Abstract
The slug Arion subfuscus produces a mucus-based defensive secretion that is remarkably tough. This glue appears to be a double network hydrogel, gaining its toughness through the synergistic actions of two networks of polymers, a relatively stiff network and a relatively deformable network. The double network mechanism has great potential to guide the development of synthetic adhesives. Mechanical tests were performed to analyse key predictions of the mechanism. Stress relaxation tests and tensile tests support the presence of stable cross-links. Cyclic stress–strain tests demonstrate that the glue dissipates a great deal of energy through the failure of these cross-links as sacrificial bonds. Energy dissipation by failure of sacrificial bonds rather than viscous processes is supported by the minimal effect of the time course of the experiments on the measured properties. These sacrificial bonds appear able to reform within minutes after failure. Finally, the glue's stiffness decreases at pH values below 5.5, whereas magnesium and calcium rapidly dissociate from the glue at all pH values tested. Thus, these ions may not be the primary cross-linkers generating the glue's stiffness.
This article is part of the theme issue ‘Transdisciplinary approaches to the study of adhesion and adhesives in biological systems’.
Keywords: double network, hydrogel, gastropod, glue, sacrificial bonds, mucus
1. Introduction
The slug Arion subfuscus produces a defensive secretion that has remarkable adhesive and cohesive properties. This glue is different from the mucus produced by the ventral surface during locomotion, and it is different from typical gastropod mucus [1]. It is secreted from the dorsal surface in large quantities when the slug is threatened, and sets within seconds into a tough gel. This glue would coat and adhere strongly to the surface of an attacking organism. The glue combines stiffness with great extensibility [2]. Thus, a large amount of energy is necessary to break it [3]. Because the glue adheres strongly, and is so difficult to break, it would presumably allow the slug time to move away.
The striking feature of A. subfuscus glue is that it is remarkably tough given that it is a dilute hydrogel [2]. The secretion contains only a few per cent protein and carbohydrate by weight [1]. Most dilute hydrogels are excellent lubricants. The glue appears to gain its toughness through the synergistic effects of two separate networks, working together as a double-network hydrogel [3]. Such gels are typically two to three orders of magnitude tougher than comparable single network gels [4]. In A. subfuscus, the stiffness appears to be due to a cross-linked network of proteins. This is interpenetrated by a network of large polysaccharides that would presumably hold the glue together as it experiences extensive deformation [3]. Instead of brittle failure in a simple fracture plane, the extension of the deformable network forces the stiff network to be extensively disrupted through a large volume before the glue eventually fails. This requires the fracture of a much greater number of cross-links, requiring greater energy [5,6].
For the double network mechanism to work, several features must be true. First, the gel would be viscoelastic due to the combination of a stiff and a deformable network, but it should be markedly stiffer than typical mucus. This is due to the number of stable cross-links, which provide most of the resistance to deformation [6]. Second, these cross-links must serve as sacrificial bonds. Sacrificial bonds are cross-links within the stiff network that fail before the polymers themselves fail, absorbing energy while allowing the glue to continue to deform [6]. The number of sacrificial bonds should correlate with the amount of energy dissipated.
Slug glue has several possible candidates for sacrificial bonds. Metal ions are excellent candidates since they can form electrostatic or coordinate covalent bonds that are strong enough to provide good stability, however they will fail before the covalent bonds of the polymer backbone [7,8]. In slug glue, calcium (40 mM), magnesium (approx. 25 mM), and zinc (approx. 1 mM) are unusually abundant, and iron, manganese and copper are present (less than 1 mM) [9,10]. Furthermore, the proteins in the glue have a large number of metal-binding domains. In particular, many of the proteins are matrilin-like, containing epidermal growth factor and von Willebrand factor A domains [11]. These domains bind to divalent ions such as calcium and magnesium, and are used by matrilins to form intermolecular cross-links [12,13]. There is also at least one other protein with an unusually histidine-rich domain, which would be likely to bind to zinc [11]. Similarly, some of the key proteins that distinguish the glue from lubricating mucous secretions appear to be C-lectins [11]. C-lectins use calcium ion binding domains to bind to other proteins or carbohydrates [14].
The abundance of metal ions and metal-binding domains, however, does not prove that they form the sacrificial bonds. The charge on these ions will contribute to the overall structure and integrity of the gel. The charge affects the swelling of the gel [15], and the ions would presumably interact with the polyanionic polymers in the glue. This would be especially true of the carbohydrates, which appear to be heavily sulfated glycosaminoglycans [3,10]. Thus, it is possible that calcium and magnesium ions contribute to structural integrity and balance charge to control swelling, but do not serve as the primary sacrificial bonds. There appear to be other bonds that could also play a central role as sacrificial bonds. Many of the proteins in the glue are heavily oxidized [16], and there is evidence that the carbonyl groups resulting from oxidation form imine bonds [10,16]. These are covalent, but readily reversible. Finally, many of the proteins that distinguish the glue from lubricating mucus (Asmp15a-k) [1], are positively charged and carry an unusually high number of conserved aromatic amino acid residues [11]. The interaction between the electron-rich pi orbitals of aromatic rings and positive charges also can form strong but reversible bonds [17,18].
The goals of this paper are to test predictions of the double network mechanism for A. subfuscus glue, and to gain a better understanding of the glue's toughness and cross-linking. It is important to demonstrate the presence of stable cross-links that can act as sacrificial bonds. Furthermore, clarifying the nature of those bonds will lead to greater understanding of the glue. Because of the glue's toughness and ability to adhere strongly to wet, irregular surfaces, it has great potential to guide the biomimetic or bioinspired development of medical adhesives. Slug glue has already shown its potential to guide the development of a synthetic glue [19]. This synthetic adhesive took advantage of the ability of a double-network gel to deform extensively and absorb energy rather than fail catastrophically, and was able to generate far greater adhesive toughness than currently available medical adhesives [19].
2. Results
(a). Stress relaxation
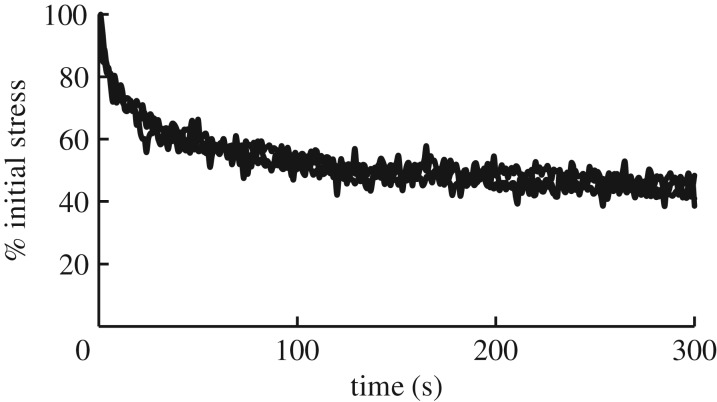
Mechanical tests were performed on test strips (1.5 × 1.5 × 5 mm) that were cut from masses of glue, each collected from a single slug (detailed Methods are in electronic supplementary material). Because the glue sets within seconds after collection into a firm ball, it can be cut by a pair of conjoined razor blades into roughly rectangular test strips with known dimensions [3]. This is unlike typical gastropod mucus, which is usually a slime and does not hold its shape. Thus, samples of the glue could be tested using a tensometer. In stress relaxation tests, the glue behaved as a viscoelastic material (figure 1). When held at a constant strain of 2 after rapid extension, the stress dropped roughly 40% in the first 30 s, then it levelled off and remained relatively constant. In the final 2 min of the test, the average per cent of initial stress was 46.1% ± 1.6 (mean ± s.e.m.) for all three samples combined. The stress relaxation profile had the same shape when samples were stretched to strains of 1, 2, 3, 4 or 5 in preliminary tests.
Figure 1.
Stress relaxation of A. subfuscus glue. Samples were rapidly stretched to a strain of 2, and then held at constant strain. Three trials from separate glue samples are shown superimposed. The initial stress at a strain of 2 was 42 ± 8.5 kPa.
(b). Tensile tests and strain rate
Strips cut from the glue were tested to failure in a tensometer. As described in previous work [3], the glue had a linear stress–strain curve up to a clearly defined yield point, where the sample began to fail by necking. At this point, the stress dropped to a value substantially below the peak stress but then stayed constant at a plateau as the glue continued to extend before its final failure.
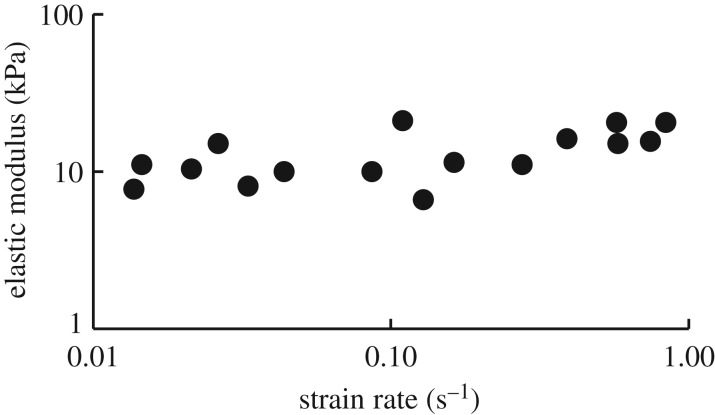
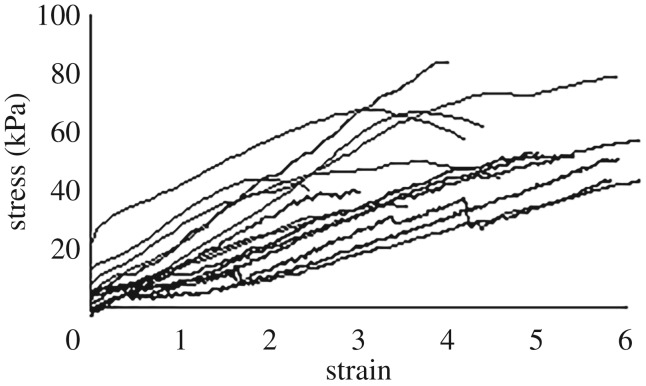
In the linear part of the stress–strain curve, the elastic modulus was somewhat dependent on strain rate. The modulus increased from roughly 10 kPa to 20 kPa as the strain rate increased over two orders of magnitude (ANOVA, p = 0.006) (figure 2). In contrast, yield strain (when necking began) and peak stress were independent of strain rate (ANOVA, p = 0.22, p = 0.28 respectively). The average yield strain was 5.0 ± 0.41 (i.e. the glue stretched to six times its initial length before visibly yielding). The peak stress when the sample yielded was 53.1 ± 3.7 kPa. After yielding, the samples typically continued to extend at relatively constant stress up to a final strain of 6.6 ± 0.5. The magnitudes of the modulus and yield strain values were consistent with those of Wilks et al. (see electronic supplementary material). Regardless of strain rate, the glue had a linear stress–strain response up to the yield point (figure 3). Some samples were also tested in a dynamic rheometer, although because the glue set into firm, elastic balls after collection, it had to be deformed markedly in order to be tested in this way. The rheometry results were consistent with the previous measurements (electronic supplementary material, figure S1). There was a relatively small effect of strain rate, and the storage modulus (elastic contribution) was substantially greater than the loss modulus (viscous contribution) at all strain rates tested (0.01–50 rad s−1).
Figure 2.
The effect of strain rate on glue stiffness. The elastic modulus was measured in the linear region of the stress–strain curve, before the yield point.
Figure 3.
The linear regions of stress–strain curves for slug glue. Data is shown up to the point where the sample yielded visibly. Strain rate ranged from 0.01 to 0.7 s−1. The data was smoothed using a running average such that each point represents an average of a set of points comprising roughly 0.5 to 1% of the total data.
(c). Cyclic stress–strain tests
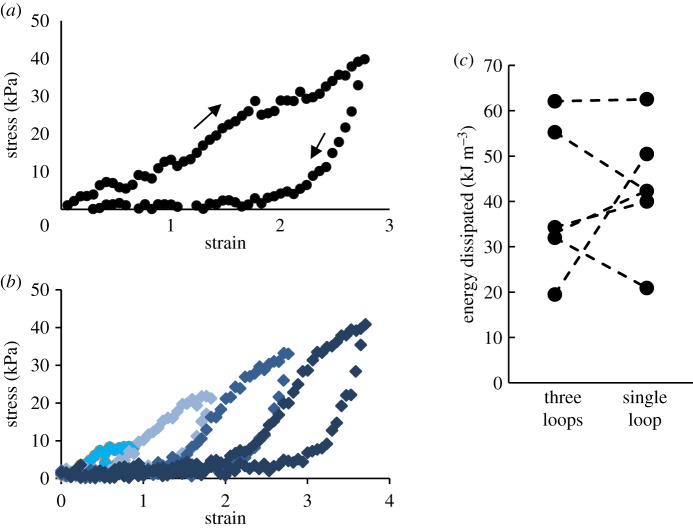
Test strips were also subjected to cycles of extension followed by return to resting length. Although the glue had a linear stress–strain curve during elongation up to a strain of 5, it showed marked hysteresis when unloaded (figure 4a). This occurred despite not reaching the yield point at which necking started. There was a linear increase in stress as strain increased, but when the strain was reversed, the stress dropped to a value between 0 and 5 kPa. Notably, despite the measured hysteresis, the glue did not show any visible signs of plastic deformation. Throughout the entire return to resting length, the sample appeared taut. In fact, when the glue was manually stretched, then released, it snapped back to its original shape almost instantly, as an elastic band would. Note that these findings were for samples that were not taken past the yield point at a strain of 5. The linearity during extension, the extent of hysteresis, and the shape of the stress–strain curve were also the same in preliminary trials at a strain rate that was roughly ten times greater (0.5–1 s−1).
Figure 4.
The results of cyclic stress–strain tests on A. subfuscus glue. (a) A single sample of slug glue stretched to a strain of 2.8, then returned to its resting length. Arrows indicate loading and unloading. (b) A single sample of glue stretched through progressively larger strains, with a return to the original length after each extension. In this trial, the sample was stretched through four cycles to a peak strain of 3.6. (c) The effect of strain history on the total energy dissipated. The group of points on the left shows the total energy dissipated in samples taken through three progressively larger strain cycles (similar to part b). The group of points on the right shows the energy dissipated in paired samples taken to the same ultimate strain in a single cycle (similar to part a). Dashed lines link the results from pairs cut from the same sample. Samples were extended to strains of 0.9, 1.8 then 2.7 or directly to 2.7 with no previous extensions. N = 6. (Online version in colour.)
The energy dissipation occurred continuously during extension and was not the result of reaching a critical value. This can be seen by the fact that the hysteresis occurred regardless of what strain the glue reached. When stretching a sample through progressively larger strains, with a return to the original length after each extension, the same extent of hysteresis was seen at all strains (figure 4b). Interestingly, every time the sample was re-stretched after returning to the original length, the stress while re-extending matched the low values of the previous return phase. Then when it reached the peak strain of the previous extension, the stress was again linear, following the same slope as the extension phase of the previous loop. In other words, when re-extending through strains it had previously undergone, the sample exactly matched the loss of stiffness due to hysteresis, yet once it reached a strain that it had not previously experienced, it once again showed the same modulus as the original sample. This was true up to the point at which the sample began necking.
The amount of energy dissipated was measured by the area enclosed by the stress–strain loops. The dissipation when a strip of glue was extended to a strain of 2.7 and back in one single loop was not significantly different from the total dissipation that occurred when a matched strip of glue cut from the same sample was stretched to strains of 0.9, then 1.8 then 2.7 with a return to the original length after each extension (paired t-test, p = 0.59) (figure 4c). The mean energy dissipated was 39.4 kJ m−3 (three loops) and 43.1 kJ m−3 (single loop). In addition, the elastic moduli were the same during the extension phase. When samples were extended to a strain of 2.7 in a single cycle, the elastic modulus was 12.5 ± 1.5 kPa, whereas the elastic modulus when samples were taken to the same ultimate strain through three progressively longer extensions was 14.0 ± 2.0 kPa (N = 6, p = 0.45, paired t-test).
The glue showed evidence of recovering its stiffness when allowed to rest at the original length between trials (figure 5). After performing the series of progressively longer extensions in five of the previous experiments, samples were allowed to rest at their original length for an average of 7 min (minimum 5 min, maximum 10 min), and then extended to a strain of 2.7 again. Longer rest periods were not tested, due to the difficulty in ensuring comparable hydration. The difference between the stress for any given strain was compared between the unloading (return) phase of a previous loop and the extension phase of the subsequent loop, with or without recovery. As noted above, when re-extending with no recovery after one loop, the stress–strain curve exactly matched the previous unloading loop, reflecting the loss of stiffness. The unloading and re-extending curves were compared for the strain range between 1 and 1.5 for cycles that reached an ultimate strain of 2, and between 2 and 2.5 for cycles that reached a final strain of 3. These ranges contained nine points each and were chosen to minimize discrepancies at the extremes of the loop.
Figure 5.
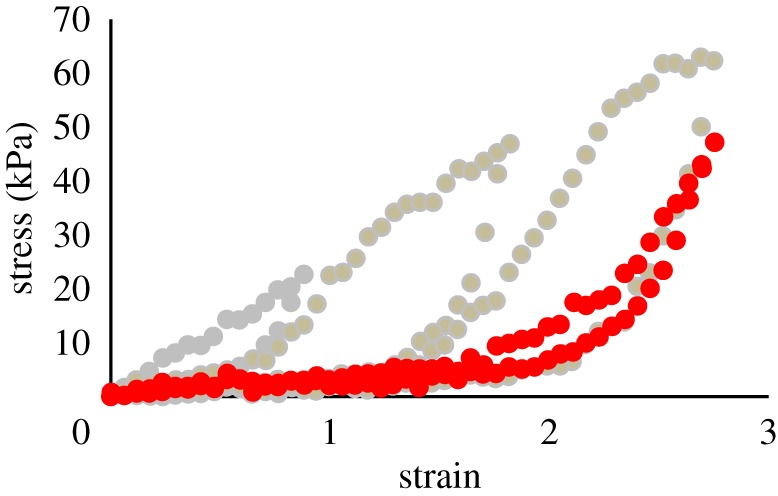
Partial recovery of stiffness after a brief rest period. This shows the results of one trial where a sample was extended to strains of 0.9, 1.8, then 2.7 with no rest. The sample was then allowed to remain at zero strain for 6 min and re-extended to a strain of 2.7. The red data points show this final post-recovery loop. (Online version in colour.)
During the initial stress–strain loop, the hysteresis described in the previous test was apparent, with the stresses during the unloading phase being 27.3% ± 1.1 of those during extension (N = 5). When a sample did not have time to recover (loop 2 return versus loop 3 extension), there was no significant difference between the stresses for any give strain. The average difference in stress was −0.2 ± 0.7 kPa over the range of strains from 2 to 2.5 (paired t-test, p = 0.75). In contrast, when the sample had 5–10 min to recover (loop 3 return versus re-extension after 5–10 min), the stress was significantly higher during the re-extension than during the previous unloading phase. The average difference in stress was 4.4 ± 0.9 kPa (paired t-test, p = 0.01). This amounted to recovering 19.9% of what was lost due to hysteresis (figure 5). After re-extension, when the sample was returned back to the original strain, there was no difference between the original unloading phase and the unloading phase after recovery (paired t-test, p = 0.25, average difference in stress of −0.9 ± 0.6 kPa).
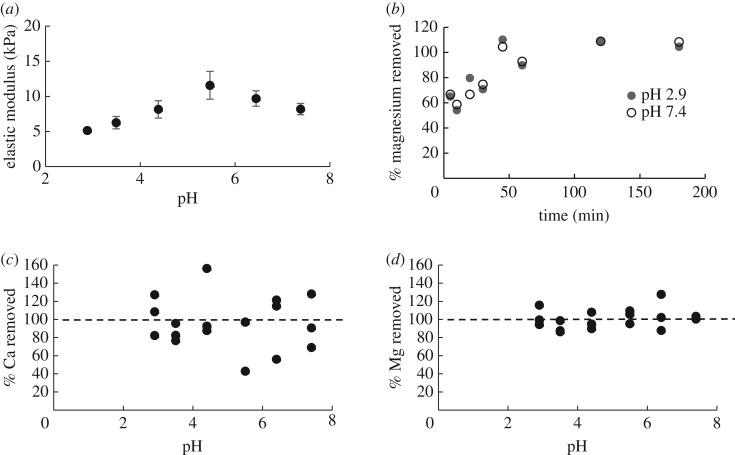
(d). The effect of pH on glue stiffness and metal content
Test strips of glue were soaked briefly in different pH acetate buffers, then tested in a tensometer. The elastic modulus of glue samples was affected by pH (figure 6a, ANOVA, p = 0.008). The glue showed a loss in stiffness when exposed to low pH buffers. The decrease in modulus appears to begin at pH 5.5, and continues with each decrease in pH. When a post hoc test was performed, the modulus of samples below pH 5 was significantly lower than the modulus of samples above pH 5 (t-test, p = 0.001). The control, unsoaked, samples had an elastic modulus of 15.3 ± 1.7 kPa, which was consistent with that of comparable samples tested on the other devices. For context, the pH of homogenized glue samples was between 5.8 and 6.0, so the normal glue pH falls within the range where the glue is stronger.
Figure 6.
The effect of pH on A. subfuscus glue mechanical properties and ion content. (a) The elastic modulus after soaking for 5 min in different acetate buffers. Data shows mean ± s.e.m. (N = 6 for each pH value). (b) Time course of magnesium diffusion from the glue at neutral and acidic pH. (c) The amount of calcium removed from the glue in 1 h of soaking in acetate buffers of different pH. (d) The amount of magnesium removed from the glue in 1 h of soaking in acetate buffers of different pH. All ion content values are given as a percentage of the starting concentration, which was measured after 1 h incubation in 0.7% nitric acid (1901 ± 132 ppm calcium, 593 ± 88 ppm magnesium).
The extent of calcium and magnesium ion dissociation was tested at different pH values, to see if there was a correlation between the decrease of stiffness at low pH and the loss of these ions. Samples were soaked in different buffers and the amount of metal ions removed was measured with an atomic absorption spectrometer. Neither of these ions were bound tightly to the gel. A rapid, uniform dissociation of calcium and magnesium was observed at all of the pH values tested (figure 6b–d; electronic supplementary material, figure S2). There was no difference in dissociation among the pH values (figure 6c,d; ANOVA, p = 0.75 for calcium, p = 0.60 for magnesium), and the average amount of calcium or magnesium removed in 1 h across all pH values was 96% ± 6.5 for calcium, and 101% ± 5.9 for magnesium. To investigate this further, the amount of metal ions removed was measured over shorter time increments. The time course was the same at pH 2.9 and 7.4 (figure 6b; electronic supplementary material, figure S2). In fact, at both extremes of pH tested, more than half of the magnesium diffused out of the glue in merely 5 min, and roughly 20 min for calcium.
3. Discussion
These results provide further support for the hypothesis that slug glue is a double network hydrogel with a stiff network and a deformable network acting synergistically. The stiff network provides a large number of cross-links, and is primarily responsible for bearing the stress. These cross-links form sacrificial bonds that are intended to break before the material as a whole fails. The deformable network holds the gel together through extreme extension, and during extension redistributes stress to a large volume of the sacrificial bond network. The combination of the two networks gives stiffness with great extensibility, so that it requires orders of magnitude more energy to break the gel than either network would provide on its own [6]. The hypothesized double network structure of slug glue is shown in figure 7.
Figure 7.
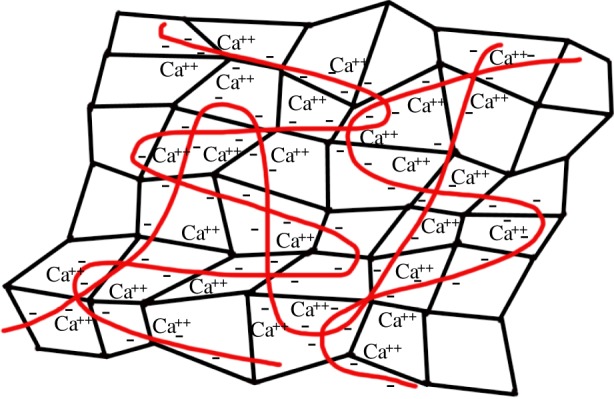
Hypothesized structure of the glue. Slug glue contains a network of proteins and a separate network of polysaccharides [3]. The proteins (black) are hypothesized to form a highly cross-linked network of sacrificial bonds, while the polysaccharides (red) are hypothesized to form a highly extensible second network. The polysaccharides carry a strong negative charge, and may associate with ions such as calcium and magnesium, which are loosely bound to ligands in the glue and may contribute by electrostatic interactions that allow the polysaccharides to fold up. (Online version in colour.)
The results support several key predictions of the double network mechanism. As predicted, slug glue is markedly stiffer than typical mucous secretions [20,21], and is heavily cross-linked. While most forms of mucus are so deformable that they must be tested in shear between rotating plates, and have shear moduli on the order of 10 Pa or less [20,21], strips of slug glue can be cut and tested in tension, and have elastic moduli on the order of a thousand times greater. Slug glue has a linear stress–strain curve during extension up to a yield point at a strain of 5, and the physical properties are not greatly affected by strain rate. Stress relaxation experiments show that after an initial relaxation, the stress stays constant for an extended period of time as is typical for stably cross-linked, visco-elastic materials [22]. The stability of the primary cross-links is further demonstrated by dynamic tests with a rheometer, confirming that the material behaves in a primarily elastic manner across a large range of strain rates. Furthermore, the supramolecular bond lifetime can be estimated by identifying the critical strain rate where the storage modulus and loss modulus are equal [23,24]. This critical strain rate was below the lowest frequency tested, and may be in the order of 0.001 rad s−1 based on a rough extrapolation of the data. A critical frequency lower than 0.01 rad s−1 would mean that the supramolecular bond lifetime of the primary bonds was greater than 628 s [24]. This is longer than other non-covalent, metal-based interactions that have been reported [23,24]. Because the dynamics of molecular cross-links govern the properties of supramolecular networks, cross-links with long bond lifetimes typically lead to stronger materials [25].
Also as predicted, these cross-links act as sacrificial bonds that fail continuously throughout extension. The shape of the loading–unloading curve in cyclic tests and the response to repeated tests at progressively increasing strain is characteristic of double networks [26,27]. These tests demonstrate marked hysteresis due to the failure of sacrificial bonds. While the stress linearly increases during extension, it drops precipitously to near zero on the return phase. This indicates that the glue has dissipated most of its stored energy through failure of specific cross-links. This pattern occurs when the glue is extended to any strain between 0 and 5, indicating that failure of sacrificial bonds is continuous rather than occurring at a specific yield point. Thus, as the material extends, sacrificial bonds break, such that the material is much less stiff when returned to its initial length.
When repeated tests are performed to progressively larger strains, a striking pattern emerges. When re-extended after a cycle, the curve matches the previous unloading phase, right up to the maximum extension of the previous loop. Essentially, once the sacrificial bonds are broken in a given strain range, there is a weak, residual elastic component that behaves consistently in the tensile test. Going beyond the previous maximum extension appears to engage new cross-links, and the modulus is once again the same as the original modulus. This continues until the extension is reversed again. Notably, the total area under the curves only depends on the ultimate strain; it does not depend on how many loading–unloading cycles were used to reach that strain. Since the area under the curve corresponds to the total energy dissipated during extension, and the dissipation appears to be due to the failure of sacrificial bonds, then one can conclude that the glue behaves as if there are a finite number of sacrificial bonds. Once these sacrificial bonds are broken, they no longer contribute to stiffness.
There is good evidence that the energy dissipation is due to failure of cross-links between the polymers, and not due to viscous processes. First, as explained above, the precise pattern of the hysteresis curves when taken through cyclic tests suggests a finite number of sacrificial bonds [26,27]. It is inconsistent with untangling interactions and flow, which would not follow such a predictable path. Second, the mechanics of the glue are not markedly rate-dependent. This relative independence from the effects of loading rate is characteristic of stable cross-links, whereas untangling interactions and viscous processes involving large polymers are more rate-dependent. In addition, there are other lines of evidence supporting stable cross-links rather than tangling-based physical linkages and viscous dissipation. Up to a strain of 5, samples show no evidence of plastic deformation when returned to rest. This strongly argues against viscous dissipation due to untangling and relative movement of polymers. Also, the time scale at which viscous processes occur in the glue is too long to account for the glue's behaviour. During stress relaxation tests, it takes roughly a minute for the glue to relax to a relatively constant stress, and the stress only decreases 23% in the first 10 seconds. In contrast, preliminary cyclic stress–strain tests were performed in several seconds. Furthermore, the strain rate used in the preliminary stress–strain loops was at least ten times greater than the later tests, and yet showed the exact same pattern. If viscous processes were the main source of energy dissipation, this would not be true.
While previous work had suggested that calcium and magnesium may form sacrificial bonds in slug glue [9,10], the results in this paper suggest that they play a less prominent role. Previous evidence for calcium cross-linking is its abundance [10], the weakening of the glue in the presence of ligands that compete for hard Lewis acids such as calcium [9,10], and the fact that most of the proteins in the glue have calcium-binding sites [11]. In this study, calcium and magnesium readily and completely dissociate from the glue at all pH values tested. The presence of acetate would presumably accelerate the dissociation, as it is a ligand for these ions. Nevertheless, these metal ions seem unlikely to serve as the primary cross-links, given that they dissociate rapidly while the primary cross-links have a relatively long bond lifetime.
Perhaps the abundance of calcium and magnesium merely reflects the need to balance charge on the polyanionic polysaccharides and to control glue structure. The ability of divalent ions to balance charge may allow the polyanionic polysaccharides to fold up extensively, providing hidden length (figure 7). Because of electrostatic interactions, these ions would contribute somewhat to the glue's mechanics. This would be consistent with the fact that glue samples soaked for 5 min, which have lost a substantial fraction of their calcium and magnesium, have lower stiffness than unsoaked glue. Furthermore, the failure of these interactions may be responsible for the initial drop in stress during stress relaxation experiments. The presence of similar amounts of calcium and matrilin-like calcium-binding proteins in adhesive and non-adhesive forms of mucus, such as the ventral mucus used in locomotion, would be consistent with the idea that these ions contribute to mechanics, but do not form the primary sacrificial bonds of the glue [11,28].
Because the calcium and magnesium are not bound tightly into the glue, other bonds may form the sacrificial bonds that are responsible for most of the glue's stiffness. Transition metals such as iron and manganese typically form much stronger interactions than calcium [29]. In that context, it is important to note that the lectin-like proteins in the glue (Asmp15a-k) bind to iron [9], and their presence constitutes the defining structural difference between adhesive and non-adhesive slug mucus. Iron is also extremely difficult to remove from the glue [5]. Notably, while loss of calcium and magnesium is correlated only with swelling and some loss of stiffness, treatment with the metal chelator ethylenediaminetetraacetic acid (EDTA) caused complete liquification of the glue [5,9]. This suggests that metal ions such as iron and manganese, with similar ligand preferences but much higher affinity, are involved. Coordination by multiple ligands could further increase the stability. These factors could explain the relatively long bond lifetimes in slug glue.
Aside from coordination of transition metals, other types of bonds may form the primary cross-links. Many proteins in the glue are heavily oxidized, and there is evidence that this leads to imine bonds [16], which could be the main cross-links. Alternatively, cation–pi interactions are strong non-covalent bonds that are likely to occur in the glue [11]. Likewise, other electrostatic or ionic interactions could play a role. Many of the primary glue proteins are basic, and could form charge-based interactions with the anionic polymers of the glue [11,28]. The effect of pH on glue stiffness would be consistent with an electrostatic effect. Previous work found that high salt concentrations, which disrupt electrostatic interactions, weakened the glue [10]. This was complicated by the fact that high salt would also markedly change the structure of the glue by reducing repulsion between charged polymers. It is significant that in a previous study, high salt concentration did not disrupt the gel-stiffening activity of the primary glue proteins, and did not help break up complexes in the glue sufficiently to solubilize the proteins [30].
An unusual feature of the glue is that, despite showing marked hysteresis in cyclic stress–strain curves up to a strain of 5, the material still recoils to its original shape in an elastic manner after stretching. In fact, it stays taut through the entire unloading cycle. Visually, it appears as elastic as a rubber band and the energy dissipation is only apparent when viewing the measured stress relative to strain. One explanation for this could be if the deformable network or an additional component of the glue behaves as an elastomer. In a double-network, while the stiff network is constantly disrupted to dissipate energy, the deformable network is extending to redistribute stresses. This extension might involve a relatively small amount of energy to drive molecular rearrangement, unfolding or straightening of the polymers. This would be much smaller than the energy necessary to disrupt the sacrificial bonds of the stiff network. When the stress is removed, however, the extended polymers may be able to revert back to their original condition causing elastic recoil, albeit at a much lower stress. This is similar to what has been described for mussel byssus [8]. In mussel byssus, metal-coordination bonds involving zinc provide the sacrificial bonds and primary stiffness of the byssus, but unfolding of crossed β-sheets provides hidden length to allow much greater extension. The unfolding requires less energy than disrupting metal-based interactions, but it would provide sufficient driving force to refold when the stress is removed. A similar mechanism could explain the behaviour of slug glue in cyclic tests, except that slug glue is a hydrogel rather than a more structured, solid material. Such a model would be consistent with the shape of the stress–strain loops. When the material is re-extended after one loop, the unfolding or extension of the polymers would provide exactly the same low resistance to extension as was seen during the unloading part of the previous cycle, up until the peak extension of the previous cycle. At this point, the remaining unbroken elements in the stiff network would become engaged and the material would once again behave as if it were a much stiffer material.
4. Conclusion
The glue produced by the terrestrial slug A. subfuscus behaves as a stiff, visco-elastic material. Its performance is consistent with the double network mechanism. It is stably cross-linked, creating a markedly stiff gel, yet these cross-links break continuously as the gel is stretched, dissipating energy. Because the glue must be stretched to high strains before failure, the continuous failure of sacrificial bonds leads to a large energy dissipation and thus a high fracture energy. These sacrificial bonds begin to reform within minutes of failure. Although calcium and magnesium are highly abundant, they do not appear to contribute as the primary sacrificial bonds. These ions are rapidly removed from the glue at all pH values tested. There appear to be other pH-sensitive bonds that play a more prominent role.
Supplementary Material
Supplementary Material
Acknowledgements
We are grateful to Julie Hasenwinkel and the Syracuse Biomaterials Institute for access to materials testing equipment.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
T.-M.F., C.G.L. and A.M.S. contributed equally to the data collection. T.-M.F. and C.G.L. drafted sections of the article, and contributed to the analysis and interpretation. A.M.S. conceived and designed the work, drafted sections of the article, contributed to analysis and interpretation of data, and edited and revised the final article.
Competing interests
We have no competing interests.
Funding
A.M.S. was supported by a summer research grant from Ithaca College.
References
- 1.Pawlicki JM, Pease LB, Pierce CM, Startz TP, Zhang Y, Smith AM. 2004. The effect of molluscan glue proteins on gel mechanics. J. Exp. Biol. 207, 1127–1135. ( 10.1242/jeb.00859) [DOI] [PubMed] [Google Scholar]
- 2.Smith AM. 2016. The biochemistry and mechanics of gastropod adhesive gels. In Biological adhesives (ed. Smith AM.), pp. 177–192. Cham, Switzerland: Springer. [Google Scholar]
- 3.Wilks AM, Rabice SR, Garbacz HS, Harro CC, Smith AM. 2015. Double-network gels and the toughness of terrestrial slug glue. J. Exp. Biol. 218, 3128–3137. ( 10.1242/jeb.128991) [DOI] [PubMed] [Google Scholar]
- 4.Gong JP. 2010. Why are double network hydrogels so tough? Soft Matter 6, 2583–2590. ( 10.1039/b924290b) [DOI] [Google Scholar]
- 5.Brown HR. 2007. A model of the fracture of double network gels. Macromolecules 40, 3815–3818. ( 10.1021/ma062642y) [DOI] [Google Scholar]
- 6.Haque MA, Kurokawa T, Gong JP. 2012. Super tough double network hydrogels and their application as biomaterials. Polymer 53, 1805–1822. ( 10.1016/j.polymer.2012.03.013) [DOI] [Google Scholar]
- 7.Ashton NN, Stewart RJ. 2015. Self-recovering caddisfly silk: energy dissipating, Ca2+-dependent, double dynamic network fibers. Soft Matter 11, 1667–1676. ( 10.1039/C4SM02435D) [DOI] [PubMed] [Google Scholar]
- 8.Reinecke A, Bertinetti L, Fratzl P, Harrington MJ. 2016. Cooperative behavior of a sacrificial bond network and elastic framework in providing self-healing capacity in mussel byssal threads. J. Struct. Biol. 196, 329–339. ( 10.1016/j.jsb.2016.07.020) [DOI] [PubMed] [Google Scholar]
- 9.Werneke SW, Swann C, Farquharson L, Hamilton KS, Smith AM. 2007. The role of metals in molluscan adhesive gels. J. Exp. Biol. 210, 2137–2145. ( 10.1242/jeb.006098) [DOI] [PubMed] [Google Scholar]
- 10.Braun M, Menges M, Opoku F, Smith AM. 2013. The relative contribution of calcium, zinc and oxidation-based cross-links to the stiffness of Arion subfuscus glue. J. Exp. Biol. 216, 1475–1483. ( 10.1242/jeb.077149) [DOI] [PubMed] [Google Scholar]
- 11.Smith AM, Papaleo C, Reid CW, Bliss JM. 2017. RNA-Seq reveals a central role for lectin, C1q and von Willebrand factor A domains in the defensive glue of a terrestrial slug. Biofouling 33, 741–754. ( 10.1080/08927014.2017.1361413) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rao Z, Handford P, Mayhew M, Knott V, Brownlee GG, Stuart D. 1995. The structure of a Ca2+-binding epidermal growth factor-like domain: its role in protein-protein interactions. Cell 82, 131–141. ( 10.1016/0092-8674(95)90059-4) [DOI] [PubMed] [Google Scholar]
- 13.Whittaker CA, Hynes RO. 2002. Distribution and evolution of von Willebrand/integrin A domains: widely dispersed domains with roles in cell adhesion and elsewhere. Mol. Biol. Cell 13, 3369–3387. ( 10.1091/mbc.e02-05-0259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drickamer K. 1988. Two distinct classes of carbohydrate-recognition domains in animal lectins. J. Biol. Chem. 263, 9557–9560. [PubMed] [Google Scholar]
- 15.Tanaka T. 1981. Gels. Sci. Am. 244, 124–138 ( 10.1038/scientificamerican0181-124) [DOI] [PubMed] [Google Scholar]
- 16.Bradshaw A, Salt M, Bell A, Zeitler M, Litra N, Smith AM. 2011. Cross-linking by protein oxidation in the rapidly setting gel-based glues of slugs. J. Exp. Biol. 214, 1699–1706. ( 10.1242/jeb.051581) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dougherty DA. 2007. Cation-π interactions involving aromatic amino acids. J. Nutr. 137, 1504S–1508S. ( 10.1093/jn/137.6.1504S) [DOI] [PubMed] [Google Scholar]
- 18.Gebbie MA, Wei W, Schrader AM, Cristiani TR, Dobbs HA, Idso M, Chmelka BF, Waite JH, Israelachvilli JN. 2017. Tuning underwater adhesion with cation-π interactions. Nat. Chem. 9, 473–479. ( 10.1038/nchem.2720) [DOI] [PubMed] [Google Scholar]
- 19.Li J, et al. 2017. Tough adhesives for diverse wet surfaces. Science 357, 378–381. ( 10.1126/science.aah6362) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Celli JP, Turner BS, Afdhal NH, Ewoldt RH, McKinley GH, Bansil R, Erramilli S. 2007. Rheology of gastric mucin exhibits a pH-dependent sol-gel transition. Biomacromolecules 8, 1580–1586. ( 10.1021/bm0609691) [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Su H, Lv W, Du M, Song Y, Zheng Q.. 2015. Complex rheological behaviors of loach (Misgurnus anguillicaudatus) skin mucus. J. Rheol. 59, 51–62. ( 10.1122/1.4902928) [DOI] [Google Scholar]
- 22.Denny MW. 1983. Molecular biomechanics of molluscan mucous secretions. In The Mollusca, vol. I (eds Wilbur K, Simkiss K, Hochachka PW), pp. 431–465. New York, NY: Academic Press. [Google Scholar]
- 23.Grindy SC, Learsch R, Mozhdehi D, Cheng J, Barrett DG, Guan Z, Messersmith PB, Holten-Andersen N. 2015. Control of hierarchical polymer mechanics with bioinspired metal-coordination bonds. Nat. Mater. 14, 1210–1216. ( 10.1038/nmat4401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bose RK, Hohlbein N, Garcia SJ, Schmidt AM, van der Zwaag S.. 2015. Connecting supramolecular bond lifetime and network mobility for scratch healing in poly(butyl acrylate) ionomers containing sodium, zinc and cobalt. Phys. Chem. Chem. Phys. 17, 1697–1704. ( 10.1039/C4CP04015E) [DOI] [PubMed] [Google Scholar]
- 25.Yount WC, Loveless DM, Craig SL. 2005. Strong means slow: dynamic contributions to the bulk mechanical properties of supramolecular networks. Angew. Chem. Int. Ed. 44, 2746–2748. ( 10.1002/anie.200500026) [DOI] [PubMed] [Google Scholar]
- 26.Webber RE, Creton C, Brown HR, Gong JP. 2007. Large strain hysteresis and Mullins effect of tough double-network hydrogels. Macromolecules 40, 2919–2927. ( 10.1021/ma062924y) [DOI] [Google Scholar]
- 27.Ducrot E, Chen Y, Bulters M, Sijbesma RP, Creton C. 2014. Toughening elastomers with sacrificial bonds and watching them break. Science 344, 186–189. ( 10.1126/science.1248494) [DOI] [PubMed] [Google Scholar]
- 28.Smith AM. 2013. Multiple metal-based cross-links: protein oxidation and metal coordination in a biological glue. In Biological and biomimetic adhesives: challenges and opportunities (eds Santos R, Aldred N, Gorb S, Flammang P), pp. 3–15. Cambridge, UK: Royal Society of Chemistry. [Google Scholar]
- 29.Holten-Andersen N, Mates TE, Toprak MS, Stucky GD, Zok FW, Waite JH. 2009. Metals and the integrity of a biological coating: the cuticle of mussel byssus. Langmuir 25, 3323–3326. ( 10.1021/la8027012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith AM, Robinson TM, Salt MD, Hamilton KS, Silvia BE, Blasiak R. 2009. Robust cross-links in molluscan adhesive gels: testing for contributions from hydrophobic and electrostatic interactions. Comp. Bioch. Physiol. B 152, 110–117. ( 10.1016/j.cbpb.2008.10.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.