Abstract
Light irradiation has been used in clinical therapy for several decades. In this context, photobiomodulation (PBM) modulates signaling pathways via ROS, ATP, Ca2+, while photodynamic therapy (PDT) generates reactive oxygen species by excitation of a photosensitizer. NO generation could be an important tool when combined with both kinds of light therapy. By using a metal-based compound, we found that PBM combined with PDT could be a beneficial cancer treatment option. We used two types of ruthenium compounds, ([Ru(Pc)], Pc = phthalocyanine) and trans- [Ru(NO)(NO2)(Pc)]. The UV–vis spectra of both complexes displayed a band in the 660 nm region. In the case of 0.5 μM trans-[Ru(NO)(NO2)(Pc)], light irradiation at the Q-band reduced the percentage of viable human melanoma (A375) cells to around 50% as compared to [Ru(Pc)]. We hypothesized that these results were due to a synergistic effect between singlet oxygen and nitric oxide. Similar experiments performed with PDT (660 nm) combined with PBM (850 nm) induced more photocytotoxicity using both [Ru (Pc)] and trans-[Ru(NO)(NO2)(Pc)]. This was interpreted as PBM increasing cell metabolism (ATP production) and the consequent higher uptake of the ruthenium phthalocyanine compounds and more efficient apoptosis. The use of metal-based photosensitizers combined with light therapy may represent an advance in the field of photodynamic therapy.
Keywords: Light irradiation therapy, Photobiomodulation, Nitrosyl ruthenium-phthalocyanine complex, Metal-based drug
1. Introduction
Light irradiation is a non-invasive clinical therapy that can be used to treat several diseases, as attested by recent reports [1]. Cancer, for example, can be treated by using the modality named photodynamic therapy (PDT) [2], which essentially combines light irradiation, photosensitizer, and oxygen to generate reactive oxygen species (ROS) [3]. Although PDT has been employed to treat many types of cancer [4], its effectiveness seems to depend on the disease stage. The earlier the cancer treatment is initiated, the better the PDT efficacy [5]. ROS photo-production depends on oxygen concentration, which is probably one of the main factors limiting the general clinical application of PDT. Compared to normal tissues, oxygen levels in the area surrounding the cancerous cells are significantly much lower and fall along time [6,7], resulting in poor tumor response to PDT treatment. New strategies have been developed to improve treatment efficacy and to enhance therapeutic strategies, so as to obtain better clinical outcomes without substantially increasing PDT toxicity [8]. Some of the most frequently applied approaches that have been combined with PDT are chemotherapy [9–11], immunotherapy [12–14], gene therapy [15], anti-angiogenesis [16], among others. Nowadays scientists are exploring combinations of different types of light.
Photobiomodulation (PBM) is a kind of light therapy that uses non-ionizing light sources in the red or near infrared spectral region (700–1100 nm) (without any addition of photosensitizer) [17], PBM is usually used to stimulate, heal or regenerate damaged or dying tissues, which is the opposite of PDT that tends to kill cells. PBM therapy combined with PDT has been less frequently described as a possibility for enhancing cancer treatment [18], but in some cases PBM plus PDT has demonstrated increased reduction in cancer cell viability compared to PDT alone [19]. The process seems to be related to increased PDT efficiency as a result of PBM inducing higher cellular ATP levels, which leads to higher photosensitizer uptake and more efficient apoptosis [20,21]. In this report we describe a photochemotherapy process combining PBM with PDT using ruthenium phthalocyanine systems as photosensitizers. Taking advantage of coordination compounds based on ruthenium(II) systems, which form an octahedral or a tetrahedral complex depending on the ligands, we synthesized [Ru(Pc)] and trans-[Ru(NO)(NO2)(Pc)] (Pc = phthalocyanine) complexes (Fig. 1A and B). The preparation of ruthenium phthalocyanine complexes have been described previously and photochemical properties well studied [22,23]. The [Ru(Pc)] complex produces reactive oxygen species (ROS) [22,23], whereas trans-[Ru(NO)(NO2)(Pc)] simultaneously generates reactive oxygen species and reactive nitrogen species (RONS) [24]. Recently, enhancement of PDT has been claimed by insertion of photodonors on the treatment process [25,26].
Fig. 1.
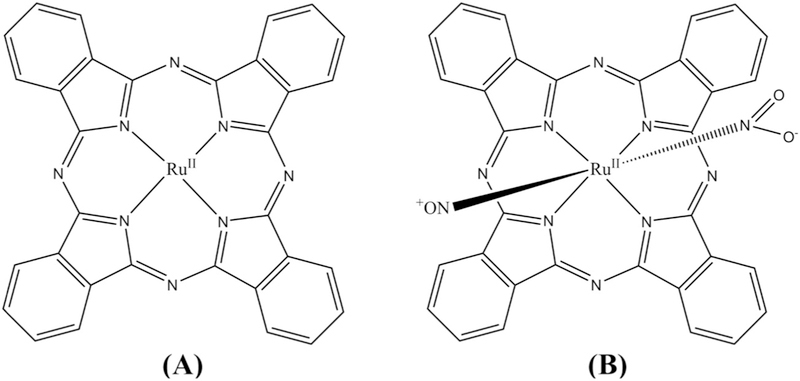
Structure of the ruthenium complexes [Ru(Pc)] (A) and irans-[Ru(NO)(NO2)(Pc)] (B).
This paper evaluates the synergistic effect of PBM combined with PDT using the ruthenium phthalocyanine-based complexes as photosensitizers. This strategy makes the use of coordination compounds as an approach for combination therapy using a single molecule. PBM likely activates NO release from trans-[Ru(NO)(NO2)(Pc)], which, following light irradiation at 660 nm, decreases A375 cell viability by 50% as compared to [Ru(Pc)], which does not release NO.
2. Experimental
2.1. Synthesis of ruthenium phthalocyanine complexes
2.1.1. [Ru(Pc)]
The [Ru(Pc)] complex was synthesized as previously described [27] with some modifications. Briefly, 0.0450 g (0.076 mmol) of cis-[RuCl2(DMSO)4] was dissolved in 15 mL of dry pentanol and charged into a 100 mL flask with a magnetic stir bar. The system was heated to a 140 °C, measured externally in the oil bath, for five hours under argon atmosphere. Next, 3.4000 g (26.5 mmol) of 1,2-dicyanobenzene and 0.5 mL of 1,8-diazabicyclo [5.4.0] undec-7ene (DBU) were added. The mixture was allowed to react at 140 °C under argon for 24 h. The crude product was purified by column chromatography on silica gel; the mobile phase consisted of a mixture of dichloromethane/methanol (10:1). The eluate was concentrated to dryness in a rotary evaporator. A blue solid was obtained. Maldi-Tof analysis show m/z = 614.248 (Supplementary Material: Fig. S1). Yield: 45.0%.
2.1.2. trans-[Ru(NO)(NO2)(Pc)]
The title compound was synthesized with slight modification according to procedures reported earlier [24,28]. Briefly, the trans-[Ru(NO)(NO2)(Pc)] complex was obtained by bubbling nitric oxide (NO) into 32 mL of a previously de-aerated chloroform/ethanol/water (1:5:0.4) solution containing 0.1 g (1.63 × 10−1mol) of the precursor [Ru(Pc)] at room temperature, under an argon atmosphere. This procedure was repeated three times each day, and the reaction was allowed to occur over a period of three consecutive days. The reaction mixture was concentrated to dryness under vacuum. Yield: 90.0%.
2.2. Cell culture
A human melanoma cell line A375 was cultured in RPMI 1640 medium (Invitrogen, USA) supplemented with 10% fetal bovine serum (Invitrogen, USA) and 1% penicillin-streptomycin (Invitrogen, USA) at 37 °C and 5% CO2.
2.3. Light sources
A LEDLB-24E-IR infrared LED light bar (Larson Eletronics, USA) was used for PBM, 850 nm. The average power was 1.0 mW and the irradiance were 0.3180 mW/cm2. The power density for PBM was 4.6 J/cm2. For PDT assay was used a 660 nm Red 96 well LED array (Elétron Comercial, BRA), 660 nm. The average power was 7.0 mW and the irradiance were 2.3 mW/cm2. The power densities of both light sources were measured with a power meter (Thorlab, USA).
2.4. Cell viability and PDT treatment
Cell viability was evaluated by using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) in a colorimetric assay. A375 melanoma cells were seeded at a density of 2.0 × 104 cells per well in a 96-well culture plate (Fisher Sicentific, USA) at 37 °C for 24 h under 5% CO2. The cells were incubated with different [Ru(Pc)] or trans-[Ru(NO)(NO2)(Pc)] concentrations (0.1–1.0μM) for 24 h. After incubation, the cells were washed and subjected to PDT treatment at 660 nm with different fluences: 1.0J/cm2, 3.0J/cm2, or 6.0J/cm2. The same procedure was performed under light irradiation and in the dark controls.
2.5. Photobiomodulation and PDT treatment
A375 melanoma cells were seeded at a density of 2.0 × 104 cells per well in a 96-well culture plate (Fisher Scientific, USA) at 37 °C for 24 h under 5% CO2. The cells were incubated with [Ru(Pc)] or trans-[Ru(NO)(NO2)(Pc)] for 4 h under NIR-LLLT irradiation at 850 nm, 4.6 J.cm−2. After incubation, the cells were washed and subjected to PDT at 660 nm, 3.0 J.cm−2. Cell viability was evaluated by using the MTT assay.
2.6. ATP assay
The ATP activity assay was performed according to the CellTiter-Glo® Luminescent Cell Viability Assay. To this end, A375 cells (5 × 104 units) were seeded in a 96-well culture plate and incubated at 37 °C overnight under 5% CO2. A375 cells were treated with 1.0 μM [Ru (Pc)] or 1.0 μM trans-[Ru(NO)(NO2)(Pc)]. A375 cells were evaluated with or without irradiation with 4.6 J.cm−2 light at 850 nm for 4 h, and the ATP level was measured 22 min after PBM. To determine ATP, 50 μL of CellTiter-Glo® Reagent was added to 50 μL of RPMI. The contents were mixed on an orbital shaker for 2 min to induce cell lysis. The plate was incubated at room temperature for 10 min to stabilize the luminescent signal, and the luminescence was recorded.
2.7. Cell uptake
The [Ru(Pc)] and trans-[Ru(NO)(NO2)(Pc)] uptake by A375 cells was evaluated through the Agilent 7900 ICPMS system to measure the ruthenium metal ions. Cells at a density of 7.0 × 105 were plated in T-25 flasks overnight. Next, the cells were treated with 1.0 μM [Ru(Pc)] or 1.0 μM trans-[Ru(NO)(NO2)(Pc)] and incubated for 2, 4, 8, 12, or 24 h. After incubation, the flasks were washed with PBS, and the cells were collected with 1.0% trypsin. Then, 50 μL of pure nitric acid was added to digest the cells, and the samples were stored in a freezer. Next, 1 ppb of rhenium solution with 2% of nitric acid was added to each sample as a positive control. The experiment was conducted in triplicate. A ruthenium calibration curve was constructed by using a standard ruthenium chloride solution at the following concentrations: 1000 ppm, 1000 ppb, 100 ppb, 10ppb, 1 ppb, 100 ppt, and 10ppt.
2.8. Comet assay
The Lysis Solution was prepared and chilled at 4 °C or on ice for at least 20 min before use. LM Agarose was melted in a beaker containing boiling water for 5 min, with the cap loosened. The bottle was placed in a water bath at 37 °C for at least 20 min to cool. The agarose temperature is critical as the cells may undergo heat shock. The cells at 1 × 105/mL were combined with molten LM Agarose (at 37 °C) at a ratio of 1:10 (v/v), and 75 μL of the mixture was immediately pipetted onto a CometSlide™. The flat slide was placed at 4 °C in the dark (in a refrigerator) for 10–30 min. A 0.5-mm clear ring appeared at the edge of the CometSlide™ area. The slide was immersed in pre-chilled Lysis Solution and left on ice or at 4 °C for between 30- and 60-min. Excess buffer was tapped off from the slide, which was immersed in freshly prepared alkaline solution, pH > 13. CometSlide™ was left in the alkaline solution at room temperature for between 20 and 60 min, in the dark. The slide was transferred from the alkaline solution to a horizontal electrophoresis apparatus; the voltage was about 1 Volt/cm. The current was approximately 300 mA, and electrophoresis was carried out for 20–40 min. Excess electrophoresis solution was gently tapped off, and the slide was rinsed by dipping several times in H2O, which was followed by immersion in 70% ethanol for 5 min. The sample was stored at room temperature to dry and then analyzed by epifluorescence microscopy. (SYBR® Green I’s maximum excitation and emission are respectively 494nm/521 nm. A fluorescein filter is adequate).
2.9. Statistical analysis
All the results are expressed as the mean ± standard error mean. Statistical analyses were accomplished using Two-way ANOVA and Bonferroni’s correction to evaluate the interaction of factors; statistical significance was obtained by One-Way ANOVA and Newman-Keuls post-hoc test (p < .05).
3. Results and discussion
The [Ru(Pc)] and trans-[Ru(NO)(NO2)(Pc)] complexes described in this work are stable in the dark at room temperature in non-aqueous solvents or in a mixture of water based on the observed unchangeable UV–visible spectrum up to 24 h. The UV–vis absorption spectrum of the [Ru(Pc)] complex in chloroform displayed a Soret band at 314 nm (log ε = 4.32) and a Q band at 642 nm (log ε = 4.24), which are characteristic of ruthenium phthalocyanine compounds [29]. The absorption spectrum of the trans-[Ru(NO)(NO2)(Pc)] complex in chloroform presented Q and Soret bands at 682 nm (log ε = 4.44) and 355 nm (ε = 4.39), respectively. This spectrum is slightly different of previous published procedure [28]. It may suggest different spatial structure of the trans-[Ru(NO)(NO2)(Pc)] perhaps with binding of nitrite to Ru(II) by N atom coordination. The FTIR spectra of both complexes contained the typical bands of phthalocyanine from around 1600 to 1200 cm−1, which entirely agreed with the bands reported for the metal-free phthalocyanine core [30]. The FTIR spectrum of trans-[Ru(NO)(NO2)(Pc)] exhibited one extra strong band in the terminal nitrosyl region at 1888 cm−1, which indicated the presence of the NO+ group. Fluorescence spectroscopy in dichloromethane revealed weak emission bands at 695 and 674 nm for [Ru(Pc)] and trans-[Ru(NO)(NO2)(Pc)], respectively, upon excitation at 650 nm. The MALDI-TOF spectrum of isolated [Ru(Pc)] showed m/z = 614.248, in agreement with the proposed formulae. For trans-[Ru(NO)(NO2)(Pc)], loss of nitrogen oxide derivative ligands gave the same fragment.
We conducted photobiology studies of both complexes using human melanoma cells (A375) by incubating the cells with one of the complexes at eight different concentrations ranging from 0.1 to 1.0 μM. We searched for the optimal light dose for [Ru(Pc)] and trans-[Ru(NO)(NO2)(Pc)] by testing light irradiation doses between 1.0 and 6.0J.cm−2 and verified light dose-dependent cytotoxicity. The light source had a wavelength maximum around 660 nm. The MTT reduction assay demonstrated that the ruthenium phthalocyanine compounds exerted concentration-dependent, light dose-dependent and incubation time-dependent cytotoxicity effects on the A375 cell line. A significant effect was achieved at 6.0J.cm−2 for both complexes. Ruthenium complexes at 1 μM provided maximum cell killing after 24 h of incubation. In the absence of ruthenium phthalocyanine complexes, different light doses did not produce any cytotoxicity as compared to untreated controls and to the dark control. Fig. 2A and B illustrate the [Ru(Pc)] and trans-[Ru(NO)(NO2)(Pc)] cytotoxicity in A375 cells. Irradiation with 660-nm light at a fluence of 6.0 J.cm−2 caused 1 μM [Ru (Pc)] and 1 μM trans-[Ru(NO)(NO2)(Pc)] to induce up to 80 and 95% cell death, respectively.
Fig. 2.
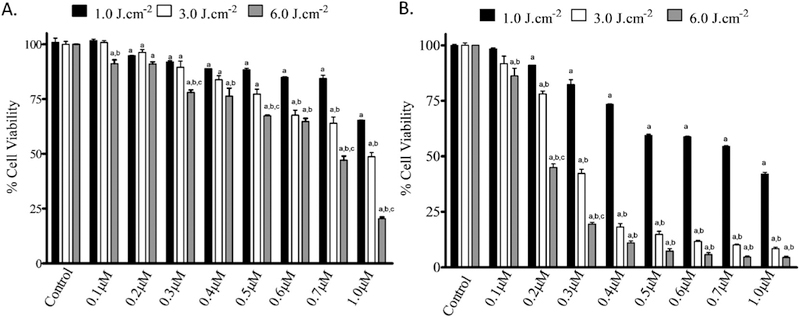
Effect of [Ru(Pc)] (A) and trans-[Ru(NO)(NO2)(Pc)] (B) on A375 cell viability. Cells were incubated with different concentrations (from 0.1 to 1.0 μM) of either of the complexes for 24 h and were irradiated with 660 nm light at different fluences (1.0, 3.0, or 6.0 J.cm−2). Data are the mean ± SEM with p value < .05. “a” represents a significant difference in relation to the control in the dark; “b” represents significant difference in relation to the 1 J.cm−2 group; and “c” represents significant difference in relation to the 3 J.cm−2 group. All the results in this figure are representative of independent experiments performed in quadruplicate.
The PDT efficacy before and after PBM was also evaluated by incubating A375 cells with one of the ruthenium phthalocyanine complexes at six different concentrations (from 0.1 to 1.0 μM). Four parallel experiments were carried out and analyzed by the student t-test; the data expressed as mean ± standard error. In the first group, we incubated the cells with one of the complexes and kept them in the dark (without light irradiation). In the second group, we irradiated the cells with 660nm light at 3.0 J.cm−2 (PDT alone). In the third group, we subjected the cells to PBM using irradiation with 850-nm light for 4 h (PBM alone). In the last group, we subjected the cells to irradiation with 850 nm light for 4 h and then carried out PDT with light at 3.0 J.cm−2 (PBM + PDT). Neither the ruthenium phthalocyanine complexes nor laser irradiation alone showed any cytotoxicity (Fig. 3). A375 cell viability decreased as a function of the ruthenium phthalocyanine complex concentration under irradiation with 660 nm light. Cytotoxicity was more pronounced in the cells treated with [Ru(Pc)] and trans-[Ru(NO)(NO2)(Pc)] that had been first irradiated with near infrared 850 nm light then followed by PDT with red 660 nm light (PBM + PDT group) as compared to the cells treated with PDT only at the same dose. For example, in the presence of 0.4 μM [Ru(Pc)], A375 cell viability was 13% in the PBM + PDT group as compared to 35% in the PDT alone group. Trans-[Ru(NO)(NO2)(Pc)] afforded similar results: 31 and 44% for the PBM + PDT and PDT groups, respectively (Fig. 3). In other words, combined PDT and PBM treatment enhanced A375 cell death.
Fig. 3.
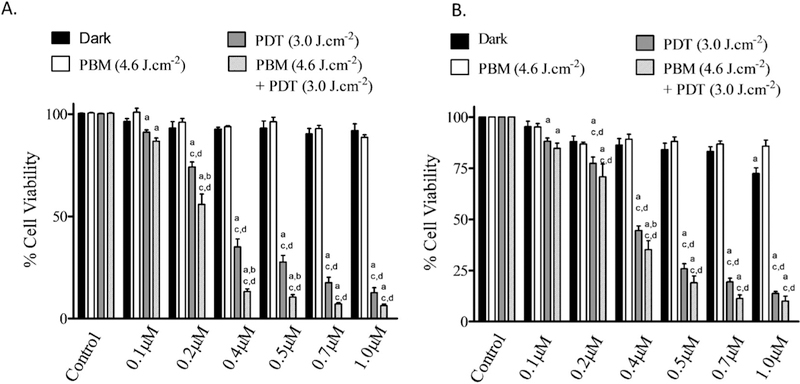
Cell viability studies of [Ru(Pc)] (A) or trans-[Ru(NO)(NO2)(Pc)] (B) for 4 h incubation time in A375 cell line; “a” represents a significant difference in relation to the dark control; “b” represents a significant difference in relation to the PDT group; and “c” represents a significant difference in relation to the PBM group. All the results in this figure are representative of independent experiments performed in quadruplicate. An asterisk represents p value < .05 as a significant difference in relation to the dark control.
Photosensitizers based on ruthenium complexes have emerged as candidates for use in PDT [23,31,32]. Some of those complexes have shown no cytotoxicity on the dark but it becomes cytotoxic when it is irradiated with light [33]. Here, we studied photobiological applications of ruthenium phthalocyanine species. The [Ru(Pc)] complex was synthesized in a straightforward manner as previously described. The trans-[Ru(NO)(NO2)(Pc)] was obtained by sequential NO addition to the [Ru(Pc)] complex [24]. NO is a ligand that undergoes redox reactions when it is bound to Ru(II): it stabilizes as RuII-NO+, giving rise to a very strong bond due to back-bonding involving the metal dπ orbitals and the ligand pπ* orbital. The high π-acceptor character of the nitrosyl ligand removes an excessive amount of electron density from the ruthenium(II). Subsequently, a second bond RuII-NO+ formed in the presence of excess NO undergoes basic hydrolysis to give a Ru-NO2 bond [34].
Both ruthenium phthalocyanine complexes synthesized here presented intense absorption in the UV–vis region, the so- called Q-band (in the visible region) and B-band (in the ultraviolet region). These bands are attributed to π-π* transitions centered on the macrocycle [35]. The metal-ion coordination affects the phthalocyanine structure. Ruthenium(II) insertion in the phthalocyanine cycle slightly shifts the Q-band and the B-band toward the blue as compared to the free ligand. This shift happens because the spatial structure changes, consequently stabilizing the molecular orbital involved in the electronic transitions. Both ruthenium phthalocyanine complexes prepared herein are highly stable in aqueous solution in the dark, as judged from the fact that their UV–Vis spectra remain unaltered for at least a week. The [Ru(Pc)] and trans-[Ru(NO)(NO2)(Pc)] complexes were sensitive to external stimuli. Recently, we reported that [Ru(Pc)] can generate singlet oxygen (1O2) with a quantum yield of 0.35 under irradiation with 660 nm light [24]. On the other hand, trans-[Ru(NO)(NO2)(Pc)] has been described as a RuII-NO+ system on the basis of its FTIR spectrum. Reduction drives dissociation of two NO molecules, as depicted in eqs. I-V as previously described based on the spectroelectrochemistry experiment [24]. In principle, the ruthenium species remaining after the electrochemical process could generate singlet oxygen under light irradiation.
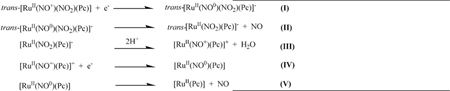
The NO molecule has been implicated in various pharmacological processes including signaling events related to cancer [36]. At nano-molar concentration, NO promotes tumorigenesis; and at concentrations above 100 nanomolar, NO exerts tumoricidal effects [37]. Thus, researchers have actively searched for compounds that can deliver NO to the target site in a controlled way [38,39]. The trans-[Ru(NO)(NO2)(Pc)] complex seems to be a good candidate: one mole of this complex can deliver two NO molecules via a chemical reduction process (eqs. I-V) as previously described [24], which was also observed in cell culture as described in Fig. S2 (Supplementary Material).
Phototoxicity results obtained for [Ru(Pc)] and trans-[Ru(NO)(NO2)(Pc)] in the presence of A375 cells indicate that the coordination compounds can be useful in a combinatory cancer therapeutic approach. As expected, [Ru(Pc)] and trans-[Ru(NO)(NO2)(Pc)] cytotoxicity requires irradiation with 660 nm light. The cytotoxicity results show that the amount of ROS and RONS generated during the process depends on the incident light dose. Fig. 4 is a typical threshold light dose-response curve for the synthesized ruthenium phthalocyanine complexes at 1.0 μM. Unsurprisingly, A375 cell viability decreases with increasing light exposure and consequent ROS and RONS formation. The [Ru(Pc)] and trans-[Ru(NO)(NO2)(Pc)] light-dose response curves can be correlated with a polynomial function. For all the studied systems, trans-[Ru(NO)(NO2)(Pc)] is more cytotoxic to A375 cells than [Ru (Pc)]. Intracellular NO release due to the reduction process described in eqs. (I-V) may improve the ruthenium phthalocyanine complex efficiency in killing cancer cells. Besides that, PBM shows tendency to increase the cytotoxicity of both ruthenium-phthalocyanine complexes.
Fig. 4.
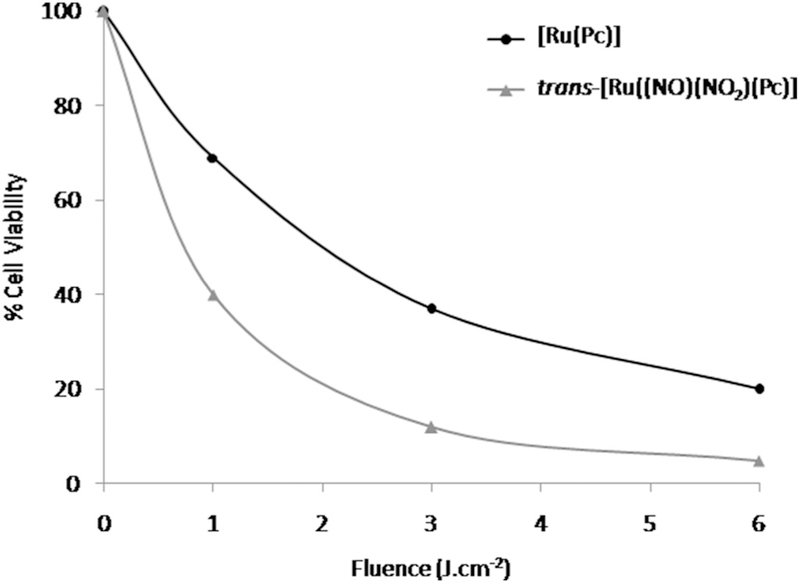
Cell viability evaluation of 1.0 μM [Ru(Pc)] or trans-[Ru(NO)(NO2)(Pc)] on A375 cell line as a function of 660 nm light fluency.
Fig. 5 depicts the concentration-effect curve regarding the PBM effect on cell viability obtained by using light at 3.0 J.cm−2. Both ruthenium-phthalocyanine complexes present pronounced potentiation when PDT was applied after PBM. We have hypothesized that PBM probably increases the cellular adenosine triphosphate (ATP) uptake, to allow apoptosis to occur more efficiently. In this way, we have evaluated how PBM affects the ATP level in A375 cells by cytosolic measurements in the presence and absence of the ruthenium phthalocyanine complexes. Fig. 6 reveals that PBM induces increasing in ATP levels.
Fig. 5.
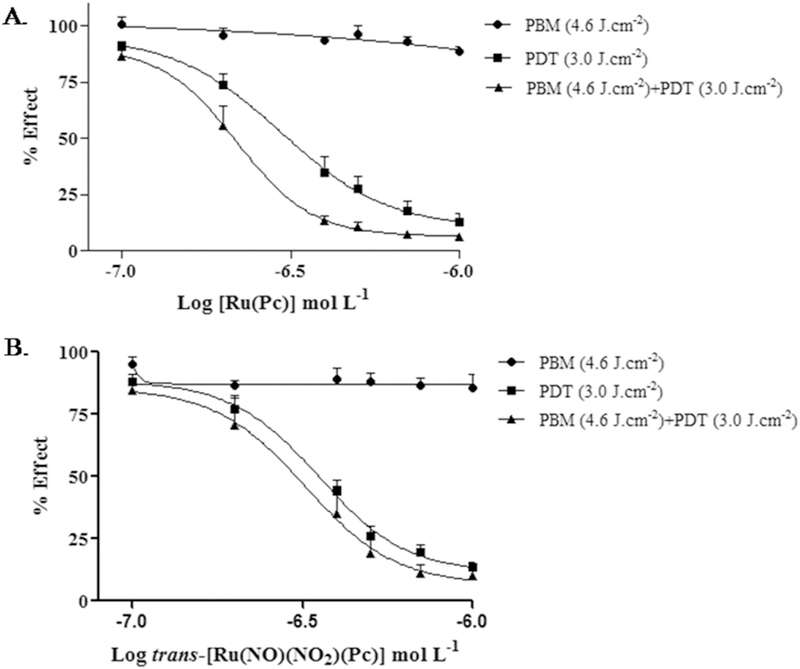
Concentration-effect curve comparing the effect of 3.0 J.cm−2 PDT (660 nm) under photobiomodulation stimulus at 850 nm and of 3.0 J.cm−2 PDT (660 nm) alone for [Ru(Pc)] (A) and trans-[Ru(NO)(NO2)(Pc)] (B).
Fig. 6.
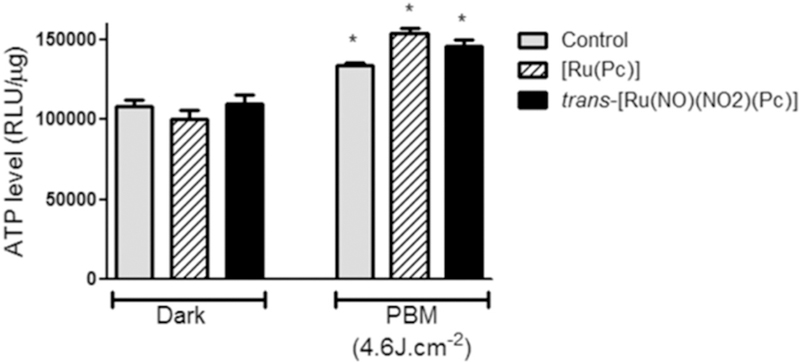
ATP production measurement in A375 cell line. The cells were incubated with 1.0 μM of ruthenium phthalocyanine complexes irradiated in 850nm (4.6 J.cm−2). Data are the mean ± SEM. Asterisk represents the p value < .05 as a significant difference in relation to control. All the results in this figure are representative of independent experiments performed in triplicate.
Therefore, interventions that increase ATP levels may be important to improve [Ru(Pc)] and trans-[Ru(NO)(NO2)(Pc)] cellular uptake, which is supported by the Inductively Coupled Plasma Mass Spectrometry (ICP-MS) results of ruthenium uptake after 4h of incubation (Table 1).
Table 1.
Ruthenium concentration (ppb) in A375 cells after 4-h incubation with [Ru (Pc)] and trans-[Ru(NO)(NO2)(Pc)] and 4h under PBM (4.6J.cm−2) with 850 nm light.
| Ru concentration (ppb) | ||
|---|---|---|
| [Ru(Pc)] | trans-[Ru(NO)(NO2)(Pc)] | |
| After 4 h | 2.10 | 2.23 |
| After 4 h under PBM | 2.19 | 2.31 |
Besides that, researchers have recently shown that PBM also involves stimulating mitochondria and other organelles with low-level light therapy to produce ROS, Ca2+, and NO, to modulate the signaling pathways [40]. Some secondary effects include changes in stress signaling, cell metabolism and proliferation, and increased cell survival [41–43]. Apparently, all these effects help to increase the cytotoxicity of the ruthenium phthalocyanine complexes.
Morphology of the A375 treated with 660 nm light and [Ru(Pc)] or trans-[Ru(NO)(NO2)(Pc)] remains unchanged as compared to control cells; membrane integrity is maintained. However, cells subjected to light irradiation bear a resemblance to cellular shrinkage that is typical of an apoptosis-mediated pathway. DNA integrity assessment could provide information about the cell death mechanism. On the basis of the comet assay, the ruthenium phthalocyanine compounds show DNA damage in the A375 cell line, as shown in Fig. 7. The DNA damage and the comet tails observed after irradiation with 660 nm light suggest that apoptosis is the cell killing mode as apoptosis produces DNA fragmentation.
Fig. 7.
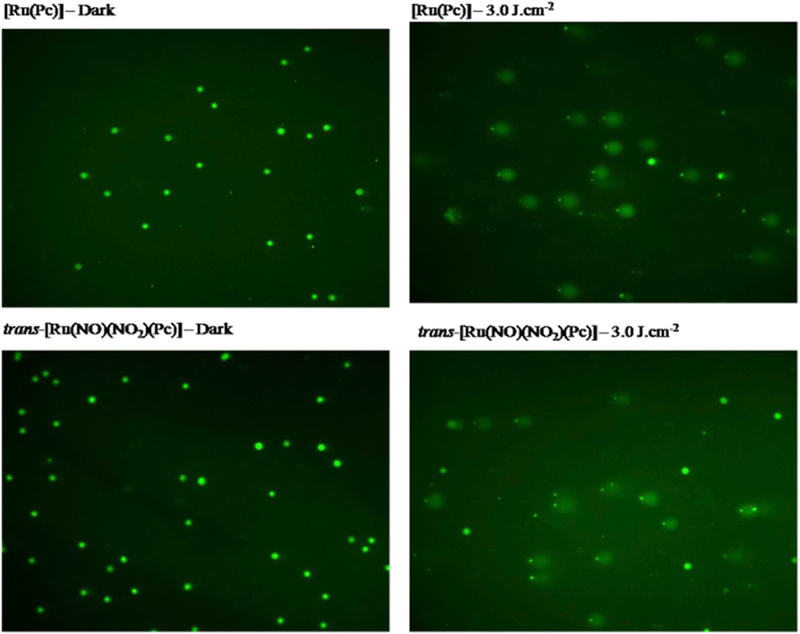
The comet assay in A375 cells for [Ru(Pc)] and trans-[Ru(NO)(NO2)(Pc)] complexes in the dark and after PDT at 660 nm (3.0 J.cm−2).
4. Conclusions
The use of ruthenium complexes could provide a photo-chemotherapeutic approach to kill melanoma cancer cells. The synergistic effect between PBM and PDT modifies the tumor micro-environment, promoting enhancing ruthenium compound uptake and increased anticancer efficacy. Controlled NO release from trans-[Ru(NO)(NO2)(Pc)] improves cytotoxicity through RONS production upon light irradiation. The very low ruthenium complex uptake attests to the impressive cytotoxicity results, showing that ruthenium complexes can effectively be used to kill the A375 cell line with very low EC50. These results strongly suggest that ruthenium phthalocyanine compounds have potential application as photosensitizers in clinical therapy. Up to millimolar concentrations, ruthenium complexes are not toxic to healthy cells, which favors the clinical use of this new class of metal-based drugs.
Supplementary Material
Acknowledgements
This work was supported by CNPq, CAPES, and FAPESP grant 2016/12707-0 and scholarship 2015/03746-9 (Laisa Bonafim Negri). MRH was supported by US NIH grants R01AI050875 and R21AI121700, Air Force Office of Scientific Research grant FA9550-13-1-0068, US Army Medical Research Acquisition Activity grant W81XWH-09-1-0514, and US Army Medical Research and Materiel Command grant W81XWH-13-2-0067.
Footnotes
Declaration of Competing Interest
There are no conflicts to declare.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jphotobiol.2019.111564.
References
- [1].Castano AP, Demidova TN, Hamblin MR, Mechanisms in photodynamic therapy: part one—photosensitizers, photochemistry and cellular localization, Photodiagn. Photodyn. Ther. 1 (2004) 279–293, 10.1016/S1572-1000(05)00007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dougherty TJ, Activated dyes as antitumor agents, J. Natl. Cancer Inst. 52 (1974) 1333–1336, 10.1093/jnci/52.4.1333. [DOI] [PubMed] [Google Scholar]
- [3].Dougherty TJ, Kaufman JE, Goldfarb A, Weishaupt KR, Boyle D, Mittleman A, Photoradiation therapy for the treatment of malignant tumors, Cancer Res. 38 (1978) 2628–2635 10.0008-5472/78/0038. [DOI] [PubMed] [Google Scholar]
- [4].Kinsella TJ, Colussi VC, Oleinick NL, Sibata CH, Photodynamic therapy in oncology, Expert. Opin. Pharmacother. 2 (2001) 917–927, 10.1517/14656566.2.6.917. [DOI] [PubMed] [Google Scholar]
- [5].Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q, Photodynamic therapy J Natl. Cancer Inst. 90 (1998) 889–905, 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].McKeown SR, Defining normoxia, physoxia and hypoxia in tumours-implications for treatment response, Br. J. Radiol. 87 (2014) 20130676, , 10.1259/bjr.20130676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Graeber TG, Osmanian C, Jacks T, Housman DE, Koch CJ, Lowe SW, Giaccia AJ, Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours, Nature. 379 (1996) 88–91, 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- [8].Spring BQ, Rizvi I, Xu N, Hasan T, The role of photodynamic therapy in over-coming cancer drug resistance, Photochem. Photobiol. Sci. 14 (2015) 1476–1491, 10.1039/c4pp00495g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gangopadhyay M, Singh T, Behara KK, Karwa S, Ghosh SK, Singh NDP, Coumarin-containing-star-shaped 4-arm-polyethylene glycol: targeted fluorescent organic nanoparticles for dual treatment of photodynamic therapy and chemotherapy, Photochem. Photobiol. Sci. 14 (2015) 1329–1336, 10.1039/c5pp00057b. [DOI] [PubMed] [Google Scholar]
- [10].Candido NM, de Melo MT, Franchi LP, Primo FL, Tedesco AC, Rahal P, Calmon MF, Combining photodynamic therapy and chemotherapy: improving breast cancer treatment with nanotechnology, J. Biomed. Nanotechnol. 14 (2018) 994–1008, 10.1166/jbn.2018.2558. [DOI] [PubMed] [Google Scholar]
- [11].Guo Y, Jiang K, Shen Z, Zheng G, Fan L, Zhao R, Shao J, A small molecule Nanodrug by self-assembly of dual anticancer drugs and photosensitizer for synergistic near-infrared cancer theranostics, ACS Appl. Mater. Interfaces 9 (2017) 43508–43519, 10.1021/acsami.7b14755. [DOI] [PubMed] [Google Scholar]
- [12].Kwitniewski M, Juzeniene A, Glosnicka R, Moan J, Immunotherapy: a way to improve the therapeutic outcome of photodynamic therapy? Photochem. Photobiol. Sci. 7 (2008) 1011, 10.1039/b806710d. [DOI] [PubMed] [Google Scholar]
- [13].Garg AD, Nowis D, Golab J, Agostinis P, Photodynamic therapy: illuminating the road from cell death towards anti-tumour immunity, Apoptosis. 15 (2010) 1050–1071, 10.1007/s10495-010-0479-7. [DOI] [PubMed] [Google Scholar]
- [14].Kleinovink JW, van Driel PB, Snoeks TJ, Prokopi N, Fransen MF, Cruz LJ, Mezzanotte L, Chan A, Löwik CW, Ossendorp F, Combination of photodynamic therapy and specific immunotherapy efficiently eradicates established tumors, Clin. Cancer Res. 22 (2016) 1459–1468, 10.1158/1078-0432.CCR-15-0515. [DOI] [PubMed] [Google Scholar]
- [15].Christie C, Pomeroy A, Nair R, Berg K, Hirschberg H, Photodynamic therapy enhances the efficacy of gene-directed enzyme prodrug therapy, Photodiagn. Photodyn. Ther. 18 (2017) 140–148, 10.1016/j.pdpdt.2017.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Oku N, Ishii T, Antiangiogenic photodynamic therapy with targeted liposomes, Methods Enzymol. 465 (2009) 313–330, 10.1016/S0076-6879(09)65016-3. [DOI] [PubMed] [Google Scholar]
- [17].Tsai S-R, Hamblin MR, Biological effects and medical applications of infrared radiation, J. Photochem. Photobiol. B Biol. 170 (2017) 197–207, 10.1016/j.jphotobiol.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tsai S-R, Yin R, Huang Y-Y, Sheu B-C, Lee S-C, Hamblin MR, Low-level light therapy potentiates NPe6-mediated photodynamic therapy in a human osteosarcoma cell line via increased ATP, Photodiagn. Photodyn. Ther. 12 (2015) 123–130, 10.1016/j.pdpdt.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Luo T, Zhang Q, Lu Q-B, Combination of near infrared light-activated photo-dynamic therapy mediated by Indocyanine green with etoposide to treat non-small-cell lung cancer, Cancers (Basel). 9 (2017) 63, 10.3390/cancers9060063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Liu Y, Miyoshi H, Nakamura M, Nanomedicine for drug delivery and imaging: a promising avenue for cancer therapy and diagnosis using targeted functional nanoparticles, Int. J. Cancer 120 (2007) 2527–2537, 10.1002/ijc.22709. [DOI] [PubMed] [Google Scholar]
- [21].Anselmo AC, Mitragotri S, An overview of clinical and commercial impact of drug delivery systems, J. Control. Release 190 (2014) 15–28, 10.1016/j.jconrel.2014.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rawling T, Xiao H, Lee S-T, Colbran SB, McDonagh AM, Optical and redox properties of ruthenium phthalocyanine complexes tuned with axial ligand sub-stituents, Inorg. Chem. 46 (2007) 2805–2813, 10.1021/IC061866U. [DOI] [PubMed] [Google Scholar]
- [23].Rodríguez-Morgade MS, Plonska-Brzezinska ME, Athans AJ, Carbonell E, de Miguel G, Guldi DM, Echegoyen L, Torres T, Synthesis, characterization, and photoinduced electron transfer processes of orthogonal ruthenium phthalocyani-ne–fullerene assemblies, J. Am. Chem. Soc. 131 (2009) 10484–10496, 10.1021/ja902471w. [DOI] [PubMed] [Google Scholar]
- [24].Carneiro ZA, De Moraes JCB, Rodrigues FP, De Lima RG, Curti C, Da Rocha ZN, Paulo M, Bendhack LM, Tedesco AC, Formiga ALB, Da Silva RS, Photocytotoxic activity of a nitrosyl phthalocyanine ruthenium complex - a system capable of producing nitric oxide and singlet oxygen, J. Inorg. Biochem. 105 (2011) 1035–1043, 10.1016/j.jinorgbio.2011.04.011. [DOI] [PubMed] [Google Scholar]
- [25].Fraix A, Sortino S, Combination of PDT photosensitizers with NO photodononors, Photochem. Photobiol. Sci. 17 (2018) 1709–1727, 10.1039/C8PP00272J. [DOI] [PubMed] [Google Scholar]
- [26].Sortino S, Light-controlled nitric oxide delivering molecular assemblies, Chem. Soc. Rev. 39 (2010) 2903–2913, 10.1039/b908663n. [DOI] [PubMed] [Google Scholar]
- [27].Adeloye AO, Ajibade PA, Synthesis, characterization, electrochemistry, and spectroscopic properties of some anthracenyl functionalized phthalocyanine complexes of ruthenium(II), J. Chem. 2014 (2014) 1–8, 10.1155/2014/257206. [DOI] [Google Scholar]
- [28].Rocha ZN, Lima RG, Doro FG, Tfouni E, da Silva RS, Photochemical production of nitric oxide from a nitrosyl phthalocyanine ruthenium complex by irradiation with light in the phototherapeutic window, Inorg. Chem. Commun. 11 (2008) 737–740, 10.1016/j.inoche.2008.03.019. [DOI] [Google Scholar]
- [29].Da Rocha ZN, Marchesi MSP, Molin JC, Lunardi CN, Miranda KM, Bendhack LM, Ford PC, Da Silva RS, The inducing NO-vasodilation by chemical reduction of coordinated nitrite ion in cis-[Ru(NO2)L(bpy)2] + complex, Dalton Trans. (2008) 4282–4287, 10.1039/b803441a. [DOI] [PubMed] [Google Scholar]
- [30].Ziminov AV, Ramsh SM, Terukov EI, Trapeznikova IN, Shamanin VV, Yurre TA, Correlation dependences in infrared spectra of metal phthalocyanines, Semiconductors 40 (2006) 1131–1136, 10.1134/S1063782606100022. [DOI] [Google Scholar]
- [31].Schmitt F, Govindaswamy P, Süss-Fink G, Ang WH, Dyson PJ, Juillerat-Jeanneret L, Therrien B, Ruthenium porphyrin compounds for photodynamic therapy of cancer, J. Med. Chem. 51 (2008) 1811–1816, 10.1021/jm701382p. [DOI] [PubMed] [Google Scholar]
- [32].Charlesworth P, Truscotta TG, Brooksb RC, Wilsonc BC, Photophysical properties of a ruthenium-substituted phthalocyanine, J. Photochem. Photobiol. B 26 (1994) 277–282, 10.1016/1011-1344(94)07048-2. [DOI] [Google Scholar]
- [33].Heinrich TA, Tedesco AC, Fukuto JM, Da Silva RS, Production of reactive oxygen and nitrogen species by light irradiation of a nitrosyl phthalocyanine ruthenium complex as a strategy for cancer treatment, Dalton Trans. 43 (2014) 4021–4025, 10.1039/c3dt52217b. [DOI] [PubMed] [Google Scholar]
- [34].Gorelsky SI, da Silva SC, Lever ABP, Franco DW, Electronic spectra of trans-[Ru(NH3)4(L)NO]3+/2+ complexes, Inorganica Chim. Acta. 300–302 (2000) 698–708, 10.1016/S0020-1693(99)00611-8. [DOI] [Google Scholar]
- [35].Soares LA, Trsic M, Berno B, Aroca R, Electronic spectra of metal phthalocya-nines and configuration interaction ZINDO calculations for tetra-azaporphyrin complexes with the first transition metal series, Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 52 (1996) 1245–1253, 10.1016/0584-8539(96)01659-5. [DOI] [Google Scholar]
- [36].Somasundaram V, Basudhar D, Bharadwaj G, No JH, Ridnour LA, Cheng RYS, Fujita M, Thomas DD, Anderson SK, McVicar DW, Wink DA, Molecular mechanisms of nitric oxide in cancer progression, signal transduction, and metabolism, Antioxid. Redox Signal. 30 (2019) 1124–1143, 10.1089/ars.2018.7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ridnour LA, Thomas DD, Switzer C, Flores-Santana W, Isenberg JS, Ambs S, Roberts DD, Wink DA, Molecular mechanisms for discrete nitric oxide levels in cancer, Nitric Oxide 19 (2008) 73–76, 10.1016/j.niox.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Evans MA, Huang P-J, Iwamoto Y, Ibsen KN, Chan EM, Hitomi Y, Ford PC, Mitragotri S, Macrophage-mediated delivery of light activated nitric oxide prodrugs with spatial, temporal and concentration control, Chem. Sci. 9 (2018) 3729–3741, 10.1039/C8SC00015H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Rose MJ, Mascharak PK, Photoactive ruthenium nitrosyls: effects of light and potential application as NO donors, Coord. Chem. Rev. 252 (2008) 2093–2114, 10.1016/j.ccr.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Karu TI, Mitochondrial signaling in mammalian cells activated by red and near-IR radiation, Photochem. Photobiol. 84 (2008) 1091–1099, 10.1111/j.1751-1097.2008.00394.x. [DOI] [PubMed] [Google Scholar]
- [41].Rojas JC, Gonzalez-Lima F, Neurological and psychological applications of transcranial lasers and LEDs, Biochem. Pharmacol. 86 (2013) 447–457, 10.1016/j.bcp.2013.06.012. [DOI] [PubMed] [Google Scholar]
- [42].Shapiro MG, Homma K, Villarreal S, Richter C-P, Bezanilla F, Infrared light excites cells by changing their electrical capacitance, Nat. Commun. 3 (2012) 736, 10.1038/ncomms1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Calles C, Schneider M, Macaluso F, Benesova T, Krutmann J, Schroeder P, Infrared a radiation influences the skin fibroblast transcriptome: mechanisms and consequences, J. Invest. Dermatol. 130 (2010) 1524–1536, 10.1038/JID.2010.9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


