Abstract
Background
Shared decision making (SDM) between families and physicians may facilitate informed, timely decisions to proceed with biologic therapy in children with inflammatory bowel disease (IBD). Our team previously developed an SDM tool to aid communication between physicians and families when considering biologic therapy for children with IBD.
Objective
We are conducting a prospective, pre-post pilot trial of a new SDM tool. The primary aim of the study is to assess feasibility of both the intervention and trial procedures for a future large-scale trial.
Methods
We are enrolling physicians with experience prescribing biologic therapy in the past year and families of children with IBD. Families in the intervention arm receive a 3-step intervention including a letter sent before trial consent or clinic appointment, an in-clinic decision tool and a follow-up phone call. Our primary trial outcome is a measure of feasibility, with measures of clinical and decision outcomes secondary. We seek to enroll 27 families in each of 2 arms (usual-care and intervention) and plan data collection at the time of the initial visit or hospital stay, and at 1 week, 3 months, and 6 months after the initial visit.
Conclusion
This study protocol is designed to demonstrate that integrating novel consent procedures, including timing and multiple versions of written consent, may increase trial feasibility while maintaining scientific rigor and full protection of study participants.
Keywords: Pediatrics, Inflammatory bowel disease, Pilot trials, Consent
1. Background
Decision making about treatment with biologic therapies is a complex process for families of children with inflammatory bowel disease (IBD). This process has been shown to cause more uncertainty and decisional conflict in families than some end-of-life decisions parents make for children [1,2]. The emotional investment in deciding about biologic therapy is understandable given the challenges of weighing the benefits of timely, effective treatment against the potential risks of therapy, such as infusion reactions, infections, and cancer [[3], [4], [5], [6]]. Additionally, families may hesitate to proceed with costly therapies that require painful injections or visits to infusion centers for intravenous administration of therapy. Lengthy decision processes are common, and delaying treatment may lead to greater disease complications, while also foregoing potential benefits of early treatment including growth optimization and improved steroid-free remission rates [[7], [8], [9]].
Promoting shared decision making (SDM) between physician and family members often leads to improved outcomes such as less uncertainty about the decision, more efficient decision making, and potentially better health outcomes [10,11]. Facilitating SDM about the use of biologic therapy requires acknowledging that decision making about therapy for IBD occurs across settings and is a prolonged process, beginning before patients are in the office discussing options with their physician and extending beyond the clinical encounter [12]. Prior to discussing treatment options with their physician, families often seek input from online sources, family members, and friends, and begin to consider risks and benefits of starting a new therapy [[12], [13], [14]]. By the time conversations happen in the clinic, families have often already begun the decision-making process.
As the decision-making moves into the clinical encounter, conversations with physicians and physician guidance become an integral component of the process [12]. The content and structure of in-office discussions are therefore important as means of supporting families, correcting misinformation and facilitating SDM, which has been reported to occur in less than 50% of decisions about biologic therapy in IBD [2,15]. In order to address these deficits, we developed a multi-component decision support intervention aimed at facilitating SDM between physician and family. This intervention will be tested in a pilot trial utilizing novel consent processes designed to increase overall trial efficiency while maintaining full protection of study participants.
2. Methods
2.1. Setting and ethics
This study is being conducted at Cincinnati Children's Hospital Medical Center (CCHMC), an urban, tertiary care center where gastrointestinal specialty care is routinely provided for children with IBD. The institutional review board at CCHMC approved the study protocol.
2.2. Study design and duration
We are conducting a prospective clinical trial with repeated measures in a pre-post study design including physicians and families of children with IBD. This design controls for variation in communication style between physicians. Specifically, by having all physicians participate in both study arms, we avoid teaching a new communication skill and then asking physicians to selectively apply it to half of their patients. Physicians will participate for the full length of the trial and in both usual care and intervention arms. Parents and patients (hereafter referred to together as families) will participate for six months from the time of their clinic visit in which treatment with biologic therapy is discussed. Each family will be assigned to either the usual care or the intervention arm based on the timing of their enrollment, with enrollment of the usual care arm to be completed prior to initiating enrollment of the intervention arm.
2.3. Population
We are enrolling physicians caring for children with IBD and families of such children. Physicians are eligible if they care for children with IBD and have prescribed biologic therapy to more than one patient with IBD in the preceding year. Families are eligible to participate if their physician anticipates discussing starting biologic therapy at their scheduled clinic visit or during an acute inpatient hospitalization and was consented to participate in the trial. We are excluding families of patients over age 17 years because such patients do not legally require the input of parents for medical decision making. Additional exclusion criteria for families include: previous treatment with biologic therapy, inability to read or speak English, clinical encounters not conducted in English, major mental illness in the parent or patient, medical instability, or prior participation in the study.
2.4. Recruitment and consent
2.4.1. Physicians
Eligible physicians are invited to participate in the study via presentations at division faculty meetings and individual e-mails. We explain the study to each physician at the time of consent, including a requirement to participate, mid-trial, in training on use of the intervention. All physicians meeting enrollment criteria will be included in the study. As new physicians enter the practice they will be considered for inclusion, as well.
2.4.2. Families: usual-care group
We identify families eligible for enrollment through consultation with gastroenterology physicians in pre-clinic planning conferences and review of clinic schedules of consented physicians. After obtaining permission from the physician, we are approaching families in the clinic setting prior to their appointment to explain the study and invite participation. Families are consented using a written consent that states that the study is being conducted “to learn whether the way treatment decisions are made affects outcomes.” Because the trial does not involve randomization, participants in the usual care arm will have no opportunity to be exposed to the intervention. Due to concern that providing information about the intervention may unintentionally influence usual care, the consent process and documents for this study arm make no mention of an intervention. The consent process does address how the study will be conducted with regard to recording of clinical visits and follow-up data collection. Institutional policies require that children over the age of 7 also provide assent as a complement to traditional parental consent. All families for the usual-care group will be recruited from outpatient settings.
2.4.3. Families: intervention group
We identify families eligible for enrollment using the same processes used for the usual care group plus reviewing inpatient admissions for possible eligible families. Although our initial plan included recruitment from the outpatient setting only, when enrollment lagged our approach evolved to include enrolling patients in the intervention group during hospitalization. We contact outpatient families eligible to participate by phone if their appointment is at least 48 h away, provide basic information about the study and assess their interest. If the appointment is less than 48 h away, a member of the study staff contacts the family in person at the time of their appointment. All eligible families we identify at least 24 h before an outpatient visit receive intervention part A (see section 2.5.2.1 below). We approach families for consent/assent at the time of their appointment. We approach eligible families of hospitalized children during their inpatient stay and obtain consent at that time. All families in the intervention group receive a consent stating each of the interventions that will occur. The consent contains specific information about study procedures including recording of the clinic visit or inpatient treatment discussion and follow up data collection.
2.5. Interventions
2.5.1. Physicians
Following enrollment of the usual care arm, a 1-h in-person training will be provided to all physicians, at a time convenient for them, on fundamentals of SDM, a video demonstrating incorrect and correct use of the SDM support tool and role-playing practice with the in-clinic tool. See section 2.5.2.2 for a description of the tool. Subsequently, all physicians will be videotaped using the SDM tool with a previously scheduled clinic patient, the video will then be evaluated by trained observers, and feedback offered, via email, on their interaction with the family. Training was developed to be of minimal burden by using existing patient encounters as opportunities to practice using the tool. Once the prescribing physicians demonstrate interventional fidelity with the SDM process, they will be free to begin using the tool without feedback from study staff. We will provide additional feedback, based on review of study recordings, every 2 months to ensure fidelity of the intervention.
2.5.2. Families
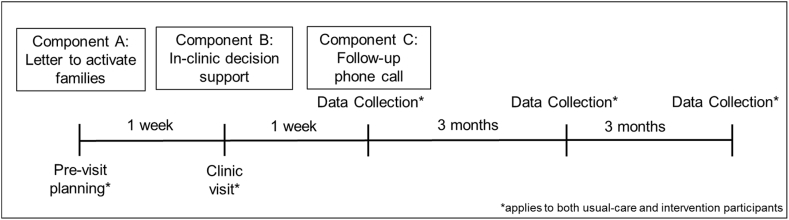
All families included in the trial have visits with their physician recorded, including families in the usual-care group. We are implementing three intervention components directed at families of children with IBD in the intervention arm of the trial. These are described below according to timing of the interventions relative to consent (Fig. 1).
Fig. 1.
Timing of interventions and follow-up for data collection.
2.5.2.1. Component A: pre-consent family activation
Prior to obtaining consent, we send families eligible for the intervention arm a family activation letter via email or priority mail, based on availability of information in the medical record. The activation letter prompts families to consider their goals for treatment for IBD, the ways they anticipate treatment may affect their child's life and any concerns they may have about IBD treatment. Additionally, this letter encourages families to incorporate friends and relatives into their decision making about IBD treatment prior to their clinic visit. Because the letter does not discuss specific treatment options, it requires no response from families. Families will receive this letter on hospital letterhead signed from “The IBD Team.” In most cases, it is not feasible to get consent prior to sending the activation letter, and the local IRB approved sending the letter prior to obtaining consent.
2.5.2.2. Component B: in-person decision support
This intervention component is based upon a well-established model of facilitating SDM conversations between physicians and patients [[16], [17], [18], [19]]. as well as our team's prior work focused on understanding how treatment decisions are made in IBD [13,15,16,[20], [21], [22]]. We developed an IBD specific tool, based on a similar intervention developed for pediatric arthritis [16]. We used images and plain language, to ensure understanding in both adolescent patients and their parents. The tool was tested and refined iteratively through focus groups with patients, parents and physicians, as well as in clinic testing by an IBD physician.
The intervention consists of a series of cards which highlight various characteristics of treatment options (e.g. side effects, how quickly it starts to work, costs) (Fig. 2). Each card provides information on that characteristic for all treatment options. Patients and parents are invited to pick the card they first want to discuss and then continue the conversation with further choices. Cards can be laid side by side to facilitate comparison of treatments across characteristics. The cards contain minimal information as they are designed to be used in conjunction with the physician's expertise; however, a PDF copy of the cards is available for physicians, at their discretion, to give to families to take home.
Fig. 2.
IBD treatment option cards.
2.5.2.3. Component C: follow-up phone call
One week after the appointment or hospital discharge (for inpatients), we call families in the intervention group at a time and number arranged during their consent to participate. The purpose of this phone call is to facilitate ongoing communication between families and their provider, allow families to articulate their decision process and concerns and to offer to forward questions to their care team. Specifically, families are told, “If you have questions that might help you make a decision, I'm happy to take a message and pass it along to [your doctor's] nurse. Then she'll get back to you with some answers.” Any questions they have are sent to their care team as a message in the electronic health record.
2.6. Outcomes
We chose outcomes pertaining to acceptability and feasibility of our SDM tool, clinical outcomes and decision outcomes to explore the practicality of this tool and clinical and psychological implications of its use. All decision outcome measures will be based on the first treatment decision each family makes following study enrollment.
2.6.1. Feasibility and acceptability
We will have two measures of feasibility as our primary outcomes, percent of participants who receive all three interventions and the length of each clinic visit. We are defining length of the visit as the length of time from the physician entering the exam room until the physician leaves for the final time, which may include interruptions. We will consider our intervention feasible if 80% of the participants receive all three components of the intervention and the average clinic visit length does not increase by more than 10%. We determined our target for feasibility (80%) based on previous work in SDM [23]. We selected a 10% increase as our measure of feasibility for length of clinic visit based on dialogue with physicians working in our IBD clinic. We will determine acceptability using a measure adapted from other trials of decision support interventions [19,24]. With regard to physician participation, we will assess feasibility by the percent of eligible physicians who complete training with the SDM tool.
2.6.2. Clinical outcomes
We will assess effectiveness of our interventions with our primary clinical outcome, the 3-month change in disease control score as measured by the pediatric ulcerative colitis activity index (PUCAI) [25] for patients with Ulcerative Colitis, or the short pediatric Crohn's disease activity index (short PCDAI) [26] for patients with Crohn's Disease, and the patient disease activity rating on the current Physician Global Assessment (PGA) [27]. We chose these measures to assess our primary clinical outcome as they are commonly used in studies evaluating pediatric IBD treatment [7,28,29]. Each of these measures is either directly reported in the medical record or can be calculated from other data available in the medical record.
Secondary clinical outcomes will include time to treatment initiation, medication adherence and quality of life. We will measure time to treatment initiation as the number of days from the recorded clinic appointment until the patient receives the first dose of biologic therapy, which always occurs in the clinic, or a prescription for a non-biologic treatment. For treatments given at home, adherence will be measured using pharmacy records to calculate a medication possession ratio (MPR) [30]. Adherence to infusions will be determined based on a modified-MPR calculated as the number of days expected between infusions over the actual number of days between infusions, based on review of the medical record. Like the MPR, this value is capped at 1. We will measure quality of life from the PedsQL generic core, using parent report for patients under age 12 and both parent and patient report for those 12–17 years [31].
2.6.2. Decision outcomes
Our decision outcomes will be parental decisional conflict[32] and decision regret scores[33], as well as the extent of SDM, as measured by two scales. First, we will use the OPTION(5) scale, a widely used, validated tool for assessing SDM in a video-recorded/audio recorded encounter [34,35]. Second, we will use the Shared Decision Making Questionnaire-9 (SDMQ-9) which assesses patient/parent perception of SDM [36]. We will include both measures to assist in future multi-center trial development. Specifically, we are considering the feasibility of OPTION scoring, which requires recording clinic visits and reviewing them. We will compare the OPTION score to the SDQ9 to determine if, in this setting, they appear to measure the same construct.
Additional decision outcomes will include the expectancy scale which measures an individual's thoughts and beliefs regarding what will happen when a new treatment is started, and physician's use of engagement behaviors, as measured by an 8-question item adapted by our group, and used in a prior study from an Institute of Medicine report [37]. Examples of such engagement behaviors include “taking the time to understand your goals and concerns” and “listening to you.” We will collect all measures from parents, as well as patients if they are at least 12 years old.
2.7. Data collection
2.7.1. Families
We will collect data at four time points, during the initial visit, at 1 week, 3 months and 6 months after the initial visit (see Table 1). During the initial visit we will collect information via recording. For patients recruited while hospitalized, physicians will complete the PGA as outlined above. One week after the initial visit we will contact families by telephone to obtain data from parents and children about quality of life and decision making. For families enrolled in the intervention arm, this occurs concurrently with component C of the intervention. Three months after the initial recorded visit a second data collection will occur by phone interview to collect quality of life and decision making data. At this time, we will also use medical record data from the outpatient clinic visit closest to 3 months after enrollment to assess disease control and medication adherence. We will also collect data for the PGA from the medical record. Finally, we will collect medical record data from the outpatient clinic visit closest to 6 months after enrollment and perform our final assessment of disease control and quality of life.
Table 1.
Data collection.
| Visit | 1 wk | 3 mo | 6 mo | |
|---|---|---|---|---|
| Demographics | P | |||
| Acceptability | P,A | P,A | ||
| Disease control (PUCAI, short PCDAI) | M | M | M | |
| Adherence (MPR) | M | M | ||
| Quality of Life (PedsQL) | P, A | P, A | P, A | |
| Time to treatment initiation | M | |||
| Shared Decision Making – Observed (OPTION) | x | |||
| Shared Decision Making – Perceived (SDMQ-9) | P,A | P,A | ||
| Decisional Conflict Scale | P,A | P,A | ||
| Decision Regret Scale | P,A | P,A | ||
| Expectancy Measure | P,A | P,A | ||
| Physician engagement | P,A | |||
| Patient disease activity rating on current Physician Global Assessment | M | M | M |
M = medical record; P = parent; A = adolescent.
2.7.2. Physicians
For families enrolled while inpatient, we approach physicians at the time of enrollment to complete the PGA which is not typically collected inpatient. At the completion of the trial physicians will complete the acceptability survey.
2.8. Sample size
We based sample size calculations on families enrolled, not physicians, and calculated a total of 24 participants were needed in each arm to estimate a 90% feasibility and acceptability rate for each intervention component. We allowed for as much as a 10% attrition rate between enrollment and 3-month follow-up, and therefore an additional 2 to 3 participants were recruited per group, necessitating no more than 27 families per group. A total of 21 subjects are needed to identify a statistically significant difference in disease control between intervention and usual-care groups.
2.9. Analysis
We will generate descriptive statistics for all relevant variables in the data set. Standard proportions complete with 95% confidence intervals will be used for acceptability and intervention component receipt. We will use a Wilcoxon rank-sum test of location to compare differences between the two arms on all clinical and decision outcomes. For repeated measures, including decisional conflict, decision regret and disease control, we will look at the change from baseline to both three and six month follow-up.
3. Discussion
The design of our pilot trial, evaluating an SDM tool for families of children with IBD considering biologic therapy, is novel in regard to consent and timing of interventions.
This pilot trial represents a key step-forward in two different domains of clinical trials. First, the focus of the trial, SDM in pediatric IBD, represents an under-developed area of research. Most trials of SDM interventions have focused on decisions that are likely to occur only once, for example elective surgeries or screening tests [11]. Decisions in chronic conditions are, by their very nature, inherently different as they occur over time and may be revisited [12,38]. Second, while more than 100 trials of decision aids have been conducted(11) only a very small number have been conducted in pediatric settings [39]. Decision making in pediatrics is inherently complicated due to the involvement of a decision triad (physician, parent, patient) and evolving decision skills of the patient as they grow older and take a more active role in medical decision making [40]. Thus, our focus on a pediatric chronic condition represents a crucial step forward in the field of SDM trials.
Second, this current trial would not be feasible without novel approaches to consent. The lack of randomization in this study introduces challenges to consent as mentioning the intervention may bias the usual care group. As a single center pilot study, randomization is not feasible given the limited number of physicians. Additionally, randomization by patient is not realistic given the nature of the interventions. It is impossible to train physicians to use SDM strategies, which should influence long-term communication skills, and then ask them to apply that skill with only some patients. Therefore, we utilized two different written consents, one for the usual care group and one for the intervention group.
Our prior research demonstrated the longitudinal nature of decision making in pediatric IBD and emphasized that decision making starts before families discuss biologic therapy with their physician [12]. We sought to capitalize on this early phase of decision making by activating the family prior to the clinic visit. However, due to time and budget constraints consenting families prior to sending a pre-visit letter (intervention component A) was not feasible. Therefore, we worked with the IRB to design an intervention which could be delivered pre-consent, maximizing the feasibility and efficiency of our trial design.
Due to limitations arising from this being a single center study, our protocol includes recruitment from inpatient and outpatient populations. Because of slow initial study enrollment, we determined enrolling only outpatients would be prohibitively inefficient. The addition of an inpatient population would improve our recruitment potential without requiring additional resources, particularly because the same physicians work in the inpatient and outpatient settings. We considered these benefits and proceeded with this protocol modification acknowledging possible consequences including: potential introduction of bias and increased heterogeneity in our intervention group, as compared to the control group. While this was deemed to be an appropriate approach for a single-center study, a future multi-center trial will need to carefully consider consistency and efficiency in recruiting patients.
Pilot trials to test the feasibility of interventions and study procedures are an essential part of the clinical trials process [41], particularly in new areas of intervention such as SDM in pediatric chronic conditions. We plan to conduct a future, multi-center trial that benefits from our learnings, including point estimates we generate from this pilot study on feasibility. Specifically, a cluster randomized, factorial design may be better poised to further delineate the effects of each component of our intervention. However, the nature of pilot trials creates challenges due to small sample sizes and limited resources. This trial protocol is designed to demonstrate that the use of novel consent procedures, specifically different consents for usual care and intervention arms of the trial, and timing of consent, allow for an efficient and feasible pilot trial that maintains full protection of all study participants.
Funding
This work was supported by the Crohn's and Colitis Foundation. The sponsor had no role in study design, in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Conflicts of interest
Dr. Lipstein has an institutional grant for other work from Pfizer, Inc.
Dr. Denson has an institutional grant for other work from Janssen.
The remaining authors have no relevant conflicts of interest.
References
- 1.Knapp C., Huang I.C., Madden V., Vadaparampil S., Quinn G., Shenkman E. An evaluation of two decision-making scales for children with life-limiting illnesses. Palliat. Med. 2009;23(6):518–525. doi: 10.1177/0269216309104892. PubMed PMID: 19346274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lipstein E.A., Lovell D.J., Denson L.A., Kim S.C., Spencer C., Ittenbach R.F., Britto M.T. High levels of decisional conflict and decision regret when making decisions about biologics. J. Pediatr. Gastroenterol. Nutr. 2016;63(6):e176–e181. doi: 10.1097/MPG.0000000000001425. Epub 2016/10/18. PubMed PMID: 27749390; PMCID: PMC5123667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoentjen F., van Bodegraven A.A. Safety of anti-tumor necrosis factor therapy in inflammatory bowel disease. World J. Gastroenterol. 2009;15(17):2067–2073. doi: 10.3748/wjg.15.2067. Epub 2009/05/07. PubMed PMID: 19418577; PMCID: 2678575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown S.L., Greene M.H., Gershon S.K., Edwards E.T., Braun M.M. Tumor necrosis factor antagonist therapy and lymphoma development: twenty-six cases reported to the Food and Drug Administration. Arthritis Rheum. 2002;46(12):3151–3158. doi: 10.1002/art.10679. Epub 2002/12/17. PubMed PMID: 12483718. [DOI] [PubMed] [Google Scholar]
- 5.Diak P., Siegel J., La Grenade L., Choi L., Lemery S., McMahon A. Tumor necrosis factor alpha blockers and malignancy in children: forty-eight cases reported to the Food and Drug Administration. Arthritis Rheum. 2010;62(8):2517–2524. doi: 10.1002/art.27511. Epub 2010/05/28. PubMed PMID: 20506368. [DOI] [PubMed] [Google Scholar]
- 6.Dulai P.S., Thompson K.D., Blunt H.B., Dubinsky M.C., Siegel C.A. Risks of serious infection or lymphoma with anti–tumor necrosis factor therapy for pediatric inflammatory bowel disease: a systematic review. Clin. Gastroenterol. Hepatol. 2014;12(9):1443–1451. doi: 10.1016/j.cgh.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 7.Walters T.D., Kim M.O., Denson L.A., Griffiths A.M., Dubinsky M., Markowitz J., Baldassano R., Crandall W., Rosh J., Pfefferkorn M., Otley A., Heyman M.B., LeLeiko N., Baker S., Guthery S.L., Evans J., Ziring D., Kellermayer R., Stephens M., Mack D., Oliva-Hemker M., Patel A.S., Kirschner B., Moulton D., Cohen S., Kim S., Liu C., Essers J., Kugathasan S., Hyams J.S., Group P.-K.R. Increased effectiveness of early therapy with anti-tumor necrosis factor-alpha vs an immunomodulator in children with Crohn's disease. Gastroenterology. 2014;146(2):383–391. doi: 10.1053/j.gastro.2013.10.027. PubMed PMID: 24162032. [DOI] [PubMed] [Google Scholar]
- 8.Forrest C.B., Crandall W.V., Bailey L.C., Zhang P., Joffe M.M., Colletti R.B., Adler J., Baron H.I., Berman J., del Rosario F. Effectiveness of anti-TNFα for Crohn disease: research in a pediatric learning health system. Pediatrics. 2014;134(1):37–44. doi: 10.1542/peds.2013-4103. PubMed PMID: 24935993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kugathasan S., Denson L.A., Walters T.D., Kim M.O., Marigorta U.M., Schirmer M., Mondal K., Liu C., Griffiths A., Noe J.D., Crandall W.V., Snapper S., Rabizadeh S., Rosh J.R., Shapiro J.M., Guthery S., Mack D.R., Kellermayer R., Kappelman M.D., Steiner S., Moulton D.E., Keljo D., Cohen S., Oliva-Hemker M., Heyman M.B., Otley A.R., Baker S.S., Evans J.S., Kirschner B.S., Patel A.S., Ziring D., Trapnell B.C., Sylvester F.A., Stephens M.C., Baldassano R.N., Markowitz J.F., Cho J., Xavier R.J., Huttenhower C., Aronow B.J., Gibson G., Hyams J.S., Dubinsky M.C. Prediction of complicated disease course for children newly diagnosed with Crohn's disease: a multicentre inception cohort study. Lancet. 2017;389(10080):1710–1718. doi: 10.1016/S0140-6736(17)30317-3. Epub 2017/03/06. PubMed PMID: 28259484; PMCID: PMC5719489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shay L.A., Lafata J.E. Where is the evidence? A systematic review of shared decision making and patient outcomes. Med. Decis. Mak. 2015;35(1):114–131. doi: 10.1177/0272989X14551638. PubMed PMID: 25351843; PMCID: 4270851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stacey D., Legare F., Lewis K., Barry M.J., Bennett C.L., Eden K.B., Holmes-Rovner M., Llewellyn-Thomas H., Lyddiatt A., Thomson R., Trevena L. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst. Rev. 2017;4(4) doi: 10.1002/14651858.CD001431.pub5. Epub 2017/04/13. PubMed PMID: 28402085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lipstein E.A., Britto M.T. Evolution of pediatric chronic disease treatment decisions: a qualitative, longitudinal view of parents' decision-making process. Med. Decis. Mak. 2015;35(6):703–713. doi: 10.1177/0272989X15581805. Epub 2015/04/23. PubMed PMID: 25899248; PMCID: PMC4618270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lipstein E.A., Lovell D.J., Denson L.A., Moser D.W., Saeed S.A., Dodds C.M., Britto M.T. Parents' information needs in tumor necrosis factor-alpha inhibitor treatment decisions. J. Pediatr. Gastroenterol. Nutr. 2013;56(3):244–250. doi: 10.1097/MPG.0b013e31827496c3. Epub 2012/10/13. PubMed PMID: 23059648. [DOI] [PubMed] [Google Scholar]
- 14.Lipstein E.A., Lovell D.J., Denson L.A., Kim S.C., Spencer C., Britto M.T. Parents’ information needs and influential factors when making decisions about TNF-alpha inhibitors. Pediatr Rheumatol Online J. 2016;14(1) doi: 10.1186/s12969-016-0113-5. PMID: PMC5024421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lipstein E.A., Dodds C.M., Britto M.T. Real life clinic visits do not match the ideals of shared decision making. J. Pediatr. 2014;165(1):178–183 e1. doi: 10.1016/j.jpeds.2014.03.042. PubMed PMID: 24795203; PMCID: PMC4106460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brinkman W.B., Lipstein E.A., Taylor J., Schoettker P.J., Naylor K., Jones K., Vora S.S., Mims C.C., Roth-Wojcicki E., Gottlieb B., Griffin N., Lannon C., Morgan E. Design and implementation of a decision aid for juvenile idiopathic arthritis medication choices. Pediatr. Rheumatol. Online J. 2017;15(1):48. doi: 10.1186/s12969-017-0177-x. PubMed PMID: 28583183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breslin M., Mullan R.J., Montori V.M. The design of a decision aid about diabetes medications for use during the consultation with patients with type 2 diabetes. Patient Educ. Couns. 2008;73(3):465–472. doi: 10.1016/j.pec.2008.07.024. Epub 2008/09/06. PubMed PMID: 18771876. [DOI] [PubMed] [Google Scholar]
- 18.Mullan R.J., Montori V.M., Shah N.D., Christianson T.J., Bryant S.C., Guyatt G.H., Perestelo-Perez L.I., Stroebel R.J., Yawn B.P., Yapuncich V., Breslin M.A., Pencille L., Smith S.A. The diabetes mellitus medication choice decision aid: a randomized trial. Arch. Intern. Med. 2009;169(17):1560–1568. doi: 10.1001/archinternmed.2009.293. PubMed PMID: 19786674. [DOI] [PubMed] [Google Scholar]
- 19.Brinkman W.B., Hartl Majcher J., Poling L.M., Shi G., Zender M., Sucharew H., Britto M.T., Epstein J.N. Shared decision-making to improve attention-deficit hyperactivity disorder care. Patient Educ. Couns. 2013;93(1):95–101. doi: 10.1016/j.pec.2013.04.009. PubMed PMID: 23669153; PMCID: 3759588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipstein E.A., Dodds C.M., Lovell D.J., Denson L.A., Britto M.T. Making decisions about chronic disease treatment: a comparison of parents and their adolescent children. Health Expect. 2016;19(3):716–726. doi: 10.1111/hex.12210. Epub 2014/06/04. PubMed PMID: 24889468; PMCID: PMC5055230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dodds C.M., Britto M.T., Denson L.A., Lovell D.J., Saeed S., Lipstein E.A. Physicians' perceptions of shared decision making in chronic disease and its barriers and facilitators. J. Pediatr. 2016;171:307–309. doi: 10.1016/j.jpeds.2015.12.071. Epub 2016/01/29. PubMed PMID: 26817588; PMCID: PMC4808590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lipstein E.A., Muething K.A., Dodds C.M., Britto M.T. "I'm the one taking it": adolescent participation in chronic disease treatment decisions. J. Adolesc. Health. 2013;53(2):253–259. doi: 10.1016/j.jadohealth.2013.02.004. Epub 2013/04/09. PubMed PMID: 23561895. [DOI] [PubMed] [Google Scholar]
- 23.Carroll S.L., McGillion M., Stacey D., Healey J.S., Browne G., Arthur H.M., Thabane L. Development and feasibility testing of decision support for patients who are candidates for a prophylactic implantable defibrillator: a study protocol for a pilot randomized controlled trial. Trials. 2013;14:346. doi: 10.1186/1745-6215-14-346. Epub 2013/10/24. PubMed PMID: 24148851; PMCID: PMC4015905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weymiller A.J., Montori V.M., Jones L.A., Gafni A., Guyatt G.H., Bryant S.C., Christianson T.J., Mullan R.J., Smith S.A. Helping patients with type 2 diabetes mellitus make treatment decisions: statin choice randomized trial. Arch. Intern. Med. 2007;167(10):1076–1082. doi: 10.1001/archinte.167.10.1076. PubMed PMID: 17533211. [DOI] [PubMed] [Google Scholar]
- 25.Turner D., Otley A.R., Mack D., Hyams J., de Bruijne J., Uusoue K., Walters T.D., Zachos M., Mamula P., Beaton D.E., Steinhart A.H., Griffiths A.M. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multicenter study. Gastroenterology. 2007;133(2):423–432. doi: 10.1053/j.gastro.2007.05.029. PubMed PMID: 17681163. [DOI] [PubMed] [Google Scholar]
- 26.Shepanski M.A., Markowitz J.E., Mamula P., Hurd L.B., Baldassano R.N. Is an abbreviated pediatric crohn's disease activity index better than the original? J. Pediatr. Gastroenterol. Nutr. 2004;39(1):68–72. doi: 10.1097/00005176-200407000-00014. PubMed PMID: 15187784. [DOI] [PubMed] [Google Scholar]
- 27.Otley A., Loonen H., Parekh N., Corey M., Sherman P.M., Griffiths A.M.J.G. Assessing Activity of Pediatric Crohn's Disease: Which Index to Use? vol. 116. 1999. pp. 527–531. 3. [DOI] [PubMed] [Google Scholar]
- 28.Hyams J., Crandall W., Kugathasan S., Griffiths A., Olson A., Johanns J., Liu G., Travers S., Heuschkel R., Markowitz J., Cohen S., Winter H., Veereman-Wauters G., Ferry G., Baldassano R., Group R.S. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn's disease in children. Gastroenterology. 2007;132(3):863–873. doi: 10.1053/j.gastro.2006.12.003. quiz 1165-6. PubMed PMID: 17324398. [DOI] [PubMed] [Google Scholar]
- 29.Kim M.J., Lee J.S., Lee J.H., Kim J.Y., Choe Y.H. Infliximab therapy in children with Crohn's disease: a one-year evaluation of efficacy comparing 'top-down' and 'step-up' strategies. Acta Paediatr. 2010 doi: 10.1111/j.1651-2227.2010.01938.x. Epub 2010/07/16. PubMed PMID: 20626362. [DOI] [PubMed] [Google Scholar]
- 30.Steiner J.F., Koepsell T.D., Fihn S.D., Inui T.S. A general method of compliance assessment using centralized pharmacy records. Description and validation. Med. Care. 1988;26(8):814–823. doi: 10.1097/00005650-198808000-00007. PubMed PMID: 3398608. [DOI] [PubMed] [Google Scholar]
- 31.Varni J.W., Seid M., Kurtin P.S. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med. Care. 2001;39(8):800–812. doi: 10.1097/00005650-200108000-00006. PubMed PMID: 11468499. [DOI] [PubMed] [Google Scholar]
- 32.O'Connor A.M. Validation of a decisional conflict scale. Med. Decis. Mak. 1995;15(1):25–30. doi: 10.1177/0272989X9501500105. Epub 1995/01/01. PubMed PMID: 7898294. [DOI] [PubMed] [Google Scholar]
- 33.Brehaut J.C., O'Connor A.M., Wood T.J., Hack T.F., Siminoff L., Gordon E., Feldman-Stewart D. Validation of a decision regret scale. Med. Decis. Mak. 2003;23(4):281–292. doi: 10.1177/0272989X03256005. Epub 2003/08/21. PubMed PMID: 12926578. [DOI] [PubMed] [Google Scholar]
- 34.Barr P.J., O'Malley A.J., Tsulukidze M., Gionfriddo M.R., Montori V., Elwyn G. The psychometric properties of Observer OPTION(5), an observer measure of shared decision making. Patient Educ. Couns. 2015;98(8):970–976. doi: 10.1016/j.pec.2015.04.010. PubMed PMID: 25956069. [DOI] [PubMed] [Google Scholar]
- 35.Elwyn G., Edwards A., Wensing M., Hood K., Atwell C., Grol R. Shared decision making: developing the OPTION scale for measuring patient involvement. Qual. Saf. Health Care. 2003;12(2):93–99. doi: 10.1136/qhc.12.2.93. Epub 2003/04/08. PubMed PMID: 12679504; PMCID: 1743691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kriston L., Scholl I., Holzel L., Simon D., Loh A., Harter M. The 9-item Shared Decision Making Questionnaire (SDM-Q-9). Development and psychometric properties in a primary care sample. Patient Educ. Couns. 2010;80(1):94–99. doi: 10.1016/j.pec.2009.09.034. Epub 2009/11/03. doi: S0738-3991(09)00450-9 [pii] 10.1016/j.pec.2009.09.034. PubMed PMID: 19879711. [DOI] [PubMed] [Google Scholar]
- 37.Lipstein E.A., Lovell D.J., Denson L.A., Kim S.C., Spencer C., Britto M.T. Parents' information needs and influential factors when making decisions about TNF-alpha inhibitors. Pediatr. Rheumatol. Online J. 2016;14(1):53. doi: 10.1186/s12969-016-0113-5. PubMed PMID: 27641835; PMCID: PMC5024421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Montori V.M., Gafni A., Charles C. A shared treatment decision-making approach between patients with chronic conditions and their clinicians: the case of diabetes. Health Expect. 2006;9(1):25–36. doi: 10.1111/j.1369-7625.2006.00359.x. Epub 2006/01/27. PubMed PMID: 16436159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wyatt K.D., List B., Brinkman W.B., Prutsky Lopez G., Asi N., Erwin P., Wang Z., Domecq Garces J.P., Montori V.M., LeBlanc A. Shared decision making in pediatrics: a systematic review and meta-analysis. Acad. Pediatr. 2015;15(6):573–583. doi: 10.1016/j.acap.2015.03.011. Epub 2015/05/20. PubMed PMID: 25983006. [DOI] [PubMed] [Google Scholar]
- 40.Lipstein E.A., Brinkman W.B., Fiks A.G., Hendrix K.S., Kryworuchko J., Miller V.A., Prosser L.A., Ungar W.J., Fox D. An emerging field of research: challenges in pediatric decision making. Med. Decis. Mak. 2015;35(3):403–408. doi: 10.1177/0272989X14546901. Epub 2014/08/26. PubMed PMID: 25145576; PMCID: PMC4336633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kistin C., Silverstein M. Pilot studies: a critical but potentially misused component of interventional research. J. Am. Med. Assoc. 2015;314(15):1561–1562. doi: 10.1001/jama.2015.10962. PubMed PMID: 26501530. [DOI] [PMC free article] [PubMed] [Google Scholar]