content imaging (Fig. 1c). The percentage of round cells among KLF4-KD HCECs was significantly lower than that among control HCECs. Further quantification analyses of morphology revealed that on day 7, KLF4-KD mean cell size was twice that of HCEC controls, and the number of KLF4-KD HCECs was half that of control HCECs (Fig. 1c), over an equal surface area.
Fig. 1.
KLF4 knockdown (KD) altered the morphology and increased cell size of human corneal epithelial cells (HCECs). (a) Compared to HCECs transfected with control siRNA, complete KLF4 KD was confirmed via qRT-PCR (biological n = 8, Mann Whitney test), and at the protein level via immunoblot and immunofluorescence (representative of three independent experiments; scale bars, 20 μm). (b) Morphology of HCECs transfected with control siRNA (left panel) and siRNA against KLF4 (KLF4 siRNA; right panel). KLF4 KD led to the loss of the classical polygonal HCEC morphology, forming asymmetrical and elongated cells (representative of three independent experiments; scale bars, 50 μm). (c) The percentage of round cells in KLF4-KD HCECs was significantly lower than that in control siRNA transfected HCECs (left panel). In addition, the mean size was significantly higher (middle panel) and cell number was significantly lower (right panel) in KLF4-KD HCECs, than in control siRNA transfected HCECs (biological n = 6, Mann Whitney test). *P < 0.05, **P < 0.01, and ***P < 0.001.
3.2. KLF4 KD promoted EMT in corneal epithelia
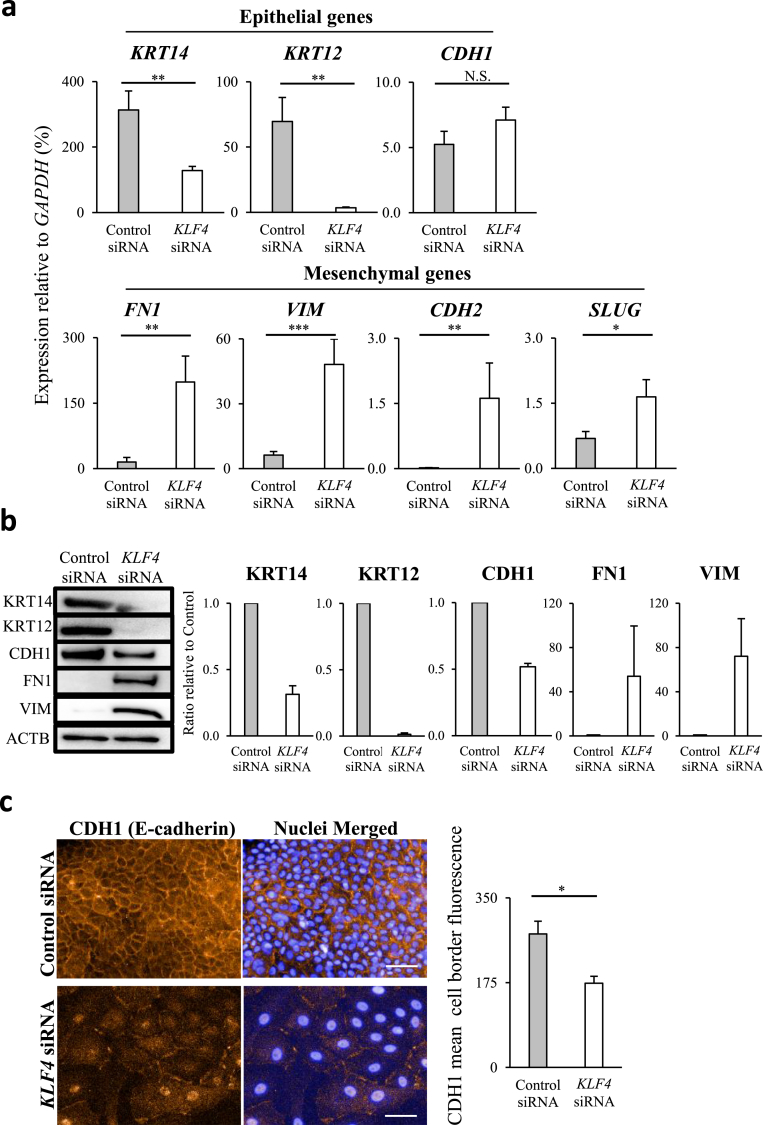
As KLF4 KD altered the morphology of HCECs, indicating EMT, we examined the gene expression of EMT-associated markers. In KLF4-KD HCECs, the epithelial genes keratin 14 (KRT14) and keratin 12 (KRT12) were significantly downregulated, whereas E-cadherin (CDH1) showed no significant changes (Fig. 2a). In addition, all the mesenchymal genes analysed, namely fibronectin 1 (FN1), vimentin (VIM), N-cadherin (CDH2), and SLUG, were significantly upregulated (Fig. 2a). Immunoblotting revealed that KRT14, KRT12, and CDH1 protein levels were reduced in KLF4-KD HCECs, compared to those in the control cells, and that FN1 and VIM proteins were expressed only in KLF4-KD HCECs (Fig. 2b). Immunocytochemical analysis showed that CDH1 adherens junction decreased in KLF4-KD HCECs (Fig. 2c). Immunofluorescence quantification revealed that membrane CDH1 in KLF4-KD HCECs was significantly lower than that in the controls (Fig. 2c bar graph).
Fig. 2.
KLF4 knockdown (KD) promoted epithelial to mesenchymal transition in corneal epithelia. (a) KLF4 KD in human corneal epithelial cells (HCECs) significantly downregulated the epithelial genes KRT14 and KRT12 but had no significant effect on CDH1. Conversely, the mesenchymal genes FN1, VIM, CDH2, and SLUG were significantly upregulated (biological n = 8, Mann Whitney test, N.S., not significant). (b) In KLF4-KD HCECs, KRT14, KRT12, and CDH1 protein levels decreased and FN1 and VIM protein was detected. Immunoblots are representative of each sample that was used for densitometric analyses (bar graph), relative to control siRNA (biological n = 3). (c) Immunofluorescence staining for CDH1 (E-cadherin) adherens junction did not show a clearly defined border in KLF4-KD HCECs (left panels; scale bars, 50 μm). Immunofluorescence quantification revealed that membrane CDH1 intensity in KLF4-KD HCECs was significantly lower than that in control siRNA transfected HCECs (right bar graph; biological n = 5, Mann Whitney test). *P < 0.05, **P < 0.01, and ***P < 0.001.
3.3. KLF4 inhibits EMT via activation of TGF-β signalling pathway in corneal epithelia
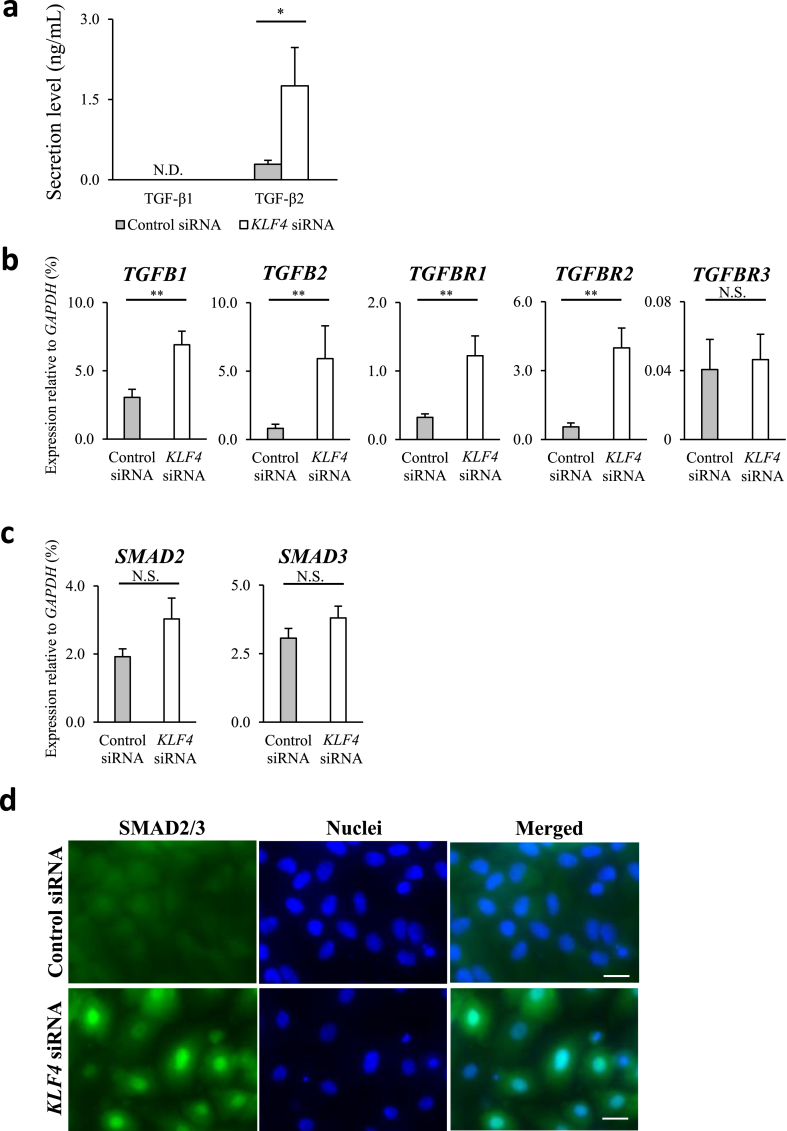
The TGF-β signalling pathway plays a major role in EMT [18], [19], and we observed EMT in KLF4-KD HCECs; therefore, we further investigated the involvement of the TGF-β canonical pathway. TGF-β1 was not detectable in control or KLF4-KD HCECs but TGF-β2 expression in KLF4-KD HCECs was significantly higher than that in the control cells (Fig. 3a). In addition, the expression of TGFB1, TGFB2, TGFBR1, and TGFBR2 increased significantly in KLF4-KD HCECs, compared to that in control cells (Fig. 3b). Interestingly, the upregulation of TGFB1, TGFB2, TGFBR1, and TGFBR2 had a trend to be repressed by SB431542, a potent and specific inhibitor of TGF-β type I receptor kinases (Supplementary Fig. 6). The expression of SMAD2/3, the main signal transduction molecules in the TGF-β signalling pathway, was higher in KLF4-KD HCECs than in the control cells; however, the difference was not statistically significant (Fig. 3c). Immunocytochemistry analyses revealed the nuclear localisation of SMAD2/3 proteins in KLF4-KD HCECs, compared to the diffuse nuclear and cytoplasmic localisation in control HCECs (Fig. 3d).
Fig. 3.
KLF4 inhibited epithelial to mesenchymal transition via TGF-β2 activation in HCECs. (a) TGF-β2 secretion by human corneal epithelial cells (HCECs) increased significantly in KLF4-knockdown (KLF4-KD) HCECs (biological n = 5; Mann Whitney test, N.D., not detected). (b) Expression of TGFB1, TGFB2, TGFBR1, and TGFBR2 was significantly upregulated in KLF4-KD HCECs (biological n = 8, Mann Whitney test, N.S., not significant). (c) Expression of SMAD2 and SMAD3 was not significantly different (biological n = 8, Mann Whitney test, N.S., not significant). (d) Immunocytochemistry for SMAD2/3 displayed intense nuclear staining in KLF4-KD HCECs but diffused staining in control siRNA transfected HCECs. (representative of three independent experiments; scale bars, 20 μm). *P < 0.05 and **P < 0.01.
3.4. KLF4 overexpression enhanced corneal epithelial characteristics
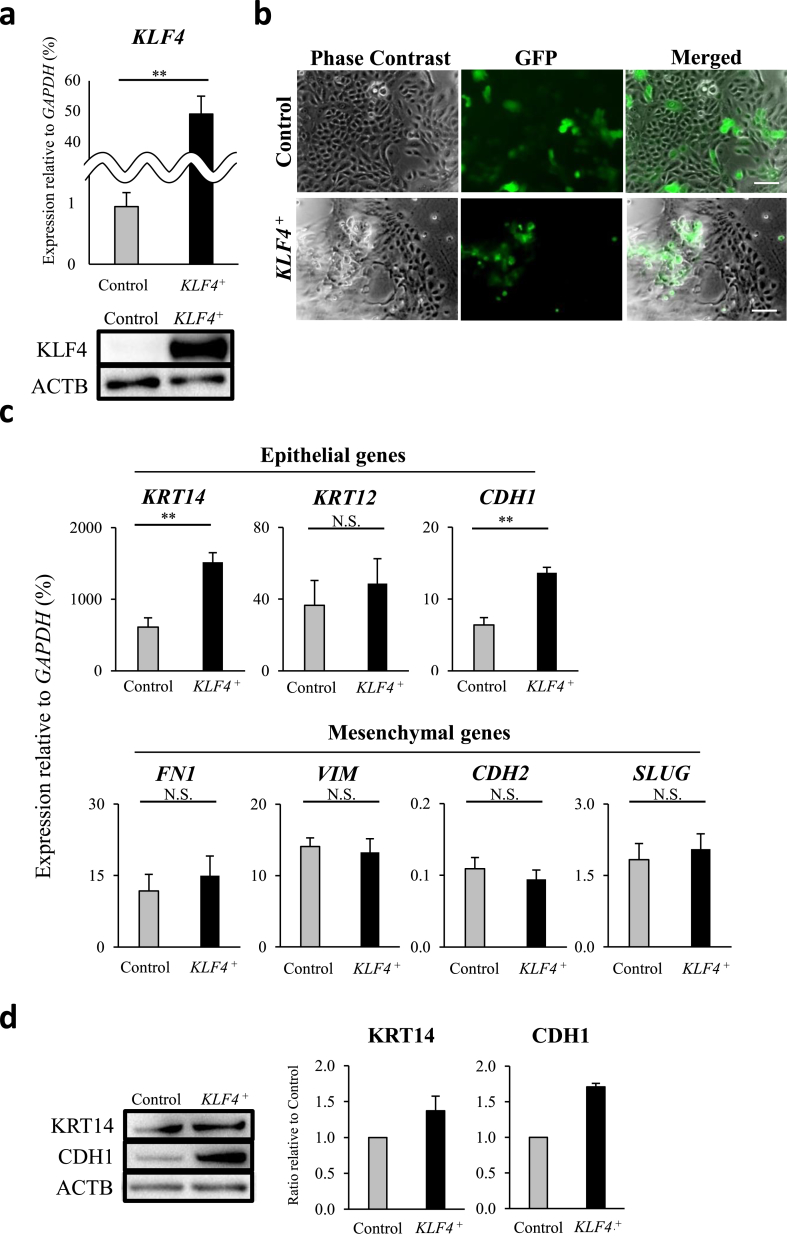
As previously demonstrated, KLF4 is highly expressed in HCECs in vivo [4], whereas expression in vitro is comparatively low. As such, cultivated HCECs were transfected with KLF4 or control lentivirus to elucidate the specific contribution toward corneal epithelium homeostasis. We generated KLF4-overexpressing (KLF4+) HCECs and confirmed KLF4 overexpression using qRT-PCR, immunoblotting, and immunocytochemistry (Fig. 4a and Supplementary Fig. 7). GFP-positive KLF4+ HCECs appeared slightly bulging compared with GFP-positive control HCECs (Fig. 4b). Epithelial genes KRT14 and CDH1 were upregulated in KLF4+ HCECs (Fig. 4c upper panels), and the same tendency was detected at the protein level (Fig. 4d). Mesenchymal genes revealed no significant changes in KLF4+ HCECs (Fig. 4c lower panels).
Fig. 4.
KLF4 enhanced human corneal epithelium characteristics. (a) HCECs were transfected with control vector (Control) or KLF4 vector (KLF4+). KLF4 overexpression was confirmed by qRT-PCR (biological n = 5, Mann Whitney test) and immunoblotting (biological n = 3). (b) Lentiviral-positive GFP-KLF4+ cells appeared slightly bulging compared to GFP-control HCECs (representative of three independent experiments; scale bars, 100 μm). (c) Epithelial markers KRT14 and CDH1 were significantly upregulated in KLF4+ HCECs, whilst mesenchymal markers displayed no relevant differences (biological n = 6, Mann Whitney test, N.S., not significant). (d) KRT14 and CDH1 protein levels were increased by KLF4 overexpression (biological n = 3). **P < 0.01.
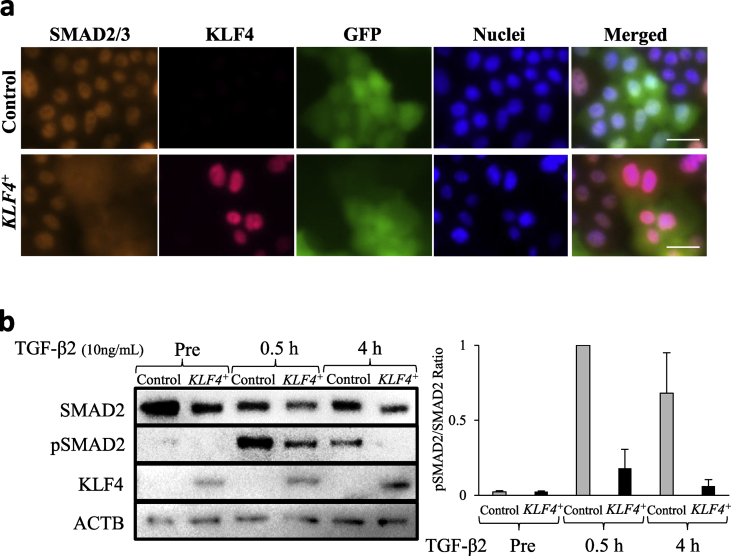
3.5. Nuclear localisation of SMAD2/3 induced by TGF-β2 treatment was inhibited in KLF4-overexpressing HCECs
As TGF-β2 is known to be released from corneal epithelial cells after basement membrane injury [22], [23], we hypothesised that KLF4 inhibits the TGF-β2–induced EMT in the corneal epithelium by inhibiting the nuclear localisation of SMAD2/3. Therefore, we treated KLF4+ HCECs with TGF-β2. Immunocytochemistry revealed that the nuclear localisation of SMAD2/3 proteins post–TGF-β2 treatment was inhibited in the GFP-KLF4+ HCECs, compared to that in the GFP-control HCECs (Fig. 5a). As it is well known that TGF-β activates SMAD2/3 phosphorylation and its nuclear localization [18], [19], we further investigated the phosphorylation level of SMAD2 in KLF4+ HCECs to identify the precise TGF-β signalling event inhibited by KLF4. SMAD2 phosphorylation was detected as early as 30 min post–TGF-β2 treatment in control and KLF4+ HCECs; however, the phosphorylation level in KLF4+ HCECs was less than 20% of that in the control HCECs. At 4 h post–TGF-β2 treatment, phosphorylation levels returned to pre-treatment levels in KLF4+ HCECs, but were reduced to approximately half in the control cells (Fig. 5b). These results indicate that KLF4+ HCECs were resistant to TGF-β2-induced EMT.
Fig. 5.
TGF-β2 treatment-induced nuclear localisation of SMAD2/3 was inhibited in KLF4-overexpressing (KLF4+) HCECs. (a) Immunocytochemistry revealed the nuclear localisation of SMAD2/3 proteins after TGF-β2 treatment was inhibited in GFP-KLF4+ HCECs, compared with that in the GFP-control HCECs (representative of three independent experiments; scale bars, 10 μm). (b) Following TGF-β2 treatment (10 ng/mL), levels of phosphorylated SMAD2 (pSMAD2) decreased in KLF4+ HCECs, compared with that in the control. After 4 h, the levels returned to the pre-treatment levels in KLF4+ HCECs (ACTB reference control; biological n = 3).
4. Discussion
In the current study, we investigated the involvement of KLF4 in EMT in HCECs and elucidated the mechanisms underlying the role of KLF4 in homeostasis maintenance, suggesting that the use of KLF4 may serve as a novel approach for EMT suppression in the field of regenerative medicine in maintaining cultivated corneal epithelial cell sheets. We also elucidated that KLF4 prevents EMT by suppressing TGF-β2 secretion, which has a vital role in corneal wound healing, via the canonical TGF-β signalling pathway. We demonstrated EMT in HCECs by KLF4 KD via morphological and gene expression analysis. EMT is generally detected as changes in gene expression or protein level; however, we additionally demonstrated that cell morphology analysis using high-content imaging assay can be a useful tool for quantification of EMT.
Here, we showed that KLF4 KD in HCECs decreased the gene expression and protein levels of the epithelial markers KRT12 and KRT14. These results support the previous findings that KLF4 directly binds and transactivates the promoter of KRT12 [24], and that KLF4 overexpression upregulates KRT14 expression [4]. Although CDH1 expression remained unchanged in KLF4-KD HCECs, membrane CDH1 protein levels were significantly decreased as well as total protein levels of CDH1, suggesting that KLF4 may regulate the expression of CDH1 or the post-transcriptional recruitment of CDH1 to the cell membrane via other factors.
On the other hand, upregulation of mesenchymal markers in KLF4-KD HCECs was observed to occur via promotion of the TGF-β signalling pathway. We demonstrated increased secretion of TGF-β2 by KLF4-KD HCECs due to activation of the TGF-β signalling pathway, implying that KLF4 KD promoted EMT, which is known to be maintained by an autocrine TGF-β loop [25], [26], [27]. Contrary to previous reports, we did not detect TGF-β1 secretion [28], [29]; however, we and others previously reported that TGF-β2 plays a major role in normal corneal epithelial maintenance and enhances corneal epithelial wound healing [30], [31], [32]. During corneal epithelial wound healing after epithelial debridement, KLF4 expression is lost in the cells migrating for repair, compared with that in the normal corneal epithelial cells [2]; therefore, our result, together with this aforementioned finding, indicates that higher secretion of TGF-β2 by migrating cells could enhance corneal epithelial wound healing. Following corneal injury with disruption of the basement membrane, TGF-β2 is released by the corneal epithelium into the corneal stroma, inducing a subpopulation of keratocytes to undergo transformation into myofibroblasts and form a fibrotic scar [22], [23] that may directly cause vision impairment. Therefore, attenuation of TGF-β expression and signalling may provide means to counteract fibrotic changes, and KLF4 may play an important role in penetrating wound healing essential for preventing corneal scar formation by repressing TGF-β2 secretion.
In this study, we did not investigate the detailed mechanism underlying the function of KLF4 as a transcription factor in suppressing phosphorylation and nuclear localisation of SMAD2/3, the main signal transduction molecule in the TGF-β signalling pathway. However, since recent reports demonstrated that KLF4 suppresses TGF-β signalling by transcriptional activation of SMAD7, an inhibitory SMAD [33], [34], we can speculate that KLF4 KD led to SMAD7 downregulation, causing nuclear localisation of SMAD2/3 in KLF4-KD HCECs, whilst KLF4 overexpression led to SMAD7 upregulation, causing inhibition of nuclear localisation of SMAD2/3 or decrease of SMAD2 phosphorylation in KLF4+ HCECs treated with TGF-β2.
5. Conclusions
Our findings demonstrate that KLF4 prevented EMT via inhibition of the TGF-β signalling pathway in human corneal epithelia, resulting in the suppression of TGF-β2 secretion, thereby providing a foundation for developing KLF4-based approaches to prevent corneal scar formation.
Acknowledgements
The authors thank Yuki Ishikawa, Kimihito Nomi, Yuki Kobayashi, Shun Shibata, Yu Yoshinaga, and Masahito Yoshihara for their technical assistance and Hiroyuki Miyoshi, from RIKEN BioResource Center, for provision of lentiviral vectors. This work was supported by Osaka Eye Bank, Osaka, JAPAN, and by Integrated Frontier Research for Medical Science Division, Institute for Open and Transdisciplinary Research Initiatives, Osaka University, JAPAN.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.reth.2019.08.003.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Kitazawa K., Hikichi T., Nakamura T., Mitsunaga K., Tanaka A., Nakamura M. OVOL2 maintains the transcriptional program of human corneal epithelium by suppressing epithelial-to-mesenchymal transition. Cell Rep. 2016;15:1359–1368. doi: 10.1016/j.celrep.2016.04.020. [DOI] [PubMed] [Google Scholar]
- 2.Tiwari A., Loughner C.L., Swamynathan S., Swamynathan S.K. KLF4 plays an essential role in corneal epithelial homeostasis by promoting epithelial cell fate and suppressing epithelial-mesenchymal transition. Investig Ophthalmol Vis Sci. 2017;58:2785–2795. doi: 10.1167/iovs.17-21826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayashi R., Ishikawa Y., Sasamoto Y., Katori R., Nomura N., Ichikawa T. Co-ordinated ocular development from human iPS cells and recovery of corneal function. Nature. 2016;531:376–380. doi: 10.1038/nature17000. [DOI] [PubMed] [Google Scholar]
- 4.Sasamoto Y., Hayashi R., Park S.J., Saito-Adachi M., Suzuki Y., Kawasaki S. PAX6 isoforms, along with reprogramming factors, differentially regulate the induction of cornea-specific genes. Sci Rep. 2016;6:20807. doi: 10.1038/srep20807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shields J.M., Christy R.J., Yang V.W. Identification and characterization of a gene encoding a gut-enriched Kruppel-like factor expressed during growth arrest/ J Biol Chem. 1996;271:20009–20017. doi: 10.1074/jbc.271.33.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen X., Johns D.C., Geiman D.E., Marban E., Dang D.T., Hamlin G. Kruppel-like Factor 4 (Gut-enriched Kruppel-like Factor) inhibits cell proliferation by blocking G1/S progression of the cell cycle. J Biol Chem. 2001;276:30423–30428. doi: 10.1074/jbc.M101194200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen X., Whitney E.M., Gao S.Y., Yang V.W. Transcriptional profiling of Kruppel-like factor 4 reveals a function in cell cycle regulation and epithelial differentiation. J Mol Biol. 2003;326:665–677. doi: 10.1016/S0022-2836(02)01449-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 9.Yoshihara M., Sasamoto Y., Hayashi R., Ishikawa Y., Tsujikawa M., Hayashizaki Y. High-resolution promoter map of human limbal epithelial cells cultured with keratinocyte growth factor and rho kinase inhibitor. Sci Rep. 2017;7:2845. doi: 10.1038/s41598-017-02824-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohnishi S., Ohnami S., Laub F., Aoki K., Suzuki K., Kanai Y. Downregulation and growth inhibitory effect of epithelial-type Krüppel-like transcription factor KLF4, but not KLF5, in bladder cancer. Biochem Biophys Res Commun. 2003;308:251–256. doi: 10.1016/s0006-291x(03)01356-1. [DOI] [PubMed] [Google Scholar]
- 11.Guan H., Xie L., Leithäuser F., Flossbach L., Möller P., Wirth T. KLF4 is a tumor suppressor in B-cell non-Hodgkin lymphoma and in classic Hodgkin lymphoma. Blood. 2010;116:1469–1478. doi: 10.1182/blood-2009-12-256446. [DOI] [PubMed] [Google Scholar]
- 12.Yu F., Li J., Chen H., Fu J., Ray S., Huang S. Kruppel-like factor 4 (KLF4) is required for maintenance of breast cancer stem cells and for cell migration and invasion. Oncogene. 2011;30:2161–2172. doi: 10.1038/onc.2010.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leng Z., Tao K., Xia Q., Tan J., Yue Z., Chen J. Krüppel-like factor 4 acts as an oncogene in colon cancer stem cell-enriched spheroid cells. PLoS One. 2013;8:56082. doi: 10.1371/journal.pone.0056082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swamynathan S., Kenchegowda D., Piatigorsky J., Swamynathan S. Regulation of corneal epithelial barrier function by Kruppel-like transcription factor. Invest Opthalmol Vis Sci. 2011;52:1762–1769. doi: 10.1167/iovs.10-6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delp E.E., Swamynathan S., Kao W.W., Swamynathan S.K. Spatiotemporally regulated ablation of Klf4 in adult mouse corneal epithelial cells results in altered epithelial cell identity and disrupted homeostasis. Investig Ophthalmol Vis Sci. 2015;56:3549–3558. doi: 10.1167/iovs.15-16463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandler H.L., Colitz C.M., Lu P., Saville W.J., Kusewitt D.F. The role of the slug transcription factor in cell migration during corneal re-epithelialization in the dog. Exp Eye Res. 2007;84:400–411. doi: 10.1016/j.exer.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Aomatsu K., Arao T., Abe K., Kodama A., Sugioka K., Matsumoto K. Slug is upregulated during wound healing and regulates cellular phenotypes in corneal epithelial cells. Investig Ophthalmol Vis Sci. 2012;53:751–756. doi: 10.1167/iovs.11-8222. [DOI] [PubMed] [Google Scholar]
- 18.Massagué J. TGFβ signalling in context. Nat Rev Mol Cell Biol. 2012;13:616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Massagué J. TGFbeta in cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyashita H., Yokoo S., Yoshida S., Kawakita T., Yamagami S., Tsubota K. Long-term maintenance of limbal epithelial progenitor cells using rho kinase inhibitor and keratinocyte growth factor, Stem Cells Transl. Med. 2013;2:758–765. doi: 10.5966/sctm.2012-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hara S., Tsujikawa M., Maruyama K., Nishida K. STAT3 signaling maintains homeostasis through a barrier function and cell survival in corneal endothelial cells. Exp Eye Res. 2019;179:132–141. doi: 10.1016/j.exer.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 22.West-Mays J.A., Dwivedi D.J. The keratocyte: corneal stromal cell with variable repair phenotypes. Int J Biochem Cell Biol. 2006;38:1625–1631. doi: 10.1016/j.biocel.2006.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torricelli A.A., Wilson S.E. Cellular and extracellular matrix modulation of corneal stromal opacity. Exp Eye Res. 2014;129:151–160. doi: 10.1016/j.exer.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swamynathan S.K., Katz J.P., Kaestner K.H., Ashery-Padan R., Crawford M.A., Piatigorsky J. Conditional deletion of the mouse Klf4 gene results in corneal epithelial fragility, stromal edema, and loss of conjunctival goblet cells. Mol Cell Biol. 2007;27:181–194. doi: 10.1128/MCB.00846-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oft M., Peli J., Rudaz C., Schwarz H., Beug H., Reichmann E. TGF-β1 and Ha-Ras collaborate in modulating the phenotypic plasticity and invasiveness of epithelial tumor cells. Genes Dev. 1996;10:2462–2477. doi: 10.1101/gad.10.19.2462. [DOI] [PubMed] [Google Scholar]
- 26.Gotzmann J., Huber H., Thallinger C., Wolschek M., Jansen B., Schulte-Hermann R. Hepatocytes convert to a fibroblastoid phenotype through the cooperation of TGF-beta1 and Ha-Ras: steps towards invasiveness. J Cell Sci. 2002;115:1189–1202. doi: 10.1242/jcs.115.6.1189. [DOI] [PubMed] [Google Scholar]
- 27.Rodón L., Gonzàlez-Juncà A., Inda Mdel M., Sala-Hojman A., Martínez-Sáez E., Seoane J. Active CREB1 promotes a malignant TGFβ2 autocrine loop in glioblastoma. Cancer Discov. 2014;4:1230–1241. doi: 10.1158/2159-8290.CD-14-0275. [DOI] [PubMed] [Google Scholar]
- 28.Kawakita T., Espana E.M., Higa K., Kato N., Li W., Tseng S.C. Activation of Smad-mediated TGF-β signaling triggers epithelial-mesenchymal transitions in murine cloned corneal progenitor cells. J Cell Physiol. 2013;228:225–234. doi: 10.1002/jcp.24126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benito M.J., Calder V., Corrales R.M., García-Vázquez C., Narayanan S., Herreras J.M. Effect of TGF-β on ocular surface epithelial cells. Exp Eye Res. 2013;107:88–100. doi: 10.1016/j.exer.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 30.Nishida K., Kinoshita S., Yokoi N., Kaneda M., Hashimoto K., Yamamoto S. Immunohistochemical localization of transforming growth factor-beta 1, -beta 2, and -beta 3 latency-associated peptide in human cornea. Investig Ophthalmol Vis Sci. 1994;35:3289–3294. [PubMed] [Google Scholar]
- 31.Nishida K., Sotozono C., Adachi W., Yamamoto S., Yokoi N., Kinoshita S. Transforming growth factor-beta 1, -beta 2 and -beta 3 mRNA expression in human cornea. Curr Eye Res. 1995;14:235–241. doi: 10.3109/02713689509033520. [DOI] [PubMed] [Google Scholar]
- 32.Er H., Uzmez E. Effects of transforming growth factor-beta 2, interleukin 6 and fibronectin on corneal epithelial wound healing. Eur J Ophthalmol. 1998;8:224–229. doi: 10.1177/112067219800800404. [DOI] [PubMed] [Google Scholar]
- 33.Tiwari A., Swamynathan S., Alexander N., Gnalian J., Tian S., Kinchington P.R. KLF4 regulates corneal epithelial cell cycle progression by suppressing canonical TGF-β signaling and upregulating CDK inhibitors P16 and P27. Investig Ophthalmol Vis Sci. 2019;60:731–740. doi: 10.1167/iovs.18-26423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun H., Peng Z., Tang H., Xie D., Jia Z., Zhong L. Loss of KLF4 and consequential downregulation of Smad7 exacerbate oncogenic TGF-β signaling in and promote progression of hepatocellular carcinoma. Oncogene. 2017;25:2957–2968. doi: 10.1038/onc.2016.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.