Abstract
The rapid control of surface attachment is a key feature of natural adhesive systems used for locomotion, and a property highly desirable for man-made adhesives. Here, we describe the challenges of adhesion control and the timescales involved across diverse biological attachment systems and different adhesive mechanisms. The most widespread control principle for dynamic surface attachment in climbing animals is that adhesion is ‘shear-sensitive’ (directional): pulling adhesive pads towards the body results in strong attachment, whereas pushing them away from it leads to easy detachment, providing a rapid mechanical ‘switch’. Shear-sensitivity is based on changes of contact area and adhesive strength, which in turn arise from non-adhesive default positions, the mechanics of peeling, pad sliding, and the targeted storage and controlled release of elastic strain energy. The control of adhesion via shear forces is deeply integrated with the climbing animals’ anatomy and locomotion, and involves both active neuromuscular control, and rapid passive responses of sophisticated mechanical systems. The resulting dynamic adhesive systems are robust, reliable, versatile and nevertheless remarkably simple.
This article is part of the theme issue ‘Transdisciplinary approaches to the study of adhesion and adhesives in biological systems’.
Keywords: peeling, directional adhesion, active and passive control, strain energy
1. Introduction
In contrast to conventional man-made glues, the adhesive systems of many animals can be switched rapidly between strong attachment and easy detachment, enabling locomotion in environments that require firm surface attachment, such as the canopy of forests, or the intertidal zone. Throughout the lifetime of a climbing animal, such cycles between strong attachment and rapid detachment have to occur millions of times with no loss of adhesive force [1]. The ability to control adhesion is therefore a fundamental property of natural adhesive systems. Indeed, four out of the seven benchmark properties for the performance of gecko adhesives defined by Autumn [2] relate to the controllability of adhesion (namely anisotropic attachment, low detachment force, non-sticky default state, high pull-off to preload ratio; the remaining three are self-cleaning, anti-self-matting and material independence). As controllability is also a highly desirable feature for synthetic adhesives, animal adhesive structures have become models for worldwide efforts to fabricate controllable ‘biomimetic’ adhesives, which may have a wide range of applications, including but not limited to industrial pick up-and-release manipulation at the macro- and microscale, and climbing robots (for recent reviews, see [3–5]).
An adhesive system is ‘controllable’ if large variations in adhesive force can be achieved via the variation of system parameters, and ‘dynamic’ if such changes can be realized within short periods of time. In biological adhesive systems, these changes are not merely binary, but many animals can adjust their attachment systems in a gradual manner to respond to external forces resulting from climbing on substrates with various slopes, and from waves, wind or additional loads [6–12]. In this article, we provide a brief overview of the mechanisms which allow adhesion control in biological adhesive pads.
2. Control of adhesion: from permanent glues to dynamic adhesives
Attachment and detachment of adhesive contacts is a fundamental requirement for locomotion. Protraction of one body part, such as a leg or part of a foot, requires other body parts to remain in contact, in order to resist gravity or other external forces, and to produce the forward thrust which powers locomotion. The feet of moving animals go through a coordinated cycle of ‘stance’ and ‘swing’, and at the start and end of each stance phase, the adhesive contacts will have to be formed and broken.1
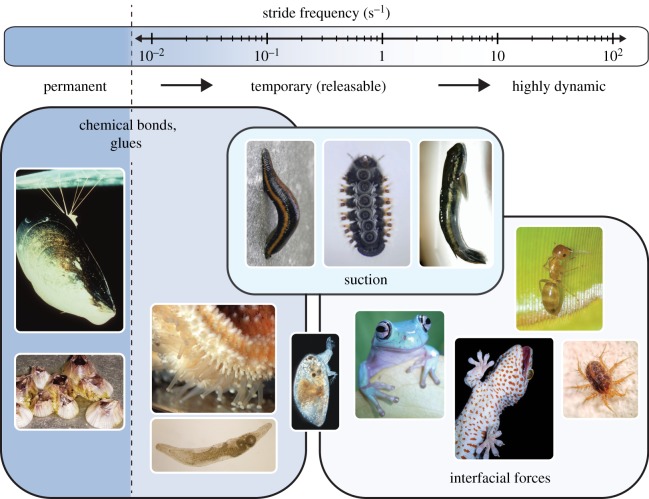
Although the time required to form and release adhesive contacts may often represent only a fraction of the stance and swing phase, it is likely that at least in some animals, the speed of attachment and detachment imposes a limit to the stepping frequency and hence the speed of movement. Animal adhesive systems range from permanent to highly dynamic, reflecting the animals’ lifestyle and speed of locomotion. The limited data available on the timing of stance–swing cycles in animals that use adhesion during locomotion are summarized in the electronic supplementary material, table S1. Temporary adhesion is used by animals differing in ‘stride frequency’ (defined here as the inverse of the time of one complete pad attachment–detachment cycle) by more than two orders of magnitude (see figure 1). This large variation in stride frequencies may be based on several factors, including the medium in which the adhesive organs operate (air or water), the adhesive mechanism and the dimensions of individual adhesive contacts.
Figure 1.
Animal adhesive systems range from permanent to temporary and highly dynamic. While permanent adhesive systems are glue-based, slow temporary adhesive systems use releasable glues or suction, and the most dynamic adhesive systems employ interfacial forces. Image sources are provided in the electronic supplementary material. (Online version in colour.)
Permanent attachment is predominantly achieved by glues, which allow animals to remain firmly attached in the same place for extended periods of time (e.g. sessile marine animals including sponges, cnidarians, cirripede crustaceans, bivalves, polychaetes, bryozoans and tunicates, but also terrestrial phoretic mites and insect pupae that attach themselves to substrates, see [14–16]). Glues may be defined as secretions, often consisting of multiple components, which are applied in liquid form, but then solidify in contact with the substrate. Well-studied systems include the byssus thread of mussels and barnacle cement [17]. However, glues do not have to be permanent, and indeed are also used for locomotion in temporary underwater adhesive systems. For example, flatworms and echinoderms achieve repeated attachment and detachment by the subsequent release of adhesive and de-adhesive secretions, each produced by distinct glands or cells [14,18–20]. Glue-based adhesion and de-adhesion require (i) the secretion of the adhesive, (ii) contact formation, (iii) solidification, (iv) secretion of the release agent, (v) its diffusion into the adhesive, and (vi) reaction with it. Contact formation in particular is a key challenge for adhesive systems employed in water, as it requires water to be removed beneath the adhesive organ. Water is initially squeezed out via hydrodynamic forces, but complete removal by dewetting requires the thin remaining water films to be thermodynamically unstable, which is unlikely for many polar natural substrates [21]. Some mussels and cyprid larvae can displace water via the secretion of lipids into the contact zone instead [22,23]. As an alternative, viscous secretions such as the glycoprotein footprints of temporarily adhering cyprid larvae may strengthen underwater adhesion by effectively replacing the water film [24].
The numerous steps involved in attachment–release cycles of glue-based adhesives are time consuming, and probably only feasible if diffusion distances are short; even for microscopic contacts they may therefore impose a speed constraint on attachment–detachment cycles. Water displacement, secretion of lipids or glycoproteins, and viscous adhesion will result in further speed constraints, together explaining why glue-based adhesive systems of aquatic animals are generally less dynamic than those of terrestrial animals that do not rely on glues (electronic supplementary material, table S1). One strategy to reduce the time needed for underwater attachment and detachment may be the miniaturization of individual adhesive contacts (as seen in flatworms and cyprid larvae; electronic supplementary material, table S1), which helps to accelerate both fluid drainage and diffusion-based processes [24,25].
A potentially faster type of controllable attachment is suction, which lacks the speed and size constraints of diffusion. A clear definition of suction for biological attachment systems is still missing (and beyond the scope of this review); here we use the term to refer to attachment produced by reducing the pressure beneath the attachment organ, excluding pure capillary or viscous adhesion. Suction in this sense is used by diverse primarily aquatic animals including limpets, leeches, clingfish, remora fish, water-fall climbing gobies, octopus, squid, net-winged midge larvae and diving beetles [26–34]. These animals produce suction either by muscular action (active suction), or by the recoil of elastic elements (passive suction). Both strategies have in common that they tend to expand the volume underneath the suction organ. Suction organs share with other underwater adhesive systems the need to drain water from the outer rim of the contact zone, in order to achieve a tight seal. However, they are probably more tolerant to small amounts of residual water, as even leaky suction organs can allow for sufficient attachment over the timescales required for locomotion, and leakage rates can be reduced by the secretion of mucus [34]. In fact, the almost exclusive occurrence of suction among aquatic animals probably arises because the presence of an incompressible fluid such as water or mucus beneath the suction cup has the advantage that large variations in pressure can be produced by miniscule displacements. Whereas suction is limited by atmospheric pressure when air is in the cavity of the suction organ, water can also resist tensile forces, so that even negative pressures can be achieved [27].
Little is known about the mechanisms of detachment in natural suction organs. Generally, voluntary detachment takes place once the low pressure inside the sucker cavity is neutralized; the fastest way for this to occur may be by relaxation of the muscle(s) that produce(s) the suction for ‘active’ suction systems, but movements by other muscles might be needed to release passive suction. In net-winged midge larvae (Blephariceridae), attachment is achieved by raising a ‘piston’ in the centre of the suction disc. Detachment, in turn, can occur by rapidly ‘flooding’ the sucker through the opening of a V-shaped notch located at the anterior rim of the disc, with the piston still in its upper position [35].
By far the most dynamic control of adhesion occurs in adhesive systems which rely on interfacial forces, including both ‘dry’ van der Waals interactions and ‘wet’ capillary forces. Most terrestrial climbing animals belong to this category (a contribution of van der Waals forces has also been discussed for the temporary underwater adhesion of barnacle cyprid larvae, see [24]). There has been substantial convergence both in the morphology of the adhesive systems of terrestrial climbing animals, and in the control mechanisms they employ [12,36–47]. Detachment in these adhesive systems neither requires chemical release agents (as for glues), nor muscular action to neutralize pressure gradients (as for suction). Instead, rapid control of adhesion is achieved through mechanical systems. The universal strategy for rapidly reversible attachment is the control of adhesion via shear forces, which we review in detail in the following sections.2
3. Control of adhesive forces in dynamic biological adhesive systems
The maximum force an adhesive can carry is the product of its adhesive strength, and the area of contact. Animals could thus control how well they adhere in two ways: first, they could alter the fraction of the available adhesive area which they bring into contact; second, they could vary the strength of the contact, i.e. alter the force required to detach a unit area of their sticky pads.
In dynamic biological adhesive systems, the universal control parameter for both variables is shear force, i.e. a force acting parallel to the adhesive interface. The typical effects of shear force on contact area, adhesive strength and hence net adhesive force can be summarized as follows: ‘pushing’ pads away from the body results in an unstable contact, reflected in a rapid decrease in contact area and thus effortless detachment, whereas ‘pulling’ pads towards the body results in a stable or increasing contact area, and strong attachment [48–54]. This ‘shear-sensitivity’ of adhesion is widespread across terrestrial climbing animals, including flies [48,55,56], beetles [43,51], leafhoppers [57], bushcrickets [58], stick insects [51,54,59], cockroaches [46,52], ants [11,49,60], bees [49], spiders [12,61], bats [62], tree frogs [59,63,64], and geckos [2,65–67]. Climbing animals can therefore control attachment simply by shearing their adhesive pads along the surface; pulling results in strong attachment, whereas pushing enables easy detachment (an important exception to this rule are ‘friction pads’; see below).
(a). Control of adhesive strength
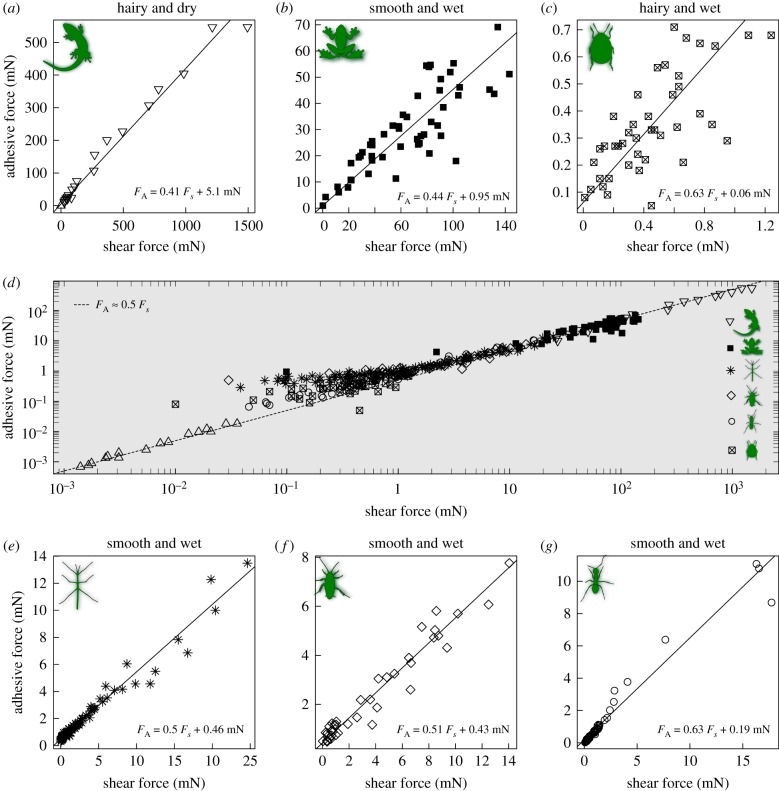
In all adhesive pads of climbing animals tested to date, the adhesive force, i.e. the perpendicular force required to detach the pads, increases when pads are pulled towards the body [46,54,59,60,62,66]. As a rule of thumb, the adhesive force is approximately half the shear force applied during detachment (figure 2, [60,62]). This empirical rule holds for large and small, ‘wet’ and ‘dry’, ‘smooth’ and ‘hairy’ adhesive systems tested with various methods, suggesting the presence of a universal control mechanism that is independent of contact size, adhesive mechanism, pad morphology and experimental method (figure 2). Importantly, the increase of adhesion with shear force arises from an increase in adhesive strength, and not solely from changes in contact area [46,54,66,70]. While this characteristic shear-sensitivity of biological adhesive pads is empirically well-established, the exact mechanisms through which shear forces increase contact strength are still unclear. Why is this relationship approximately linear, and why is the slope of this linear relationship 0.5? To the best of our knowledge, there is currently no theoretical model which predicts these peculiar features from first principles [60,62].
Figure 2.
In all non-aquatic animals climbing with adhesive pads tested to date, adhesion, FA, is approximately half of the shear force, FS, acting during detachment (see panel (d)); the dashed line visualizes this approximate ‘rule of thumb’, which appears to hold for (a) geckos (Gekko gecko, seta, array and toe-level data; [59,60]), (b) tree frogs (Litoria caerulea, whole-body data; [66]), (c) dock beetles (Gastrophysa viridula, single-pad data, D Labonte & JMR Bullock 2015, unpublished data), (e) stick insects (Carausius morosus, single-pad data; [62]), (f) cockroaches (Nauphoeta cinerea, single-pad data; [68]) and (g) ants (Oecophylla smaragdina, single-pad data; [69]). Shear force therefore appears to be a universal control mechanism independent of pad morphology (smooth or hairy), adhesive mechanism (wet or dry) or contact size. Detailed regression results for (a–c) and (e–g) can be found in the electronic supplementary material. (Online version in colour.)
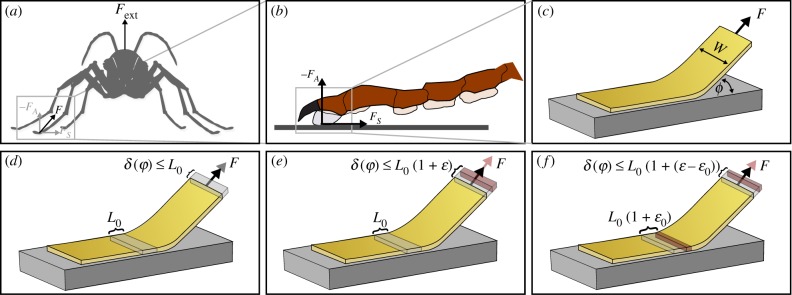
The most successful theoretical attempts at explaining the shear-sensitivity of biological adhesive pads have been based on tape peeling theory, which likens the pads to thin strips of adhesive tape ([62,66,71–73], figure 3a–c). Based on peeling theory, the effect of shear forces may be understood qualitatively as follows (for a quantitative discussion, see [62]): breaking adhesive contacts increases the system’s total energy, as it creates new surfaces. This energy per unit area, G, is ‘paid for’ by the work done when the point of force application moves by a distance δ while detaching a unit length L0 of tape (figure 3d). In this framework, climbing animals can hence increase the adhesive force FA by (i) increasing G or (ii) reducing δ. Actively applying a shear force makes good use of both options in at least three different ways.
Figure 3.
(a) Owing to the sprawled-leg posture of most climbing animals, externally applied attachment forces result in the application of both a normal and a shear force at the level of individual pads (illustrated in (b); climbing animals can also apply shear forces actively). These shear forces make it harder to detach the pads, and this ‘shear-sensitivity’ can be partially understood through peeling models (c), which liken adhesive pads to thin strips of adhesive tape, with width w, peeled at an angle ϕ. (d) As a unit length L0 of the tape is peeled, the point of force application moves by a fraction of this distance. Because this fraction decreases with decreasing peeling angle (or increasing shear force component), more force needs to be supplied to do the required work, leading to an apparent ‘strengthening’ of the contact. (e) Biological adhesive pads are thin and soft, and therefore probably stretch upon detachment (strain ɛ). This stretching increases the work done upon detachment, so reducing the effect outlined in (d). (f) The negative effect of pad stretching can be circumvented if the tape is stretched prior to detachment (‘pre-strain’ ɛ0). Storing strain energy in attached parts of the tape can not only make involuntary detachment less likely, but also aid rapid voluntary detachment. A more detailed discussion is provided in the text. (Online version in colour.)
First, as the shear component of the applied force is increased, the pads peel at lower angles ϕ. For a perpendicular pull-off, δ = L0, but for pull-offs at lower angles, the point of force application only moves by a fraction of the peeled tape length, L0 (1 − cos(ϕ)), so that δ ≤ L0 ([74], figure 3d). Thus, a larger force F must be supplied to provide the same amount of work. To resist detachment, animals can hence reduce δ/L0 = 1 − cos(ϕ) by decreasing ϕ, i.e. by actively pulling their pads inwards. This effect is based purely on geometry.
Unfortunately, there is a limit to this strategy: detached parts of the pads stretch, and this stretch increases δ ([75], figure 3e). As the amount of work done for stretching the pad is larger than the associated increase in elastic strain energy [75], stretching reduces the force required to peel off the pads. For soft and thin biological adhesive pads, this effect would severely limit the positive effect of applying a shear force.
It is here where the second effect comes in: large shear forces eventually result in pad sliding, which also strains attached parts of the pads prior to detachment ([62], figure 3f). Upon detachment, these ‘pre-stretched’ pads then stretch less than unstretched pads, reducing the negative impact of stretching outlined above. This second effect is thus based on ‘energy dissipation’ [61,62,76]: the work done for pad stretching does not help to create new surfaces, but is instead balanced by the frictional work done when pads are stretched while still in contact with the surface [62,76]. Here, shear-sensitive biological adhesives differ fundamentally from many commercial high-strength adhesives, as they dissipate energy at the interface (via friction) instead of the bulk (via cavitation, viscoelastic fingering, etc.) [61,62,77].
The effect of (i) a decrease in peel angle and (ii) stretching attached parts of the tape have both been included in quantitative models [62,73–76,78], which show good agreement with experimental data on biological adhesive systems [62,66]. However, data for stick insects showed that this agreement was limited to peel angles larger than approximately 30°. For smaller peel angles, adhesive forces systematically exceeded theoretical predictions [62]. There hence must be a third effect. In contrast to the first two effects, which reduce δ, this effect must reflect an increase in G, which may be understood as follows: the positive effects of pad sliding are bounded by the geometrical limit δ ≤ L0 − L0 cosϕ, i.e. the distance moved for a unit length of tape which does not stretch at all upon detachment (a more formal proof is presented in the electronic supplementary material). While pad sliding can thus make even thin and soft pads behave as if they were effectively inextensible [62], the peeling model for such tapes only predicts a square-root dependency of adhesion on shear force (in the limit of large shear forces, see [46,62]). As the observed relationship is linear, the only way to reconcile peeling theory with the experimental data is therefore a shear-induced increase in G.
In stick insects, the departure from peeling theory coincided with the onset of whole-pad sliding [70], so that it appears plausible that sliding results in an increase of G. Two hypothetical mechanisms could explain such an increase: first, sliding may result in triboelectric charging. However, the relationship between adhesion and friction remained unaltered on conducting surfaces [70], suggesting that triboelectric effects do not play an important role; second, sliding can rapidly deplete liquid films in the contact zone [79], and such changes in film thickness may cause an increase in G [62,77]. Indeed, recent experiments suggested that the contact-mediating secretion found in all insects studied to date acts as a ‘release layer’, consistent with this hypothesis [77,80]. However, fluid depletion should only occur in animals with wet adhesive systems, so that we would still be lacking an explanation for identical data on dry adhesive systems [60].
Clearly, more theoretical and experimental work is required to quantitatively explain the approximately linear relationship between adhesion and friction in biological adhesive systems. The sharp drop of adhesion for peel angles more than 30° is biologically important, as it allows animals to use relatively small movements to switch from weak to strong adhesion. Clarifying the basis for this most fundamental adhesion control mechanism across climbing animals should therefore be a core area for future research.
(b). Control of contact area
Changes in contact area occur by definition during attachment and detachment, but as any soft object increases its contact area when pressed against a substrate, and decreases it during pull-off, not all such changes correspond to an active control mechanism. In the following discussion, we will therefore focus on non-trivial contact area changes in two scenarios: in the context of attachment, we will describe active and passive adjustments of the contact area which occur rapidly as a direct result of increased loading requirements. In the context of voluntary detachment, we will discuss strategies through which the contact can be broken by other means than a perpendicular pull-off.
(i). Active and passive control of attachment
In technical adhesives, contact formation is typically achieved via the application of a force perpendicular to the interface. In sharp contrast, the contact area of dynamic biological adhesive systems can be controlled via shear forces. An increase of contact area in response to shear forces towards the body has been found across most dynamic adhesive systems of climbing animals studied to date, despite the striking diversity of adhesive pad morphologies. For example, the smooth footpads of ants and bees can unfold passively (without any muscular action) when the retracted pad in surface contact is dragged towards the body [49,64]. The adhesive footpad of stick insects is not foldable, but possesses an internal fibrillar ultrastructure, which hydraulically translates longitudinal pulls into a lateral expansion of the adhesive contact zone [81]. The hairy adhesive pads of lizards, many arachnids and diverse insects, in turn, possess spatula-shaped tips with a non-adhesive ‘default’ (non-contact) position, i.e. they are not parallel to the substrate. Only when setae are sheared towards the body do the tips bend and come into full contact [43,60,82,83]. Importantly, the increase of contact area with shear force is not an all-or-nothing reaction. Stronger shear forces generally lead to larger contact areas, until the contact zone has reached its maximum size [49,65], allowing a gradual adjustment to external loads. Such gradual contact area adjustments can be made actively, i.e. via the contraction of muscles pulling feet inwards (or pushing them outwards), but they can also arise passively. Because of the sprawled posture of climbing arthropods and vertebrates, legs are pulled inwards automatically by the animal’s body weight during inverted climbing (or pushed outwards during horizontal locomotion); during vertical climbing, legs above the body centre of gravity (CoG) will be pulled automatically, whereas those below will be pushed. External forces resulting from wind, rain or from carrying loads, further add to the shear force arising from the animal’s own body weight.
Because shear forces arise passively in situations where strong attachment is required, shear-sensitivity ensures an ‘automatic’ engagement and activation of the adhesive organs. Indeed, it is no coincidence that adhesion control is both active and passive. Neuromuscular control of attachment and detachment is essential for climbing, and for adjusting to different environmental conditions, substrate geometries and textures [84,85]. However, passive mechanisms simplify the complexity of the active feedback control that needs to be mastered for successful climbing [86], and a purely mechanical response triggered by shear forces can result in extremely rapid increases in adhesive contact area. For example, the pad contact area of the smooth adhesive pads of weaver ants and stick insects can double within less than a millisecond of a perturbation [66]. Even for small animals such as insects, any control via active neuromuscular ‘reflexes’ would take at least an order of magnitude longer. The virtually instantaneous ‘preflex’ is hence essential for preventing detachment during rapid and unpredictable perturbations (such as raindrops or wind gusts), and avoids the need to use large contact areas and therefore high detachment forces during locomotion. In practice, contact area is thus probably controlled by a combination of passive and active loads. Indeed, in cockroaches walking upside down, loading triggers the activation of the tibial flexor muscle which mediates an inward pull of the legs [87], suggesting a coupling between active and passive control mechanisms.
While probably the dominant mechanism, shear forces are not the only way in which the adhesive contact area can be controlled. Some climbing animals are also capable of directly influencing the adhesive contact zone by muscular control. For example, contraction of the claw flexor muscle in insects and spiders can not only bring adhesive pads into surface contact, but also induce local deformations of the cuticle which increase the size of the contact zone; its relaxation in turn can drive the pad’s detachment ([6,49,56,64,86,88,89], and see next section). Recent findings show that adhesive pads of tree frogs contain bundles of smooth muscle fibres which may be involved in the direct control of adhesion [90].
(ii). Rapid detachment via release of elastic energy
Voluntary detachment during climbing locomotion has to occur rapidly, and should consume minimal amounts of energy. Both needs are at least partly met through an inbuilt release mechanism which arises as a direct consequence of pad engagement: owing to the shear-sensitivity of their pads, climbing animals need to pull their legs inwards in order to resist detachment. As outlined above, the resulting shear forces cause deformations of the attachment structures which typically increase the adhesive contact area. For example, shear forces straighten curved adhesive setae or the tarsus as a whole [43,61,64,86], bend adhesive hair tips or internal rod-like structures [43,58,81], unfold smooth pads or expand their cuticle along the transverse axis [49,81] and probably stretch pads and adhesive hair tips along the longitudinal axis [62,81,91–93]. All these deformations bring with them the storage of elastic energy, which, upon removal of the shear force, can help break the contact, and even result in spontaneous detachment of the pads in the absence of external forces [61,73,93].
An intuitive way to understand how storing elastic energy can result in spontaneous detachment is to think of a pad as a thin strip of adhesive tape, which is stretched to a ‘pre-strain’ ɛ0 prior to or during surface attachment. If the elastic energy stored in the stretched tape exceeds the decrease in surface energy associated with contact formation, the contact is unstable, because a detached but relaxed tape corresponds to a more favourable energetic state. In the electronic supplementary material, we show that spontaneous detachment of a tape requires a minimum pre-strain (here, ζ = Eh/G is a characteristic dimensionless number representing the ratio between elastic and adhesive work done during detachment; h is the thickness of the tape, and E its Young’s modulus). If this strain is exceeded, the tape can only adhere if an external force is applied (see also [73]). The somewhat complex relationship between contact stability and pre-strain can be visualized in ‘stability envelopes’, encompassing combinations of pre-strain and applied force for which stable attachment is possible (figure 4). For ɛ0 < ɛmin, these plots only have an upper bound, corresponding to the critical peel force. In this regime, detaching the tape always requires the application of a force. If ɛ0 > ɛmin, however, the envelopes also have a lower bound, corresponding to the force required to stabilize the tape against spontaneous detachment (see the electronic supplementary material and [73]). In this regime, voluntary detachment can simply be triggered by reducing the applied force below this lower bound, causing the tape to peel spontaneously. Remarkably, pre-stretching hence not only enhances the resistance against forced detachment, but also provides a mechanism for fast and effortless voluntary detachment [73]. Storage of elastic energy during attachment to recover it for detachment helps to balance the contradictory demands of a strong yet easy-to-detach adhesive system, and may therefore be a key principle enabling controllable adhesion.
Figure 4.
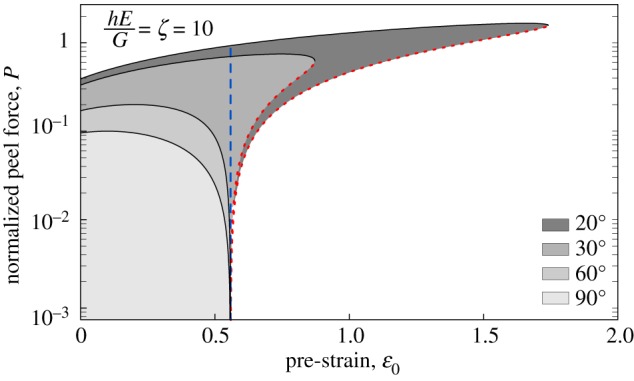
‘Stability envelopes’ for tape peeling at varying tape pre-strains and peel angles ϕ. If the strain exceeds a minimum strain ɛmin (dashed, vertical blue line), stable attachment requires the application of the minimum force to stabilize the contact (dotted red line). In this regime, spontaneous detachment occurs whenever the applied force drops below this lower bound, providing a rapid and efficient detachment mechanism. (Online version in colour.)
As compelling as these arguments may be, the extent to which climbing animals use ‘pre-strain’ to ease detachment remains unclear. Benefiting from the release mechanism described above requires ɛ0 > ɛmin. For biological adhesive pads, the required strain levels are probably unrealistically large (for an estimated range of 1 < ζ < 100, ; we provide a more detailed discussion of the limits of this model in the electronic supplementary material). Thus, longitudinally stretched pads are unlikely to be the sole provider of the elastic energy which drives detachment, but other deformations in pads and legs may be involved (see above). The compliance of the pads or such external structures needs to be fine-tuned to the system’s demands, so as to enable sufficient elastic energy storage without requiring excessive stresses or strains [80]. More generally, adhesive pads may be designed so that unloading does not cause complete detachment but only facilitates it, as maintaining some active control over detachment may be adaptive to protect against unwanted detachment by perturbations. An example of an actively controlled detachment that does not appear to rely on the release of elastic energy is the detachment of gecko toes by digital hyperextension [94].
(c). Contact mechanics affect organismal-level locomotion, and vice versa
Locomotion constrains how adhesive pads can be attached and detached, but surface attachment also influences locomotion. Comparisons of animals climbing on vertical, horizontal and inverted surfaces, as well as on non-slippery versus slippery substrates have revealed clear differences. Flies walking upside down showed a higher duty factor (average proportion of legs in surface contact) than when walking horizontally. While flies mainly used a tripod gait for horizontal walking, they switched to gaits with four or more legs in surface contact when climbing [95,96]. Similar kinematic adjustments are seen in insects climbing on slippery substrates (e.g. waxy plant stems; [97]), or following pad contamination [98]. In both cases, insects showed an increase in the duty factor, accompanied by a decrease in step frequency and walking speed. The detailed causes triggering these kinematic changes are still unclear; they may include both direct physical effects of gravity or slipping on locomotion, and sensory detection of substrate orientation, substrate texture or leg slip, followed by active adjustment of locomotion. Indeed, numerous sensors have been identified on the legs and tarsi of insects, which can detect substrate engagement and leg slip, and trigger the activation of the grip-enhancing claw flexor and tibial flexor muscles [85,99,100]. Sensory feedback is doubtlessly essential for adapting locomotion to changes in load and environmental conditions [87,101].
The higher number of legs in surface contact during inverted climbing may simply provide insects with a proportional increase in adhesive capacity, but it may also be critical for attaching and detaching their shear-sensitive adhesive pads. A simple geometric rule that may always hold during slow inverted walking is that the projection of the body CoG onto the surface must be located within the polygon formed by the feet in contact. If this condition is met, all the legs in stance can be under tension and sheared inwards simultaneously. This ‘inverted stability’ rule is equivalent to the rule for static stability during upright walking, which demands that the CoG has to fall within the polygon of support in order to avoid toppling [102]. In contrast to the rule of static stability, the minimum number of legs in surface contact for achieving stable inward shear is two [103]. However, under quasi-static conditions (and assuming that small insect pads can only produce negligible torques around their contact zones), at least four legs must be in surface contact to allow detachment of one leg by unloading or pushing. Hence, the higher number of legs in contact during inverted walking may not only increase adhesion, but also enable controlled detachment, and as such may arise as a direct consequence of the control mechanisms on the level of single adhesive contacts.
Pad detachment can be driven by the controlled release of elastic energy, but it can also be achieved by an increase of the peel angle, which strongly reduces adhesion as predicted by tape peeling models. As joint torques in running animals are typically minimized by keeping force vectors approximately aligned along the legs [104,105], changing from a low to a high peel angle for detachment would require a corresponding movement of the leg. Such a ‘rolling’ motion (lifting the tarsus from the proximal side) is a ‘normal’ part of walking and running for the forward-oriented front legs of lizards and insects, but is less natural for middle and hind legs, as it would require the animals to walk sideways or backwards, respectively. Indeed, observations on ants and flies suggest that rolling is commonly used only by the front legs, whereas middle and hind legs mostly detach without such an angle change [69,106].
While the shear sensitivity of adhesion allows animals to efficiently switch adhesion on and off during locomotion, it also leads to constraints. As adhesive pads detach easily when pushed, they are not suitable for transmitting forces in this direction. Both during horizontal running and vertical climbing, however, at least some of the legs have to produce pushing forces. Vertically climbing tree frogs and geckos can solve this problem by adjusting the orientation of their limbs and digits for head-up, head-down or lateral climbing, so that for each leg some toes are pointing upwards, in the correct orientation to support the body weight by pulling [107–109]. Climbing arthropods, however, possess only one tarsus per leg. Similar to geckos and frogs, vertically climbing insects can re-orient their legs to some extent so that a larger proportion of their body weight is supported by legs above the body centre of gravity, where the adhesive pads are in the correct pulling orientation [110,111]. However, using only the legs above the body CoG for climbing would impose a severe constraint on locomotion.
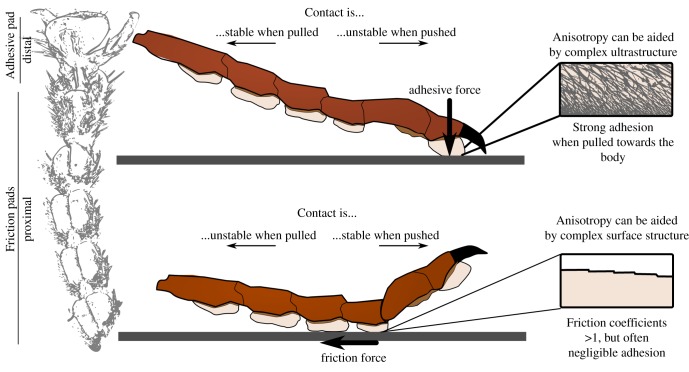
Many arthropods have therefore evolved distinct types of attachment devices on their tarsus, which allow them to push (figure 5). For example, vertically climbing cockroaches engage the tarsal pads (euplantulae) in legs below the body CoG, but use the distal adhesive pad in legs above the CoG [52]. Similar observations have been made in beetles, stick insects and crickets [54,112,113]. The ability to produce large pushing forces is particularly essential for insects performing explosive jumps by rapidly extending their hind limbs [65]. As pushing is typically coupled with positive normal forces, the proximal tarsal pads do not need to produce adhesion, but only high traction forces; they have therefore been termed friction pads (figure 5). Single-pad force measurements in stick insects demonstrate that the biomechanical properties of friction pads can differ fundamentally from those of adhesive pads [54,114]: while adhesive pads produce high adhesion when activated by shear forces, friction pads produce little adhesion, but high friction coefficients when activated by normal forces [54]. Because of the sprawled posture of arthropods, adhesive pads therefore automatically increase adhesion when exposed to pull-off forces, whereas friction pads automatically increase friction when loaded during natural locomotion. Therefore, both types of pad may be thought of as ‘self-stabilizing’. Their mechanisms of attachment and detachment are also analogous: both store elastic energy during contact formation, the release of which then drives detachment.
Figure 5.
Comparison of adhesive pads and friction pads in insects. Both pad types are specialized for resisting forces in different directions, and thereby serve fundamentally different functions. Adhesive pads are located distally on the foot, and produce high adhesion when activated by shear (pulling) forces, whereas friction pads are located proximally on the foot, and produce high coefficients of friction even when pressed only gently against the substrate. (Online version in colour.)
The different functional adaptations of proximal friction pads and distal adhesive pads are also reflected in their anatomical position, which can be explained by the chain-like flexibility of the arthropod tarsus. As the tarsus buckles easily, the tibia can transmit large pushing forces only to the friction pads on the proximal tarsus, but not to the distal adhesive pads. However, the tarsus is a tensile structure, and can easily transmit a strong pull to the distal adhesive pads. As a result, pulling legs towards the body increases contact area for (distal) adhesive pads, but decreases the contact area of (proximal) friction pads; the opposite holds when legs are pushed [51,52]. Because this anisotropy is at least partly based on the structure of the insect leg, it can be reduced by immobilizing the tarsus and pre-tarsus. Such experimental manipulation strongly reduces or even reverses anisotropy in insects with smooth adhesive pads (tested in cockroaches and stick insects, [51,52,115]), but in some cases, directionality is retained, probably owing to anisotropic surface sculptures or the complex fibrous ultrastructure characteristic of smooth pads ([58,81,116], figure 5). In hairy pads, in contrast, the reduction is much weaker because of the direction-dependence at the level of individual setae, which is based on their angled orientation and non-parallel tips [12,51].
4. Conclusion
The ability of many animals to climb on vertical and even inverted surfaces has struck scientists with awe for centuries, and is a hallmark of dynamic biological adhesive systems. Strong adhesion is achieved by maximizing the amount of energy needed for the creation of new surfaces; rapid detachment, in turn, requires the exact opposite. Controlling adhesion on short timescales hence requires combining two opposing demands. The shear-sensitivity of dynamic biological adhesive systems is an ingenious strategy to solve this conundrum, as it uses the same mechanism to achieve both: during attachment, elastic energy is stored in the attachment systems, but does not drive detachment, as the active application of a stabilizing force renders continued pad attachment energetically favourable. During voluntary detachment, climbing animals can actively use the stored strain energy to trigger pad detachment, and thus effortlessly detach their feet. Further research should uncover more of the fundamental physical principles underlying this core property, which will enable the efficient design of strong yet highly dynamic bio-inspired adhesives.
Supplementary Material
Acknowledgements
We thank Jonas Wolff for pointing us towards the data for spiders included in the electronic supplementary material, table S2.
Endnotes
During the locomotion of some gastropods, muscular waves moving along the underside of the foot switch different parts of the foot between stationary adhesive contact (stance) and forward sliding over mucus (swing), so that the protracted part is not completely detached but remains in surface contact [13].
Many insects possess several attachment pads per leg. Unless otherwise stated, we are referring to distal adhesive pads in this review, and only briefly discuss the role of proximal friction pads.
Data accessibility
This article has no additional data.
Competing interests
We declare we have no competing interests.
Funding
This study was supported by a research grant by the Biotechnology and Biological Sciences Research Council (B/R017360/1) to D.L., and the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement no. 642861 to W.F. We are grateful for support from the European Network of Bioadhesion Expertise, financed by the European COST programme, Action CA 15216.
References
- 1.Peattie A. 2009. Functional demands of dynamic biological adhesion: an integrative approach. J. Comp. Physiol. B 179, 231–239. ( 10.1007/s00360-008-0310-8) [DOI] [PubMed] [Google Scholar]
- 2.Autumn K. 2006. Properties, principles, and parameters of the gecko adhesive system. In Biological adhesives (eds Smith A, Callow J), pp. 225–256. Berlin, Germany: Springer. [Google Scholar]
- 3.Cutkosky MR. 2015. Climbing with adhesion: from bioinspiration to biounderstanding. Interface Focus 5, 20150015 ( 10.1098/rsfs.2015.0015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eisenhaure J, Kim S. 2017. A review of the state of dry adhesives: biomimetic structures and the alternative designs they inspire. Micromachines 8, 125 ( 10.3390/mi8040125) [DOI] [Google Scholar]
- 5.Croll AB, Hosseini N, Bartlett MD. In press. Switchable adhesives for multifunctional interfaces. Adv. Mater. Technol. ( 10.1002/admt.201900193) [DOI] [Google Scholar]
- 6.Heming B. 1971. Functional morphology of the thysanopteran pretarsus. Can. J. Zool. 49, 91–108. ( 10.1139/z71-014) [DOI] [PubMed] [Google Scholar]
- 7.Stork N. 1980. Experimental analysis of adhesion of Chrysolina polita (Chrysomelidae: Coleoptera) on a variety of surfaces. J. Exp. Biol. 88, 91–107. [Google Scholar]
- 8.Eisner T, Aneshansley D. 2000. Defense by foot adhesion in a beetle (Hemisphaerota cyanea). Proc. Natl Acad. Sci. USA 97, 6568–6573. ( 10.1073/pnas.97.12.6568) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellem GK, Furst JE, Zimmerman KD. 2002. Shell clamping behaviour in the limpet Cellana tramoserica. J. Exp. Biol. 205, 539–547. [DOI] [PubMed] [Google Scholar]
- 10.Frantsevich L, Gorb S. 2002. Arcus as a tensegrity structure in the arolium of wasps (Hymentoptera: Vespidae). Zoology 105, 225–237. ( 10.1078/0944-2006-00067) [DOI] [PubMed] [Google Scholar]
- 11.Federle W, Endlein T. 2004. Locomotion and adhesion: dynamic control of adhesive surface contact in ants. Arthropod. Struct. Dev. 33, 67–75. ( 10.1016/j.asd.2003.11.001) [DOI] [PubMed] [Google Scholar]
- 12.Wolff JO, Gorb SN. 2013. Radial arrangement of janus-like setae permits friction control in spiders. Sci. Rep. 3, 1101 ( 10.1038/srep01101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denny M. 1980. The role of gastropod pedal mucus in locomotion. Nature 285, 160–161. ( 10.1038/285160a0) [DOI] [Google Scholar]
- 14.Flammang P, Santos R, Haesaerts D. 2005. Echinoderm adhesive secretions: from experimental characterization to biotechnological applications. In Echinodermata (ed. V Matranga), pp. 201–220. Berlin, Germany: Springer. [DOI] [PubMed] [Google Scholar]
- 15.Li D, Huson MG, Graham LD. 2008. Proteinaceous adhesive secretions from insects, and in particular the egg attachment glue of Opodiphthera sp. moths. Arch. Insect Biochem. Physiol. 69, 85–105. ( 10.1002/arch.20267) [DOI] [PubMed] [Google Scholar]
- 16.Bajerlein D, Adamski Z, Kacalak W, Tandecka K, Wiesner M, Jurga S. 2016. To attach or not to attach? the effect of carrier surface morphology and topography on attachment of phoretic deutonymphs of Uropoda orbicularis (Acari). Sci. Nat. 103, 61 ( 10.1007/s00114-016-1385-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waite JH, Andersen NH, Jewhurst S, Sun C. 2005. Mussel adhesion: finding the tricks worth mimicking. J. Adhes. 81, 297–317. ( 10.1080/00218460590944602) [DOI] [Google Scholar]
- 18.Santos R, Gorb S, Jamar V, Flammang P. 2005. Adhesion of echinoderm tube feet to rough surfaces. J. Exp. Biol. 208, 2555–2567. ( 10.1242/jeb.01683) [DOI] [PubMed] [Google Scholar]
- 19.Lengerer B, Pjeta R, Wunderer J, Rodrigues M, Arbore R, Schärer L, Berezikov E, Hess MW, Pfaller K, Egger B. 2014. Biological adhesion of the flatworm Macrostomum lignano relies on a duo-gland system and is mediated by a cell type-specific intermediate filament protein. Front. Zool. 11, 12 ( 10.1186/1742-9994-11-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lengerer B, Bonneel M, Lefevre M, Hennebert E, Leclère P, Gosselin E, Ladurner P, Flammang P. 2018. The structural and chemical basis of temporary adhesion in the sea star Asterina gibbosa. Beilstein J. Nanotechnol. 9, 2071–2086. ( 10.3762/bjnano.9.196) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Persson BNJ. 2007. Wet adhesion with application to tree frog adhesive toe pads and tires. J. Phys.: Condens. Matter 19, 1–16. [Google Scholar]
- 22.Gohad NV, Aldred N, Hartshorn CM, Lee YJ, Cicerone MT, Orihuela B, Clare AS, Rittschof D, Mount AS. 2014. Synergistic roles for lipids and proteins in the permanent adhesive of barnacle larvae. Nat. Commun. 5, 4414 ( 10.1038/ncomms5414) [DOI] [PubMed] [Google Scholar]
- 23.He Y, Sun C, Jiang F, Yang B, Li J, Zhong C, Zheng L, Ding H. 2018. Lipids as integral components in mussel adhesion. Soft Matter 14, 7145–7154. ( 10.1039/C8SM00509E) [DOI] [PubMed] [Google Scholar]
- 24.Aldred N, Clare AS. 2009. Mechanisms and principles underlying temporary adhesion, surface exploration and settlement site selection by barnacle cyprids: a short review. In Functional surfaces in biology (ed. Gorb S.), pp. 43–65. Berlin, Germany: Springer. [Google Scholar]
- 25.Federle W, Barnes WJP, Baumgartner W, Drechsler P, Smith JM. 2006. Wet but not slippery: boundary friction in tree frog adhesive toe pads. J. R. Soc. Interface 3, 689–697. ( 10.1098/rsif.2006.0135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith A. 1991. Negative pressure generated by octopus suckers: a study of the tensile strength of water in nature. J. Exp. Biol. 157, 257–271. [Google Scholar]
- 27.Smith A. 1996. Cephalopod sucker design and the physical limits to negative pressure. J. Exp. Biol. 199, 949–958. [DOI] [PubMed] [Google Scholar]
- 28.Frutiger A. 1998. Walking on suckers: new insights into the locomotory behavior of larval net-winged midges (Diptera: Blephariceridae). J. North Am. Benthol. Soc. 17, 104–120. ( 10.2307/1468055) [DOI] [Google Scholar]
- 29.Kier WM, Smith AM. 2002. The structure and adhesive mechanism of octopus suckers. Int. Comp. Biol. 42, 1146–1153. ( 10.1093/icb/42.6.1146) [DOI] [PubMed] [Google Scholar]
- 30.Schoenfuss HL, Blob RW. 2003. Kinematics of waterfall climbing in hawaiian freshwater fishes (Gobiidae): vertical propulsion at the aquatic-terrestrial interface. J. Zool. 261, 191–205. ( 10.1017/S0952836903004102) [DOI] [Google Scholar]
- 31.Wainwright DK, Kleinteich T, Kleinteich A, Gorb SN, Summers AP. 2013. Stick tight: suction adhesion on irregular surfaces in the northern clingfish. Biol. Lett. 9, 20130234 ( 10.1098/rsbl.2013.0234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y, Shih MC, Wu MH, Yang EC, Chi KJ. 2014. Underwater attachment using hairs: the functioning of spatula and sucker setae from male diving beetles. J. R. Soc. Interface 11, 20140273 ( 10.1098/rsif.2014.0273) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beckert M, Flammang BE, Nadler JH. 2015. Remora fish suction pad attachment is enhanced by spinule friction. J. Exp. Biol. 218, 3551 ( 10.1242/jeb.123893) [DOI] [PubMed] [Google Scholar]
- 34.Kampowski T, Eberhard L, Gallenmüller F, Speck T, Poppinga S. 2016. Functional morphology of suction discs and attachment performance of the mediterranean medicinal leech (Hirudo verbana Carena). J. R. Soc. Interface 13, 20160096 ( 10.1098/rsif.2016.0096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frutiger A. 2002. The function of the suckers of larval net-winged midges (Diptera: Blephariceridae). Freshw. Biol. 47, 293–302. ( 10.1046/j.1365-2427.2002.00814.x) [DOI] [Google Scholar]
- 36.Green DM. 1979. Treefrog toe pads: comparative surface morphology using scanning electron microscopy. Can. J. Zool. 57, 2033–2046. ( 10.1139/z79-268) [DOI] [Google Scholar]
- 37.Alberch P. 1981. Convergence and parallelism in foot morphology in the neotropical salamander genus Bolitoglossa. I. Function. Evolution 35, 84–100. [DOI] [PubMed] [Google Scholar]
- 38.Williams E, Peterson J. 1982. Convergent and alternative designs in the digital adhesive pads of scincid lizards. Science 215, 1509–1511. ( 10.1126/science.215.4539.1509) [DOI] [PubMed] [Google Scholar]
- 39.Stork N. 1983. A comparison of the adhesive setae on the feet of lizards and arthropods. J. Nat. Hist. 17, 829–835. ( 10.1080/00222938300770641) [DOI] [Google Scholar]
- 40.Schliemann H. 1983. Adhesive organs: frequently occurring convergences. Funkt. Biol. Med. 2, 169–177. [Google Scholar]
- 41.Gorb S. 2001. Attachment devices of insect cuticle. Dordrecht, The Netherlands: Kluwer Academic Publishers. [Google Scholar]
- 42.Scherge M, Gorb SN. 2001. Biological micro- and nanotribology: nature’s solutions. Berlin, Germany: Springer. [Google Scholar]
- 43.Federle W. 2006. Why are so many adhesive pads hairy? J. Exp. Biol. 209, 2611–2621. ( 10.1242/jeb.02323) [DOI] [PubMed] [Google Scholar]
- 44.Gorb E, Gorb S. 2009. Effects of surface topography and chemistry of Rumex obtusifolius leaves on the attachment of the beetle Gastrophysa viridula. Entomol. Exp. Appl. 130, 222–228. ( 10.1111/j.1570-7458.2008.00806.x) [DOI] [Google Scholar]
- 45.Gamble T, Greenbaum E, Jackman TR, Russell AP, Bauer AM. 2012. Repeated origin and loss of adhesive toepads in geckos. PLoS ONE 7, e39429 ( 10.1371/journal.pone.0039429) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Labonte D, Federle W. 2015. Scaling and biomechanics of surface attachment in climbing animals. Phil. Trans. R. Soc. B 370, 20140027 ( 10.1098/rstb.2014.0027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolff JO, Gorb SN. 2016. Attachment structures and adhesive secretions in arachnids. Cham, Switzerland: Springer. [Google Scholar]
- 48.Newman E. 1833. Art. lv. transactions of the Linnean society, vol. xvi, part iii. Entomol. Mag. 1, 445–450. [Google Scholar]
- 49.Federle W, Brainerd EL, McMahon TA, Hölldobler B. 2001. Biomechanics of the movable pretarsal adhesive organ in ants and bees. Proc. Natl Acad. Sci. USA 98, 6215–6220. ( 10.1073/pnas.111139298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Drechsler P, Federle W. 2006. Biomechanics of smooth adhesive pads in insects: influence of tarsal secretion on attachment performance. J. Comp. Physiol. A 192, 1213–1222. ( 10.1007/s00359-006-0150-5) [DOI] [PubMed] [Google Scholar]
- 51.Bullock JMR, Drechsler P, Federle W. 2008. Comparison of smooth and hairy attachment pads in insects: friction, adhesion and mechanisms for direction-dependence. J. Exp. Biol. 211, 3333–3343. ( 10.1242/jeb.020941) [DOI] [PubMed] [Google Scholar]
- 52.Clemente CJ, Federle W. 2008. Pushing versus pulling: division of labour between tarsal attachement pads in cockroaches. Proc. R. Soc. B 275, 1329–1336. ( 10.1098/rspb.2007.1660) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Endlein T, Federle W. 2013. Rapid preflexes in smooth adhesive pads of insects prevent sudden detachment. Proc. R. Soc. B 280, 20122868 ( 10.1098/rspb.2012.2868) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Labonte D, Federle W. 2013. Functionally different pads on the same foot allow control of attachment: stick insects have load-sensitive ‘heel’ pads for friction and shear-sensitive ‘to’ pads for adhesion. PLoS ONE 8, e81943 ( 10.1371/journal.pone.0081943) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wigglesworth V. 1987. How does a fly cling to the under surface of a glass sheet? J. Exp. Biol. 129, 373–376. [Google Scholar]
- 56.Niederegger S, Gorb S. 2003. Tarsal movements in flies during leg attachment and detachment on a smooth substrate. J. Insect Physiol. 49, 611–620. ( 10.1016/S0022-1910(03)00048-9) [DOI] [PubMed] [Google Scholar]
- 57.Clemente CJ, Goetzke HH, Bullock JMR, Sutton GP, Burrows M, Federle W. 2017. Jumping without slipping: leafhoppers (Hemiptera: Cicadellidae) possess special tarsal structures for jumping from smooth surfaces. J. R. Soc. Interface 14, 20170022 ( 10.1098/rsif.2017.0022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gorb S, Scherge M. 2000. Biological microtribology: anisotropy in frictional forces of orthopteran attachment pads reflects the ultrastructure of a highly deformable material. Proc. R. Soc. Lond. B 267, 1239–1244. ( 10.1098/rspb.2000.1133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Labonte D, Federle W. 2016. Biomechanics of shear-sensitive adhesion in climbing animals: peeling, pre-tension and sliding-induced changes in interface strength. J. R. Soc. Interface 13, 20160373 ( 10.1098/rsif.2016.0373) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Endlein T, Federle W. 2008. Walking on smooth or rough ground: passive control of pretarsal attachment in ants. J. Comp. Physiol. 194, 49–60. ( 10.1007/s00359-007-0287-x) [DOI] [PubMed] [Google Scholar]
- 61.Hill D. 1977. The pretarsus of salticid spiders. Zool. J. Linn. Soc. 60, 319–338. ( 10.1111/j.1096-3642.1977.tb00838.x) [DOI] [Google Scholar]
- 62.Riskin D, Racey P. 2010. How do sucker footed bats hold on, and why do they roost head up? Biol. J. Linn. Soc. 99, 233–240. ( 10.1111/j.1095-8312.2009.01362.x) [DOI] [Google Scholar]
- 63.Endlein T, Ji A, Samuel D, Yao N, Wang Z, Barnes WJP, Federle W, Kappl M, Dai Z. 2013. Sticking like sticky tape: tree frogs use friction forces to enhance attachment on overhanging surfaces. J. R. Soc. Interface 10, 20120838 ( 10.1098/rsif.2012.0838) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hanna G, Barnes WJP. 1991. Adhesion and detachment of the toe pads of tree frogs. J. Exp. Biol. 155, 103–125. [Google Scholar]
- 65.Autumn K, Liang Y, Hsieh S, Zesch W, Chan W, Kenny T, Fearing R, Full R. 2000. Adhesive force of a single gecko foot-hair. Nature 405, 681–685. ( 10.1038/35015073) [DOI] [PubMed] [Google Scholar]
- 66.Autumn K, Dittmore A, Santos D, Spenko M, Cutkosky M. 2006. Frictional adhesion: a new angle on gecko attachment. J. Exp. Biol. 209, 3569–3579. ( 10.1242/jeb.02486) [DOI] [PubMed] [Google Scholar]
- 67.Gravish N, Wilkinson M, Autumn K. 2008. Frictional and elastic energy in gecko adhesive detachment. J. R. Soc. Interface 5, 339–348. ( 10.1098/rsif.2007.1077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dirks JH. 2009. Mechanisms of fluid-based adhesion in insects. PhD thesis, University of Cambridge, Cambridge, UK.
- 69.Endlein T. 2007. Haftung und Fortbewegung: Kontrollmechanismen von Adhäsionskräften bei Ameisen. PhD thesis, Julius-Maximilians-Universität Würzburg, Germany.
- 70.Labonte D, Clemente CJ, Dittrich A, Kuo CY, Crosby AJ, Irschick DJ, Federle W. 2016. Extreme positive allometry of animal adhesive pads and the size limits of adhesion-based climbing. Proc. Natl Acad. Sci. USA 113, 1297–1302. ( 10.1073/pnas.1519459113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tian Y, Pesika N, Zeng H, Rosenberg K, Zhao B, McGuiggan P, Autumn K, Israelachvili J. 2006. Adhesion and friction in gecko toe attachment and detachment. Proc. Natl Acad. Sci. USA 103, 19 320–19 325. ( 10.1073/pnas.0608841103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pesika N, Tian Y, Zhao B, Rosenberg K, Zeng H, McGuiggan P, Autumn K, Israelachvili J. 2007. Peel-zone model of tape peeling based on the gecko adhesive system. J. Adhes. 83, 383–401. ( 10.1080/00218460701282539) [DOI] [Google Scholar]
- 73.Chen B, Wu P, Gao H. 2009. Pre-tension generates strongly reversible adhesion of a spatula pad on substrate. J. R. Soc. Interface 6, 529–537. ( 10.1098/rsif.2008.0322) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rivlin R. 1944. The effective work of adhesion. Paint Technol. 9, 215–216. [Google Scholar]
- 75.Kendall K. 1975. Thin-film peeling: the elastic term. J. Phys. D: Appl. Phys. 8, 1449–1452. ( 10.1088/0022-3727/8/13/005) [DOI] [Google Scholar]
- 76.Begley MR, Collino R, Israelachvili JN, McMeeking RM. 2013. Peeling of a tape with large deformations and frictional sliding. J. Mech. Phys. Solids 61, 1265–1279. ( 10.1016/j.jmps.2012.09.014) [DOI] [Google Scholar]
- 77.Labonte D, Federle W. 2015. Rate-dependence of ‘wet’ biological adhesives and the function of the pad secretion in insects. Soft Matter 11, 86618673 ( 10.1039/C5SM01496D) [DOI] [PubMed] [Google Scholar]
- 78.Jagota A, Hui C. 2011. Adhesion, friction, and compliance of bio-mimetic and bio-inspired structured interfaces. Mat. Sci. Eng. R. 72, 253–292. [Google Scholar]
- 79.Hutt W, Persson B. 2016. Soft matter dynamics: accelerated fluid squeeze-out during slip. J. Chem. Phys. 144, 124903 ( 10.1063/1.4944384) [DOI] [PubMed] [Google Scholar]
- 80.Betz O, et al. 2017. Adhesion and friction of the smooth attachment system of the cockroach Gromphadorhina portentosa and the influence of the application of fluid adhesives. Biol. Open 6, 589–601. ( 10.1242/bio.024620) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dirks J, Li M, Kabla A, Federle W. 2012. In vivo dynamics of the internal fibrous structure in smooth adhesive pads of insects. Acta Biomater. 8, 2730–2736. ( 10.1016/j.actbio.2012.04.008) [DOI] [PubMed] [Google Scholar]
- 82.Autumn K, Hansen W. 2006. Ultrahydrophobicity indicates a non-adhesive default state in gecko setae. J. Comp. Physiol. 192, 1205–1212. ( 10.1007/s00359-006-0149-y) [DOI] [PubMed] [Google Scholar]
- 83.Gernay S, Federle W, Lambert P, Gilet T. 2016. Elasto-capillarity in insect fibrillar adhesion. J. R. Soc. Interface 13, 20160371 ( 10.1098/rsif.2016.0371) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zill SN, Keller BR, Chaudhry S, Duke ER, Neff D, Quinn R, Flannigan C. 2010. Detecting substrate engagement: responses of tarsal campaniform sensilla in cockroaches. J. Com. Physiol. A 196, 407–420. ( 10.1007/s00359-010-0526-4) [DOI] [PubMed] [Google Scholar]
- 85.Zill SN, Chaudhry S, Exter A, Büschges A, Schmitz J. 2014. Positive force feedback in development of substrate grip in the stick insect tarsus. Arthropod. Struct. Dev. 43, 441–455. ( 10.1016/j.asd.2014.06.002) [DOI] [PubMed] [Google Scholar]
- 86.Frazier SF, Larsen GS, Neff D, Quimby L, Carney M, DiCaprio RA, Zill SN. 1999. Elasticity and movements of the cockroach tarsus in walking. J. Comp. Physiol. 185, 157–172. ( 10.1007/s003590050374) [DOI] [Google Scholar]
- 87.Larsen G, Frazier S, Fish S, Zill S. 1995. Effects of load inversion in cockroach walking. J. Comp. Physiol. A 176, 229–238. ( 10.1007/BF00239925) [DOI] [PubMed] [Google Scholar]
- 88.Dunlop JA. 1994. Movements of scopulate claw tufts at the tarsus tip of a tarantula spider. Neth. J. Zool. 45, 513–520. ( 10.1163/156854295X00447) [DOI] [Google Scholar]
- 89.Frantsevich L, Gorb S. 2004. Structure and mechanics of the tarsal chain in the hornet, Vespa crabro (Hymenoptera: Vespidae): implications on the attachment mechanism. Arthropod Struct. Dev. 33, 77–89. ( 10.1016/j.asd.2003.10.003) [DOI] [PubMed] [Google Scholar]
- 90.Langowski JKA, Schipper H, Blij A, van den Berg FT, Gussekloo SWS, van Leeuwen JL. 2018. Force-transmitting structures in the digital pads of the tree frog hyla cinerea: a functional interpretation. J. Anat. 233, 478–495. ( 10.1111/joa.12860) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Niederegger S, Gorb S, Jiao Y. 2002. Contact behaviour of tenent setae in attachment pads of the blowfly Calliphora vicina (Diptera, Calliphoridae). J. Comp. Physiol. 187, 961–970. ( 10.1007/s00359-001-0265-7) [DOI] [PubMed] [Google Scholar]
- 92.Gorb SN. 2008. Biological attachment devices: exploring nature’s diversity for biomimetics. Phil. Trans. R. Soc. A 366, 1557–1574. ( 10.1098/rsta.2007.2172) [DOI] [PubMed] [Google Scholar]
- 93.Cheng QH, Chen B, Gao HJ, Zhang YW. 2011. Sliding-induced non-uniform pre-tension governs robust and reversible adhesion: a revisit of adhesion mechanisms of geckos. J. R. Soc. Interface 9, 283–291. ( 10.1098/rsif.2011.0254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Russell AP. 2002. Integrative functional morphology of the gekkotan adhesive system (reptilia: Gekkota). Integr. Comp. Biol. 42, 1154–1163. ( 10.1093/icb/42.6.1154) [DOI] [PubMed] [Google Scholar]
- 95.Gorb S. 2005. Uncovering insect stickiness: structure and properties of hairy attachment devices. Am. Entomol. 51, 31–35. ( 10.1093/ae/51.1.31) [DOI] [Google Scholar]
- 96.Mendes CS, Rajendren SV, Bartos I, Márka S, Mann RS. 2014. Kinematic responses to changes in walking orientation and gravitational load in Drosophila melanogaster. PLoS ONE 9, e109204 ( 10.1371/journal.pone.0109204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Federle W, Brüninig T. 2006. Ecology and biomechanics of slippery wax barriers and waxrunning in Macaranga-ant mutualisms. In Ecology and biomechanics: a mechanical approach to the ecology of animals and plants (eds Herrel A, Speck T, Rowe NP), pp. 163–185. Boca Raton, FL: CRC Press. [Google Scholar]
- 98.Amador GJ, Endlein T, Sitti M. 2017. Soiled adhesive pads shear clean by slipping: a robust self-cleaning mechanism in climbing beetles. J. R. Soc. Interface 14, 20170134 ( 10.1098/rsif.2017.0134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ridgel AL, Frazier SF, Dicaprio RA, Zill SN. 1999. Active signaling of leg loading and unloading in the cockroach. J. Neurophysiol. 81, 1432–1437. ( 10.1152/jn.1999.81.3.1432) [DOI] [PubMed] [Google Scholar]
- 100.Ridgel AL, Frazier FS, Zill SN. 2001. Dynamic responses of tibial campaniform sensilla studied by substrate displacement in freely moving cockroaches. J. Com. Physiol. A 187, 405–420. ( 10.1007/s003590100213) [DOI] [PubMed] [Google Scholar]
- 101.Duysens J, Clarac F, Cruse H. 2000. Load-regulating mechanisms in gait and posture: comparative aspects. Physiol. Rev. 80, 83–133. ( 10.1152/physrev.2000.80.1.83) [DOI] [PubMed] [Google Scholar]
- 102.Ting L, Blickhan R, Full RJ. 1994. Dynamic and static stability in hexapedal runners. J. Exp. Biol. 197, 251–269. [DOI] [PubMed] [Google Scholar]
- 103.Heepe L, Raguseo S, Gorb SN. 2017. An experimental study of double-peeling mechanism inspired by biological adhesive systems. Appl. Phys. A 123, 124 ( 10.1007/s00339-016-0753-9) [DOI] [Google Scholar]
- 104.Full R, Blickhan R, Ting L. 1991. Leg design in hexapedal runners. J. Exp. Biol. 158, 369–390. [DOI] [PubMed] [Google Scholar]
- 105.Wöhrl T, Reinhardt L, Blickhan R. 2017. Propulsion in hexapod locomotion: how do desert ants traverse slopes? J. Exp. Biol. 220, 1618–1625. ( 10.1242/jeb.137505) [DOI] [PubMed] [Google Scholar]
- 106.Endlein T, Federle W, Sitti M. 2015. Directional adhesion and locomotion: insects detach adhesive pads of front and hind legs in fundamentally different ways. In Society for Experimental Biology Annual Main Meeting, Prague, 2015. p. A11.17. [Google Scholar]
- 107.Barnes WJP, Oines C, Smith J. 2006. Whole animal measurements of shear and adhesive forces in adult tree frogs: insights into underlying mechanisms of adhesion obtained from studying the effects of size and scale. J. Comp. Physiol. A 192, 1179–1191. ( 10.1007/s00359-006-0146-1) [DOI] [PubMed] [Google Scholar]
- 108.Birn-Jeffery AV, Higham TE. 2014. Geckos significantly alter foot orientation to facilitate adhesion during downhill locomotion. Biol. Lett. 10, 20140456 ( 10.1098/rsbl.2014.0456) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Russell AP, Oetelaar GS. 2016. Limb and digit orientation during vertical clinging in Bibron’s gecko, Chondrodactylus bibronii (A. Smith, 1846) and its bearing on the adhesive capabilities of geckos. Acta Zool. 97, 345–360. ( 10.1111/azo.12128) [DOI] [Google Scholar]
- 110.Endlein T, Federle W. 2015. On heels and toes: how ants climb with adhesive pads and tarsal friction hair arrays. PLoS ONE 10, e0141269 ( 10.1371/journal.pone.0141269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dallmann CJ, Dürr V, Schmitz J. 2019. Motor control of an insect leg during level and incline walking. J. Exp. Biol. 222, 188748 ( 10.1242/jeb.188748) [DOI] [PubMed] [Google Scholar]
- 112.Bullock JMR, Federle W. 2009. Division of labour and sex differences between fibrillar, tarsal adhesive pads in beetles: effective elastic modulus and attachment performance. J. Exp. Biol. 212, 1876–1888. ( 10.1242/jeb.030551) [DOI] [PubMed] [Google Scholar]
- 113.Grohmann C, Henze MJ, Nørgaard T, Gorb SN. 2015. Two functional types of attachment pads on a single foot in the Namibia bush cricket Acanthoproctus diadematus (Orthoptera: Tettigoniidae). Proc. R. Soc. B 282, 20142976 ( 10.1098/rspb.2014.2976) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Labonte D, Williams J, Federle W. 2014. Surface contact and design of fibrillar ‘friction pads’ in stick insects (Carausius morosus): mechanisms for large friction coefficients and negligible adhesion. J. R. Soc. Interface 11, 20140034 ( 10.1098/rsif.2014.0034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bußhardt P, Wolf H, Gorb S. 2012. Adhesive and frictional properties of tarsal attachment pads in two species of stick insects (Phasmatodea) with smooth and nubby euplantulae. Zoology 115, 135–41. ( 10.1016/j.zool.2011.11.002) [DOI] [PubMed] [Google Scholar]
- 116.Clemente C, Dirks J, Barbero D, Steiner U, Federle W. 2009. Friction ridges in cockroach climbing pads: anisotropy of shear stress measured on transparent, microstructured substrates. J. Comp. Physiol. A 195, 805–814. ( 10.1007/s00359-009-0457-0) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.