Abstract
Malignant struma ovarii presenting with follicular carcinoma is extremely rare, and its mechanism of tumorigenesis remains unknown. Here, we present a case of malignant struma ovarii with peritoneal dissemination of follicular carcinoma, for which a molecular analysis for major oncogenic gene alterations in follicular thyroid carcinoma was performed. A 39-year-old nulliparous woman was referred with a diagnosis of highly differentiated follicular carcinoma of ovarian origin. Primary thyroid cancer was not diagnosed, and she had a normal thyroid function. 123I scintigraphy revealed multiple peritoneal dissemination that was surgically resected. Histologically, the tumor consisted of numerous follicles without nuclear features of papillary thyroid carcinoma. Tumor samples were investigated for 50 cancer-related genes, including RAS, BRAF, and p53, and PPARg-PAX8 gene fusion by targeted DNA sequencing and fluorescence in situ hybridization, respectively. No major oncogenic gene alterations were detected. These negative findings suggest a different mechanism of tumorigenesis from that of adult-type follicular thyroid carcinoma.
Keywords: Malignant struma ovarii, Follicular carcinoma, RAS, PPARgamma, BRAF
Highlights
-
•
Malignant struma ovarii presenting with follicular carcinoma (MSOFC) is rare and often shows long-latency recurrence.
-
•
Detailed molecular investigation of MSOFC was firstly performed to identify its specific molecular characteristics.
-
•
Lack of common molecular alterations suggests MSOFC may have different tumorigenesis from that of adult thyroid counterpart.
1. Introduction
Struma ovarii is a specialized or monodermal teratoma predominantly composed of mature thyroid tissue, and its malignant counterpart has thyroid cancer components. Malignant struma ovarii (MSO) presenting with follicular-type carcinoma is extremely rare. Most MSO lesions histologically appear as papillary carcinoma; approximately 50 cases of MSO with follicular-type carcinoma have been reported in the English literature (Table 1). Recently, driver gene alterations of thyroid cancer have been elucidated, and a few reports showed that MSO presenting with papillary carcinomas harbored BRAF or RAS gene mutations (Gobitti et al., 2017; Tan et al., 2015) that are frequently observed in papillary thyroid carcinoma (Cancer Genome Atlas Research, 2014). However, MSO with follicular carcinoma (MSOFC) has not been assessed for known major molecular alterations in follicular thyroid carcinoma such as RAS gene mutations and PPARg-PAX8 fusion (Raman and Koenig, 2014; Vasko et al., 2003).
Table 1.
Previously reported cases of malignant struma ovarii with follicular carcinoma.
| # | First author | Year | Age | Extraovarian spread | Treatment of extraovarian disease | Prognosis and follow-up |
|---|---|---|---|---|---|---|
| 1 | De Graaff | 1983 | 72 | Peritoneal dissemination | Omentectomy | NED, 3 yrs |
| 2 | Willemse | 1987 | 36 | Peritoneal dissemination | RAI | Rec, 4 mo |
| 3 | O'Connell | 1990 | 35 | No | None | NED |
| 4 | Zakhem C1 | 1990 | 52 | No | None | NED, 1.7 yrs |
| 5 | Zakhem C2 | 1990 | 30 | No | None | NED, 2.7 yrs |
| 6 | Kragel | 1991 | 37 | Peritoneal dissemination | None | NED, 5 yrs |
| 7 | Thomas C1 | 1992 | 17 | Peritoneal dissemination | RAI | NED, 2 yrs |
| 8 | Thomas C2 | 1992 | 35 | Peritoneal dissemination | Omentectomy | NA |
| 9 | Ito | 1992 | 36 | Bone metastasis | Chemotherapy | NED, 1 mo |
| 10 | Balasch | 1993 | 36 | Peritoneal dissemination | RAI to be considered | NA |
| 11 | Ayhan | 1993 | 66 | Peritoneal dissemination | Omentectomy | AWD, 8 yrs |
| 12 | Tokuda | 1993 | 25 | Cranial metastasis | Resection | NED |
| 13 | Karseladze | 1994 | 49 | Peritoneal dissemination | Chemotherapy | NED, 16.3 yrs |
| 14 | Piana | 1994 | 26 | No | None | NED 1 yr |
| 15 | Brenner | 1996 | 49 | Urinary bladder metastsis | RAI | NED, 3.2 yrs |
| 16 | Tennvall C1 | 1997 | 50 | Peritoneal dissemination | Omentectomy, RAI | NED, 6 yrs |
| 17 | Tennvall C2 | 1997 | 43 | Peritoneal dissemination | None | Died(GBC), 4 mo |
| 18 | Mango | 1997 | 47 | Pelvic bone metastasis | RAI | AWD, 4 mo |
| 19 | Barrande | 1997 | 31 | No | RAI | NED, 6 mo |
| 20 | Bhansali | 1999 | 47 | No | Radiation | NED, 4 yrs |
| 21 | Takeuchi C1 | 2000 | 43 | Peritoneal dissemination | Omentectomy | NED, 7 yrs |
| 22 | Takeuchi C2 | 2000 | 70 | Peritoneal dissemination | Omentectomy, PLND | NED, 2 yrs |
| 23 | Rotman-Pikielny | 2000 | 46 | Liver metastasis | RAI | AWD, 6 mo |
| 24 | Konez | 2000 | 45 | Liver metastasis | RAI | NA |
| 25 | Chan | 2001 | 27 | Bone metastasis | RAI | AWD, 8 mo |
| 26 | Checrallah | 2001 | 38 | Lung and bone metastasis | RAI | AWD, 6 yrs |
| 27 | DeSimone | 2003 | 32 | No | RAI | NED, 1.2 yrs |
| 28 | Kdous | 2003 | 45 | No | None | NED, 1 yr |
| 29 | Brogsitter | 2004 | 50 | Peritoneal dissemination | RAI | NED, 6 mo |
| 30 | Ihalagama | 2004 | 27 | Pritoneal cyology positive | RAI | NED 1.5 yrs |
| 31 | Garcia | 2005 | 22 | Invasion to adjucent tissue | Resection | NED, 6 yrs |
| 32 | McDougall | 2006 | 17 | Liver and bone metastasis | RAI | AWD, 4 yrs |
| 33 | Zekri | 2006 | 26 | Lung and bone metastasis | RAI | AWD, 15 yrs |
| 34 | Roth, C1 | 2008 | 32 | Peritoneal dissemination | RAI | Rec, 26 yrs |
| 35 | Roth, C2 | 2008 | 49 | Peritoneal dissemination | Chemotherapy | NED, 16.3 yrs |
| 36 | Roth, C3 | 2008 | 50 | Peritoneal dissemination | Resection & RAI | NED, 6 yrs |
| 37 | Roth, C4 | 2008 | 70 | Peritoneal dissemination | RAI | DOD, 3 yrs |
| 38 | Prasad | 2008 | 40 | Pelvic mass | Resection & RAI | NED, 4 yrs |
| 39 | Kim | 2009 | 49 | Peritoneal dissemination | Omentectomy | NA |
| 40 | Michels | 2010 | 41 | Peritoneal dissemination | RAI | AWD, 3 mo |
| 41 | Selvaggi | 2012 | 50 | Peritoneal dissemination | Omentectomy | NED, 1 yr |
| 42 | Shirimali, C1 | 2012 | 52 | Vaginal vault mass | RAI | NA |
| 43 | Shirimali, C2 | 2012 | 59 | Peritoneal dissemination | RAI | NA |
| 44 | Shirimali, C3 | 2012 | 53 | Peritoneal dissemination | RAI | NA |
| 45 | Carey | 2014 | 70 | Peritoneal dissemination | RAI | NA |
| 46 | Ukita | 2014 | 45 | Lung and bone metastasis | Chemotherapy | AWD, 20 yrs |
| 47 | Cong | 2015 | 38 | Lung metastasis | RAI | AWD, 3 yrs |
| 48 | Kobayashi | 2015 | 49 | Spinal metastasis | Radiation | AWD, 9 mo |
| 49 | Ranade | 2015 | 55 | Peritoneal dissemination | RAI | AWD, 3 mo |
| 50 | Park | 2015 | 35 | Peritoneal dissemination | RAI | NED, 25 mo |
| 51 | Anagnostou | 2016 | 64 | Peritoneal dissemination | Omentectomy | NED, 4 yrs |
| 52 | Riggs | 2018 | 32 | Peritoneal dissemination | Laparoscopic resection | NED, 1 yr |
| 53 | Present case | 2019 | 39 | Peritoneal dissemination | Resection & RAI | AWD, 2 yrs |
Abbreviations; RAI, radioactive iodine therapy; NED, no evidence of disease; Rec, recurrence; NA, not available; AWD, alive with disease; GBC, gallbladder cancer; DOD, death of disease; C# denotes case# in the same report; PLND, pelvic lymph node dissection.
The complete list of all the reference above is provided as Supplementary material.
The histological diagnosis of MSOFC is often challenging, because it is frequently impossible to evaluate capsular invasion in the ovary. In these cases, a diagnosis of MSOFC is based on the evidence of malignant behaviors such as the presence of vascular invasion, dissemination, or metastasis. Therefore, the identification of a specific molecular alteration in MSOFC can be a useful biomarker for the prediction of malignant behavior of MSOFC even when confined to the ovary.
Herein, we report a case of MSOFC presenting with peritoneal dissemination for which we performed molecular analysis including DNA sequencing, immunohistochemistry, and fluorescent in situ hybridization (FISH) for major pathogenic molecular alterations in thyroid follicular carcinoma. To the best of our knowledge, this is the first report of MSOFC with detailed molecular analysis.
2. Case presentation
A 39-year-old nulliparous woman was referred with a diagnosis of highly differentiated follicular carcinoma of ovarian origin (HDFCO). She underwent left salpingo-oophorectomy for mature cystic teratoma with thyroid tissue in the left ovary 16 years ago and cystectomy for right struma ovarii 8 years ago. Two years ago, an approximately 4 cm wide ovarian mass was detected that had increased in diameter up to 7 cm. Furthermore, other new lesions were found in the pelvic cavity. These lesions were surgically resected and histologically diagnosed as peritoneal dissemination of HDFCO. She had no primary thyroid cancer, and thyroid function test results (serum T3, T4, and TSH) were normal. At our hospital, 123I scintigraphy revealed multiple lesions in the abdominopelvic cavity and she underwent an abdominal hysterectomy, right salpingo-oophorectomy, and partial omentectomy for residual tumors. All macroscopic tumors were removed. She was discharged without any complications. Although no macroscopic residual disease was found intraoperatively, intraperitoneal microscopic residual disease was detected via 123I scintigraphy performed 6 weeks after the surgery. Additional radioactive iodine therapy (RAI) was planned for the residual disease, and total thyroidectomy was performed in preparation for RAI. Pathological examination revealed an adenomatous goiter without any malignant features. Two months after thyroidectomy, RAI was initiated. After completing 4 cycles of RAI, the patient has been alive with residual disease for 2 years after the gynecologic surgery.
2.1. Pathological findings
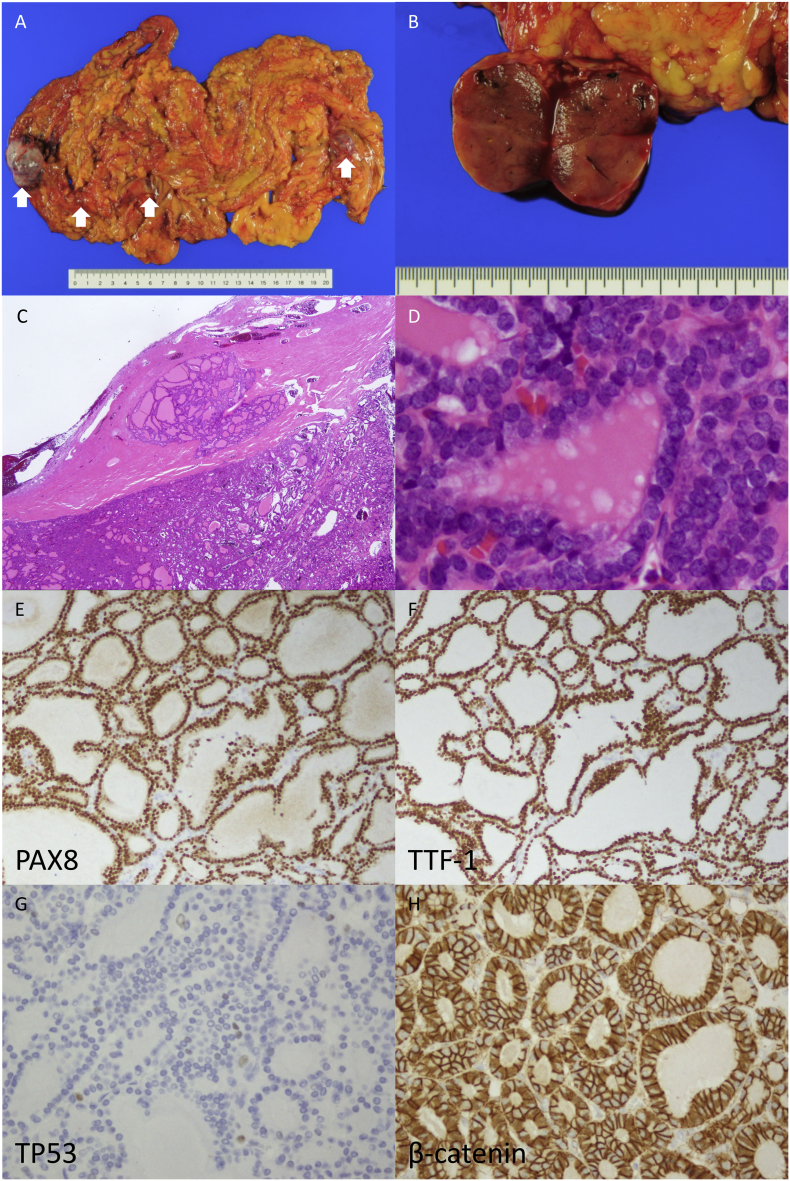
Macroscopically, there were multiple tan-colored solid tumors on the omentum (Fig. 1A and B), and the largest mass was 4 × 3 × 2.5 cm in size. Microscopically, there were many follicles of various sizes composed of tumor cells without nuclear features of papillary thyroid carcinoma such as overlapping nuclei, irregular contours, nuclear grooves, pseudo inclusions, and chromatin clearing. Microscopic lesions were also found in the right ovary and on the uterine serosa. No carcinoid component was observed. Tumor cells showed mild nuclear atypia and formed numerous micro- to normal-sized follicles, resembling a thyroid follicular tumor rather than non-neoplastic thyroid tissue. Immunohistochemically, tumor cells showed diffuse positivity for TTF-1 and PAX8 (Fig. 1E and F). The final pathological diagnosis was peritoneal dissemination of MSOFC.
Fig. 1.
Malignant struma ovarii presenting with follicular carcinoma. (A) There are multiple tumor nodules on the omentum. (B) The cut surface of the tumor is tan-colored and solid. (C) At low magnification, there are follicles of various sizes containing pink-colored colloid resembling thyroid follicular tumor. (D) At higher magnification, no nuclear features suggesting papillary carcinoma, nuclear groove, ground-glass appearance, and intranuclear cytoplasmic inclusion are observed. Immunohistochemically, tumor cells show diffuse positivity for PAX8 (E) and TTF-1 (F). Scattered p53 positive tumor cells showing a wild-type staining pattern (G). Nuclear accumulation of β-catenin is not observed. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.2. Molecular analysis
All molecular analyses were approved by the institutional review board of our hospital. A representative section of an omental lesion was selected for all subsequent analyses. Immunohistochemical analyses for p53, β-catenin, BRAFV600E, and PTEN and split FISH assay for PPARgamma rearrangement were performed on formalin-fixed paraffin-embedded specimens. Targeted sequencing of 50 cancer-related genes (the Ion Ampliseq™ Cancer Hotspot Panel version 2) was performed using DNA extracted from the resected tumor tissue. All the targeted genes are listed in the supplementary materials and methods. These 50 genes included well-known proto-oncogenes and tumor suppressor genes such as BRAF, EGFR, ERBB2, HRAS, KIT, KRAS, NRAS, PIK3CA, PTEN, APC, CTNNB1, RET, and TP53. Details of the methods of molecular analysis mentioned above are provided in the supplementary materials and methods.
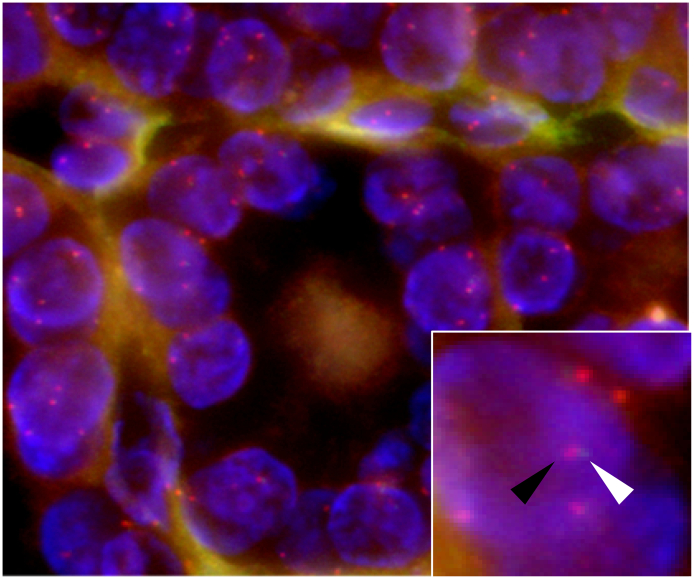
The results of these analyses are described below. Immunohistochemically, tumor cells showed scattered positivity for p53, the so-called wild-type pattern (Fig. 1G). Nuclear accumulation of β-catenin was not observed (Fig. 1H). The tumor cells tested negative for BRAFV600E. Split probe FISH assay for PPARgamma showed no split signals in the nuclei of tumor cells (Fig. 2). Target sequencing revealed no pathogenic/oncogenic mutations in the 50 cancer-related genes, including BRAF, EGFR, HRAS, KRAS, NRAS, KIT, CTNNB1, and TP53.
Fig. 2.
Split probe fluorescence in situ hybridization assay for PPAR-gamma gene rearrangement. Red signal (distal gene region) and green signal (proximal gene region) were not split in the nuclei of tumor cells. The black and white arrowheads indicate red and green signals, respectively (inset). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3. Discussion
We, herein, reported a rare case of MSOFC presenting with peritoneal dissemination for which we performed a molecular analysis. The major known driver gene alterations in follicular thyroid carcinoma were not detected, which implied the possibility of a different mechanism of tumorigenesis for MSOFC.
MSOFC is extremely rare, and about 50 cases have been reported, including HDFCO (the so-called peritoneal strumosis) and MSO with typical follicular carcinoma (Table 1). To date, there has been only one case of death due to this tumor with a poorly differentiated follicular carcinoma component (Roth et al., 2008). Most cases did not show dismal prognoses; however, the long-span of recurrent disease deteriorates the quality of patients' lives.
Making a diagnosis of MSOFC is challenging, especially for cases confined to the ovaries, and histomorphological features play a limited role in predicting subsequent malignant behavior. The present case was diagnosed as mature teratoma with thyroid tissue 16 years ago and the same tumor cells formed multiple lesions in the right ovary 8 years ago, on the peritoneum, and omentum 2 years ago. Unless peritoneal dissemination, evidence of malignant behavior, was detected, a diagnosis of MSOFC could not be rendered. This is because the tumor confined to the ovary showed no histological evidence of malignancy such as vascular invasion. Therefore, any specific molecular abnormalities in MSOFC would become useful biomarkers for predicting peritoneal dissemination or metastasis.
Driver gene mutations of thyroid cancer have been well investigated, and a correlation with histological subtypes has also been reported (Cancer Genome Atlas Research, 2014; Raman and Koenig, 2014; Vasko et al., 2003). The majority of papillary thyroid cancers are reported to have BRAFV600E, RAS, and RET genetic alterations, which are most often mutually exclusive (Cancer Genome Atlas Research, 2014). Moreover, follicular thyroid cancers harboring either RAS mutations or PAX8/PPARG gene fusion comprise approximately 80% of cases (Gianoukakis et al., 2011). Furthermore, less differentiated carcinomas, such as anaplastic carcinomas, have been reported to harbor p53 mutations or abnormalities in β-catenin signaling. The phosphatidylinositide 3-kinase (PI3K)/AKT pathway is also affected and can occur by activating mutations in PIK3CA or AKT1, or loss of PTEN. A few reports showed that BRAF mutations frequently occur in MSO with papillary carcinoma but not benign tumors (Schmidt et al., 2007), and RAS mutations were reported in MSO with follicular variants of papillary carcinoma (Tan et al., 2015). These authors postulated that MSO with papillary carcinoma might share a mechanism of carcinogenesis with papillary thyroid carcinoma. Recently, Park et al. first reported a case of MSOFC without mutation of BRAFV600E, RAS (HRAS codon 61, NRAS codon 61, and KRAS codon 12/13), and PAX8/PPARG gene fusion (Park et al., 2015). Unfortunately, detailed materials and methods of the analysis are not given in the article. In line with this previous report, we did not detect any major molecular alterations in this case, regardless of more extensive analyses, including mutation analyses of 50 cancer-related genes. These negative findings may suggest different mechanisms of tumorigenesis in MSOFC. Interestingly, infrequent RAS mutations and PPARgamma rearrangement is reported as a unique feature of pediatric follicular thyroid carcinoma. Vuong et al. reported that only 12.2% (5/41) of NRAS mutations and no PPARgamma rearrangements occurred in 41 pediatric cases of follicular thyroid carcinoma (Vuong et al., 2017). We, therefore, speculate that MSOFC is a type of malignant transformation of monodermal teratoma and may be similar to pediatric follicular thyroid carcinoma rather than the adult-type.
Since we investigated only one case and analyzed a limited number of gene alterations, a strong conclusion could not be drawn. We expect that our findings will be validated in another cohort of MSOFC cases and evoke additional analyses. The identification of new driver gene alterations may require more extensive investigations such as whole-exome sequencing.
Summarily, we described a case of MSOFC without the major pathogenic molecular alterations of adult-type follicular thyroid cancer. These results imply the possibility of a different tumorigenesis pathway of MSOFC from that of thyroid follicular carcinoma. Further studies are needed to identify biomarkers for predicting malignant behavior.
Consent
Written informed consent was obtained from the patient for the publication of this case report.
Author contributions
Dr. Tsukada and Dr. Yoshida drafted and revised the manuscript and prepared the figures. Dr. Tsukada and Dr. Ishilawa collected the clinical data. Dr. Shiraishi and Dr. Asami performed all the mutation analysis. Dr. Ishikawa and Dr. Kato revised the manuscript. All the authors have read and approved the final manuscript.
Declaration of Competing Interest
We have no conflict of interest to declare. This work was supported by grants-in-aid from the Mitsui Life Social Welfare Foundation and the Public Foundation of the Vaccination Research Center.
Acknowledgments
We would like to thank Editage (www.editage.jp) for English language editing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gore.2019.100498.
Appendix A. Supplementary data
Supplementary material 1
Supplementary material 2
References
- Cancer Genome Atlas Research N Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159(3):676–690. doi: 10.1016/j.cell.2014.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianoukakis A.G., Giannelli S.M., Salameh W.A., McPhaul L.W. Well differentiated follicular thyroid neoplasia: impact of molecular and technological advances on detection, monitoring and treatment. Mol. Cell. Endocrinol. 2011;332(1–2):9–20. doi: 10.1016/j.mce.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Gobitti C., Sindoni A., Bampo C., Baresic T., Giorda G., Alessandrini L., Canzonieri V., Franchin G., Borsatti E. Malignant struma ovarii harboring a unique NRAS mutation: case report and review of the literature. Hormones (Athens, Greece) 2017;16(3):322–327. doi: 10.14310/horm.2002.1750. [DOI] [PubMed] [Google Scholar]
- Park M.J., Kim M.A., Shin M.K., Min H.S. Follicular proliferative lesion arising in struma ovarii. J. Pathol. Transl. Med. 2015;49(3):262–266. doi: 10.4132/jptm.2015.03.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman P., Koenig R.J. Pax-8-PPAR-gamma fusion protein in thyroid carcinoma. Nat. Rev. Endocrinol. 2014;10(10):616–623. doi: 10.1038/nrendo.2014.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth L.M., Miller A.W., 3rd, Talerman A. Typical thyroid-type carcinoma arising in struma ovarii: a report of 4 cases and review of the literature. Int. J. Gynecol. Pathol. 2008;27(4):496–506. doi: 10.1097/PGP.0b013e31816a74c6. [DOI] [PubMed] [Google Scholar]
- Schmidt J., Derr V., Heinrich M.C., Crum C.P., Fletcher J.A., Corless C.L., Nose V. BRAF in papillary thyroid carcinoma of ovary (struma ovarii) Am. J. Surg. Pathol. 2007;31(9):1337–1343. doi: 10.1097/PAS.0b013e31802f5404. [DOI] [PubMed] [Google Scholar]
- Tan A., Stewart C.J., Garrett K.L., Rye M., Cohen P.A. Novel BRAF and KRAS mutations in papillary thyroid carcinoma arising in Struma Ovarii. Endocr. Pathol. 2015;26(4):296–301. doi: 10.1007/s12022-015-9394-3. [DOI] [PubMed] [Google Scholar]
- Vasko V., Ferrand M., Di Cristofaro J., Carayon P., Henry J.F., de Micco C. Specific pattern of RAS oncogene mutations in follicular thyroid tumors. J. Clin. Endocrinol. Metab. 2003;88(6):2745–2752. doi: 10.1210/jc.2002-021186. [DOI] [PubMed] [Google Scholar]
- Vuong H.G., Kondo T., Oishi N., Nakazawa T., Mochizuki K., Miyauchi A., Hirokawa M., Katoh R. Paediatric follicular thyroid carcinoma - indolent cancer with low prevalence of RAS mutations and absence of PAX8-PPARG fusion in a Japanese population. Histopathology. Nov 2017;71(5):760–768. doi: 10.1111/his.13285. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1
Supplementary material 2