Abstract
Neutrophil recruitment is vital for host defense, but also relevant in pathological inflammatory reactions such as sepsis. Model systems have been established to examine different steps of the leukocyte recruitment cascade in vivo and in vitro under inflammatory conditions. Recently, tissue-specific recruitment patterns have come into focus, requiring modification of formerly generalized assumptions. The aim of this review is to summarize existing models of neutrophil recruitment and to point out recent discoveries in organ-specific recruitment patterns. New techniques show that previously-stated assumptions of integrin activation and tissue invasion may need revision. Similarly, neutrophil recruitment to specific organs can rely on different organ properties, adhesion molecules and chemokines. To advance our understanding of neutrophil recruitment, organ-specific intravital microscopy methods are needed.
The Classical Neutrophil Recruitment Cascade: A Time for Revision
Inflammatory reactions involve the recruitment of a wide variety of cells to sites of action. This generates a local inflammatory response necessary to tackle pathogen invasion, or an overshooting inflammatory reaction resulting in unwanted organ damage, such as in septic patients [1]. In each case, neutrophils play a key role. The tightly regulated recruitment of these polymorphonuclear cells has been a matter of research for many years, ultimately resulting in a mammalian model of step-wise leukocyte recruitment, i.e. the leukocyte recruitment cascade [2] (Box 1). In this generalized model, neutrophils exit free flow within the circulation, interact with vessel walls, roll along the activated endothelium, and firmly adhere to the endothelium. This is followed by a slower motion of the cells, termed crawling, ultimately resulting in para- or transcellular transmigration and tissue-infiltration, with ensuing migration within the infiltrated tissue. The recruitment of neutrophils appears mainly in post-capillary venules within the microcirculation, while recruitment under pathologic inflammatory conditions within the arterial system, has also been observed in mammals, as in the case of atherosclerosis [3].
Box 1: The Leukocyte Activation Cascade.
The first steps of this cascade, namely capturing and rolling, are mediated by interaction of neutrophil receptors, prominently PSGL-1 and CD44, with selectins on activated endothelial cells [2]. While fast rolling relies mainly on endothelial P-selectin-dependent interactions, slower rolling has been shown to be E-selectin mediated [154]. Receptor-binding can initiate the activation of intracellular signaling pathways in neutrophils, ultimately resulting in the activation of β2- and α4-integrins [116]. Selectin engagement results in the extended conformation of β2-integrins, chemokines further activate neutrophils by G-protein coupled receptor (GPCR)-mediated signaling cascades, for example via CXCR2 or FPR1 signaling. This results in the open-headpiece conformation of the integrin and ultimately the β2-integrin mediated neutrophil arrest. A bent- open headpiece conformation of integrins was recently discovered, capable of inhibiting adhesion [6]. The mechanisms underlying this conformation are not fully understood, whereas kindlin-3, talin-1, Rap1 and Rap1-GTP-interacting adaptor molecule (RIAM) are involved in full integrin activation [22]. Adherent neutrophils crawl to sites of extravasation, a step mediated mainly by the integrin Mac-1 on neutrophils [155]. This is followed by extravasation of the neutrophil, a step requiring the chemokine guiding signals CXCL1 for luminal and subendothelial crawling and CXCL2 for breaching of the endothelial barrier [57].
Figure I within Box 1.
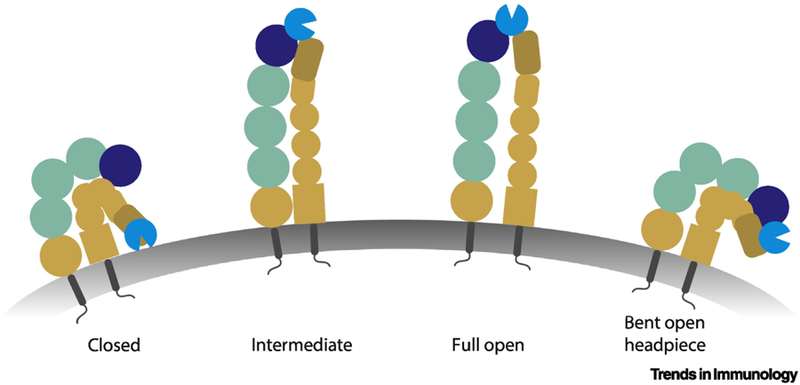
Integrin Conformations in Neutrophils
Integrins can exist in different conformations. These include up to current knowledge a closed, intermediate and high affinity conformation. The high affinity conformation includes an extension of the integrin with opening of the binding site on its headpiece. The intermediate conformation is an extended conformation but with a closed headpiece. Recently, also a closed-open-headpiece (= bent-open) conformation was reported.
The cascade model of neutrophil recruitment has recently been amended and challenged, resulting in a paradigm change [4–6]. Specifically, it appears that the previously generalized recruitment assumptions cannot be extrapolated to all activation steps within all organs, hereby challenging long-held beliefs. New organ-specific inflammatory models have revealed that leukocyte activation and recruitment steps within different organs (e.g. kidney and lung) might not equally involve either selectin or integrin-dependent recruitment steps; this might contrast assumptions based on the presumed requirement for both selectins and integrins in mediating recruitment, as postulated in the classical cascade model [5]. In addition, changes due to differential expression of endothelial surface molecules, chemokines, distinct vascular properties or flow conditions have been uncovered, resulting in organ-specific neutrophil dynamics [7]. Furthermore, species-specific differences have to be accounted for; for example, the ITIM domain-containing natural killer (NK) cell receptor Ly49Q was previously shown to be an important regulator of neutrophil activation, and pulmonary immune defense in the mouse, whereas the molecular analog remains to be discovered in humans [8]. Reverse neutrophil migration has also recently come into focus [4]. Among multiple integrin conformations -- some of which are crucial for functional neutrophil recruitment-- is the ‘bent-open headpiece’ conformation, that actually hinders the adhesion of human neutrophils in vitro, and mouse neutrophils, in vivo [6]. Equally important to advance our understanding of neutrophil recruitment is the cellular interplay that occurs during this process, and that may alter, or extend, our view of the pre-existing classical model. Most prominently, platelets participate in the inflammatory process as linkers between neutrophils and activated endothelial cells and thus, represent an additional mechanism for organ-specific neutrophil recruitment in mammals [9]. In mice, platelets are capable of guiding leukocytes to extravasation sites [10]; they regulate ICAM-1 expression on endothelial cells [11], and furthermore, platelet-derived P-selectin is crucial for neutrophil recruitment in the kidney [12]. Another important aspect to consider is that recruitment of neutrophils appears to be time-dependent according to circadian rhythms, thus impacting the immune defense capacity of the organism [13, 14]. Consequently, whole organism-wide phenomena of neutrophil recruitment and recirculation have to be taken into account in order to increase our understanding of inflammatory and autoimmune responses within individual organs. By the same token, biomechanical properties related to neutrophil recruitment have to be analyzed in more detail to fully understand mechanisms underlying the efficient invasion of these inflammatory cells into tissue. For example, recently, nucleus positioning within leukocytes was acknowledged for its relevance in modulating ‘poresize discrimination’ during leukocyte migration [15]. Appropriate model systems will help define basic mechanisms and dissimilarities in the recruitment of neutrophils. Reflecting on these aims, in this review, we highlight specific experimental models used in neutrophil research and link these to recent areas of interest relevant to organ-related neutrophil infiltration and neutrophil homeostasis. An ultimate aim is to improve our understanding of immune defense and inflammation in mammalian organisms.
Overview of Experimental Model Systems
Analysis of neutrophil recruitment requires usage of different experimental models to corroborate hypotheses and fully understand the results. On the one hand, in vitro approaches can be used to simulate and dissect several steps of the leukocyte recruitment cascade (Table 1). On the other hand, in vivo models resemble a complete biological environment, but are more complex and thus, likely to be perturbed by confounders or inter-experimental variations (Table 2).
Table 1:
Common In Vitro and Ex Vivo Models of Neutrophil Function
| Assay | Application and Benefit | How to | Advantage (+) / Disadvantage (−) | Recruitment Step | Platforms or Readouts |
|---|---|---|---|---|---|
| In vitro flow chamber assays | - Analysis of rolling and adhesion or crawling on specific receptor ligands or endothelial cells with flow dynamics | Small rectangular glass capillaries or commercial system coated with ligands or endothelial cells | (+) detailed biomechanical analysis possible (−) isolated neutrophils |
Rolling, adhesion, spreading and migration under flow | - 20×200μm [116] or 40×400μm glass capillaries [117] - Side view flow chamber [17] - Commercial chambers with HUVEC coating [118] - Spreading under flow in commercial chambers [119] |
| Autoperfused whole blood flow chamber | - Analysis of rolling and adhesion in whole blood | Canulate carotid artery of mouse and connect to coated glass capillary flow chamber | (+) whole blood including all plasmatic components (−) clotting (−) blood pressure variation |
- Rolling and adhesion | - Autoperfused glass flow chamber [44] |
| Transmigration / Migration assays | - Analysis of barrier crossing with usage of defined stimuli - Pattern tracking of neutrophil chemotactic motility |
- Transwell inserts - Either only chemokine gradient or - Seeded endothelial cell layer - Possibility of combination of endothelial / epithelial cells |
(+) seeding of endothelial cells possible to mimic tissue environment (−) high variation depending on confluence of endothelial cells and chemokine gradient |
- Transmigration and chemotaxis | - Filter transmigration [120] - 3D chemotaxis [32] - Transwell with murine immortalized brain endothelial (bend5) cells [121] |
| Flow cytometric assays | - Independent analysis of specific receptors - Expression vs. activation |
- Isolation of neutrophils, labeling for expression analysis or: - Activation, incubation with ligands, labeling with neutrophil markers, analysis of ligand binding |
(+) diverse applications (−) sample preparation needed and thus (−) no analysis of direct inorgan effects |
- Integrin activation and receptor expression | - Expression 121] - Activation [122] |
| Neutrophil effector functions | - Assessment of neutrophil functionality | - Activation of neutrophils required - ROS: Seeding of neutrophils on coated (e.g. polyRGD) plates, stimulation, measurement of superoxide dismutase inhibitable reduction of cytochrome C by absorption on plate reader - Phagocytosis: incubation with fluorescent bacterial particles |
(+) functional relevance (+) diverse stimuli possible |
-Effector functions: Outside-in signaling (ROS), phagocytosis, NET formation | - Outside-in ROS [123] - Phagocytosis [124] |
| Platelet-neutrophil interaction | - Cell-cell-interactions | - In vitro NET formation - Aggregate formation - Co-activation |
(−) sample clotting (−) no shear condition |
-Thrombus formation cascade - Activation -Extravasation / Migration / Bacterial bundling |
- Aggregate formation [125] - NET formation [126, 127] |
Abbreviations Table 1: ROS: Reactive oxygen species; NET: Neutrophil extracellular traps
Table 2:
Common In vivo Models of Neutrophil Function.
| Assay | Type, Application or Benefit | How to | Difficulties | Reference |
|---|---|---|---|---|
| Cremaster (IVM) | - Most common for studying recruitment cascade - Only transillumination needed, possibly fluorescence imaging and RLOT - Administration of inflammatory stimuli possible (trauma vs. TNFα vs. fMLP vs. LPS) → local inflammation - CLP for systemic inflammation |
- Inject stimulus (e.g. TNFα intrascrotally) - Wait 2h - Anesthetize mouse - Mobilize cremaster |
- Tissue damage and flow reduction by stretching of the muscle - Bleeding |
- [128] |
| Lung (IVM) | Intravital microscopy | - Anesthetize mouse, mechanical ventilation - Thoracic window preparation |
- Motion artefacts - Bleeding - Tissue damage due to thoracic window |
- [129] |
| Lung inflammation | - Different disease models ((A) ventilator induced VILI vs. (B) acid induced ALI vs. (C) pneumonia) | - (A) Mechanical ventilation (VILI) - (B) Application of acid (ALI) - (C) Application of bacteria (intratracheal injection) - (C) Bacterial burden (plating of tissue samples) - (A–C) Flow cytometry of whole organ lysates and bronchoalveolar lavage for inflammatory cell recruitment |
- Inhomogeneous injection of bacteria (left vs. right lung) | - (A) ALI. [130] - (B) Bacterial pneumonia:. [11] - (C) LPS induced lung injury: [69] |
| Heart (IVM) | Intravital microscopy | - Anesthetize animal - Intubation and mechanical ventilation - Preparation of thorax - Connect ECG - Positioning of suction device or imaging-holder - ECG-triggered imaging |
- Motion artefacts - Bleeding - Tissue damage |
- Heterotopic heart transplant: [43] - Tissue stabilization and acquisition gating: [131] |
| Heart inflammation | - LAD occlusion - Lipotoxic cardiomyopathy |
- Anesthesia - Thorax preparation - Ligation of the LAD - Re-opening of LAD if wanted |
- Inconsistent vessel ligation | - LAD occlusion: [93] - Myocarditis: [132] - Lipotoxic cardiomyopathy: [133] - Sepsis related cardiac dysfunction: [134] - Model guidelines: [135] |
| Kidney (IVM) | Intravital microscopy | - Anesthesia - Preparation of kidney - Intravital microscopy |
- Bleeding - Non-flat surface for imaging |
- [136] |
| Kidney inflammation | (A) Ischemia reperfusion injury vs. (B) Sepsis related acute kidney injury vs. (C) Chronic kidney failure |
(A) - Clamping of both kidneys (B) - Uterine ligation and bacterial inoculation or - CLP (C) - 5/6 nephrectomy |
(A) - Bleeding, organ damage (B) - Inconsistent cecal puncturing or bacterial burden (C) - Inconsistent nephrectomy size |
- (A) IRI: [44] - (B) Sepsis related: Uterine ligation and bacterial inoculation:. [137] - (B) Sepsis related: CLP: [138] - (C) CKD: [124] |
| Brain (IVM) | Intravital microscopy, cranial window | - Anesthesia - Disinfect and prepare skin - Thinning of skull bone with micro-drill - Multiphoton imaging |
- Tissue damage, compression artefacts - Bleeding |
- [139] |
|
Brain inflammation |
(A) CLP vs. (B) EAE vs. (C) Stroke |
(A) Prepare abdomen, ligate and puncture the cecum (B) Subcutaneous injection of Mog35–55 peptide in CFA with 1 mg/ml killed Mycobacterium tuberculosis H37Ra; double injection of i.v. pertussis toxin. (C) MCA electrocoagulation or small filament insertion |
(A) Inconsistent ligation and puncture (B) Scoring difficulties, incongruent resemblance of human MS disease (C) Inconsistent advancement of filament or tissue damage by electrocoagulation |
- (A) [140][91] - (B) [141] - (C) MCA electrocoagulation: [89]; Filament insertion: [142] |
| Liver (IVM) | Intravital microscopy | - Anesthesia - Opening of abdominal cavity - Mobilization of liver lobe - Tissue preparation and coverslip attachment - Time lapse microscopy |
- Immobilization - Drying of liver tissue - Duration of imaging |
- [143] |
| Liver inflammation | (A) Bacterial vs. (B) Sterile heat injury |
(A) CLP (compare above) or injection of bacteria (B) - Anesthesia - Opening of abdominal cavity - Mobilization of liver lobe - Heat wire injury - Time lapse microscopy |
(A) Compare above (B) Inconsistent injury size; excessive tissue damage |
-(A) MRSA infection: [76] -(B) [41] |
| Intestinal inflammation | Colitis (A) Bacterial (B) Dextran sodium sulfate (DSS)-induced |
(A) Antibiotic treatment, followed by gavage of bacteria (B) 1.5 to 3.5% DSS in drinking water for 5 to 9 days |
- Motility - Inconsistent inflammation |
- (A) [144] -(B) [145, 146] |
| Fetal (Yolk-sac) (IVM) | Yolk-sac vs. skin vs. skull | - Anesthesia - Prepare abdomen - Exteriorize and incise uterus - Mobilize fetus - Imaging |
- Drying - Tilted surface - Pressure onto vessels |
- [147] |
| Joints | Arthritis model | - Serum separation from K/BxN mice - Injection of serum (150–250 μl) intraperitoneally on days 0 and 2 - Clinical scoring |
- Inconsistent injections - Inconsistent blood collection and serum separation from donor mice |
- [148][149] |
| Skin (IVM) | Intravital microscopy |
- Anesthesia - Prepare imaging area, for example ear stage - Multiphoton microscopy |
- Movement artefacts - Pigmentation - Z-deviation |
- [150] |
| Skin inflammation | (A) Bacterial vs. (B) Allergic skin inflammation |
(A) Intradermal injection in small volume (0.5 – 4 μl) into pinnae of ear or back skin of mice (B) Shave skin, tape-strip 6 times, place patch with chicken egg ovalbumin on skin; sensitization for 7 weeks, challenge on days 0 and 3 after sensitization by application of ovalbumin to shaved, tape-stripped, nonharmed skin area |
- Inconsistent stimulation - Injection of amount of bacteria imprecise or displacement of ovalbumin patch |
-(A) [151] -(B) [152] |
| Atherosclerosis | ApoE knockout model | - ApoE−/− mice - High fat diet containing 21% fat (ssniff) |
- Dissimilar lipid metabolism to humans (VLDL vs. LDL) - Possible non-atherosclerosis related inflammatory effects |
- [153] |
| Venous thrombosis | Flow restriction model | - Anesthesia - Median laparotomy - Placement of space holder on inferior vena cava - Application of narrowing ligature below left renal vein - Removal of space holder - Flow measurements (reduction by approx. 80% desired) |
- Injury of the vessel wall and subsequent thrombus induction due to endothelial damage - Bleeding - Complete occlusion of the vessel |
- [126] |
| Systemic hypoxia | Normobaric hypoxia supply | - Expose animals to hypoxic conditions for set time periods (e.g. 4 hours) - Can be combined with other disease models (such as bacterial pneumonia) |
- Inconsistent oxygen supply - Changes in respiratory rate - Differences in gas-mixtures used for hypoxia induction |
- [50][48] |
Abbreviations Table 2: CFA: Complete Freund’s adjuvant; CLP: Cecal ligation puncture; DSS: Dextran sulfate sodium; EAE: Experimental autoimmune encephalomyelitis; LAD: Left anterior descending; MCA: Middle cerebral artery; MRSA: Methicillin resistant staphylococcus aureus; MS: Multiple sclerosis; RLOT: Reflected light oblique transillumination; VILI: Ventilator induced lung injury, ALI: acute lung injury.
In Vitro Flow Chamber Assays and Microscopy
In past decades, ex vivo and in vitro flow chamber systems have been established to examine rolling and arrest of leukocytes on either immobilized proteins or isolated endothelial cells. These approaches showed that examining adhesion under flow is important, since neutrophils require different signals to migrate under static and flow conditions [16]. Under flow, neutrophils form tethers and slings --membrane protrusions at the front (sling) or back (tether) - visible as ‘tears’ when reaching contact with the endothelium, after which the cells start to roll. Novel microscopy techniques using fluorescent membrane dyes and sideview flow chambers and isolated mouse neutrophils have shown that slings result from detached tethers [17]. These structures are known to stabilize the rolling process by offering anchoring points for substrate-cell interactions, as a high number of tethers in P-selectin-coated flow chambers perfused with isolated human neutrophils correlated with a slower rolling velocity than controls, and breaking tethers resulted in a ‘skipping behavior’ in cells [18, 19]. Slings provide an adhesive substrate, as shown in vitro and in vivo in mice, enabling rolling at high shear rates [20]. One specific technique is quantitative dynamic footprinting (qDF)-- a modification of total internal reflection fluorescence microscopy; experiments using this method led to the conclusion that ex vivo and in vitro neutrophil tethers, were anchored when PSGL-1 (the major neutrophil selectin-receptor) bound P-Selectin (the endothelial ligand) on the surface of coated flow chamber systems [21]. However, these findings were obtained using an artificial model system that could only mimic some of the processes relevant for tethering and rolling in vivo in mice [21]. Shear rates were constant (no pulsatility) and only one specific endothelial interaction partner was coated, lacking a multitude of interaction partners, activators, and also, distinct flow patterns visible within the mammalian organism. qDF was advanced into a multichannel fluorescence setup, to determine the activation states of neutrophil β2-integrins [6]. β2 integrins had been previously thought to exist only in ‘bent-closed’, ‘intermediate’ and ‘full open’ conformations (Box 1) [22]. As mentioned, two studies utilizing human in vitro flow chamber assays and mouse neutrophils in vivo revealed that neutrophil integrins can exist in a ‘bent open-headpiece’ conformation, hindering adhesion [6, 23]. The results were obtained by dual color qDF imaging and assessed by usage of conformation-specific antibodies. [23]. This conformation was needed for in cis ICAM (intercellular adhesion molecule)-binding, which resulted in anti-adhesive properties, effectively reducing integrin interaction with binding partners on other surfaces, aside from the same cell itself. This ICAM-mediated effect led to increased rolling velocity relative to controls, thus limiting adhesion of neutrophils in the mouse cremaster muscle model [6]. These findings contradict the classic ‘switchblade’ model of integrin activation, which relies on the aforementioned three major integrin conformation/activation states, thus leading us to reconsider the ‘deadbolt’ model of integrin conformation, where regulation of integrin affinity does not require integrin extension. This model was previously established by theoretical and molecular modeling, and more recently amended by preliminary data stemming from all-atom dynamic simulations, network modeling and experimental ligand binding measurements [24, 25]. Nonetheless, the debate about both models (switchblade vs. deadbolt) is still ongoing [26].
In Vitro Migration and Chemotaxis
Neutrophils ‘sense’ inflammation by following chemokine gradients in a process termed chemotaxis, resulting in the slow directed migration of these cells towards a chemokine source [27, 28]. This active movement on a substrate-adhesive surface follows rolling and adhesion of neutrophils and leads to relocalization of the neutrophil, intravascularly and (crossing the endothelial barrier) perivascularly [2]. To study this phenomenon, different approaches can be chosen, including non-flow and flow-conditions, artificial surfaces coated with interaction partners, or chamber preparations seeded with stimulated endothelial cells. As a readout, either the relocation of a neutrophil relative to its point of origin (distance), its directionality (straightness towards a chemokine source), or the number of transmigrated cells (for transmigration purposes), can be assessed. 2-dimensional and 3-dimensional assays have been historically applied in neutrophil research to study chemotaxis and migration. Neutrophil polarization and migration can significantly differ in 2D versus 3D environments. For example, relative to control short hairpin (sh)RNA-transfected cells, shRNA-mediated kindlin-3 knockdown in differentiated neutrophil-like PLB-985 cells led to chemotaxis-competent cells within a 3D environment, yet these cells are non-directional in a 2D environment [29]. Kindlin-3 is involved in integrin activation and loss of Kindlin-3 in cells results in impaired integrin activation and transendothelial migration in mice in vivo (Box 1) [30]. Thus, these findings together with migration experiments in integrin- depleted murine leukocytes, suggest that integrin-dependent and -independent processes can differentially manifest in 2D vs. 3D migratory environments [31]. Aside from commercially-available chambers utilizing transmigration- or collagen-based chemotaxis assays [32], a planar gradient system was recently developed to image neutrophil chemotaxis in 3D within a collagen matrix; using this system, the precise positioning and force generation of cells in the 3D z-direction (in addition to the x and y axes) could be assessed [33]. This was accomplished by preparation of a lightpenetrable collagen-medium, in which chemokine diffusion could be measured, accessible by confocal microscopy in combination with traction-force microscopy. By contrast, previous preparations utilizing 3D collagen matrices had mainly relied only on measurements of x-y directionality, velocity or percentage of migrating cells [34]. Adding neutrophil force measurements might thus lead to the identification of formerly underappreciated mechanisms of neutrophil invasion and motility.
Most in vitro assays serve to analyze an overall neutrophil functional state, such as an inhibited or overshooting activation (organ non-specific). By contrast, mimicking in-organ conditions requires the use of more complex experimental methods, such as neutrophil-pulmonary endothelial cell co-culture systems [35]. In-organ conditions might include the analysis of parameters such as shear rate, the characterization of distinct endothelial cells isolated from specific organs, or the assessment of chemokine gradients specific to chemokine concentrations physiologically measured in organs. Such approaches might help uncover more specific organ relevant neutrophil recruitment patterns. Accordingly, organoids can be powerful tools to assess neutrophil invasion -- for example in the analysis of pulmonary inflammation [36]. Indeed, the transition from a mechanistically-isolated in vitro environment towards a clinically relevant pathophysiological or physiological environment appears to be on its way, but must be further advanced and validated by in vivo methods.
In Vivo Bacterial Infection Models
To investigate the complex cellular interplay within a living organism, in vivo models are important for basic as well as translational research (Table 2). Here, we describe commonly used mouse models and their human correlates.
An inflammatory reaction can be triggered by bacteria, leading to either a systemic (e.g. cecal ligation and puncture, CLP) or localized (e.g. skin) neutrophil response. Each pathogen elicits a unique immune response. It is thus crucial to examine different pathogens to demonstrate pathogen-related neutrophil recruitment differences. Recent data show that methicillin resistant Staphylococcus aureus (MRSA) homes primarily to the murine liver and thus, can be used to observe neutrophil defense mechanisms in this organ [37]. Neutrophil bactericidal functions differ dependent on pathogen size, with larger pathogens, such as Candida albicans hyphae, resulting in the release of neutrophil extracellular traps (NETs), whereas smaller microbes such as C. albicans yeast (in this study), fail to induce NETs [38]. Intratracheal administration of Escherichia coli bacteria in mice was recently utilized to reveal significant neutrophil-platelet interactions in host defense in the lung; specifically, depletion of platelets resulted in impaired neutrophil infiltration and worsened animal survival relative to controls [11]. Cryptococcus neoformans administration exposed a unique defense mechanism in the murine brain, where neutrophils could transport bacteria away from the brain vasculature, as evidenced from intravital microscopy and analysis of colony forming units in brain homogenates [39]. A bacterial bundling and transport function of platelets was also observed in the liver of mice following E. coli or MRSA-infection by intravital (two-photon) microscopy (sepsis) and in vitro co-culture experiments with neutrophils; the findings suggested that platelets were capable of presenting bacteria to neutrophils, facilitating their anti-bacterial responses [40].
In Vivo Sterile Inflammation
Inflammation can also result from autoimmune responses or from the release of damageassociated molecular patterns (DAMPs) due to tissue injury. Models used to analyze such responses include the sterile heat injury (liver) or laser injury (skin, cremaster) mouse models [41, 42]. In the liver, neutrophil recruitment following sterile heat injury relies on a chain of chemotactic gradients, involving a chemokine-dependent intravascular phase and an FPR1-Mac1-dependent extravascular migration phase in the necrotic zone of the damaged liver [41, 42]. Another model of sterile inflammation is that of ischemia-reperfusion-injury, established for studies of liver, kidney and heart [43–46]. This model links local hypoxia and inflammation [47]. Additional models focus on hypoxic conditions resulting from reduced systemic oxygen supply [48]. Hypoxia itself can trigger inflammation, which in turn, can result in hypoxia due to consumption of oxygen, in this case, by neutrophils [49]. On the one hand, acute hypoxia, induced by acute exposure to 10% FiO2 can impair immune defense against S. aureus and Staphylococcus pneumoniae infections and survival in mice compared to normoxic mice; by contrast, chronic hypoxia in preconditioned animals exposed to 10% O2 over 7 days prior to infection, resulted in protective effects under similar (S. aureus and S. pneumoniae infections) pathologic conditions [50]. Of relevance, remote ischemic preconditioning (RIPC) experiments have demonstrated an interdependence between local hypoxic insults, such as brief forearm or leg ischemia in humans, and systemic anti-inflammatory priming of leukocytes. Specifically, in a study of children with cardiopulmonary bypass, RIPC prevented CD11b upregulation and changed antiinflammatory (e.g., increased blood urinary proteinase inhibitor concentrations with RIPC relative to controls) and proinflammatory mediator concentrations (increased complement C3 6h post RIPC (tetralogy of Fallot cardiac repair study) relative to controls [51–54]. In addition, increased generation of endothelial nitric oxide (NO) in RIPC-treated mice and humans relative to non-RIPC treated controls has been noted [51–54]. This is relevant as NO has been shown to dampen neutrophil rolling, adhesion and migration (and hence inflammation) in mice, as well as in isolated human neutrophils [55]. In summary, sterile inflammation leads to neutrophil activation, which can be induced by tissue damage and release of DAMPs. This process can be examined using different models, including heat or ischemiareperfusion injury, with the latter linking hypoxia to inflammation.
Intravital Microscopy
Independent of the primary stimulus, the inflammatory response can be imaged by intravital microscopy. Detailed examination of each step of the recruitment cascade can reveal organ-specific differences in neutrophil mechanics. A selection of in vivo models is summarized in Table 2. Even though these models are well advanced, important challenges remain, such as reproducibility, prevention of unwanted tissue damage, analysis of physiologic processes and reduction of animal numbers. Regarding tissue damage, recent work emphasized the limitations of murine brain models requiring the removal of the skull for brain imaging. In one mouse model of stroke, using a cell-site specific labeling technique in combination with advanced confocal imaging, vascular channels of neutrophil transfer between skull and brain were reported [56]. These channels connected the skull marrow with the dura and led to increased probability of neutrophil influx from adjacent regions (skull) relative to neutrophils from distant regions (tibia) [56]. Thus, models involving the removal of the skull and, thereby, breaking of the vascular channels for neutrophil transfer, possibly alter the appropriate recruitment environment; consequently, this type of approach might lead to false experimental results and conclusions in intravital microscopy studies examining inflammatory responses in animal brains (Box 2). This example is aligned with the current reassessment of intravital imaging approaches [56]. Modern intravital microscopy uses deep-penetration and label-free imaging approaches such as multiphoton microscopy [172]. However, currently, the invasiveness of preparation procedures can still hinder examination of physiologic phenomena and inaccessible sites, and this needs to be taken into account.
Box 2: Neutrophil Homeostasis vs. Inflammation:
Each preparation (except some skin models) involves the induction of surgical trauma, thus resulting in the activation and stimulation of endothelial cells and subsequent inflammatory cell recruitment (compare trauma cremaster muscle model). Recent questions concerning neutrophil homeostasis and recruitment in niches (and beyond) pose difficult obstacles to overcome. Physiologic observations are generally hard to obtain by invasive microscopy techniques, and histological analyses or in vitro assays only provide limited insight. Chronic imaging modalities, such as the dorsal skinfold chamber or the cranial window are both equally associated with surgical preparation or manipulation, possibly leading to stimulatory artifacts in data. Thus, future endeavors have to focus on obtaining data from a non-traumatic environment as well, by using for example single cell MRI or comparable methods.
Recent Advances in Tissue-Specific Neutrophil Recruitment
Intravital microscopy models led to progress in the understanding of neutrophil recruitment to different organs [5]. Here, we focus on specific advances using in vivo and in vitro mammalian models of tissue-specific neutrophil recruitment, providing a short overview, but also aiming to spark discussion in the field (Figure 1).
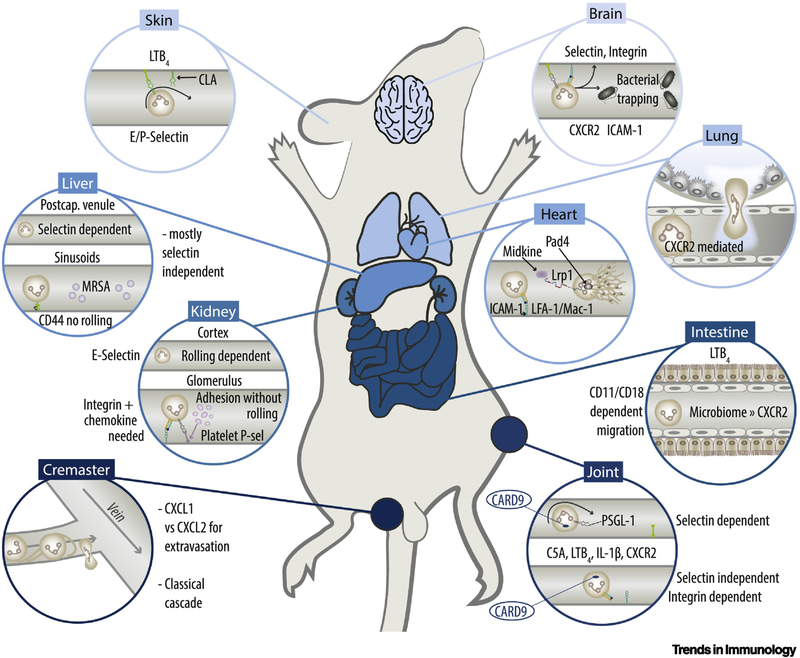
Figure 1. Tissue-Specific Mammalian Neutrophil Recruitment.
Recruitment in the skin is mediated by CLA, E- and P-Selectin while both integrin-dependent and –independent recruitment can occur (compare Box 2). In the brain microvasculature both selectins (green), PSGL-1 (grey), integrins (dark-green/orange), CXCR2 and ICAM-1 (turquoise) are involved. In the liver, recruitment appears mostly selectin independent through CD44/hyaluronan-dependent-interaction, whereas in postcapillary venules also select independent recruitment can occur. In the kidney, E-selectin has been implicated in neutrophil recruitment in kidney injury models. Within glomerular vessels, immediate arrest occurs without rolling and is mediated by platelet derived P-selectin. The cremaster model was used to demonstrate the classical recruitment cascade implying tether and sling formation. In joints CARD9 was implicated in the recruitment of neutrophils. Both selectin-dependent and – independent recruitment can be observed. The intestine relies on a LTB4-driven, CD11/CD18 dependent extravasation. Microbiome mediated neutrophil recruitment relies on CXCR2. The heart features integrin (LFA-1/Mac-1)-ICAM-1 dependent recruitment. NET release relies on Midkine, LRP1 and PAD4. Recruitment in the lung appears in small capillaries. Both selectin dependent and –independent recruitment can occur. Recruitment depends on CXCR2 and TREM1/3. CXCR2: CXC-motive chemokine receptor; ICAM-1: Intercellular adhesion molecule 1; CD44: Cluster of differentiation 44; MRSA: Methicillin resistant staphylococcus aureus; CXCL: CX-C motif ligand; PAD4: Protein arginine deiminase 4; LFA-1: Lymphocyte function-associated antigen 1; Mac-1: Macrophage-1 antigen; LRP1: Low Density Lipoprotein Receptor-related protein 1; CARD9: Caspase recruitment domain-containing protein 9; C5a: Complement component 5a; LTB4: leukotriene B4; IL-1: Interleukin-1;PSGL-1: P-selectin glycoprotein ligand-1.
Symbols:  Bacteria;
Bacteria;  Neutrophil;
Neutrophil;  Platelets;
Platelets;  Megakaryocyte;
Megakaryocyte;  MRSA;
MRSA;  Lung epithelial cell;
Lung epithelial cell;  Endothelial cell;
Endothelial cell;  NET;
NET;  Selectin;
Selectin;  PSGL-1;
PSGL-1;  CD44;
CD44;  Hyaluronan;
Hyaluronan;  Integrin;
Integrin;  ICAM.
ICAM.
Cremaster
Neutrophil recruitment in mouse cremaster venules follows the classical adhesion cascade [2], recently refined by the discovery that endothelial cell- and pericyte-derived CXCL1 could mediate neutrophil crawling, as evidenced from in vivo analysis of systemic or locally-injected blocking-antibody treated mice [57]. Using atypical chemokine receptor 1 Ackr1−/− mice and binding experiments with recombinant murine CXCL2, this report also showed that neutrophil derived CXCL2 could be bound by ACKR1. This in turn led to increased expression and retention of CXCL2 at endothelial cell junctions. Furthermore, utilizing Cxcl2−/− bone marrow chimeric mice, the authors demonstrated that such CXCL2-ACKR1-mediated events promoted paracellular neutrophil transendothelial migration [57]. Using transwell CXCL1 and/or CXCL2 chemotaxis assays, exposure of isolated neutrophils to a first soluble chemokine gradient in the upper chamber led to reduced chemotaxis towards a second soluble gradient in the lower chamber, contrasting to exposure to immobilized chemokines in the upper chamber. Moreover, using a 4D in vivo migration model in the mouse cremaster muscle, these researchers previously showed that pericytes also contributed to subendothelial neutrophil crawling [58]. Pericyte stimulation led to pericyte shape changes resulting in enhanced pericyte gap formation, as well as induced expression of integrin ligand ICAM-1 on pericytes. This in turn, supported the active motion of neutrophils on pericytes, as evidenced from local injection of blocking antibodies. However, it is not known whether this mechanism is also relevant in other organs [58].
Lung
Neutrophil recruitment within the lung occurs in small capillaries rather than in venules [7]. In the lung, it is clearly chemokine-dependent, as inhibition of CXCR2 has resulted in attenuation of acute lung injury in mice [59]. By contrast, the selectin and integrin dependence of neutrophil recruitment appears to be model-specific. For instance, in a model of acute lung injury in sheep, provoked by third-degree burns and cotton smoke inhalation, blockade of P-selectin did not improve injury; by contrast, cobra venom factor-mediated lung injury appeared to be P-selectin dependent [60–62]. Transepithelial migration of neutrophils in the mouse lung has been reported to rely on neutrophil-triggering receptors expressed on myeloid cells (TREM)-1/3. This was visualized using in vitro transmigration assays of TREM-1/3 deficient neutrophils across primary airway epithelia [63]. In addition, TREM-1/3 deficiency resulted in decreased in vivo infiltration of neutrophils into the airway of Pseudomonas aeruginosa infected mice relative to wildtype (WT) mice (pneumonia model). By contrast, transendothelial migration, (also evidenced by in vitro transmigration across primary endothelial cell monolayers), was not affected by loss of TREM-1/3 [63]. Like many organs, the lung contains two very distinct barriers -- endothelial and epithelial – that neutrophils need to breach before reaching the alveolar space. Using bone marrow transplantation experiments from and into Cxcr2−/− mice vs control WT mice, a study showed that non-hematopoietic CXCR2 significantly contributed to neutrophil airway infiltration following LPS-inhalation [64]. Irradiated WT mice reconstituted with Cxcr2−/− bone marrow presented approximately 50% reduction in neutrophil recruitment into the bronchoalveolar lavage (BAL) fluid. Cxcr2−/− mice with Cxcr2−/− bone marrow showed no neutrophil recruitment, and Cxcr2−/− mice reconstituted with WT bone marrow still exhibited a marked reduction in neutrophil recruitment; these data supported the role of CXCR2 in transendothelial and/or transepithelial migration steps [64]. In a model of primary lung allograft dysfunction in mice, CXCL2 was upregulated in isolated donor-derived non-classical monocytes[65]. Using intravital two-photon microscopy, depletion of donor intravascular non-classical monocytes abolished neutrophil extravasation after lung transplantation, and neutralization of CXCL2 inhibited neutrophil influx into murine lung transplants by over 50% [65]. Thus, it appears that in the mouse lung, neutrophil, monocyte, endothelial and epithelial CXC-receptors are involved in the recruitment of neutrophils. Nonclassical recruitment and activation are also important: The neuronal guidance molecule netrin1 -- a member of the laminin-related secreted protein family -- has been implicated in the regulation of pulmonary inflammation. Indeed, Ntn1+/− mice have been shown to exhibit increased neutrophil infiltration after LPS-inhalation, whereas exogenous reconstitution of netrin-1 led to reduced neutrophil infiltration into alveolar spaces relative to controls [66].
Neutrophil recruitment into the lung also requires neutrophil interactions with platelets. Platelet-neutrophil interactions in pulmonary tissue allow shuttling of neutrophil-derived arachidonic acid to platelets, which results in thromboxane A2-triggered endothelial ICAM-1 expression; this in turn, is responsible for neutrophil recruitment into mouse lungs in an Escherichia coli pneumonia model [11]. Neutrophil-platelet-interplay appears to rely on specific enzymes, including inositol hexakisphosphate kinase 1 (IP6K1). In Ip6k1−/− mice, enhanced bacterial killing and reduced neutrophil accumulation was noted in E. coli or Staphylococcus aureus-mediated lung infection models relative to WT [67]. In vitro coincubation of neutrophils and platelets (both from WT and/or Ip6k1-null mice) revealed that the formation of platelet-neutrophil complexes relied on platelet and not neutrophil-expressed Ip6k1 [67]. Thus, regulation of neutrophil-platelet interactions can boost efficient host defense; nevertheless, other data indicate that neutrophil interactions with other cells (e.g. platelets) can also be detrimental for the host. For instance, in a mouse model of gut ischemia-reperfusion injury, a devastating pulmonary thrombotic disorder ensues, characterized by large thrombi that contain leukocyte clusters [68]. Accordingly, as assessed by confocal microscopy, polysaccharide positive stimulated and ‘spread’ platelets led to the formation of the neutrophil macroaggregates in vitro in this study [68]. These findings suggest that neutrophil recruitment in the mouse lung i) occurs in capillaries, is ii) model-specific, iii) pathogen specific and iv) can be amplified by platelet-neutrophil interactions.
In the mouse lung, a marginated pool of neutrophils can mediate a fast CD11b-dependent neutrophil response (crawling velocity prior to LPS-challenge: 20 µm/10 min vs. crawling velocity 20 minutes after LPS stimulation: 50 µm/10 min) [69]. Quantification of neutrophils by flow cytometry has revealed higher percentages of neutrophils-to-total leukocytes in lung compared to liver, with the majority of neutrophils being localized intravascularly. Moreover, intravital imaging confirmed a capillary pool of crawling, tethering, or adherent leukocytes under baseline conditions, with rapid crawling upon LPS exposure. Furthermore, injection of fluorescently-labeled E. coli into mice led to neutrophil-dependent capture and phagocytosis of bacteria [69]. The idea of an organ-specific immune defense reservoir poses a new challenge for the field, with difficulties in distinguishing circulating from resident neutrophil pools, and where the concept of neutrophil recirculation has to be re-considered (Figure 2). Accordingly, there is evidence that different neutrophil subpopulations may exist within the lung, as a higher amount of aged, CD11bhigh, L-selectinlow, CXCR4+ neutrophils was been detected in the murine lung compared to peripheral circulation [70]. Also, infection of mice with the helminth Nippostrongylus brasiliensis has been found to lead to increased transcription of Il13, Il33, Igf1, Retnla, and Chi313, and the generation of “N2 neutrophils” relative to controls, in contrast to models of LPS-activation, characterized by the activation of “N1 neutrophils” with upregulated Il6 and Il12b [71]. These findings suggest that organ-residing neutrophil pools and functionally-distinct subpopulations exist, and thus, these populations will need to be examined in the context of different disease phenotypes [72]. With such knowledge, attempts to modulate inflammation may become more precise. Ideally, treatments could aim to target outcome-deteriorating inflammatory neutrophil subpopulations, without affecting overall host defense. Of note, while neutrophils are mostly regarded as pro-inflammatory [61, 73], their anti-inflammatory modes of function have to be considered as well. Recent work using in vitro co-cultures of human or murine primary alveolar epithelial cells with isolated neutrophils showed that shuttling of the miRNA miR-223 to lung epithelial cells could pose a novel putative anti-inflammatory mechanism. Specifically, in a model of S. aureus infection of hemizygous miR-223−/y mice, acute lung injury was worsened relative to WT, with increased myeloperoxidase concentrations in bronchoalveolar lavage (BAL) fluid and deteriorated animal survival [74]. Overall, the lung harbors intrinsic anatomic (capillary recruitment) and cellular (platelet–neutrophil complex formation) characteristics, and demonstrates the impact of specific neutrophil subsets. This implies that in addition to neutrophil localization and age, functional differences leading to differential pro- versus anti-inflammatory effects need to be considered when embarking on translational research endeavors. Indeed, it appears that hypotheses that rely on one general phenotype or activation state, among the multitude of neutrophil subsets, might potentially miss out on the significance and impact of neutrophil heterogeneity.
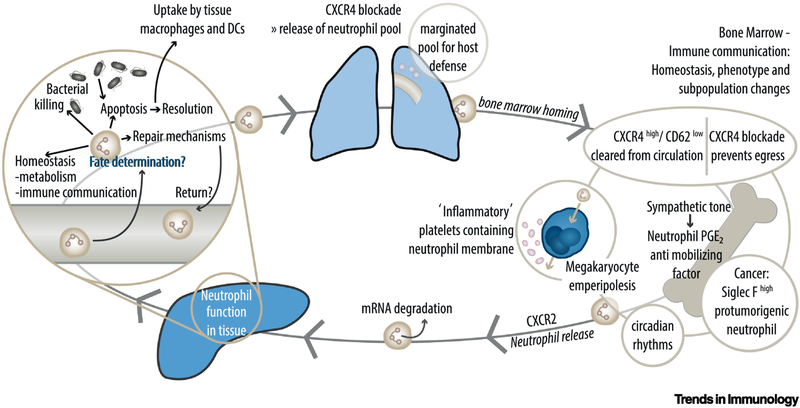
Figure 2. The Mammalian Neutrophil Feedback Loop.
Following exertion of their functions in the liver, neutrophils pass through the lung to return to the bone marrow. The invasion and extravasation of neutrophils into and from the bone marrow relies on CXCR4 and CXCR2 dependent signaling cues. CXC-motif chemokine receptor (CXCR) 4 high neutrophils get cleared from the circulation in the bone marrow. At the same time, CXCR4 is responsible for the maintenance of the marginated pool in the lung and the regress of neutrophils into the bone marrow. CXCR2 leads to mobilization of neutrophils from the bone marrow niche. In a cancer environment, protumorigenic neutrophils are released from the bone marrow and sent to the lung. In an inflammatory setting, neutrophils modulate the hematopoietic stem and progenitor cell environment in the bone marrow by releasing of prostaglandin E2. Neutrophils returning into the bone marrow can be emperipolized by megakaryocytes. Emperipolesis describes a process in which neutrophils are “ingested” by megakaryocytes without phagocytosing the cell. This step leads to the transfer of membrane fragments from neutrophils to newly produced platelets, possibly resulting in a population of ‘inflammatory’ platelets. Neutrophils can then return into circulation and exert their functions again.  Bacteria;
Bacteria;  Neutrophil;
Neutrophil;  Platelets;
Platelets;  Megakaryocyte
Megakaryocyte
Liver
In the liver, postcapillary venules feature selectin-dependent recruitment similar to that of the classical recruitment cascade, while sinusoidal neutrophil recruitment -- the most common recruitment mechanism in the liver [75] -- appears to be independent of selectins and rolling. In mice, eighty percent of leukocytes adhere within sinusoids in response to fMLP; this response is not reduced in P-selectin-deficient mice, P-/E-selectin-deficient mice or in mice receiving a blocking L-selectin antibody [7, 75]. Sterile inflammation in the mouse liver relies on a specific chemokine gradient, and Bruton’s tyrosine kinase (BTK) signaling has been implicated in fMLP mediated Mac-1 neutrophil activation [41]. Specifically, using the heat injury mouse model, WT and BTK-deficient neutrophils have been shown to exhibit differential migration potentials. This study was also supported by analysis of fMLP-mediated neutrophil arrest in cremaster venules, as well as flow cytometric binding assays of the Mac-1 ligand fibrinogen [41]. In mouse models of systemic bacterial infection -- such as via intravenously injected MRSA -- CD44-dependent neutrophil recruitment within the liver has been noted, where NETs can promote liver damage [76]. In this, in NETosis-deficient [peptidylarginine deiminase 4 (Pad4)−/− mice] mice smaller focal necrotic areas and lower serum alanine transaminase concentrations have been reported relative to WT [76]. However, in a model of polymicrobial sepsis (CLP) NET-depletion via rhDNase led to increased lung and peritoneal cavity bacterial colony forming units (CFU) relative to WT, suggesting that NETosis can also act as a ‘double-edged sword’ in modulating inflammation[77]. NETs have also been shown to influence nonalcoholic steatohepatitis (NASH)-- a common and clinically relevant form of sterile inflammation. In this study, using a mouse model of streptozotocin- and high-fat diet-induced liver inflammation, treatment with DNase, or usage of Pad4−/− mice, altered this inflammation, as evidenced by a decreased NASH activity score, IL-6 concentrations and liver-infiltrating macrophages relative to controls; moreover, this interference with NETs even reduced tumor burden in hepatocellular carcinoma progression of NASH relative to controls [78]. Thus, liver neutrophil recruitment – predominantly sinusoidal -- relies on distinct chemokine patterns in sterile inflammation. Systemic bacterial infections and advanced disease models such as NASH, thus show that NETosis and cell-cell interactions are significant in modulating liver tissue damage or bacterial clearance.
Central Nervous System
While almost no neutrophils are visible in the murine brain parenchyma under basal Non-inflammatory conditions [79], during inflammation – as in the case of the EAE mouse model of multiple sclerosis -- invasion of neutrophils into the central nervous system (CNS) has been noted [80]. This recruitment appears to be model-specific, differentially relying on the engagement of selectins and ICAM-1 and ICAM-2. For instance, using blocking experiments in a mouse models of IL-1β-induced cerebral inflammation, P-selectin (but not E-selectin) appeared to be prominently involved in mediating neutrophil recruitment [81]. However, earlier data had suggested that E- and P-selectin were not directly involved in neutrophil recruitment in EAE -with P-selectin most likely being a supporting component in α4-integrin-mediated recruitment [82, 83]. The reason for these discrepancies remains unknown. β2-integrin dependent neutrophil crawling on ICAM-1 and −2 in the inflamed brain has been suggested by examining isolated primary mouse brain endothelial cells stimulated with LPS; indeed, ICAM-1 can be upregulated on endothelial cells during EAE relative to healthy mice [84, 85]. In addition, in mouse EAE, mast cell-derived TNFα can modulate inflammation-mediated neutrophil activation and CNS infiltration, contributing to meningeal invasion and inflammation [81, 86]. Moreover, since Cxcr2−/− mice are protected from EAE, CXCR2 was implicated in the inflammatory process within the CNS [87]. Of note, in EAE models, neutrophil recruitment depending on ELR+ chemokine signals (including CXCL1 and CXCL2; in this study, the latter was prominently produced by astrocytes, whereas the major source of CXCL1 and CXCL2 was unclear) appears to be necessary for inflammation and tissue damage; this occurred in the mouse brain but not in the spinal cord [88]. The mechanism for this tissue tropism was deemed to be governed by differential regulation of IL-17 and IFN-γ in these tissues, leading to varying recruitment patterns within different CNS compartments [88]. In another study, using systemic blockade of Very Late Antigen-4 (VLA-4), VLA-4-dependent neutrophil invasion into murine brain was shown, impacting behavioral outcomes in the rotarod, tight rope, and corner turn tests with VLA-4 blockade also limiting infarct size in experimental murine stroke [89]. VLA-4 blockade also limited infarct size in experimental murine stroke [89]. Similarly, in a mouse model of 5xFAD Alzheimer’s disease (AD), multiphoton microscopy revealed that neutrophil accumulation contributed to cognitive dysfunction. Specifically, relative to controls, ICAM-1 was upregulated in the brains of these mice and Aβ peptide was found in brain lesions (neuropathological feature of AD progression); this led to the activation of the neutrophil ICAM-1 ligand LFA-1 into a full-open conformation; by using Itgal−/− mice, LFA-1 was shown to be required for neutrophil recruitment into the brain [89]. In addition, another study using 3xTg-AD mice, showed that neutrophil depletion using an anti-Gr-1 antibody led to improved memory function in a Y-maze spontaneous alternation task and a contextual fear-conditioning test relative to controls; it also reduced Aβ-peptide load in brain sections. These findings support a causal role of neutrophils in memory dysfunction in this model [90]. Nevertheless, the impact of neutrophils on neurological outcomes in systemic inflammation demands extensive and robust research. Indeed, cognitive impairment following S. pneumoniae-mediated sepsis appears to be monocyte- but not neutrophil-dependent [91]. Along these lines, using a lymphocytic choriomeningitis virus (LCMV) infection mouse model and two-photon in vivo microscopy, resident meningeal macrophages were shown to interact directly with infiltrating cytotoxic CD8+ T-lymphocytes. Resident macrophages were killed, cleared and replaced with infiltrating peripheral inflammatory monocytes for long-term engraftment, thus modifying the meningeal niche; the authors suggested that this altered niche might result in heightened susceptibility to secondary infections, highlighted by a dampened neutrophil recruitment to the meninges following intracranial LPS administration. These findings emphasized a crucial role for non-neutrophilic cells in the modulation of innate immunity in the brain [173]. Another study utilized in vivo imaging to show that neutrophils could remove Cryptococcus neoformans directly from the brain vasculature in mice [39]. C. neoformans led to increased expression of Mac-1 on isolated neutrophils of infected mice compared to non-infected controls, as well as ICAM-1 upregulation on brain endothelial cells incubated with C. neoformans in vitro. Thus, aside from direct local combat, neutrophils appear to relocate the ‘battle’ to other sites, a phenomenon that might be beneficial for the protected organ, but which might also cause phenotypic neutrophil priming and inflammation in remote organs [39]. However, it is unclear whether this process relies on specific bacteria, anatomic location, or certain neutrophil subsets. Another finding likely to spark discussion, is a recent report on two different neutrophil subsets, distinguishable by ICAM-1 expression (>90% ICAM1+ neutrophils in the extravascular vs ICAM1− neutrophils in the intravascular CNS compartment, respectively); using a model of B-cell dependent autoimmune encephalomyelitis, transcriptional analysis of ICAM1+ neutrophils uncovered a profile of immunomodulatory and antigen-presenting capacity, posing possible distinct functional roles for these cells, emphasizing the importance of robustly distinguishing neutrophil subpopulations [92]. Indeed, the modulation of neutrophil subsets constitutes an area of active research (discussed in a recent review [72]). With regard to the general role of neutrophils in the brain, even though neutrophil invasion in EAE models can be frequently observed, the role of neutrophils in human MS patients is unknown. For microbial pathologies, neutrophils appear to have a more prominent role, yet research is still needed to assess the responses to a wider variety of pathogens and traumata, especially taking into account the existence of neutrophil subpopulations.
Heart
Neutrophils in the heart appear to have a dual role. On the one hand, they are among the first cells to infiltrate the tissue after myocardial ischemia in mice, and are capable of destroying cardiac tissue; on the other hand, they are needed for reparative processes following myocardial infarction [93, 94]. In the acute phase, the infarcted myocardium produces GM-CSF, a regulator of neutrophil production, attracting neutrophils to the heart, and resulting in increased neutrophil invasion [95]. It is unclear whether these recruited neutrophils originate fresh from the bone marrow, the marginated pool, or from other unidentified niches. In a heterotopic murine heart transplantation model, blockade of LFA-1, as well as Mac-1, or mutation of ICAM-1 (B6.129S4-Icam1tm1Jcgr/J) abolished neutrophil adhesion and extravasation into the transplanted heart, highlighting the relevance of integrin functionality for neutrophil invasion in the heart [43]. There are numerous studies examining the overall immune response to ischemic heart injury (summarized in [96, 97]). One recent study proposed that prevention of NETosis might be cardioprotective; specifically, Pad4−/− mice, incapable of producing NETs, were protected from myocardial ischemia-reperfusion injury (IRI) relative to WT mice [98]. This observation was supported by findings showing that the cytokine midkine could regulate NET-formation via low-density lipoprotein receptor-related protein 1 (LRP-1) – as evidenced from assays using anti-N-midkine antibody blockade, recombinant midkine stimulation, DNase treatment to abrogate NETs, and models of LRP1cKO mice or differentiated Hoxb8-LRP1cKO cells relative to control cells; these findings were then extrapolated to a murine myocarditis model [99]. In another study of myocardial IRI in the mouse, injury-related increased plasma serotonin was noted compared to baseline[100]. Using tryptophan hydroxylase-1 (Tph1)−/− mice which lack blood and platelet serotonin, a decreased infarct size and reduced neutrophil influx was found relative to controls. Also, platelet-neutrophil complexes (measured by flow cytometry) were increased following myocardial damage, and platelet serotonin led to degranulation of neutrophils. These findings underscored a dichotomy, whereby neutrophils which are known to regulate post myocardial infarction healing in mice could act as either reparative/cardioprotective or cardio-damaging (leading to increased infarct sizes) [100]. Moreover, the formation of platelet-neutrophil complexes depended on hematopoietic vasodilator-stimulated phosphoprotein, as Vasp−/− mice showed reduced platelet-neutrophil-complex formation and presence in ischemic lesions, as well as decreased myocardial IRI relative to controls [46]. Recent work using an optical clearing method to image a total AV node by confocal microscopy, showed that macrophages --primarily known for their role in inflammation and resolution, were present in the AV nodes of Cx3cr1GFP/+ mice, relative to WT[101]. Patch-clamp techniques in co-cultures of macrophages with spontaneously-beating cardiomyocytes showed relative depolarization of the macrophages relative to the cardiomyocytes, along with increased cardiomyocyte resting membrane potential in a connexin-43 (Cx43)-dependent manner. Expression of a photoactivatable channel (channelrhodopsin 2) in macrophages was then used to demonstrate that macrophages could facilitate AV node conduction in a Langendorff-perfused heart model, thus enabling electrical conduction in the heart [101]. However, it is unclear whether neutrophils --which can influence macrophage functions and/or phenotypes [93], might also impact electric excitation in the heart. These findings are relevant in that they suggest that cardiac inflammation might be linked to arrhythmic pathologies.
From another angle, recent data showed that myocardial infarction healing in mice can be impacted by circadian oscillations in cardiac neutrophil recruitment, visible using Zeitgeber time points [14], and another study focused on the circadian regulation of migratory factors in the heart [13]. Specifically, neutrophil-specific Lyz2CreBmal1flox/flox bone marrow cells were shown to lack oscillatory migratory behavior to the spleen of WT recipient mice, at specific Zeitgeber time points [13]. However, it is unknown if ablation of neutrophil BMAL1-- a circadian clock-related transcription factor-- has beneficial or detrimental effects on long-term outcomes of myocardial infarction [13, 102]. Indeed, the, regulatory mechanisms of circadian-dependent integrin functionality remain to be uncovered. In the heart, neutrophils show both pro- and antiinflammatory or reparative effects, depending on disease model and time of damage. Numerous questions thus emerge, namely, of what might be the effects of neutrophils on electric conductance; neutrophil BMAL1 depletion on myocardial infarction and healing; as well as the localized abrogation of NET-formation.
Collectively, recent work shows that tissue-specific neutrophil recruitment features certain common steps and underlying activation mechanisms in different organs; however, recruitment also relies on tissue-specific stimulatory responses and/or anatomic properties, contributing to shaping a precise and controlled inflammatory response. This has also been noted for skin and joints (Box 3).
Box 3: Kidney Neutrophil Recruitment.
Recruitment of neutrophils in the kidney depends on the vascular bed. Recruitment within the kidney cortex has been shown to rely on neutrophil rolling in an ischemia-reperfusion-injury (IRI) mouse model (visualized by intravital microscopy (IVM) of kidney cortex venules) [44]. By contrast, recruitment within the glomerulus depends on adhesion without prior rolling, as evidenced by IVM in the mouse hydronephrotic kidney following anti-glomerular basement membrane antibody injection [12]. In a mouse model, platelet depletion led to reduced P-selectin expression and reduced leukocyte recruitment in anti-GBM treated hydronephrotic kidneys, and blocking P-selectin abolished leukocyte recruitment relative to controls; thus, platelet-neutrophil interplay is also relevant for neutrophil recruitment in the kidney by providing P-selectin for neutrophil-endothelial cell interactions [12, 156]. In mouse models of sepsis-induced acute kidney injury, blocking adhesion molecules such as P-selectin, E-selectin, and integrins CD11a or CD11b, resulted in protective effects by preventing acute kidney injury (diminishing serum creatinine increases, neutrophil infiltration, and improving kidney histological scores) relative to controls [157]. Moreover, following renal ischemia-reperfusion injury (IRI) in mice, signaling adaptors SH2 domain-containing leukocyte phosphoprotein of 76 kD (SLP-76) and adhesion and degranulation promoting adaptor protein (ADAP) deficient mice showed diminished acute kidney injury and neutrophil influx compared to WT controls [157]. Also, in the mouse cremaster model, Slp-76 and ADAP regulated E-selectin-dependent integrin activation following administration of blocking substances (anti-P-selectin antibody and pertussis toxin to prevent G-protein coupled receptor activation) in bone marrow chimeric mice; rolling velocity was similar to that of WT animals having received a blocking LFA-1 antibody, and furthermore, extravasation was impaired compared to WT [44]. Thus, neutrophil integrin activation appears to be vital in the pathogenesis of kidney IRI in mice [156]. Furthermore, Mac-1 and LFA-1 activation can be regulated by the Src kinase-associated phosphoprotein 2 (SKAP-2), as binding of fibrinogen and ICAM-1 has been shown to be reduced in Skap2−/− mice relative to controls, influencing neutrophil infiltration into kidneys, also demonstrated in a model of kidney IRI as this was concomitant with changes in acute tubular necrosis and serum creatinine [121]. Thus, while the relevance of rolling, adhesion and integrin activation for neutrophil extravasation is clear, the underlying molecular mechanisms in the context of kidney pathology remain to be further elucidated. The relevance and regulation of neutrophil dwelling and retention times in the kidney vasculature remain equally elusive and may represent a fruitful area of investigation [158].
Return to Sender: Neutrophil Clearance, Phenotype Changes and Cellular Communication
Neutrophil research is evolving and requires new approaches to identify differential recruitment patterns within the body. A multi-omic technique was recently introduced, combining RNA microarrays with total and phosphoprotein mass spectrometry; it is useful for detecting disease-specific expression patterns and differences in organ-infiltrating neutrophils. Using this technique, protein and mRNA expression differences between bone marrow-derived and colitis-infiltrating neutrophils were detected in an adoptive transfer mouse model of colitis; the data suggested that mRNA transcripts might be lost during neutrophil migration from the bone marrow to the site of inflammation [103]. Whether this observation holds true for other pathologies or organ-recruited neutrophils is still unclear. For instance, in the sterile hepatic heat injury model, murine neutrophils return to the circulation after exerting regenerative effects, impacting debris clearance, collagen reorganization and macroscopic lesion reparation in thermally-damaged areas of the liver, as evidenced from systemic neutrophil depletion [104]. They enter the lung, but ultimately home back to the bone marrow [104]. Moreover, CXCR4high neutrophils represent a population of aged neutrophils, prone to home to the bone marrow to be cleared, most likely by macrophages [105]. Yet, analysis of the precise localization and phenotypic characteristics of clearance niches within the bone marrow is still ongoing. In another study, P-selectin-mediated neutrophil sequestration was noted in the rat liver and lung following LPS injection, showing a large fraction of annexin V-positive apoptotic neutrophils in the liver; similarly, increased numbers of apoptotic neutrophils in the lung were noted following inactivation of Kupffer cells in the liver via gadolinium chloride, relative to controls [106]. Thus, multiple sites may be active for neutrophil clearance from the bloodstream, with shifts occurring during endotoxemia. It is reasonable to speculate that other organs might take up clearance functions in a hierarchical manner, if primary clearance sites fail. Along these lines, CXCR4 blockade by plerixafor in mice can lead to the release of a marginated neutrophil pool from the lung, preventing regress of neutrophils into the bone marrow [107]. Specifically, this study showed increased neutrophil numbers in the blood upon CXCR4 blockade relative to controls. In addition, in vivo imaging of the murine bone marrow (tibia and skull) did not reveal direct CXCR4-dependent mobilization of neutrophils in adoptive transfer and tracing experiments in mice. As mentioned earlier, the mobilizable marginated neutrophil pool resides within a vascular niche, leading to CD11b-dependent neutrophil responses to both endotoxin and E. coli bacteria [69]. By contrast, it was reported that CXCR2 signaling was important for the positive regulation of neutrophil counts, mobilized from the bone marrow via CXCL1 stimulation or G-CSF treatment of mice (as evidenced from flow cytometry and imaging) [107]. Thus, a complex feedback loop, regulating neutrophil homeostasis under baseline and inflammation appears to exist where different chemotactic stimuli can regulate the release and regress of neutrophils to sites of clearance.
Such neutrophil circulation feedback loops might be problematic under pathologic conditions in mice, such as cancer, where osteoblasts can remotely supply lung tumors with protumoral SiglecF-high neutrophils, concomitant with the upregulation of genes involved in angiogenesis (Vegfa, Hif1a, Sema4d), tumor proliferation (Tnf, Tgfb1, Il1a), extracellular matrix remodeling (Adamdec1, Adam17, cathepsins) and immunosuppression (Cd274/PDL1, Fcgr2b, Havcr2), along with increased reactive oxygen species activity relative to controls[108]. Co-injection of T-SiglecF high neutrophils together with tumor cells in mice could promote tumor growth compared to T-SiglecF low cells [108]. Interactions within the bone marrow can also involve other cell populations. For example, using the network analysis tool ProximID following single-cell dissection of interacting structures, mature neutrophils and megakaryocytes were shown to interact within the bone marrow in mice [109]. This interaction resulted in membrane transfer from neutrophils to platelets in mice, as evidenced from distinct lipophilic staining using confocal and electron microscopy, impacting platelet production and possibly the functionality of ‘inflammatory’ platelets [110, 174]. Live cell microscopy of proplatelet generation in vitro, as well as mouse adoptive transfer of megakaryocytes co-cultured with living marrow cells, resulted in the production of more (pro-)platelets, than in mice receiving transfers devoid of marrow cells [110, 111]. Hence, it is reasonable to speculate that this sort of interaction might influence the bone marrow niche environment, possibly further shaping the interplay of processes between inflammation and coagulation/thrombosis in these models. Neutrophils can be ‘communicative’ cells, given that they can form immunological synapses with T and B cells, as shown for instance, in the CNS of mouse EAE models [92]. Thus, in order to better understand neutrophil recruitment under different conditions, aside from focusing on the detection of neutrophil numbers (e.g. in a pathological setting), it is of utmost relevance to examine whether organ-specific neutrophils interact with other cells and whether these interactions can affect such recruitment [112]. Noteworthy, alternate non-classical recruitment and activation mechanisms may exist, as is the case of neuronal guidance cues, where netrin-1 influences neutrophil infiltration and organ damage in a model of dextran sulfate sodium (DSS)-induced colitis. In this model, Ntn-1+/− mice presented exacerbated DSS-colitis (increased weight loss, colonic shortening) compared to WT controls [113]. Another neuronal guidance cue implicated in inflammation is semaphorin 7a (GPI membrane anchor), which when coupled to its receptor plexin C1, was reported to regulate hypoxia-mediated peritoneal neutrophil recruitment and inflammation in mice. Specifically, blockade of Plxnc1, as well as siRNA-mediated silencing of SEMA7A in HMEC-1 cells, led to reduced transendothelial migration of isolated neutrophils in vitro compared with controls; moreover, Plxnc1−/− mice exhibited reduced peritoneal infiltration of neutrophils in a zymosan A-induced peritonitis model relative to WT. Also, Sema7a−/− mice showed reduced hypoxia-induced influx of neutrophils into the lung and heart relative to WT [114, 115]. In summary, each tissue appears to have its very own specific patterns of neutrophil recruitment. Moreover, given that neutrophils may be involved in multiorgan regulatory processes, the outcome of neutrophil recruitment and its impact on the whole organism must be taken into account to understand the impact of this type of cellular migration.
Concluding Remarks
Different intravital microscopy and endpoint models are needed to adequately examine and describe neutrophil recruitment patterns in mouse models. Recent discoveries of neutrophil migration patterns suggest that each tissue represents a specific environment in which neutrophils behave differently. Thus, in addition to classical cascade-like recruitment of neutrophils, platelet-neutrophil interactions and other mechanisms exist. The brain features distinct recruitment patterns which appear to be mostly integrin-dependent but are still far from being fully understood. Paradigm changes from neutrophils dying in tissues towards recirculation of aged neutrophils, novel discoveries concerning restorative and anti-inflammatory functions of neutrophils, and protumorigenic neutrophil subpopulations lead to a multitude of questions (see Outstanding Questions). With recent advances in intravital microscopy and computer modeling (in silico), we can strive to establish a clearer understanding of the tissue- and organ-specific rheological differences in neutrophil recruitment and their impact in host physiology. Together, biological, genetic, transcriptomic, proteomic, biomechanic and informatic research may help to further clarify neutrophil functions in vivo.
Box 4: Joint and Skin Inflammation.
In the skin, depending on the inflammatory stimulus, integrin-dependent and –independent neutrophil recruitment can occur. For instance, a kindlin-3 mutant unable to bind β2-integrins showed no effect on neutrophil trafficking in a mouse contact hypersensitivity model relative to WT [159]. Sterile inflammation in the murine skin seems to require LTB4 for signal amplification and neutrophil swarming, whereas integrin receptors in dendritic cells, and perhaps other leukocyte populations such as neutrophils (although hypothetical), might not be needed for long-distance migration, although they appear to be crucial for neutrophil-mediated formation of a collagen-free zone at wound-centers. This was demonstrated using two-photon microscopy of the skin with a laser-induced focal skin injury in Talin-1 deficient and β2-integrin deficient (Itgb2−/− and Tln1−/−) mice [31, 160]. For restoration of skin integrity, neutrophil extracellular traps have been reported to hinder wound healing in diabetic mice, in a model of excisional skin wounding, revealed by using Pad4−/− mice relative to controls [161]. By contrast, neutrophil apoptosis at tissue injury sites might spark a ‘pro-resolution program’ associated with the release of TGFβ and IL-10 [162]. These findings demand further examination, as the resolution potential of neutrophils remains to be elucidated.
In joint inflammation, selectin-dependent and -independent mechanisms appear to exist in parallel, depending on the experimental models. Integrins, namely VLA-4 and CD18, are responsible for up to 80% of neutrophil recruitment in selectin-independent models of rat adjuvant induced arthritis, with putative overlapping roles of LFA-1 and Mac-1 – as evidenced from antibody-mediated blockade of these adhesion molecules and analysis of neutrophil recruitment into inflamed joints [163,164]. Equal effects were also noted in a model of dermal inflammation in this study. Experiments in BLT1-deficient mice showed that LTB4 and BLT1 were important for neutrophil influx, contributing to autoantibody-induced arthritis [165,166]. Leukotriene B4 (LTB4) binds to its receptor BLT1 on neutrophils; in reconstitution experiments of Ltb4r1−/− (BLT1-deficient) mice, IL-1 was delivered into joints to initiate and amplify arthritis (visualized by the development of arthritis following adoptive transfer of WT or Il1a−/−Il1b−/− neutrophils into Ltb4r1−/− mice) [149,165]. However, it is difficult to link a specific receptor or signaling molecule to definite disease pathogeneses [167]. Nonetheless, studies indicate that neutrophil expression of Fc-receptors, C5a-receptors, LFA-1, and Syk are important in the pathogenesis of autoantibody-mediated arthritis [168, 169]. Recently, using C5ar−/− mice, complement C5a was recognized as a key initiator of neutrophil adhesion in murine autoimmune arthritis [170]. Relevant to joint damage, arthritogenic isolated NET peptides can also interact with rheumatoid arthritis fibroblast-like synoviocytes (RA-FLS) (as shown by confocal microscopy), are internalized in a RAGE-TLR9-dependent manner, and lead to autoimmunity and cartilage damage in mice [171]. Again, the therapeutic applicability of these findings remains unclear and requires further examination.
Box 5: The Clinician’s Corner.
Neutrophil recruitment is necessary to combat pathogens, but can also be detrimental when over-activation occurs, resulting in self-harm, as in sepsis or autoimmune diseases.
Neutrophil recruitment, activation and function depend on the invading pathogen and the site of entry.
Neutrophil recruitment can be analyzed with a variety of model systems, yet fully translatable approaches are lacking. Novel biomarkers and imaging techniques for assessment of organ-specific neutrophil invasion as well as therapeutics targeting a precise inflammatory site are required.
Healthcare providers have to bear in mind that detrimental neutrophil effects in one organ (e.g. integrin activation in autoimmunity) have crucial importance for host defense in others (e.g. CD11b mediated bacterial defense in the lung).
Outstanding Questions Box:
How is the bent-open headpiece conformation of neutrophil integrins regulated in detail? How does this affect tissue specific recruitment of neutrophils? Do chemokines have different effects?
Are chemokine cues of equal importance in each tissue, especially those involving CXCL1 and CXCL2 differential regulation?
Mechanistically, how are organ-specific recruitment patterns bypassed under septic conditions resulting in multi-organ failure? Is this similar for all pathogens? Do neutrophil subpopulations invade all organs equally?
Which neutrophil subpopulations exist in which organs? How are neutrophil subsets determined in terms of phenotypic markers and functions?
How do neutrophils contribute to the resolution of inflammation in each tissue?
What are some neutrophil regulatory mechanisms in organ-related diseases such as cardiopulmonary bypass-associated acute kidney injury?
What neutrophil niches exist under physiological and pathological conditions in the body?
Which biomarkers can be used to measure organ-specific neutrophil activation?
Which factors determine differential fates of neutrophils?
Should neutrophils be regarded as a ‘swarming collective’ (comparable to a bee stock in which the queen instructs her workers) or as single cells?
How can more detailed temporospatial information of neutrophil activation and deactivation in specific organs be obtained in both health and disease?
Highlights Box:
Integrin activation for neutrophil extravasation into inflamed tissue is crucial only in certain tissues, as integrin independent recruitment can occur.
Neutrophil integrins can exist in a bent-open headpiece conformation, which can hinder adhesion.
Chemotactic cues guide neutrophils for extravasation, including differential chemokine CXCL1 / CXCL2 gradients.
Activation of chemokine receptors CXCR2 and CXCR4 can impact neutrophil mobilization and clearance within the bone marrow, similarly to mobilization of neutrophils from the marginated pool in the mouse lung.
Neutrophils are ‘communicative’ cells, interacting with other cell populations to exert different functions that can depend on the tissue and/or stimulus.
Acknowledgements
We thank Nina Knubel and the CIM-Cluster Muenster for help in the creation of the figures. We thank Anika Cappenberg for critical reading of the manuscript. This study was supported by interdisciplinary centre for clinical research Muenster (IZKF) grant SEED12/18 to A.M., DFG grants KFO342/1, SCHA1238/7-1, ZA428/14-1, ZA428/12-1, and INST211/604-2, and interdisciplinary centre for clinical research Muenster (IZKF) grant Za2/001/18 to A.Z., and NIH P01 HL078784 to K.L.
Glossary:
- Cecal ligation and puncture:
Experimental animal model mimicking a bacterial infection in the abdominal cavity due to puncturing of the cecum and fecal contamination.
- Chemotaxis:
The influence on the motion-direction of neutrophils, triggered by chemotactic cues, and which results in the directed migration of these cells
- Experimental autoimmune encephalomyelitis (EAE):
Mouse model used to mimic pathophysiological changes associated with multiple sclerosis.
- Flow chamber systems:
Microfluidic devices used to examine cell interactions with specific substrates.
- Free flow:
Cells flow within blood vessels without direct or constant endothelial interactions. Rheological and cellular properties lead to centralization of erythrocytes, whereas leukocytes are positioned towards the outer regions of the vessel diameter, termed ‘margination’.
- GM-CSF:
Glycoprotein implicated in the proliferation of granulocytes and macrophages.
- GPCR:
Class of receptors, including CXCR2, crucial for neutrophil activation. Binding evolves in an intracellular signaling cascade leading to chemokine induced integrin activation.
- Immunological synapse:
Contact site between two immune cells.
- Intravital (two-photon) microscopy:
Utilized to image living tissue in animal models. It uses laser excitation with large wavelength photons resulting in reduced bleaching and improved penetration depth.
- LFA-1:
One of the major integrins on neutrophils. It is a surface receptor capable of binding to ICAM-1 and can exist in different conformations.
- Lipopolysaccharide (LPS):
Part of the outer membrane of Gram-negative bacteria; can be used for assays to mimic inflammatory responses against bacterial components.
- Mac-1:
Important integrin on the neutrophil surface. It binds preferably to fibrinogen yet is also capable of binding to ICAM proteins.
- Marginated pool:
Intravascular reservoir of neutrophils (e.g. in the lung).
- Midkine:
Nonglycosylated heparin-binding chemokine.
- Mouse cremaster venules:
Small blood vessels collecting blood from capillaries in the microcirculation in the mouse cremaster muscle.
- Nonalcoholic steatohepatitis (NASH):
Form of sterile, fatty liver disease.
- Neutrophil polarization:
Here, reorganization of neutrophil intracellular components into one specific direction.
- NETs:
DNA-containing networks released by neutrophils to entrap pathogens.
- Peritonitis:
Inflammation of the peritoneal cavity triggered by e.g. bacterial infection or zymosan injection.
- Planar gradient system:
3D-oriented chemotactic gradient offering the distribution of chemokine concentration along its axes.
- Quantitative dynamic footprinting:
qDF relies on TIRF microscopy. Using cytoplasmic coloring or coloring of the plasma membrane leads to having fluorescent elements of the neutrophil outer membrane in close proximity (approx. 200 nm) to the cover slip. The fluorescence intensity is then used to estimate and correlate the z-level intensity.
- Remote ischemic preconditioning:
Remotely placed ischemic stimulus during a short time period; it is used to prime the organism for subsequent harmful challenges in other regions of the body.
- Shear rate:
Rate at which a displacement of the bloodstream layers appears. This can be calculated from centerline blood flow velocities.
- Sinusoidal / liver sinusoids:
Low pressure fenestrated capillaries
- Total internal reflection fluorescence microscopy:
Exploits a physical phenomenon where light is reflected in light-transporting-media, creating an evanescent wave of exponentially decreasing intensity within the bordering interface (glass vs. cell); this leads to high resolution fluorescence images in proximity to the cover slip.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References:
- 1.Sonego F, et al. (2016) Paradoxical Roles of the Neutrophil in Sepsis: Protective and Deleterious. Front. Immunol 7, 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nourshargh S and Alon R (2014) Leukocyte migration into inflamed tissues. Immunity 41, 694–707 [DOI] [PubMed] [Google Scholar]
- 3.Kunkel EJ, et al. (1997) TNF-alpha induces selectin-mediated leukocyte rolling in mouse cremaster muscle arterioles. Am. J. Physiol 272, H1391–1400 [DOI] [PubMed] [Google Scholar]
- 4.Nourshargh S, et al. (2016) Reverse Migration of Neutrophils: Where, When, How, and Why? Trends Immunol 37, 273–286 [DOI] [PubMed] [Google Scholar]
- 5.Maas SL, et al. (2018) Organ-Specific Mechanisms of Transendothelial Neutrophil Migration in the Lung, Liver, Kidney, and Aorta. Front. Immunol 9, 2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan Z, et al. (2016) Neutrophil recruitment limited by high-affinity bent beta2 integrin binding ligand in cis. Nat Commun 7, 12658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rossaint J and Zarbock A (2013) Tissue-specific neutrophil recruitment into the lung, liver, and kidney. J. Innate Immun 5, 348–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Margraf A, et al. (2018) The ITIM Domain-Containing NK Receptor Ly49Q Impacts Pulmonary Infection by Mediating Neutrophil Functions. J. Immunol 200, 4085–4093 [DOI] [PubMed] [Google Scholar]
- 9.Rossaint J, et al. (2018) Role of Platelets in Leukocyte Recruitment and Resolution of Inflammation. Front. Immunol 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuchtriegel G, et al. (2016) Platelets Guide Leukocytes to Their Sites of Extravasation. PLoS Biol 14, e1002459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rossaint J, et al. (2016) Directed transport of neutrophil-derived extracellular vesicles enables platelet-mediated innate immune response. Nat Commun 7, 13464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuligowski MP, et al. (2006) Leukocyte recruitment to the inflamed glomerulus: a critical role for platelet-derived P-selectin in the absence of rolling. J. Immunol 176, 6991–6999 [DOI] [PubMed] [Google Scholar]
- 13.He W, et al. (2018) Circadian Expression of Migratory Factors Establishes Lineage-Specific Signatures that Guide the Homing of Leukocyte Subsets to Tissues. Immunity 49, 1175–1190 e1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schloss MJ, et al. (2016) The time-of-day of myocardial infarction onset affects healing through oscillations in cardiac neutrophil recruitment. EMBO Mol. Med 8, 937–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Renkawitz J, et al. (2019) Nuclear positioning facilitates amoeboid migration along the path of least resistance. Nature [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fine N, et al. (2016) GEF-H1 is necessary for neutrophil shear stress-induced migration during inflammation. J. Cell Biol 215, 107–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marki A, et al. (2016) Microfluidics-based side view flow chamber reveals tether-to-sling transition in rolling neutrophils. Sci. Rep 6, 28870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marki A, et al. (2018) Rolling neutrophils form tethers and slings under physiologic conditions in vivo. J. Leukoc. Biol 103, 67–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramachandran V, et al. (2004) Dynamic alterations of membrane tethers stabilize leukocyte rolling on P-selectin. Proc. Natl. Acad. Sci. U. S. A 101, 13519–13524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sundd P, et al. (2012) ‘Slings’ enable neutrophil rolling at high shear. Nature 488, 399–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sundd P, et al. (2010) Quantitative dynamic footprinting microscopy reveals mechanisms of neutrophil rolling. Nat. Methods 7, 821–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moser M, et al. (2009) The tail of integrins, talin, and kindlins. Science 324, 895–899 [DOI] [PubMed] [Google Scholar]
- 23.Fan Z, et al. (2019) High-Affinity Bent beta2-Integrin Molecules in Arresting Neutrophils Face Each Other through Binding to ICAMs In cis. Cell Rep 26, 119–130 e115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiong JP, et al. (2003) New insights into the structural basis of integrin activation. Blood 102, 1155–1159 [DOI] [PubMed] [Google Scholar]
- 25.Bidone TC, et al. (2017) New Insights into the Conformational Activation of Full-Length Integrin. bioRxiv 203661 [Google Scholar]
- 26.Zhu J, et al. (2007) Tests of the extension and deadbolt models of integrin activation. J. Biol. Chem 282, 11914–11920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petrie Aronin CE, et al. (2017) Migrating Myeloid Cells Sense Temporal Dynamics of Chemoattractant Concentrations. Immunity 47, 862–874 e863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nuzzi PA, et al. (2007) Analysis of neutrophil chemotaxis. Methods Mol. Biol 370, 23–36 [DOI] [PubMed] [Google Scholar]
- 29.Yamahashi Y, et al. (2015) Integrin associated proteins differentially regulate neutrophil polarity and directed migration in 2D and 3D. Biomed. Microdevices 17, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moser M, et al. (2009) Kindlin-3 is required for beta2 integrin-mediated leukocyte adhesion to endothelial cells. Nat. Med 15, 300–305 [DOI] [PubMed] [Google Scholar]
- 31.Lammermann T, et al. (2008) Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature 453, 51–55 [DOI] [PubMed] [Google Scholar]
- 32.Salvermoser M, et al. (2018) Myosin 1f is specifically required for neutrophil migration in 3D environments during acute inflammation. Blood 131, 1887–1898 [DOI] [PubMed] [Google Scholar]
- 33.Stout DA, et al. (2015) Planar Gradient Diffusion System to Investigate Chemotaxis in a 3D Collagen Matrix. J Vis Exp, e52948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bzymek R, et al. (2016) Real-time two- and three-dimensional imaging of monocyte motility and navigation on planar surfaces and in collagen matrices: roles of Rho. Sci. Rep 6, 25016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lomas-Neira J, et al. (2014) Neutrophil-endothelial interactions mediate angiopoietin-2associated pulmonary endothelial cell dysfunction in indirect acute lung injury in mice. Am. J. Respir. Cell Mol. Biol 50, 193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sachs N, et al. (2019) Long-term expanding human airway organoids for disease modeling. EMBO J 38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Surewaard BG, et al. (2016) Identification and treatment of the Staphylococcus aureus reservoir in vivo. J. Exp. Med 213, 1141–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Branzk N, et al. (2014) Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat. Immunol 15, 1017–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang M, et al. (2016) Real-time in vivo imaging reveals the ability of neutrophils to remove Cryptococcus neoformans directly from the brain vasculature. J. Leukoc. Biol 99, 467–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gaertner F, et al. (2017) Migrating Platelets Are Mechano-scavengers that Collect and Bundle Bacteria. Cell 171, 1368–1382 e1323 [DOI] [PubMed] [Google Scholar]
- 41.Volmering S, et al. (2016) The Neutrophil Btk Signalosome Regulates Integrin Activation during Sterile Inflammation. Immunity 44, 73–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kurz AR, et al. (2016) MST1-dependent vesicle trafficking regulates neutrophil transmigration through the vascular basement membrane. J. Clin. Invest 126, 4125–4139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li W, et al. (2012) Intravital 2-photon imaging of leukocyte trafficking in beating heart. J. Clin. Invest 122, 2499–2508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Block H, et al. (2012) Crucial role of SLP-76 and ADAP for neutrophil recruitment in mouse kidney ischemia-reperfusion injury. J. Exp. Med 209, 407–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khandoga A, et al. (2005) Junctional adhesion molecule-A deficiency increases hepatic ischemia-reperfusion injury despite reduction of neutrophil transendothelial migration. Blood 106, 725–733 [DOI] [PubMed] [Google Scholar]
- 46.Kohler D, et al. (2011) Phosphorylation of vasodilator-stimulated phosphoprotein prevents platelet-neutrophil complex formation and dampens myocardial ischemia-reperfusion injury. Circulation 123, 2579–2590 [DOI] [PubMed] [Google Scholar]
- 47.Bartels K, et al. (2013) Hypoxia and inflammation are two sides of the same coin. Proc. Natl. Acad. Sci. U. S. A 110, 18351–18352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morote-Garcia JC, et al. (2008) HIF-1-dependent repression of adenosine kinase attenuates hypoxia-induced vascular leak. Blood 111, 5571–5580 [DOI] [PubMed] [Google Scholar]
- 49.Taylor CT and Colgan SP (2017) Regulation of immunity and inflammation by hypoxia in immunological niches. Nat. Rev. Immunol 17, 774–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thompson AA, et al. (2017) Hypoxia determines survival outcomes of bacterial infection through HIF-1alpha dependent re-programming of leukocyte metabolism. Sci Immunol 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Konstantinov IE, et al. (2004) The remote ischemic preconditioning stimulus modifies inflammatory gene expression in humans. Physiol. Genomics 19, 143–150 [DOI] [PubMed] [Google Scholar]
- 52.Hepponstall M, et al. (2015) Remote ischemic preconditioning (RIPC) modifies the plasma proteome in children undergoing repair of tetralogy of fallot: a randomized controlled trial. PLoS One 10, e0122778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kharbanda RK, et al. (2001) Ischemic preconditioning prevents endothelial injury and systemic neutrophil activation during ischemia-reperfusion in humans in vivo. Circulation 103, 1624–1630 [DOI] [PubMed] [Google Scholar]
- 54.Rassaf T, et al. (2014) Circulating nitrite contributes to cardioprotection by remote ischemic preconditioning. Circ. Res 114, 1601–1610 [DOI] [PubMed] [Google Scholar]
- 55.Dal Secco D, et al. (2003) Neutrophil migration in inflammation: nitric oxide inhibits rolling, adhesion and induces apoptosis. Nitric Oxide 9, 153–164 [DOI] [PubMed] [Google Scholar]
- 56.Herisson F, et al. (2018) Direct vascular channels connect skull bone marrow and the brain surface enabling myeloid cell migration. Nat. Neurosci 21, 1209–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Girbl T, et al. (2018) Distinct Compartmentalization of the Chemokines CXCL1 and CXCL2 and the Atypical Receptor ACKR1 Determine Discrete Stages of Neutrophil Diapedesis. Immunity 49, 1062–1076 e1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Proebstl D, et al. (2012) Pericytes support neutrophil subendothelial cell crawling and breaching of venular walls in vivo. J. Exp. Med 209, 1219–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zarbock A, et al. (2008) Therapeutic inhibition of CXCR2 by Reparixin attenuates acute lung injury in mice. Br. J. Pharmacol 155, 357–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mulligan MS, et al. (1993) Protective effects of oligosaccharides in P-selectin-dependent lung injury. Nature 364, 149–151 [DOI] [PubMed] [Google Scholar]
- 61.Grommes J and Soehnlein O (2011) Contribution of neutrophils to acute lung injury. Mol. Med 17, 293–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chandra A, et al. (2003) P-selectin blockade fails to improve acute lung injury in sheep. Clin. Sci. (Lond.) 104, 313–321 [DOI] [PubMed] [Google Scholar]
- 63.Klesney-Tait J, et al. (2013) Transepithelial migration of neutrophils into the lung requires TREM-1. J. Clin. Invest 123, 138–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reutershan J, et al. (2006) Critical role of endothelial CXCR2 in LPS-induced neutrophil migration into the lung. J. Clin. Invest 116, 695–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zheng Z, et al. (2017) Donor pulmonary intravascular nonclassical monocytes recruit recipient neutrophils and mediate primary lung allograft dysfunction. Sci. Transl. Med 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mirakaj V, et al. (2010) Netrin-1 dampens pulmonary inflammation during acute lung injury. Am. J. Respir. Crit. Care Med 181, 815–824 [DOI] [PubMed] [Google Scholar]
- 67.Hou Q, et al. (2018) Inhibition of IP6K1 suppresses neutrophil-mediated pulmonary damage in bacterial pneumonia. Sci. Transl. Med 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yuan Y, et al. (2017) Neutrophil macroaggregates promote widespread pulmonary thrombosis after gut ischemia. Sci. Transl. Med 9 [DOI] [PubMed] [Google Scholar]
- 69.Yipp BG, et al. (2017) The Lung is a Host Defense Niche for Immediate Neutrophil-Mediated Vascular Protection. Sci Immunol 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim JH, et al. (2018) Aged polymorphonuclear leukocytes cause fibrotic interstitial lung disease in the absence of regulation by B cells. Nat. Immunol 19, 192–201 [DOI] [PubMed] [Google Scholar]
- 71.Chen F, et al. (2014) Neutrophils prime a long-lived effector macrophage phenotype that mediates accelerated helminth expulsion. Nat. Immunol 15, 938–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Deniset JF and Kubes P (2018) Neutrophil heterogeneity: Bona fide subsets or polarization states? J. Leukoc. Biol 103, 829–838 [DOI] [PubMed] [Google Scholar]
- 73.Nikolaus S, et al. (1998) Increased secretion of pro-inflammatory cytokines by circulating polymorphonuclear neutrophils and regulation by interleukin 10 during intestinal inflammation. Gut 42, 470–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Neudecker V, et al. (2017) Neutrophil transfer of miR-223 to lung epithelial cells dampens acute lung injury in mice. Sci. Transl. Med 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wong J, et al. (1997) A minimal role for selectins in the recruitment of leukocytes into the inflamed liver microvasculature. J. Clin. Invest 99, 2782–2790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kolaczkowska E, et al. (2015) Molecular mechanisms of NET formation and degradation revealed by intravital imaging in the liver vasculature. Nat Commun 6, 6673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Meng W, et al. (2012) Depletion of neutrophil extracellular traps in vivo results in hypersusceptibility to polymicrobial sepsis in mice. Crit. Care 16, R137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van der Windt DJ, et al. (2018) Neutrophil extracellular traps promote inflammation and development of hepatocellular carcinoma in nonalcoholic steatohepatitis. Hepatology 68, 13471360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Giles JA, et al. (2018) Neutrophil infiltration to the brain is platelet-dependent, and is reversed by blockade of platelet GPIbalpha. Immunology 154, 322–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Woodberry T, et al. (2018) The Emerging Role of Neutrophil Granulocytes in Multiple Sclerosis. J Clin Med 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bernardes-Silva M, et al. (2001) Recruitment of neutrophils across the blood-brain barrier: the role of E- and P-selectins. J. Cereb. Blood Flow Metab 21, 1115–1124 [DOI] [PubMed] [Google Scholar]
- 82.Kerfoot SM, et al. (2006) Reevaluation of P-selectin and alpha 4 integrin as targets for the treatment of experimental autoimmune encephalomyelitis. J. Immunol 176, 6225–6234 [DOI] [PubMed] [Google Scholar]
- 83.Engelhardt B, et al. (1997) E- and P-selectin are not involved in the recruitment of inflammatory cells across the blood-brain barrier in experimental autoimmune encephalomyelitis. Blood 90, 4459–4472 [PubMed] [Google Scholar]
- 84.Gorina R, et al. (2014) beta2 integrin-mediated crawling on endothelial ICAM-1 and ICAM-2 is a prerequisite for transcellular neutrophil diapedesis across the inflamed blood-brain barrier. J. Immunol 192, 324–337 [DOI] [PubMed] [Google Scholar]
- 85.Steffen BJ, et al. (1994) Evidence for involvement of ICAM-1 and VCAM-1 in lymphocyte interaction with endothelium in experimental autoimmune encephalomyelitis in the central nervous system in the SJL/J mouse. Am. J. Pathol 145, 189–201 [PMC free article] [PubMed] [Google Scholar]
- 86.Christy AL, et al. (2013) Mast cell activation and neutrophil recruitment promotes early and robust inflammation in the meninges in EAE. J. Autoimmun 42, 50–61 [DOI] [PubMed] [Google Scholar]
- 87.Carlson T, et al. (2008) The Th17-ELR+ CXC chemokine pathway is essential for the development of central nervous system autoimmune disease. J. Exp. Med 205, 811–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Simmons SB, et al. (2014) Cytokine-regulated neutrophil recruitment is required for brain but not spinal cord inflammation during experimental autoimmune encephalomyelitis. J. Immunol 193, 555–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Neumann J, et al. (2015) Very-late-antigen-4 (VLA-4)-mediated brain invasion by neutrophils leads to interactions with microglia, increased ischemic injury and impaired behavior in experimental stroke. Acta Neuropathol 129, 259–277 [DOI] [PubMed] [Google Scholar]
- 90.Zenaro E, et al. (2015) Neutrophils promote Alzheimer’s disease-like pathology and cognitive decline via LFA-1 integrin. Nat. Med 21, 880–886 [DOI] [PubMed] [Google Scholar]
- 91.Andonegui G, et al. (2018) Targeting inflammatory monocytes in sepsis-associated encephalopathy and long-term cognitive impairment. JCI Insight 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Whittaker Hawkins RF, et al. (2017) ICAM1+ neutrophils promote chronic inflammation via ASPRV1 in B cell-dependent autoimmune encephalomyelitis. JCI Insight 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Horckmans M, et al. (2017) Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. Eur. Heart J 38, 187–197 [DOI] [PubMed] [Google Scholar]
- 94.Kempf T, et al. (2011) GDF-15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice. Nat. Med 17, 581–588 [DOI] [PubMed] [Google Scholar]
- 95.Anzai A, et al. (2017) The infarcted myocardium solicits GM-CSF for the detrimental oversupply of inflammatory leukocytes. J. Exp. Med 214, 3293–3310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Swirski FK and Nahrendorf M (2018) Cardioimmunology: the immune system in cardiac homeostasis and disease. Nat. Rev. Immunol 18, 733–744 [DOI] [PubMed] [Google Scholar]
- 97.Hoyer FF and Nahrendorf M (2017) Neutrophil contributions to ischaemic heart disease. Eur. Heart J 38, 465–472 [DOI] [PubMed] [Google Scholar]
- 98.Savchenko AS, et al. (2014) VWF-mediated leukocyte recruitment with chromatin decondensation by PAD4 increases myocardial ischemia/reperfusion injury in mice. Blood 123, 141–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Weckbach LT, et al. (2019) Midkine drives cardiac inflammation by promoting neutrophil trafficking and NETosis in myocarditis. J. Exp. Med [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mauler M, et al. (2018) Platelet Serotonin Aggravates Myocardial Ischemia/Reperfusion Injury via Neutrophil Degranulation. Circulation [Google Scholar]
- 101.Hulsmans M, et al. (2017) Macrophages Facilitate Electrical Conduction in the Heart. Cell 169, 510–522 e520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Scheiermann C, et al. (2013) Circadian control of the immune system. Nat. Rev. Immunol 13, 190–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lyons J, et al. (2018) Integrated in vivo multiomics analysis identifies p21-activated kinase signaling as a driver of colitis. Sci Signal 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang J, et al. (2017) Visualizing the function and fate of neutrophils in sterile injury and repair. Science 358, 111–116 [DOI] [PubMed] [Google Scholar]
- 105.Rankin SM (2010) The bone marrow: a site of neutrophil clearance. J. Leukoc. Biol 88, 241–251 [DOI] [PubMed] [Google Scholar]
- 106.Shi J, et al. (2001) Role of the liver in regulating numbers of circulating neutrophils. Blood 98, 1226–1230 [DOI] [PubMed] [Google Scholar]
- 107.Devi S, et al. (2013) Neutrophil mobilization via plerixafor-mediated CXCR4 inhibition arises from lung demargination and blockade of neutrophil homing to the bone marrow. J. Exp. Med 210, 2321–2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Engblom C, et al. (2017) Osteoblasts remotely supply lung tumors with cancer-promoting SiglecF(high) neutrophils. Science 358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Boisset JC, et al. (2018) Mapping the physical network of cellular interactions. Nat. Methods 15, 547–553 [DOI] [PubMed] [Google Scholar]
- 110.Cunin P, et al. (2018) Megakaryocyte emperipolesis mediates membrane transfer from intracytoplasmic neutrophils to platelets. bioRxiv 504555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cunin P and Nigrovic PA (2019) Megakaryocytes as immune cells. J. Leukoc. Biol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Szczerba BM, et al. (2019) Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 566, 553–557 [DOI] [PubMed] [Google Scholar]
- 113.Aherne CM, et al. (2012) Neuronal guidance molecule netrin-1 attenuates inflammatory cell trafficking during acute experimental colitis. Gut 61, 695–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Konig K, et al. (2014) The plexin C1 receptor promotes acute inflammation. Eur. J. Immunol 44, 2648–2658 [DOI] [PubMed] [Google Scholar]
- 115.Morote-Garcia JC, et al. (2012) Endothelial Semaphorin 7A promotes neutrophil migration during hypoxia. Proc. Natl. Acad. Sci. U. S. A 109, 14146–14151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stadtmann A, et al. (2013) The PSGL-1-L-selectin signaling complex regulates neutrophil adhesion under flow. J. Exp. Med 210, 2171–2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pruenster M, et al. (2015) Extracellular MRP8/14 is a regulator of beta2 integrindependent neutrophil slow rolling and adhesion. Nat Commun 6, 6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nussbaum C, et al. (2013) Neutrophil and endothelial adhesive function during human fetal ontogeny. J. Leukoc. Biol 93, 175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hepper I, et al. (2012) The mammalian actin-binding protein 1 is critical for spreading and intraluminal crawling of neutrophils under flow conditions. J. Immunol 188, 4590–4601 [DOI] [PubMed] [Google Scholar]
- 120.Romano M, et al. (1997) Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity 6, 315–325 [DOI] [PubMed] [Google Scholar]
- 121.Boras M, et al. (2017) Skap2 is required for beta2 integrin-mediated neutrophil recruitment and functions. J. Exp. Med 214, 851–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lefort CT, et al. (2012) Distinct roles for talin-1 and kindlin-3 in LFA-1 extension and affinity regulation. Blood 119, 4275–4282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Germena G, et al. (2015) Mutation in the CD45 inhibitory wedge modulates integrin activation and leukocyte recruitment during inflammation. J. Immunol 194, 728–738 [DOI] [PubMed] [Google Scholar]
- 124.Rossaint J, et al. (2016) FGF23 signaling impairs neutrophil recruitment and host defense during CKD. J. Clin. Invest 126, 962–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pircher J, et al. (2018) Cathelicidins prime platelets to mediate arterial thrombosis and tissue inflammation. Nat Commun 9, 1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.von Bruhl ML, et al. (2012) Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J. Exp. Med 209, 819–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rossaint J, et al. (2014) Synchronized integrin engagement and chemokine activation is crucial in neutrophil extracellular trap-mediated sterile inflammation. Blood 123, 2573–2584 [DOI] [PubMed] [Google Scholar]
- 128.Zarbock A, et al. (2007) Galphai2 is required for chemokine-induced neutrophil arrest. Blood 110, 3773–3779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Looney MR, et al. (2011) Stabilized imaging of immune surveillance in the mouse lung. Nat. Methods 8, 91–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zarbock A, et al. (2006) Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. J. Clin. Invest 116, 3211–3219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vinegoni C, et al. (2015) Imaging the beating heart in the mouse using intravital microscopy techniques. Nat. Protoc 10, 1802–1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mann L, et al. (2017) Quantitative Visualization of Leukocyte Infiltrate in a Murine Model of Fulminant Myocarditis by Light Sheet Microscopy. J Vis Exp [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Schilling JD, et al. (2012) Macrophages modulate cardiac function in lipotoxic cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol 303, H1366–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chen J, et al. (2018) Trimetazidine Attenuates Cardiac Dysfunction in Endotoxemia and Sepsis by Promoting Neutrophil Migration. Front. Immunol 9, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lindsey ML, et al. (2018) Guidelines for experimental models of myocardial ischemia and infarction. Am. J. Physiol. Heart Circ. Physiol 314, H812–H838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kitching AR, et al. (2009) In vivo imaging of leukocyte recruitment to glomeruli in mice using intravital microscopy. Methods Mol. Biol 466, 109–117 [DOI] [PubMed] [Google Scholar]
- 137.Maddens B, et al. (2012) Severity of sepsis-induced acute kidney injury in a novel mouse model is age dependent. Crit. Care Med 40, 2638–2646 [DOI] [PubMed] [Google Scholar]
- 138.Dear JW, et al. (2006) Sepsis-induced organ failure is mediated by different pathways in the kidney and liver: acute renal failure is dependent on MyD88 but not renal cell apoptosis. Kidney Int 69, 832–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pai S, et al. (2012) Visualizing leukocyte trafficking in the living brain with 2-photon intravital microscopy. Front. Cell. Neurosci 6, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rittirsch D, et al. (2009) Immunodesign of experimental sepsis by cecal ligation and puncture. Nat. Protoc 4, 31–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bittner S, et al. (2014) Myelin oligodendrocyte glycoprotein (MOG35–55) induced experimental autoimmune encephalomyelitis (EAE) in C57BL/6 mice. J Vis Exp [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Trotman-Lucas M, et al. (2017) An alternative surgical approach reduces variability following filament induction of experimental stroke in mice. Dis. Model. Mech 10, 931–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.McDonald B, et al. (2010) Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 330, 362–366 [DOI] [PubMed] [Google Scholar]
- 144.Sun X, et al. (2018) Microbiota-Derived Metabolic Factors Reduce Campylobacteriosis in Mice. Gastroenterology 154, 1751–1763 e1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chami B, et al. (2014) The role of CXCR3 in DSS-induced colitis. PLoS One 9, e101622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chen L, et al. (2017) NLRP12 attenuates colon inflammation by maintaining colonic microbial diversity and promoting protective commensal bacterial growth. Nat. Immunol 18, 541–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sperandio M, et al. (2013) Ontogenetic regulation of leukocyte recruitment in mouse yolk sac vessels. Blood 121, e118–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wipke BT and Allen PM (2001) Essential role of neutrophils in the initiation and progression of a murine model of rheumatoid arthritis. J. Immunol 167, 1601–1608 [DOI] [PubMed] [Google Scholar]
- 149.Chou RC, et al. (2010) Lipid-cytokine-chemokine cascade drives neutrophil recruitment in a murine model of inflammatory arthritis. Immunity 33, 266–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tong PL, et al. (2015) The skin immune atlas: three-dimensional analysis of cutaneous leukocyte subsets by multiphoton microscopy. J. Invest. Dermatol 135, 84–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Abtin A, et al. (2014) Perivascular macrophages mediate neutrophil recruitment during bacterial skin infection. Nat. Immunol 15, 45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Oyoshi MK, et al. (2012) Leukotriene B4-driven neutrophil recruitment to the skin is essential for allergic skin inflammation. Immunity 37, 747–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Doring Y, et al. (2012) Lack of neutrophil-derived CRAMP reduces atherosclerosis in mice. Circ. Res 110, 1052–1056 [DOI] [PubMed] [Google Scholar]
- 154.Kunkel EJ and Ley K (1996) Distinct phenotype of E-selectin-deficient mice. E-selectin is required for slow leukocyte rolling in vivo. Circ. Res 79, 1196–1204 [DOI] [PubMed] [Google Scholar]
- 155.Sumagin R, et al. (2010) LFA-1 and Mac-1 define characteristically different intralumenal crawling and emigration patterns for monocytes and neutrophils in situ. J. Immunol 185, 70577066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yago T, et al. (2015) Blocking neutrophil integrin activation prevents ischemia-reperfusion injury. J. Exp. Med 212, 1267–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Herter JM, et al. (2014) Adhesion molecules involved in neutrophil recruitment during sepsis-induced acute kidney injury. J. Innate Immun 6, 597–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Devi S, et al. (2013) Multiphoton imaging reveals a new leukocyte recruitment paradigm in the glomerulus. Nat. Med 19, 107–112 [DOI] [PubMed] [Google Scholar]
- 159.Savinko TS, et al. (2015) Functional Beta2-Integrins Restrict Skin Inflammation In Vivo. J. Invest. Dermatol 135, 2249–2257 [DOI] [PubMed] [Google Scholar]
- 160.Lammermann T, et al. (2013) Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature 498, 371–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wong SL, et al. (2015) Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat. Med 21, 815–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wang J (2018) Neutrophils in tissue injury and repair. Cell Tissue Res 371, 531–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Birner U, et al. (2000) The role of alpha(4) and LFA-1 integrins in selectin-independent monocyte and neutrophil migration to joints of rats with adjuvant arthritis. Int. Immunol 12, 141–150 [DOI] [PubMed] [Google Scholar]
- 164.Issekutz AC (1998) Adhesion molecules mediating neutrophil migration to arthritis in vivo and across endothelium and connective tissue barriers in vitro. Inflamm. Res 47 Suppl 3, S123132. [DOI] [PubMed] [Google Scholar]
- 165.Chen M, et al. (2006) Neutrophil-derived leukotriene B4 is required for inflammatory arthritis. J. Exp. Med 203, 837–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kim ND, et al. (2006) A unique requirement for the leukotriene B4 receptor BLT1 for neutrophil recruitment in inflammatory arthritis. J. Exp. Med 203, 829–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Futosi K, et al. (2013) Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int. Immunopharmacol 17, 638–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Monach PA, et al. (2010) Neutrophils in a mouse model of autoantibody-mediated arthritis: critical producers of Fc receptor gamma, the receptor for C5a, and lymphocyte function-associated antigen 1. Arthritis Rheum 62, 753–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Elliott ER, et al. (2011) Deletion of Syk in neutrophils prevents immune complex arthritis. J. Immunol 187, 4319–4330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Miyabe Y, et al. (2017) Complement C5a Receptor is the Key Initiator of Neutrophil Adhesion Igniting Immune Complex-induced Arthritis. Sci Immunol 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Carmona-Rivera C, et al. (2017) Synovial fibroblast-neutrophil interactions promote pathogenic adaptive immunity in rheumatoid arthritis. Sci Immunol 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.You S et al. (2018) Intravital imaging by simultaneous labelfree autofluorescence-multiharmonic microscopy. Nat. Commun 9, 2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rua R et al. (2019) Infection drives meningeal engraftment by inflammatory monocytes that impairs CNS immunity. Nat. Immunol 20, 407–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cunin P et al. (2019) Megakaryocyte emperipolesis mediates membrane transfer from intracytoplasmic neutrophils to platelets. eLife 8, e44031. [DOI] [PMC free article] [PubMed] [Google Scholar]