Abstract
Hashimoto's encephalopathy is a rare disease with nonspecific symptoms, associated with elevated levels of anti‐TPO and/or anti‐TG. It can be potentially fatal. However, it is responsive to steroid and treated in due time, it can be fully reversible.
Keywords: dementia, Hashimoto's encephalopathy, SREAT, steroid‐responsive encephalopathy associated with autoimmune thyroiditis
1. INTRODUCTION
Steroid‐responsive encephalopathy associated with autoimmune thyroiditis (SREAT), also known as Hashimoto's encephalopathy (HE), is a rare but probably underdiagnosed disease.1, 2, 3, 4 The estimated prevalence is 2.1 per 100.000.4 HE can present with various symptoms, usually with acute or subacute onset. Confusion, memory impairment, speech disorder, gait disturbance, myoclonus, hallucinations, paranoia, depression, seizures, and coma are examples of symptoms described in the literature.1, 2, 3, 4, 5, 6, 7, 8, 9, 10
The disease is associated with elevated levels of anti‐thyroid peroxidase (TPO) and/or anti‐thyroglobulin (TG).1, 2, 3, 9 The thyroid‐stimulating hormone (TSH) level is often normal. Elevated protein levels are typically seen in the cerebrospinal fluid; pleocytosis may also be present. EEG findings are often abnormal but nonspecific with generalized slowing. MRI findings can be normal or show nonspecific features.1, 2
High doses of steroids are used as first‐line treatment.1 Full or partial clinical remission is common, but some patients may experience one or several relapses and therefore require repeated treatments.1, 2
2. CASE REPORT
A 66‐year‐old man, without any prior history of neurological diseases, was referred to the regional memory clinic in March 2014 due to subacute symptoms developing over a few weeks followed by 6‐9 months of progressive symptoms. The patient had problems with performing daily practical tasks. He described memory impairment, progressive problems with his speech, dizziness, fluctuating awareness, balance problems, apraxia, twitching of the upper and lower extremities, mood changes, and hallucinations. The objective neurological examination showed discreet aphasia and dysarthria, discreet bilateral resting tremor, truncal instability, myoclonus and severe bilateral apraxia, and dysdiadochokinesia. The finger‐nose‐finger test showed accentuated bilateral tremor. The Mini‐Mental State Examination Score was 25/30 points (normally >26). The neuropsychological examination described a cognitive profile with a dysexecutive/subcortical picture. The patient fulfilled the formal criteria for dementia (see Table 1).
Table 1.
Selected neuropsychological test data before and after treatment with steroids
| June 2014 | November 2014 | |||
|---|---|---|---|---|
| Test | Score | Percentiles/scale score | Score | Percentiles/scale score |
| Continuous subtraction (100‐7) | 0/0 (135 sec, 6 errors) | 10/10 (40 sec, 0 errors) | ||
| Trail making B | Gives up after 3 min | 173 sec, 1 error | >10 pctl | |
| Stroop |
Dots: 50 sec, 0 errors Color words: 172 sec, 17 errors |
Dots: 40 sec, 0 errors Color words: 79 sec, 1 error |
||
| ADAS‐COG | Mean error score 5, recalls 6/10 | Mean error score 2, recalls 9/10 | ||
| Fluency | ||||
| animals | 14 | 8 pctl | 24 | 66 pctl |
| s‐words | 10 | 28 pctl | 15 | 64 pctl |
| Matrix Reasoning (WAIS‐IV) | 5/26 | Scale score 3 | 10/26 | Scale score 7 |
| Design fluency | Gives up | 20 | 27 pctl | |
| Picture Arrangement (WAIS‐III) | 1/22 | Scale score 2 | 7/22 | Scale score 7 |
Cerebral CT had previously excluded subdural hematoma and supplemental MRI showed normal findings. Chest X‐Ray and full‐body‐PET showed no signs of extra‐cerebral malignancy. Also, test results for paraneoplastic antibodies in blood were normal. There were negative biomarkers for Alzheimer's dementia (Aß42, F‐tau; P‐tau) in the cerebrospinal fluid, but elevated levels of both protein and leukocytes. The patient was tested negative for Lyme disease. Brain‐FDG‐PET findings showed decreased global cortical glucose metabolism (see Figure 1), interpreted as not indicative of a neurodegenerative etiology, but rather suggestive of a metabolic or toxic effect on cerebral metabolism.
Figure 1.
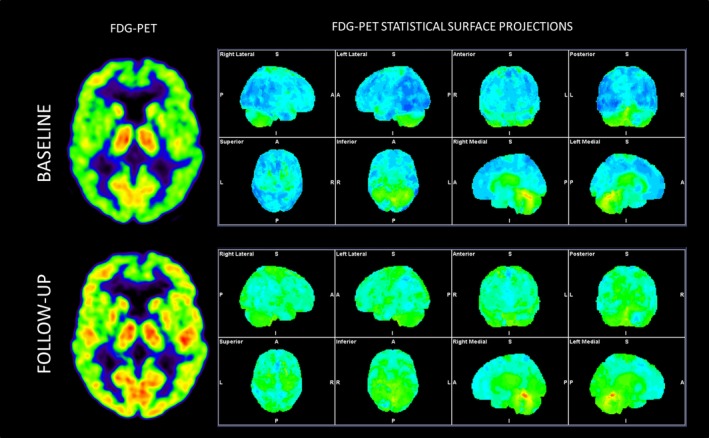
18F‐FDG‐PET before and after treatment with steroids (ca. 6 months between the two scans)
Supplemental blood tests were ordered, including thyroid antibodies. The anti‐TG level in serum was 879 (normal range <115). Other blood tests were normal, including anti‐TPO, TSH, ferroxidase, and copper. As HE was suspected, treatment with oral prednisolone was initiated.
The patient showed clinical improvement after just a few days of treatment, with approximate subjective remission after 2 weeks. A significant improvement of the cerebral glucose metabolism was seen on the brain‐FDG‐PET performed 2 months after the treatment (see Figure 1). A neuropsychological re‐examination showed remarkable improvement compared with previous examinations (see Table 1.). After the end of treatment, the patient experienced four relapses in total, in the period up to January 2016. Each time, he was treated with oral prednisolone with positive clinical response as a result. He received the last treatment in February 2016, and at the follow‐up afterward, there were no signs of further relapses. The relumbar puncture, performed due to the last relapse in 2016, showed that there were still elevated levels of protein and leukocytes in the cerebrospinal fluid prior to re‐institution of steroid treatment. Thyroid antibodies and tests for other types of autoimmune encephalitis were negative. The MRI was still normal. Screening blood tests for connective tissue disorders were also normal. In August 2017, the patient had not shown symptoms for over a year and was therefore declared cured.
3. DISCUSSION
Hashimoto's encephalopathy is a rare disease, but probably also underdiagnosed.1, 2, 3, 6 The exact relationship between the anti‐thyroid antibodies and the encephalopathy is still unknown.1, 9, 10 To our knowledge, there are no previous publications presenting follow‐up data from both FDG‐PET and neuropsychological examination, respectively, before and after treatment.
There is no diagnostic test available for HE, and the differential diagnosis is broad. Therefore, other possible etiologies must be excluded before making the diagnosis.3, 7 Etiologies that should be considered prior to making the diagnosis of HE, include CNS infection, inflammatory conditions such as systemic lupus, and neurodegenerative diseases such as CJD, paraneoplastic limbic encephalitis, and vascular causes.7, 10 The patient in our case was screened negative for vascular, paraneoplastic and infectious causes, connective tissue disorders, and other autoimmune causes. Alzheimer's disease was also ruled out.
If a patient presents with rapidly progressive dementia or subacute encephalopathy with unspecific symptoms and normal test results, without other obvious etiologies, HE should be considered, also with normal levels of TSH 1, 2, 3, 10 and no pre‐existing thyroid disorder.1 In this case report, the antibody reaction may be regarded as the triggering factor, and the elevated levels of protein and the pleocytosis in the cerebrospinal fluid were due to an ongoing inflammatory process in the central nervous system, despite negative antibodies at relapse. There are several cases described in the literature where only one of the antibodies has been elevated,1, 8 and there has not been proven a correlation between the antibody level and the severity of clinical symptoms.2, 9 The patient was treated many times due to relapses, but experienced full neurological remission, without any further relapses, after the last treatment. A fast initiation of treatment is crucial, to avoid irreversible, potentially fatal complications.6 Knowledge of the disease is important, despite its rarity, because it can be fully reversible if treated in due time.3, 8, 9
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTION
Ronja MB Lagström wrote the first draft of the article, collected comments from co‐authors, and prepared the final version. Natascha N Østerbye was responsible for the neuropsychology, prepared the table on neuropsychometrical data, and revised the draft. Otto M Henriksen was responsible for the PET‐imaging, prepared the images for the article, and revised the draft. Peter Høgh was the primary physician involved, was responsible for the clinical data, revised the draft, and was supervising the publication process.
ACKNOWLEDGMENTS
No acknowledgements to declare.
Lagström RMB, Østerbye NN, Henriksen OM, Høgh P. Hashimoto's encephalopathy: Follow‐up data from neuropsychology, lumbar puncture, and FDG‐PET. Clin Case Rep. 2019;7:1750–1753. 10.1002/ccr3.2367
REFERENCES
- 1. Laurent C, Capron J, Quillerou B, et al. Steroid‐responsive encephalopathy associated with autoimmune thyroiditis (SREAT): characteristics, treatment and outcome in 251 cases from the literature. Autoimmun Rev. 2016;15:1129‐1133. [DOI] [PubMed] [Google Scholar]
- 2. Castillo P, Woodruff B, Caselli R, et al. Steroid‐responsive encephalopathy associated with autoimmune thyroiditis. Arch Neurol. 2006;63:197‐202. [DOI] [PubMed] [Google Scholar]
- 3. Liyanage CK, Munasinghe T, Paramanantham A. Steroid‐responsive encephalopathy associated with autoimmune thyroiditis presenting with fever and confusion. Case Rep Neurol Med. 2017;2017:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ferracci F, Bertiato G, Moretto G. Hashimoto's encephalopathy: epidemiologic data and pathogenetic considerations. J Neurol Sci. 2004;217(2):165‐168. [DOI] [PubMed] [Google Scholar]
- 5. Brain L, Jellinek EH, Ball K. Hashimoto's disease and encephalopathy. Lancet. 1966;2:512‐514. [DOI] [PubMed] [Google Scholar]
- 6. Lopez‐Giovaneli J, Moreaud O, Faure P, Debaty I, Chabre O, Halimi S. Cortico‐responsive encephalopathy associated with autoimmune thyroiditis (SREAT): about two case reports characterized by a gap between the diagnosis of autoimmune thyroiditis and neurological disorders. Ann Endocrinol (Paris). 2007;68:173‐176. [DOI] [PubMed] [Google Scholar]
- 7. Lee SW, Donlon S, Caplan JP. Steroid responsive encephalopathy associated with autoimmune thyroiditis (SREAT) or Hashimoto's encephalopathy: a case and review. Psychosomatics. 2011;52(2):99‐108. [DOI] [PubMed] [Google Scholar]
- 8. Ryan SA, Kennedy C, Harrington HJ. Steroid‐responsive encephalopathy associated with autoimmune thyroiditis presenting as confusion, dysphasia, and myoclonus. Case Rep Med. 2012;2012:1‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Olmez I, Moses H, Sriram S, Kirshner H, Lagrange AH, Pawate S. Diagnostic and therapeutic aspects of Hashimoto's encephalopathy. J Neurol Sci. 2013;331(1‐2):67‐71. [DOI] [PubMed] [Google Scholar]
- 10. Creutzfeldt CJ, Haberl RL. Hashimoto encephalopathy: a do‐not‐miss in the differential diagnosis of dementia. J Neurol. 2005;252(10):1285‐1287. [DOI] [PubMed] [Google Scholar]


