Abstract
Firstly, glucocorticoids such as prednisolone can attenuate the effect of anti–PD‐1 antibody nivolumab. Secondly, malignant melanoma cells survived latently and unnoticeably in places other than those of previous metastatic lesions. Thirdly, effector T cells activated by nivolumab sustained their memory to attack malignant melanoma cells for several months.
Keywords: glucocorticoids, interstitial pneumonitis, lung metastasis, mucosal malignant melanoma, nivolumab
1. INTRODUCTION
Although it is well known that glucocorticoids have the suppressive effects on the human immune system, its effect against anti–programmed cell death‐1 (anti–PD‐1) antibodies has not yet been clearly investigated. A 66‐year‐old woman with mucosal malignant melanoma that originated from the ethmoid sinus underwent carbon‐ion radiotherapy followed by nivolumab injection for lung metastasis. The primary tumor was thought to obtain complete remission. The lung metastasis disappeared after injecting nivolumab three times. Interstitial pneumonitis was reported 22 months after the first injection of nivolumab, and cessation of nivolumab and administration of prednisolone led to newly developed metastatic lung tumors. However, decreasing the dose of prednisolone brought about remission of the lung tumors. These results indicated glucocorticoids can attenuate the effect of anti–PD‐1 antibody, malignant melanoma cells survived latently and effector T cells activated by nivolumab sustained their memory to attack malignant melanoma cells for several months.
Mucosal malignant melanoma of the head and neck is a rare disease that shows poor prognosis because of the lack of evidence of effective radiation therapy and/or chemotherapy, especially against metastatic lesions in these patients.1, 2 In some cases, recurrent or advanced stage diseases showed very good response to anti–programmed cell death‐1 (anti–PD‐1) antibodies, such as nivolumab.3, 4 However, some severe adverse events were observed in patients undergoing anti–PD‐1 antibody therapy.5, 6 Interstitial pneumonitis (IP) is one of the most crucial adverse events reported, and glucocorticoids are used to treat it.7, 8, 9, 10, 11 Although glucocorticoids are efficient in treating IP, they are known to have some side effects, of which immunosuppression is the most well‐known.12 However, immunosuppressive effects against anti–PD‐1 antibody have not yet been clearly evaluated. Here, we report a case of mucosal malignant melanoma with lung metastasis and its remission by anti–PD‐1 antibodies with a unique and unusual history, suggesting the dose‐dependent effect of glucocorticoids on anti–PD‐1 antibody and long‐acting memory of effector T cells against malignant melanoma cells.
2. CASE REPORT
A 66‐year‐old woman was referred to a hospital near her residence for a chief complaint of left orbital swelling. There was a tumor originating from the left ethmoid sinus, and biopsy from the nasal cavity revealed a histopathological diagnosis of malignant melanoma. The patient was then referred to our hospital one month later, for radical therapy against the tumor. Computed tomography (CT) image revealed that the tumor was located in the left ethmoid sinus invading the left orbital cavity and skull base (Figure 1A), and lung metastasis was suspected (data not shown).
Figure 1.
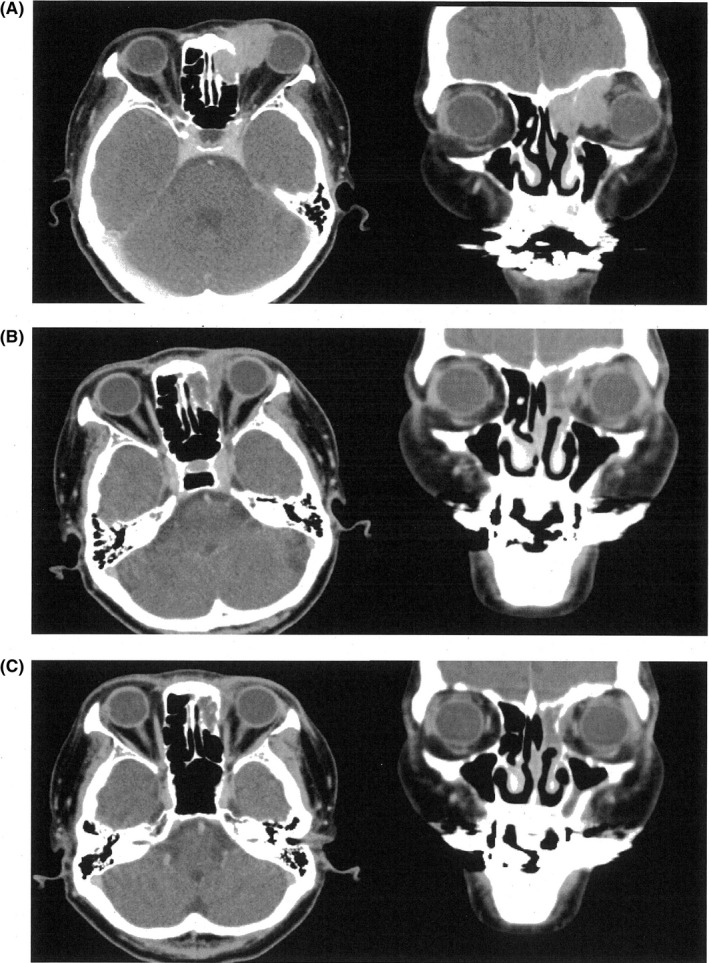
Contrast‐enhanced computed tomography images of the patient. Left panel: axial image at the orbital level; right panel: coronal image at the ethmoid sinus level. A, Images before carbon‐ion radiotherapy; B, images just after carbon‐ion radiotherapy; C, images after injecting nivolumab three times. Primary tumor in the left ethmoid sinus responded well to carbon‐ion radiotherapy, and complete response has been obtained so far
The patient underwent carbon‐ion radiotherapy (CIRT)13 in another hospital from April to May, 2016. The total dose of carbon‐ion beam was 57.6 Gy/16 fr, and 1 course of DAV (DTIC 120 mg/m2: day 1‐5, ACNU 70 mg/m2: day 1, VCR 0.7 mg/m2: day 1) was carried out during CIRT. Although the ethmoid sinus tumor showed good regression (Figure 1B), the metastases of the lung and mediastinum still progressed (Figure 2A, left panel).
Figure 2.
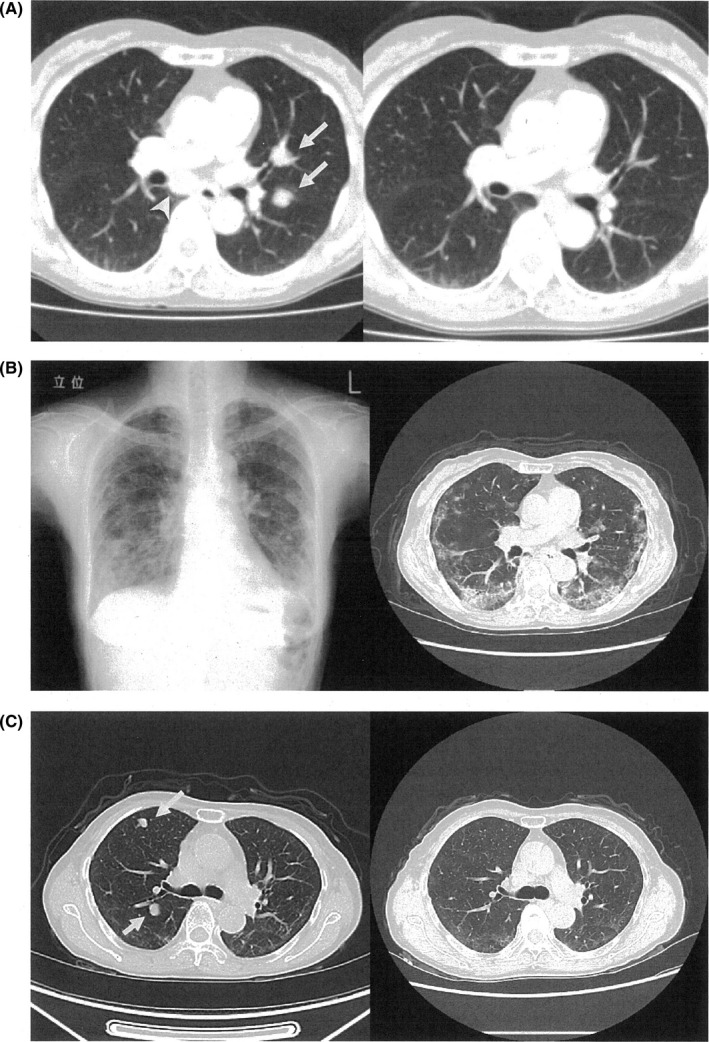
Chest images of the patient. A, Axial images of the contrast‐enhanced computed tomography (CT) at the main bronchus level. Left panel: image before nivolumab therapy; metastatic tumors visible in mediastinum [arrow‐head] and left lung [arrow]; Right panel: images after injecting nivolumab three times; metastatic tumors have disappeared. B, Left panel, chest X‐ray; Right panel, contrast‐enhanced CT image at main bronchus level after injecting nivolumab 24 times in April 2018; Reticulo‐nodular shadows visible in lower lung fields. C, axial images of the contrast‐enhanced CT at the subcarina level. Left panel: image in August 2018 shows reappearance of multiple lung metastases (arrow); Right panel: image in November represents disappearance of metastatic tumors
The patient was then referred to our hospital to treat the lung and mediastinal metastatic tumors. Nivolumab injection was administered in June 2016, and a 2 mg/kg injection (basically tri‐weekly) following the manufacturer's instruction was continued since then. The injection was administered thrice in August, and the ethmoid sinus tumor further regressed (Figure 1C) along with complete disappearance of the lung and mediastinal tumor (Figure 2A, right panel).
Occlusion of the left retinal artery due to CIRT occurred in March 2017. The patient was referred to the ophthalmologist, aspirin was prescribed, and there have been few complaints of mild epistaxis since then.
After nivolumab was injected 16 times, mild hyperthyroidism occurred and recovered in June 2017.
After injecting nivolumab 24 times in April 2018, IP occurred suddenly (Figure 2B) and nivolumab injection was stopped. Figure 3 shows the serum sialylated carbohydrate antigen (KL‐6) levels throughout the therapy. Prednisolone therapy was started with the first dose of 80 mg/d and then tapered down step‐by‐step (Figure 3).
Figure 3.
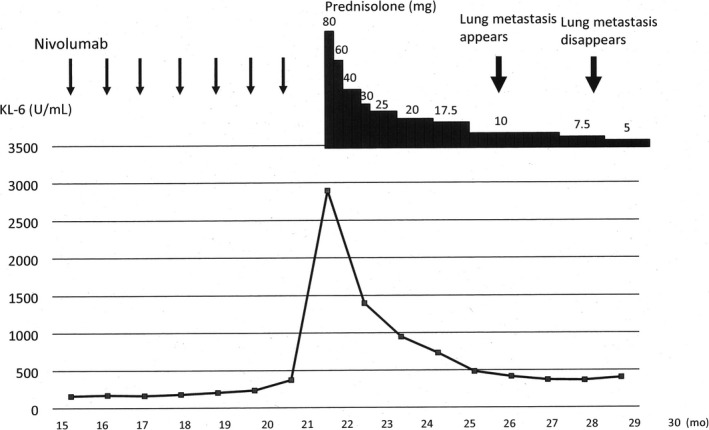
Clinical course of the patient. Horizontal axis represents the months after first injection of nivolumab. Vertical axis represents serum level of KL‐6. Amounts of prednisolone prescribed are indicated in the upper‐right area of the figure. Interstitial pneumonitis developed suddenly 22 months after first injection of nivolumab
Although the IP improved 8 weeks after the prednisolone therapy began, lung metastases reappeared in August 2018 when the prednisolone dose was 10 mg (Figure 2C, left panel and Figure 3). The locations of newly developed metastatic tumors were different from the previous metastatic tumors 2 years ago, with no recurrent tumors in the previous metastatic sites. However, these metastases disappeared again in November when the prednisolone dose was 7.5 mg/d (Figure 2C, right panel, Figure 3). Although laboratory data indicated that number of the white blood cells was within normal limits from the beginning of prednisolone administration, number of the neutrophils had been increased and number of the lymphocytes had been decreased until the dose of prednisolone was decreased. Serum levels of KL‐6 also decreased (Figure 3). The patient is currently on low dose prednisolone and is in good health without any tumor recurrence.
3. DISCUSSION
3.1. Nivolumab for mucosal melanoma
Although outcomes in patients with malignant melanomas improved with anti–PD‐1 antibodies, such as nivolumab and pembrolizumab, there was no randomized control study report of mucosal melanoma due to their rarity, which resulted in limited clinical information. A report of 35 patients with mucosal melanoma undergoing anti–PD‐1 antibody therapy described a response rate of 23% and a median progression‐free survival of 3.9 months.3 Another report described 86 patients with mucosal melanoma, who underwent nivolumab mono‐therapy and showed a response rate of 23.3% and median progression‐free survival of 3.0 months.4 On the contrary, our patient showed a progression‐free survival of nearly 3 years since undergoing nivolumab mono‐therapy.
3.2. Interstitial pneumonitis due to nivolumab
Incidence of IP in the patients receiving nivolumab was reported to be 3.24% for malignant melanoma5 and 7.2% for non–small cell lung cancer.6 In nine patients with IP, IP developed after 2‐8 courses of nivolumab administration, that is, 28‐176 days after the first administration of nivolumab.7, 8, 9, 10, 11 On the other hand, IP developed after 24 courses of nivolumab administration in 22 months (676 days) after the first administration of nivolumab in our patient. This result indicated that IP suddenly developed and was unpredictable even in the late courses. Additionally, physicians must diagnose IP and treat the patients quickly by watching the patients’ symptoms (cough, dyspnea, etc) and signs (findings of chest X‐ray serum KL‐6, etc) carefully, even if the patients appear to be in good shape during nivolumab therapy.
3.3. Amplification and attenuation of lung metastases by glucocorticoids
There was no report describing that the cessation of nivolumab and oral administration of glucocorticoids brought about the recurrence of lung metastases and decreasing the dose of glucocorticoids caused remission of the tumor again. Administration history indicated that more than 7.5 mg/d prednisolone suppressed the function of nivolumab, that is, the function of effector T cells. In fact, the number of the lymphocytes was decreased during a certain amount or more prednisolone was administrated to the patient. As physicians already know the suppressive effects of glucocorticoids on the human immune system,12, 14 clinical course of this patient was convincing. However, there were few reports that the effects of PD‐1 antibody, such as nivolumab, were suppressed by glucocorticoids. Physicians must know the suppressive effects of glucocorticoids against PD‐1 antibody function of tumor reduction. There was a question why the levels of KL‐6 could keep reduced and the IP could keep remissions after glucocorticoid tapering in spite of the antitumor immune reaction was reactivated. Although we provided no clear answers, points of actions of glucocorticoids might be different between the environment of inflammation and that of malignant tumors. Deeper understanding of the mechanisms underlying the immune‐editing process of malignant tumors can provide insights into the development of resistance to immunotherapies and the strategies to overcome such resistance.15
4. CONCLUSION
There were three crucial and noteworthy but speculative points revealed by the clinical history of this patient. Firstly, glucocorticoids such as prednisolone can attenuate the effect of anti–PD‐1 antibody nivolumab. Secondly, malignant melanoma cells survived latently and unnoticeably in places other than those of previous metastatic lesions. Thirdly, effector T cells activated by nivolumab sustained their memory to attack malignant melanoma cells for several months. Since our report is lacking in mechanical insights on how steroid can suppress the antitumor immune response, experimental data will be needed to clarify the effect of glucocorticoids against nivolumab in the patients with malignant melanoma.
CONFLICTS OF INTERESTS
The authors declare that they have no conflicts of interests.
AUTHOR CONTRIBUTIONS
KK and KS: involved in conception and design. KK, KS, DS, SO, AI, KT, and JM: involved in analysis and interpretation and data collection. KK and KS: involved in writing of the article. KS: took overall responsibility.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The patient provided full consent for participation and publication. Ethics approval for case reports was exempted by the Iwate Medical University Institutional Review Board.
ACKNOWLEDGEMENT
We thank “Editage” staffs for their help to edit our manuscript grammatically and thoroughly.
Katagiri K, Shiga K, Saito D, et al. Amplification and attenuation of lung metastases depending on glucocorticoid dosage implicating long‐acting activated memory cells induced by nivolumab against malignant melanoma. Clin Case Rep. 2019;7:1709–1713. 10.1002/ccr3.2349
REFERENCES
- 1. Shiga K, Ogawa T, Kobayashi T, et al. Malignant melanoma of the head and neck: a multi‐institutional retrospective analysis of cases in Northern Japan. Head Neck. 2012;34:1537‐1541. [DOI] [PubMed] [Google Scholar]
- 2. Lazarev S, Gupta V, Hu K, Harrison LB, Bakst R. Mucosal melanoma of the head and neck: a systematic review of the literature. Int J Radiat Oncol Biol Phys. 2014;90:1108‐1118. [DOI] [PubMed] [Google Scholar]
- 3. Shoushtari AN, Munhoz RR, Kuk D, et al. The efficacy of anti‐PD‐1 agents in acral and mucosal melanoma. Cancer. 2016;122:3354‐3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. D’Angelo SP, Larkin J, Sosman JA, et al. Efficacy and safety of nivolumab alone or in combination with ipilimumab in patients with mucosal melanoma: a pooled analysis. J Clin Oncol. 2017;35:226‐235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kiyohara Y, Uhara H, Ito Y, Matsuomoto N, Tsuchida T, Yamazaki N. Safety and efficacy of nivolumab in Japanese patients with malignant melanoma: an interim analysis of a postmarketing surveillance. J Dermatology. 2018;45:408‐415. [DOI] [PubMed] [Google Scholar]
- 6. Kato T, Masuda N, Nakanishi Y, et al. Nivolumab‐induced interstitial lung disease analysis of two phase‐II studies patients with recurrent or advanced non‐small‐cell lung cancer. Lung Cancer. 2017;104:111‐118. [DOI] [PubMed] [Google Scholar]
- 7. Nishino M, Sholl LM, Hatabu H, Ramaiya NH, Hodi FS. Anti‐PD‐1‐related pneumonitis during cancer immunotherapy. N Eng J Med. 2015;373:288‐290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nakashima K, Naito T, Omori S, et al. Organising pneumonia induced by nivolumab in a patient with metastatic melanoma. J Thorac Oncol. 2016;11:432‐433. [DOI] [PubMed] [Google Scholar]
- 9. Sano T, Uhara H, Mikoshiba Y, et al. Nivolumab‐induced organizing pneumonia in a melanoma patient. Jpn J Clin Oncol. 2016;46:270‐272. [DOI] [PubMed] [Google Scholar]
- 10. Nishino M, Chambers ES, Chong CR, et al. Anti‐PD‐1 inhibitor‐related pneumonitis in non‐small cell lung cancer. Cancer Immunol Res. 2016;4:289‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Watanabe S, Kimura H, Takato H, et al. Severe pneumonitis after nivolumab treatment in a patient with melanoma. Allergol Int. 2016;35:487‐489. [DOI] [PubMed] [Google Scholar]
- 12. Cohn LA. Glucocorticosteroids as immunosuppressive agents. Semin Vet Med Surg. 1997;12:150‐156. [DOI] [PubMed] [Google Scholar]
- 13. Koto M, Demizu Y, Saitoh J, et al. Multicenter study of carbon‐ion radiation therapy for mucosal melanoma of head and neck: Sub‐analysis of Japan carbon‐ion radiation oncology study group (J‐CROS) study (1402 HN). Int J Radiat Oncol Biol Phys. 2017;97:1054‐1060. [DOI] [PubMed] [Google Scholar]
- 14. Rogatsky I, Ivashkiv LB. Glucocorticoid modulation of cytokine signaling. Tissue Antigens. 2006;68:1‐12. [DOI] [PubMed] [Google Scholar]
- 15. O’Donnell JS, Teng M, Smyth MJ. Cancer immunoediting and resistance to T cell‐based immunotherapy. Nat Med Clin Oncol. 2019;16:151‐167. [DOI] [PubMed] [Google Scholar]


