Abstract
Ten wheat (Triticum aestivum L.) cultivars were tested in a semi-field experiment for drought response in terms of their flag leaf vigor, whole shoot growth and ultimate yield capacity. At booting stage, 25% of field capacity was held for 3 weeks, then the plants were normally irrigated. Based on split plot analysis of the pooled data, the order in which the source of variation could affect the estimated traits was watering level, then cultivar and finally the combination of both. At p ≤ 0.05, significant positive linear correlation was recorded between the drought-induced change in grain total carbohydrate content and leaf total carbohydrate content, between biological yield and each of water use efficiency for biomass and evapotranspiration efficiency as well as between economic yield and each of leaf catalase activity, water use efficiency for grain and hundred kernel mass. On contrary, significant negative correlation was recorded between the drought-induced change in shoot evapotranspiration rate and each of leaf proline content and shoot water content. Based on the drought-induced change in the estimated vegetative and yield traits, cluster analysis could sequester the concerned cultivars into drought-tolerant, moderate and sensitive ones; with Sids 13 being the most drought-tolerant cultivar as well as Shandaweel 1 and Giza 168 as the most drought-sensitive ones.
Keywords: Drought, Flag leaf, Shoot, Wheat, Yield
Introduction
Wheat (Triticum aestivum L.) is the most widely cultivated crop with an essential role in maintaining global food security. In this regard, wheat was documented to be the cereal of choice in most countries because of its nutritional virtue; where it serves as a rich source of carbohydrates and proteins (Fardet et al. 2008). However, global wheat production needs to be increased by about 50% by the year 2030 to feed the growing population (Gahlaut et al. 2017). At the same time, it was documented that at least 70% of the world’s wheat-cultivated area had experienced water stress (Monneveux et al. 2012). Thus, identifying drought-tolerant wheat cultivars along with realizing the mechanisms of their ability to cope with drought may be a strategic means to upgrade wheat productivity. Because of its wide-spread occurrence, a condensed consideration of drought revealed that it could affect the performance of wheat or any other crop plant in various aspects resulting in the most fatal economic loss in the agricultural sector. Such a stressful factor was intensively regarded to exert marked impacts on the performance of plants on cellular as well as the whole level leading to specific as well as unspecific reactions, injuries and adaptation responses (Beck et al. 2007).
For wheat, drought at heading stage had more obvious influences than at other growth stage; with marked consequences on ultimate yield (Kamel and Yazdansepas 2016). Depending on cultivar, drought was recorded to alter wheat vegetative growth by affecting flag leaf growth as indicated by its agronomy, pigment content, photosynthetic capacity and carbohydrate content (Mickky et al. 2018b). Also, the response of different cultivars to drought was found to relay on the extent of cellular reactive oxygen species (ROS) over-production and its impact on cellular membranes (Karmollachaab and Gharineh 2015). Plants with enhanced antioxidants, whether antioxidative compounds or antioxidant enzymes, could withstand in dry habitats (Boguszewska et al. 2010). Also, plant water status linked with the tendency of its cells for osmotic regulation was considered as robust traits for screening drought tolerance of different cultivars (Rachmilevitch et al. 2006). Wheat yield was also found to be linked with shoot growth as revealed by its morphology, tillering ability and water relations. Generally, alteration in shoot growth vigor in response to drought varied with the severity and duration of stress as well as the growth stage and plant cultivar (Shao et al. 2007). So, there is an urgent need to identify plant cultivars with reasonable vegetative traits contributing to acceptable yield under drought.
The use of water by a crop is normally correlated with its capacity for biomass production (biological yield) or grain productivity (economic yield); the concept of water use efficiency. Thus, water use efficiency can be based on grain yield or total biomass production; and when it is used, the yield base should be clearly indicated (Tanner and Sinclair 1983). In this context, water use efficiency was concluded in many studies as one of the main yield drivers (Reynolds and Tuberosa 2008). However, variation in the values of water use efficiency was found to be substantial even within the cultivar; mainly because of environmental conditions (Lin et al. 2012). Therefore, the current study aimed at evaluating the comparative performance of ten wheat cultivars in terms of their vegetative growth at heading stage and yield capacity under drought; with adopting some statistical procedures to reach an overall conclusion.
Materials and methods
Plant material and experimental design
Pure wheat (Triticum aestivum L.) strains (Misr 1, Misr 2, Gemmeiza 9, Gemmeiza 11, Sids 12, Sids 13, Sakha 93, Sakha 94, Shandaweel 1 and Giza 168) were obtained from the Egyptian Ministry of Agriculture. Homogeneous grains were sown within plastic pots packed with 2/1, v/v, clay/sand soil and left under natural conditions (minimum/maximum air temperature, relative humidity and light intensity of 15/27 °C, 40/77% and 10,000/76,000 lx; respectively) at midday time (12 pm) throughout the whole experimental period; with irrigation to field capacity. After 45 days from sowing, plants from each cultivar were divided into two sets; the first was further irrigated to field capacity serving as control, while irrigation was held from the second set for 21 days so that 25% of irrigation water was held. Flag leaf along with the whole shoot was sampled at heading stage when the plants were 65-day old; with yield parameters taken 115 ± 5 days after sowing.
Assessment of vegetative traits
Flag leaf specific area was computed as the leaf area divided by its dry mass (Beadle 1993); with the leaf area determined as leaf length × breadth × 0.75. After determining the amount of total chlorophyll (sum of chlorophyll a and chlorophyll b amounts) according to Kissimon (1999), chlorophyll stability index was recorded as 100 × total chlorophyll of drought sample/total chlorophyll of control sample (Sairam et al. 1997). Leaf photosynthesis rate was determined using portable gas exchange system (LCi, ADC BioScientific, UK). Leaf total carbohydrate content was colorimetrically determined using HCl as an extraction solvent and anthrone as a reagent (Sadasivam and Manickam 1996).
Leaf membrane stability index was determined as described by Mickky et al. (2019), while leaf catalase was assayed following the titration method of Devi (2007). Weight method of Smart and Bingham (1974) was adopted to determine leaf relative water content; and the colorimetric method of Bates et al. (1973) was followed to determine proline amount in leaf water extract using ninhydrin reagent. Main shoot length was recorded with the number of tillers per plant as an indication for shoot tillering ability. Shoot water content was calculated as the amount of water present in the unit shoot fresh mass. Shoot evapotranspiration rate was determined as the total amount of water added to each pot divided by the fresh mass of the potted shoot (Passioura 1977).
Assessment of yield traits
Biological yield was determined as the mass of the whole plant, while economic yield was determined as grain yield per plant. Water use efficiency for biomass or for grain was determined as biological yield or economic yield per total amount of water added; respectively (Stanhill 1987). Evapotranspiration efficiency was determined as water use efficiency for grain/harvest index (Ehdaie and Waines 1993), where harvest index is economic yield/straw yield (Beadle 1993). Hundred grains were weighed to express hundred kernel mass, while grain total carbohydrate and total protein contents were colorimetrically determined using anthrone and coomassie brilliant blue; respectively (Sadasivam and Manickam 1996).
Statistical analysis
A statistical “CoHort/CoStat” software version 6.311 was employed to analyze the replicas of collected data (five plant replicas assessed for morphological or agronomic traits and only three replicas for biochemical analyses). Analysis of variance (ANOVA) test at p ≤ 0.05 was applied to the normally-distributed data with split plot design considering cultivar as the main plot factor and watering level as the sub-plot factor. The percent change in each trait for each cultivar under the effect of drought was recorded. As a univariate algorithm, linear correlation was determined among the drought-induced changes in the estimated traits using statistical “Past” software version 3.20; and only significant correlations at p ≤ 0.05 were illustrated. As a multivariate algorithm, cluster analysis of the concerned cultivars was performed using “Minitab” software version 18 with complete linkage method and Euclidean distance measure; considering cluster centroids to represent the mean values of the drought-induced changes in vegetative and yield traits within each cluster.
Results and discussion
ANOVA results
Results in Table 1 reflect an overall picture about the relative effect of cultivar, watering level or the combination of both on vegetative growth and yield of the concerned wheat plants; taking into account the effect of the sample size or replications. According to mean squares (MS) values, replications had minor effect on most of the estimated traits (non-significant effect at p ≤ 0.05); with low percent of variation in relation to the total sources of variations (≤ 5%) except for leaf specific area and leaf photosynthesis rate where high percent of variation (up to 19%) could be recorded as a result of replications. Such effect could be reflected on the error source of variation which had minor effect on the estimated traits; with low percent of variation (≤ 2%) except for leaf specific area and leaf photosynthesis rate where high percent of variation (up to 17%) could be recorded. With respect to the solo effect of cultivar or watering level, it was recorded that watering level was the most obvious source of variation when considering all the determined traits except for leaf specific area, leaf total carbohydrate content, shoot tillering ability, water use efficiency for biomass, water use efficiency for grain and grain total protein content where the effect of cultivar was more obvious. Those traits for which the effect of cultivar as a source of variation is more pronounced can be thus considered as reliable selection criteria for drought tolerance. Matching this finding, water use efficiency was previously recorded as suitable screening trait for discriminating drought-tolerant wheat cultivars (Farshadfar et al. 2011). However, the combination of cultivar and watering level had significant effect on the estimated traits at p ≤ 0.05 except for leaf specific area, leaf relative water content, shoot length and shoot evapotranspiration rate causing low percent of variation (≤ 3%). Therefore, these four traits can be considered as constitutive traits as their expression is independent on environmental fluctuations; in other words variation in these traits among different cultivars is minimal under different watering levels. In a similar trend, leaf relative water content was recorded as a constitutive trait when considering different wheat cultivars under varied water regimes (Bayoumi et al. 2008).
Table 1.
ANOVA results based on split plot analysis of the estimated vegetative and yield traits of ten wheat cultivars under drought
| Trait | SOV | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Replication | Cultivar | Watering level | Cultivar × Watering level | Error | |||||||||||
| df | MS | % | df | MS | % | df | MS | % | df | MS | % | df | MS | % | |
| Leaf specific area | 4 | 477.23ns | 19 | 9 | 892.22** | 36 | 1 | 590.20ns | 24 | 9 | 69.32ns | 3 | 40 | 426.19 | 17 |
| Leaf chlorophyll stability index | 2 | 9.82ns | 1 | 9 | 74.86*** | 7 | 1 | 836.27*** | 83 | 9 | 74.86*** | 7 | 20 | 8.900 | 1 |
| Leaf photosynthesis rate | 2 | 11.57ns | 11 | 9 | 8.04ns | 8 | 1 | 65.29** | 62 | 9 | 14.61* | 14 | 20 | 5.067 | 5 |
| Leaf total carbohydrate content | 2 | 0.30ns | 0 | 9 | 695.79*** | 69 | 1 | 203.16*** | 20 | 9 | 104.72*** | 10 | 20 | 0.416 | 0 |
| Leaf membrane stability index | 2 | 0.13ns | 0 | 9 | 5.18*** | 1 | 1 | 467.95*** | 98 | 9 | 4.65*** | 1 | 20 | 0.634 | 0 |
| Leaf catalase activity | 2 | 0.02* | 0 | 9 | 1.68*** | 20 | 1 | 4.23*** | 49 | 9 | 2.62*** | 31 | 20 | 0 | 0 |
| Leaf relative water content | 2 | 2.33ns | 0 | 9 | 78.50*** | 8 | 1 | 885.81*** | 90 | 9 | 12.91ns | 1 | 20 | 9.636 | 1 |
| Leaf proline content | 2 | 0ns | 0 | 9 | 64.77*** | 3 | 1 | 1977.32*** | 95 | 9 | 34.37*** | 2 | 20 | 0.003 | 0 |
| Shoot length | 4 | 28.34ns | 1 | 9 | 474.35*** | 24 | 1 | 1426.57*** | 72 | 9 | 33.63ns | 2 | 40 | 22.084 | 1 |
| Shoot tillering ability | 4 | 0.27ns | 5 | 9 | 4.15*** | 70 | 1 | 0.36ns | 6 | 9 | 0.98*** | 17 | 40 | 0.145 | 2 |
| Shoot water content | 4 | 394.09ns | 1 | 9 | 5780.12*** | 13 | 1 | 36,481*** | 83 | 9 | 1015.42* | 2 | 40 | 382.405 | 1 |
| Shoot evapotranspiration rate | 2 | 1129.03ns | 3 | 9 | 2968.52** | 7 | 1 | 34,349.31*** | 85 | 9 | 1069.53ns | 3 | 20 | 745.41 | 2 |
| Biological yield | 4 | 0.61* | 0 | 9 | 12.52*** | 4 | 1 | 281.89*** | 94 | 9 | 4.26*** | 1 | 40 | 0.103 | 0 |
| Economic yield | 4 | 0.37* | 1 | 9 | 4.67*** | 10 | 1 | 38.48*** | 86 | 9 | 1.12*** | 3 | 40 | 0.065 | 0 |
| Water use efficiency for biomass | 4 | 0.02* | 3 | 9 | 0.48*** | 71 | 1 | 0ns | 0 | 9 | 0.17*** | 25 | 40 | 0.003 | 0 |
| Water use efficiency for grain | 4 | 0.01* | 4 | 9 | 0.15*** | 57 | 1 | 0.07*** | 27 | 9 | 0.03*** | 11 | 40 | 0.002 | 1 |
| Evapotranspiration efficiency | 4 | 0.02ns | 4 | 9 | 0.14*** | 26 | 1 | 0.26*** | 48 | 9 | 0.12*** | 22 | 40 | 0.003 | 1 |
| Hundred kernel mass | 4 | 0.45** | 2 | 9 | 2.86*** | 13 | 1 | 17.56*** | 83 | 9 | 0.38*** | 2 | 40 | 0.024 | 0 |
| Grain total carbohydrate content | 2 | 1.22ns | 0 | 9 | 5927.35*** | 3 | 1 | 196,424.82*** | 96 | 9 | 2598.45*** | 1 | 20 | 1.127 | 0 |
| Grain total protein content | 2 | 1.79ns | 1 | 9 | 142.72*** | 71 | 1 | 7.85ns | 4 | 9 | 47.82*** | 24 | 20 | 2.166 | 1 |
SOV source of variation, df degree of freedom, MS mean squares, ns non-significant variation, “%” MS for each SOV relative to total MS of all sources
*, ** and *** low, moderate and high degree of significant variation at p ≤ 0.05
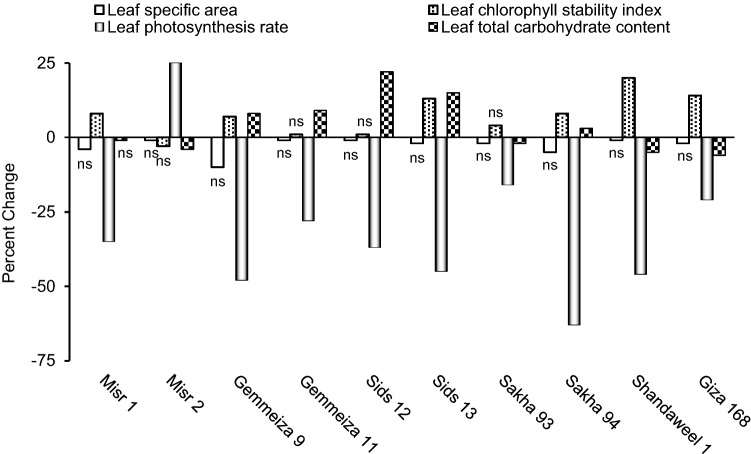
Leaf specific area, chlorophyll stability index, photosynthesis rate and total carbohydrate content
Leaf photosynthetic machinery of the studied wheat cultivars was previously considered in particular (Mickky et al. 2018b); but the percent change in leaf specific area, chlorophyll stability index, photosynthesis rate and total carbohydrate content under drought were necessarily reconsidered herein to explain yield traits. Data revealed that drought decreased leaf specific area in all cultivars although such decreases were non-significant at p ≤ 0.05 (Fig. 1). Mickky et al. (2018a) similarly recorded decrease in leaf specific area of alfalfa plants in response to drought. In this regard, it was reported that plants might have lower leaf specific area under drought to reduce transpiration (Poorter et al. 2009). From another point of view, lower leaf specific area under drought may reflect little carbon gain (Cheng et al. 2016). For leaf chlorophyll stability index, drought significantly increased it in Misr 1, Gemmeiza 9, Sids 13, Sakha 94, Shandaweel 1 and Giza 168, while drought non-significantly increased it in Gemmeiza 11, Sids 12 and Sakha 93; but non-significantly decreased it in Misr 2 (Fig. 1). Leaf chlorophyll stability index was found to reveal pigment maintenance under stressful conditions; with higher values indicating more chlorophyll availability to help plants cope with stress by enhanced photosynthesis rate with consequent better dry matter production (Ananthi et al. 2013). In this context, Surendar et al. (2013) noticed that tolerant and moderately tolerant banana cultivars showed lesser reduction in their chlorophyll stability index in response to drought, while sensitive synonyms showed higher reduction.
Fig. 1.
Effect of drought on flag leaf specific area, chlorophyll stability index, photosynthesis rate and total carbohydrate content of ten wheat cultivars; with ns referring to non-significant change at p ≤ 0.05
Also, drought was recorded herein to significantly decrease leaf photosynthesis rate in all cultivars except in Misr 2 where drought significantly decreased it (Fig. 1). Coinciding with this result, leaf photosynthesis rate of six wheat cultivars was found to decrease because of drought (Guan et al. 2015). Under drought, photosynthesis rate decreases either by stomatal or non-stomatal factors. Stomatal limitations usually result from limited CO2 availability; with stomata tending to be closed. Although stomatal closure is usually considered as a very important mechanism to protect tissues against dehydration, it can result in limited CO2 availability and/or assimilation (Biesaga-Kościelniak et al. 2014). Non-stomatal limitations however can result from; (1) little CO2 availability as a result of diffusion limitations through mesophyll, (2) inhibition of the activity of some key enzymes mainly ribulose-1,5-bisphosphate carboxylase/oxygenase, (3) damage of leaf cellular ultrastructure and/or (4) low CO2 permeability as a result of the negative effect of dehydration on leaf cell walls, plasma membranes and cuticle (Ghannoum et al. 2003; Liu et al. 2004).
In addition, the results of the present study indicated that drought non-significantly decreased leaf total carbohydrate content in Misr 1, significantly decreased it in Misr 2, Sakha 93, Shandaweel 1 and Giza 168; but significantly increased it in the remaining cultivars (Fig. 1). Drought-induced decrease in leaf total carbohydrate content was similarly recorded in two wheat cultivars (Aldesuquy et al. 2012). Such decrease could be attributed to the stress-caused drop in leaf chlorophyll content with consequent suppression of photosynthetic efficiency and carbon gain. However, accumulation of carbohydrates in different plants as a result of limited water supply was also documented (Liu et al. 2004). Accumulation of carbohydrates under stressful conditions can be regarded as a potent tolerance strategy by decreasing cellular water potential and contributing to avoidance of the ROS-induced oxidative injury (Hoekstra et al. 2001).
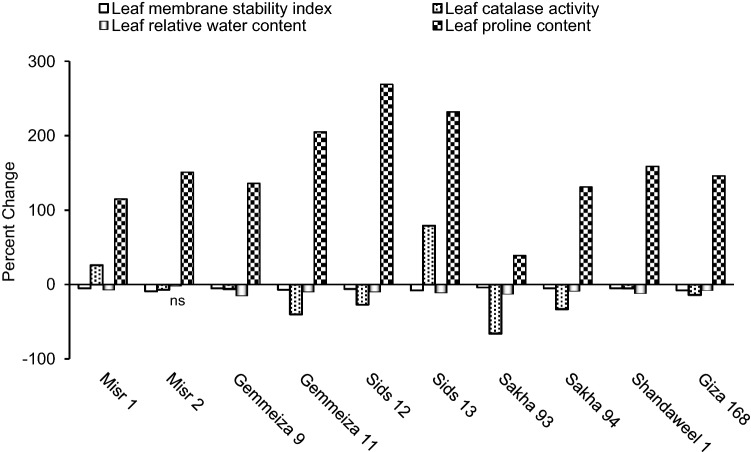
Leaf membrane stability index, catalase activity, relative water content and proline content
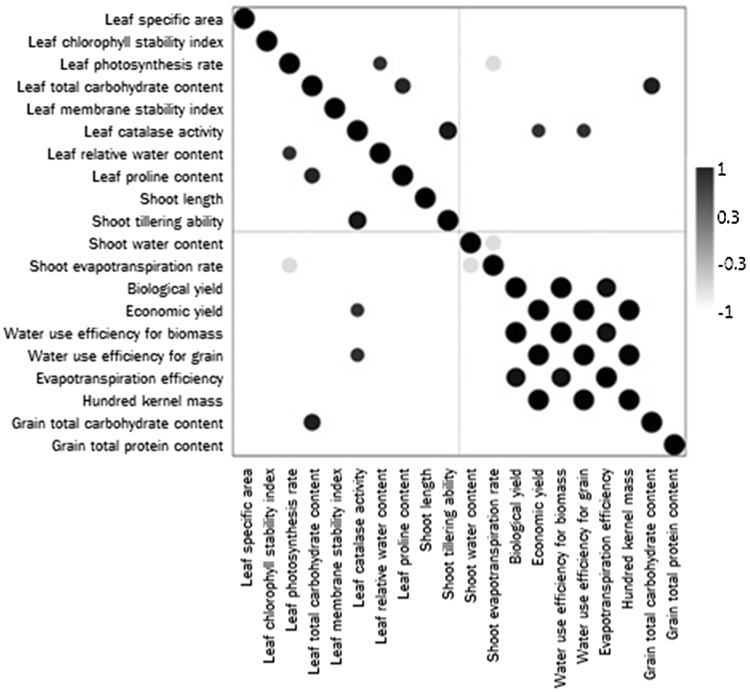
Results obtained herein indicated that drought significantly decreased leaf cellular membrane stability index in all cultivars (Fig. 2). In this connection, leaf membrane stability index was frequently used for screening the response of various plant cultivars to drought (Almeselmani et al. 2011). The decrease in leaf membrane stability index as a result of drought can be ascribed to the reverse effect of ROS accumulated under stress (Gill and Tuteja 2010). Drought was also recorded herein to significantly increase leaf catalase activity in Misr 1 and Sids 13; but significantly decreased it in the other cultivars (Fig. 2). Such alterations in leaf catalase activity match the results recorded by Mickky and Aldesuquy (2017) working on different wheat cultivars during their early growth. The drought-induced inhibition of leaf catalase activity can be ascribed to the negative effect of stress on protein synthesis or to the deficiency of some vital elements that activate this enzyme (Mickky et al. 2019). On the other hand, the drought-induced activation of leaf catalase activity recorded herein in Misr 1 and Sids 13 can possibly indicate that those cultivars could adapt to stress by scavenging H2O2. Matching this assumption, enhanced activity of CAT in drought-tolerant wheat cultivars along with inhibited activity in sensitive ones was formerly noticed (Li et al. 2010). Supporting these findings, significant positive correlation (p ≤ 0.05) could be recorded herein between the drought-induced change in leaf catalase activity and each of shoot tillering ability, economic yield and water use efficiency for grain (Fig. 6); confirming the direct link of leaf catalase activity with both vegetative growth and yield potential.
Fig. 2.
Effect of drought on flag leaf membrane stability index, catalase activity, relative water content and proline content of ten wheat cultivars; with ns referring to non-significant change at p ≤ 0.05
Fig. 6.
Black and white map of significant correlations at p ≤ 0.05 among the drought-induced change in the estimated vegetative and yield traits of ten wheat cultivars
Drought was also found to significantly decrease leaf relative water content in all cultivars except in Misr 2 where drought non-significantly decreased it (Fig. 2). Similar reduction in leaf relative water content was previously recorded in rice plants under water stress, and such decrement was ascribed to the stress-induced decrease in leaf water potential with corresponding increase in leaf temperature (Siddique et al. 2001). In the current study, the drought-induced change leaf relative water content was recorded to significantly and positively correlate with leaf photosynthesis rate (Fig. 6); indicating that the recorded suppression in leaf photosynthesis rate and leaf relative water content can be ascribed to each other. Drought was also found herein to significantly increase leaf proline content in all cultivars (Fig. 2). Matching this finding, Hajiboland et al. (2015) reported proline accumulation in the leaves of two wheat cultivars as a result of drought; but such accumulation was more pronounced in the drought-tolerant cultivar than in its sensitive relative. Proline was documented to play essential role in; (1) maintaining cytosol-vacuole pressure with well-controlled pH, (2) stabilizing cellular membranes, (3) sustaining NADP+/NADPH ratios for optimum metabolism, (4) providing potent sink for excess reductants required for maintenance of respiration and synthesis of purines and (5) contributing to cell redox balance by minimizing the level of singlet oxygen that induces lipid peroxidation (Szabados and Savouré 2010). For that, significant positive correlation could be recorded herein between the drought-induced change in leaf proline content and leaf total carbohydrate content (Fig. 6); referring to the strong relation between leaf proline content and leaf capacity to sustain more carbohydrate content.
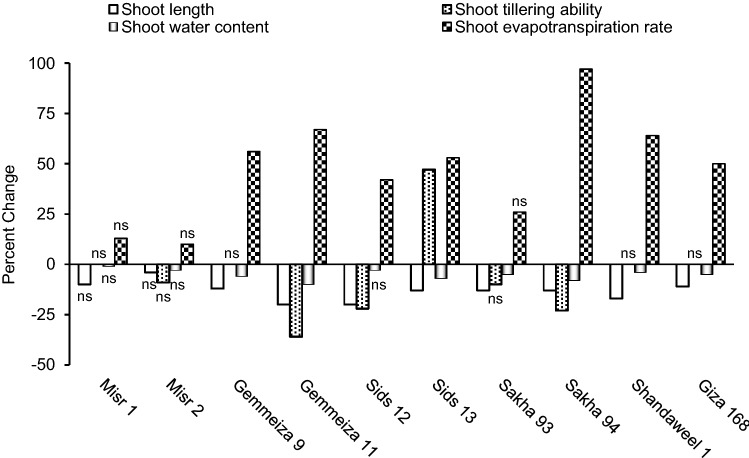
Shoot length, tillering ability, water content and evapotranspiration rate
According to data in Fig. 3, drought non-significantly decreased shoot length in the two Misr cultivars; but significantly decreased it in the remaining cultivars. In accordance with this result, Aldesuquy et al. (2012) recorded that shoot length was negatively affected in different wheat cultivars at vegetative stages as a result of drought. Reduction in shoot length could be attributed to diminished cell expansion and elongation (Jaleel et al. 2009). Also, the results of the present study indicated that shoot tillering ability significantly increased in Sids 13, did not change in Misr 1, Gemmeiza 9, Shandaweel 1 and Giza 168 but decreased in the remaining cultivars as a result of drought (Fig. 3). In this context, Waraich et al. (2007) in a field experiment on wheat recorded less number of tillers per plant under drought. However, greater tillering ability of wheat plants may be an adaptation feature to stress conditions.
Fig. 3.
Effect of drought on shoot length, tillering ability, water content and evapotranspiration rate of ten wheat cultivars; with ns referring to non-significant change at p ≤ 0.05
With respect to shoot water content, the results recorded herein indicated that drought decreased it non-significantly in the two Misr cultivars and Sids 12 but significantly in the remaining cultivars (Fig. 3). Less shoot water content in response to drought can be ascribed to little water absorption by root and/or little water translocation to shoot. Also, less shoot water content under drought can be attributed to the stress-associated increase in transpiration rate; an assumption that can be supported by the significant negative correlation recorded herein between the drought-induced change in shoot water content and shoot evapotranspiration rate (Fig. 6). Data of the present study also revealed that drought increased shoot evapotranspiration rate; non-significantly in the two Misr cultivars and Sakha 93 but significantly in the remaining cultivars (Fig. 3). Since shoot evapotranspiration rate was determined herein as the total amount of water added to each pot divided by the fresh mass of the potted plants (Passioura 1977), the recorded increase in shoot evapotranspiration rate under drought can be ascribed to the recorded drought-induced decrease in shoot biomass caused basically by reduced leaf photosynthetic capacity. Supporting this assumption, significant negative correlation could be recorded herein between the drought-induced change in shoot evapotranspiration rate and leaf photosynthesis rate (Fig. 6).
Biological yield, economic yield, water use efficiency for biomass and water use efficiency for grain
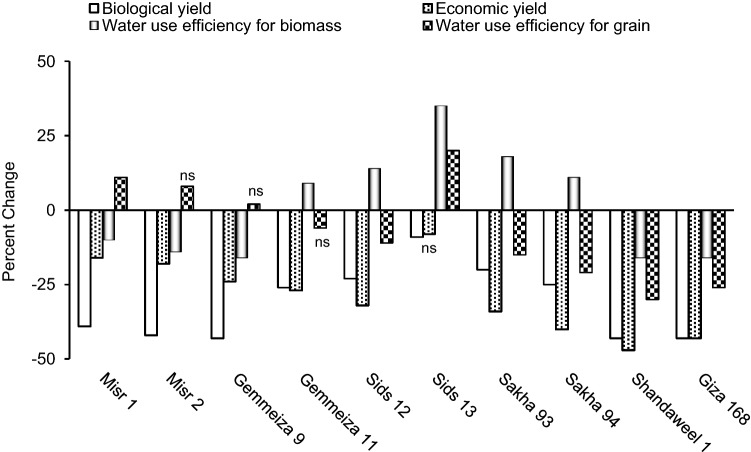
Drought significantly decreased both biological and economic yield in all cultivars except for Sids 13 in which drought had non-significant effect on its economic yield (Fig. 4). Mehraban et al. (2019) similarly noticed that drought could decrease both biological and economic yield of ten wheat cultivars. Yield loss of drought subjected plants could be ascribed to the stress-induced restriction of the plant photosynthetic capacity with consequent lower supply of photo-assimilates to the developed grains (Farooq et al. 2014). In addition, yield loss in response to drought could be attributed to high energy and carbohydrates expenses in osmotic regulation as well as the stress-induced interference with cell functions (Shani and Dudley 2001). Also, the results of the current study indicated that drought significantly decreased water use efficiency for biomass in the two Misr cultivars, Gemmeiza 9, Shandaweel 1 and Giza 168, while drought significantly increased water use efficiency for biomass in the remaining cultivars. For water use efficiency for grain, drought significantly increased it in Misr 1 and Sids 13, non-significantly increased it in Misr 2 and Gemmeiza 9 but significantly decreased it in the remaining cultivars (Fig. 4). In this context, it was assumed that drought might increase the values of water use efficiency; with many studies suggesting that cultivars with proper water use efficiency would have better drought tolerance (Varga et al. 2013). Thus, for those cultivars whose water use efficiency increased by drought, like Sids 13, such response can be considered as an indication for better drought tolerance than those cultivars whose water use efficiency decreased by drought. The recorded drought-induced change in water use efficiency for biomass can be ascribed to that in biological yield and vice versa, and the same for water use efficiency for grain whose drought-induced change can ascribed to that in economic yield and vice versa. This is clear from the significant positive correlation recorded herein between the drought-induced change in biological yield and water use efficiency for biomass as well as between economic yield and water use efficiency for grain (Fig. 6).
Fig. 4.
Effect of drought on biological yield, economic yield, water use efficiency for biomass and water use efficiency for grain of ten wheat cultivars; with ns referring to non-significant change at p ≤ 0.05
Evapotranspiration efficiency, hundred kernel mass and grain total carbohydrate and protein content
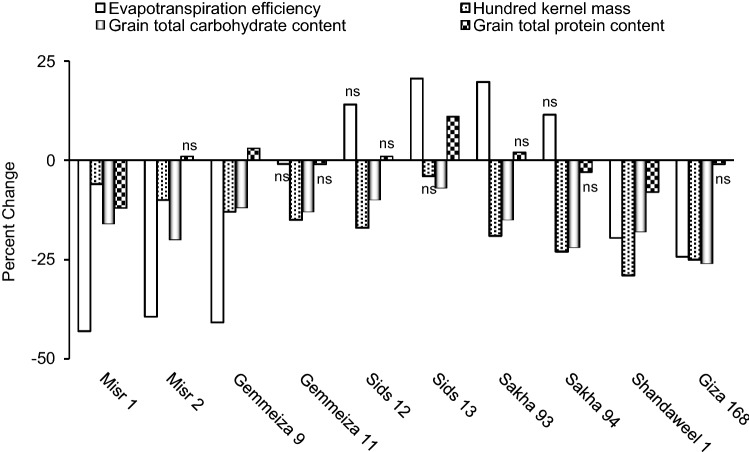
Drought non-significantly increased evapotranspiration efficiency in Sids 12 and Sakha 94, significantly increased it in Sids 13 and Sakha 93 but significantly decreased it in the remaining cultivars (Fig. 5). Abiotic stress conditions were previously recorded to greatly affect the crop evapotranspiration efficiency; water use efficiency for grain in relation to harvest index which is the ratio of economic yield to straw yield (Al-Busaidi et al. 2009). The drop in evapotranspiration efficiency in response to drought can be ascribed to little water availability for the growing plants, the reduction in their water use efficiency for grain and/or to the increase in harvest index (unenclosed data). Similar explanation was suggested by Shani and Dudley (2001) who ascribed the drought-induced drop in evapotranspiration efficiency to little water availability. However and when considering the different cultivars, the two Sids cultivars and the two Sakha ones exhibited higher evapotranspiration efficiency in response to drought; an indication for reasonable drought tolerance. Also, the drought-induced change in evapotranspiration efficiency can be linked with biological yield and consequently with water use efficiency for biomass; and this can be indicated from the significant positive correlation recorded herein between the drought-induced change in evapotranspiration efficiency and each of biological yield and water use efficiency for biomass (Fig. 6). With respect to hundred kernel mass, drought was found herein to significantly decrease it in all cultivars except for Sids 13 in which drought had non-significant effect on its hundred kernel mass (Fig. 5). The drought-induced reduction in hundred kernel mass can be ascribed to the negative impact of drought on grain filling (Farooq et al. 2009). Also, the drought-induced change in hundred kernel mass can be linked with that in economic yield and water use efficiency for grain; an assumption indicated from the significant positive correlation recorded herein between the drought-induced change in hundred kernel mass and each of economic yield and water use efficiency for grain (Fig. 6).
Fig. 5.
Effect of drought on evapotranspiration efficiency, hundred kernel mass, grain total carbohydrate content and grain total protein content of ten wheat cultivars; with ns referring to non-significant change at p ≤ 0.05
Furthermore, drought significantly decreased grain total carbohydrate content in all cultivars; but it significantly decreased grain total protein content in Misr 1 and Shandaweel 1, significantly increased it in Gemmeiza 9, and Sids 13 but non-significantly affected it in the remaining cultivars (Fig. 5). Matching these results, drought was intensively reported to hinder the accumulation of various grain constituents, mainly total carbohydrates and total proteins, in the developed grains by inhibiting the metabolic pathways leading to their synthesis (Farooq et al. 2017). Such effect of water stress is well documented since the various processes involved in grain filling as well as the reserves accumulation in the developing grains are very sensitive to environmental fluctuations. In this context, the drought-induced reduction in total carbohydrates content of the yielded grains can be attributed to the reduction in leaf growth vigor as well as the suppression in its photosynthetic capacity. Confirming such assumption, significant positive correlation could be recorded herein between the drought-induced change in grain total carbohydrate content and leaf total carbohydrate content (Fig. 6). However, the drought-induced increase or non-significant decrease in grain total protein content recorded for some cultivars may indicate better ability to withstand stress and yield grains with almost unaffected or even enhanced proteins content. The increase in grain total protein content in response to drought may presumably indicate de novo synthesis of new proteins that enhance stress tolerance.
Cluster analysis of cultivars
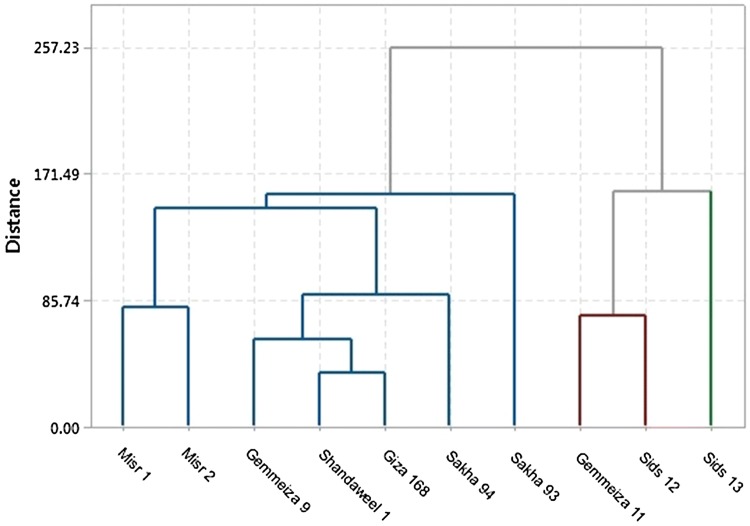
To get clear insight into the overall performance of the concerned wheat cultivars, hierarchical cluster analysis was carried out to sequester the cultivars into three clusters; drought-tolerant, moderate and sensitive cultivars. As represented in Fig. 7 and Table 2, Sids 13 was recognized as a drought-tolerant cultivar with the maximum drought-induced increase or the minimum drought-induced decrease in leaf chlorophyll stability index, leaf catalase activity, shoot tillering ability, biological yield, economic yield, water use efficiency for biomass, water use efficiency for grain, evapotranspiration efficiency, hundred kernel mass, grain total carbohydrate content and grain total protein content. Gemmeiza 11 and Sids 12 were however clustered together as drought-moderate cultivars with moderate drought-induced change in leaf photosynthesis rate, leaf membrane stability index, leaf relative water content, shoot water content, biological yield, economic yield, water use efficiency for biomass, water use efficiency for grain, evapotranspiration efficiency, hundred kernel mass, grain total carbohydrate content and grain total protein content. The remaining cultivars were thus left to be clustered as drought-sensitive cultivars with the maximum drought-induced decrease or the minimum drought-induced increase in leaf specific area, leaf total carbohydrate content, leaf proline content, shoot evapotranspiration rate, biological yield, economic yield, water use efficiency for biomass, water use efficiency for grain, evapotranspiration efficiency, hundred kernel mass, grain total carbohydrate content and grain total protein content; with Shandaweel 1 and Giza 168 identified as the most drought-sensitive cultivars. In such a way, yield traits are more homogeneously distributed among the three clusters than vegetative traits; so they can be implicated as better indicator for drought response.
Fig. 7.
Cluster analysis of ten wheat cultivars based on the drought-induced change in their vegetative and yield traits
Table 2.
Mean values of the drought-induced change (%) in vegetative and yield traits of ten wheat cultivars sequestered into three clusters; with superscripts for ranking within clusters
| Trait | Cluster 1 | Cluster 2 | Cluster 3 | Trait | Cluster 1 | Cluster 2 | Cluster 3 |
|---|---|---|---|---|---|---|---|
| Leaf specific area | − 4c | − 1a | − 2b | Shoot water content | − 5a | − 6b | − 7c |
| Leaf chlorophyll stability index | 8b | 1c | 13a | Shoot evapotranspiration rate | 45c | 55a | 53b |
| Leaf photosynthesis rate | − 29a | − 33b | − 45c | Biological yield | − 36c | − 25b | − 9a |
| Leaf total carbohydrate content | − 1c | 16a | 15b | Economic yield | − 32c | − 30b | − 8a |
| Leaf membrane stability index | − 6a | − 7b | − 8c | Water use efficiency for biomass | − 6c | 12b | 35a |
| Leaf catalase activity | − 15b | − 34c | 79a | Water use efficiency for grain | − 10c | − 9b | 20a |
| Leaf relative water content | − 9a | − 10b | − 11c | Evapotranspiration efficiency | − 19c | 7b | 21a |
| Leaf proline content | 125c | 237a | 232b | Hundred kernel mass | − 18c | − 16b | − 4a |
| Shoot length | − 11a | − 20c | − 13b | Grain total carbohydrate content | − 18c | − 12b | − 7a |
| Shoot tillering ability | − 6b | − 29c | 47a | Grain total protein content | − 3c | 0b | 11a |
Conclusion
Based on the results obtained from the current study, Sids 13 proved to be the most drought-tolerant cultivar; so it can be recommended for cultivation in dry habitats. Meanwhile, Shandaweel 1 and Giza 168 seemed to be the most drought-sensitive cultivars with the least yield capacity when facing drought.
Acknowledgements
The authors are grateful to the members of Sakha Agricultural Research Center for assistance in obtaining pure wheat strains.
Funding
This study was funded by the Scientific Research Unit of Mansoura University (Grant Number Competitive Project 8.12.2014).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Al-Busaidi A, Al-Rawahy S, Ahmed M. Response of different tomato cultivars to diluted seawater salinity. Asian J Crop Sci. 2009;1:77–86. doi: 10.3923/ajcs.2009.77.86. [DOI] [Google Scholar]
- Aldesuquy HS, Abo-Hamed SA, Abbas MA, Elhakem AH. Role of glycine betaine and salicylic acid in improving growth vigour and physiological aspects of droughted wheat cultivars. J Stress Physiol Biochem. 2012;8:149–171. [Google Scholar]
- Almeselmani M, Abdullah F, Hareri F, Naaesan M, Ammar MA, Kanbar OZ, Saud AB. Effect of drought on different physiological characters and yield component in different Syrian durum wheat varieties. J Agric Sci. 2011;3:127–133. [Google Scholar]
- Ananthi K, Vijayaraghavan H, Karuppaiya M, Anand T. Drought-induced changes in chlorophyll stability index, relative water content and yield of cotton genotypes. Insight Bot. 2013;3:1–5. doi: 10.5567/BOTANY-IK.2013.1.5. [DOI] [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water stress studies. Plant Soil. 1973;39:205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- Bayoumi TY, Eid MH, Metwali EM. Application of physiological and biochemical indices as a screening technique for drought tolerance in wheat genotypes. Afr J Biotechnol. 2008;7:2341–2352. [Google Scholar]
- Beadle C. Growth analysis. In: Hall DO, Scurlock JM, Bolhàr-Nordenkampf HR, Leegood RC, Long SP, editors. Photosynthesis and production in a changing environment: a field and laboratory manual. Dordrecht: Springer; 1993. [Google Scholar]
- Beck EH, Fettig S, Knake C, Hartig K, Bhattarai T. Specific and unspecific responses of plants to cold and drought stress. J Biosci. 2007;32:501–510. doi: 10.1007/s12038-007-0049-5. [DOI] [PubMed] [Google Scholar]
- Biesaga-Kościelniak J, Ostrowska A, Filek M, Dziurka M, Waligórski P, Mirek M, Kościelniak J. Evaluation of spring wheat (20 varieties) adaptation to soil drought during seedlings growth stage. Agriculture. 2014;4:96–112. doi: 10.3390/agriculture4020096. [DOI] [Google Scholar]
- Boguszewska D, Grudkowska M, Zagdańska B. Drought responsive antioxidant enzymes in potato (Solanum tuberosum L.) Potato Res. 2010;53:373–382. doi: 10.1007/s11540-010-9178-6. [DOI] [Google Scholar]
- Cheng J, Chu P, Chen D, Bai Y, Niu S. Functional correlations between specific leaf area and specific root length along a regional environmental gradient in Inner Mongolia grasslands. Funct Ecol. 2016;30:985–997. doi: 10.1111/1365-2435.12569. [DOI] [Google Scholar]
- Devi P. Principles and methods in plant molecular biology, biochemistry and genetics. 4. India: Agrobios; 2007. [Google Scholar]
- Ehdaie B, Waines G. Variation in water use efficiency and its components in wheat. Ι. Well-watered pot experiment. Crop Sci. 1993;33:294–299. doi: 10.2135/cropsci1993.0011183X003300020016x. [DOI] [Google Scholar]
- Fardet A, Rock E, Rémésy C. Is the in vitro antioxidant potential of whole-grain cereals and cereal products well reflected in vivo. J Cereal Sci. 2008;48:258–276. doi: 10.1016/j.jcs.2008.01.002. [DOI] [Google Scholar]
- Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SM. Plant drought stress: effects, mechanisms and management. Agron Sustain Dev. 2009;29:185–212. doi: 10.1051/agro:2008021. [DOI] [Google Scholar]
- Farooq M, Hussain M, Siddique KHM. Drought stress in wheat during flowering and grain-filling periods. Crit Rev Plant Sci. 2014;33:331–349. doi: 10.1080/07352689.2014.875291. [DOI] [Google Scholar]
- Farooq M, Gogoi N, Barthakur S, Baroowa B, Bharadwaj N, Alghamdi SS, Siddique KHM. Drought stress in grain legumes during reproduction and grain filling. J Agron Crop Sci. 2017;203:81–102. doi: 10.1111/jac.12169. [DOI] [Google Scholar]
- Farshadfar E, Rasoli V, da Silva J, Farshadfar M. Inheritance of drought tolerance indicators in bread wheat (Triticum aestivum L.) using a diallel technique. Aust J Crop Sci. 2011;5:870–878. [Google Scholar]
- Gahlaut V, Jaiswal V, Tyagi B, Singh G, Sareen S, Balyan HS, Gupta PK. QTL mapping for nine drought-responsive agronomic traits in bread wheat under irrigated and rain-fed environments. PLoS ONE. 2017;12:e0182857. doi: 10.1371/journal.pone.0182857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghannoum O, Conroy JP, Driscoll SP, Paul MJ, Foyer CH, Lawlor DW. Non-stomatal limitations are responsible for drought-induced photosynthetic inhibition in four C4 grasses. New Phytol. 2003;159:599–608. doi: 10.1046/j.1469-8137.2003.00835.x. [DOI] [PubMed] [Google Scholar]
- Gill S, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Guan XK, Song L, Wang TC, Turner NC, Li FM. Effect of drought on the gas exchange, chlorophyll fluorescence and yield of six different-era spring wheat cultivars. J Agron Crop Sci. 2015;201:253–266. doi: 10.1111/jac.12103. [DOI] [Google Scholar]
- Hajiboland R, Sadeghzadeh N, Ebrahimi N, Sadeghzadeh B, Mohammadi S. Influence of selenium in drought-stressed wheat plants under greenhouse and field conditions. Acta Agric Slov. 2015;105:175–191. doi: 10.14720/aas.2015.105.2.01. [DOI] [Google Scholar]
- Hoekstra FA, Golovina EA, Buitink J. Mechanisms of plant desiccation tolerance. Trends Plant Sci. 2001;6:431–438. doi: 10.1016/S1360-1385(01)02052-0. [DOI] [PubMed] [Google Scholar]
- Jaleel CA, Manivannan P, Wahid A, Farooq M, Somasundaram R, Panneerselvam R. Drought stress in plants: a review on morphological characteristics and pigments composition. Int J Agric Biol. 2009;11:100–105. [Google Scholar]
- Kamel M, Yazdansepas A. A study of source and sink relationships to select wheat lines and genotypes for drought tolerance. Cercet Agron Mold. 2016;49:27–38. doi: 10.1515/cerce-2016-0023. [DOI] [Google Scholar]
- Karmollachaab A, Gharineh MH. Effect of silicon application on wheat seedlings growth under water-deficit stress induced by polyethylene glycol. Iran Agric Res. 2015;34:31–38. [Google Scholar]
- Kissimon J. Analysis of the photosynthetic pigment composition. Mosonmagyarovar, Hungary: International Workshop and Training Course on Microalgal Biology and Biotechnology; 1999. [Google Scholar]
- Li X, Shen X, Li J, Eneji AE, Li Z, Tian X, Duan L. Coronatine alleviates water deficiency stress on winter wheat seedlings. J Integr Plant Biol. 2010;52:616–625. doi: 10.1111/j.1744-7909.2010.00958.x. [DOI] [PubMed] [Google Scholar]
- Lin Y, Zeng Z, Ren C, Hu Y. Water use efficiency and physiological responses of oat under alternate partial root-zone irrigation in the semiarid areas of Northeast China. Procedia Eng. 2012;28:33–42. doi: 10.1016/j.proeng.2012.01.679. [DOI] [Google Scholar]
- Liu F, Christian RJ, Mathias NA. Drought stress effect on carbohydrate concentration in soybean leaves and pods during early reproductive development: its implication in altering pod set. Field Crop Res. 2004;86:1–13. doi: 10.1016/S0378-4290(03)00165-5. [DOI] [Google Scholar]
- Mehraban A, Tobe A, Gholipouri A, Amiri E, Ghafari A, Rostaii M. The effects of drought stress on yield, yield components, and yield stability at different growth stages in bread wheat cultivar (Triticum aestivum L.) Pol J Environ Stud. 2019;28:739–746. doi: 10.15244/pjoes/85350. [DOI] [Google Scholar]
- Mickky BM, Aldesuquy HS. Impact of osmotic stress on seedling growth observations, membrane characteristics and antioxidant defense system of different wheat genotypes. Egypt J Basic Appl Sci. 2017;4:47–54. doi: 10.1016/j.ejbas.2016.10.001. [DOI] [Google Scholar]
- Mickky BM, Abbas MA, El-Shhaby OA. Alterations in photosynthetic capacity and morpho-histological features of leaf in alfalfa plants subjected to water deficit-stress in different soil types. Indian J Plant Physiol. 2018;23:426–443. doi: 10.1007/s40502-018-0383-7. [DOI] [Google Scholar]
- Mickky BM, Aldesuquy HS, Elnajar MI. Photosynthetic machinery in relation to leaf agro-histological traits of ten wheat genotypes facing drought. J Agric Forest Meteorol Res. 2018;1:25–46. [Google Scholar]
- Mickky BM, Abbas MA, Sameh NM (2019) Morpho-physiological status of fenugreek seedlings under NaCl stress. J King Saud Univ Sci (in press)
- Monneveux P, Jing R, Misra SC. Phenotyping for drought adaptation in wheat using physiological traits. Front Physiol. 2012;3:429. doi: 10.3389/fphys.2012.00429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passioura JB. Grain yield, harvest index and water use of wheat. Aust Inst Agric Sci. 1977;43:117–120. [Google Scholar]
- Poorter H, Niinemets Ü, Poorter L, Wright IJ, Villar R. Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytol. 2009;182:565–588. doi: 10.1111/j.1469-8137.2009.02830.x. [DOI] [PubMed] [Google Scholar]
- Rachmilevitch S, DaCosta M, Huang B. Physiological and biochemical indicators for stress tolerance. In: Huang B, editor. Plant–environment interactions. 3. Boca Raton, FL: CRC Press; 2006. [Google Scholar]
- Reynolds M, Tuberosa R. Translational research impacting on crop productivity in drought-prone environments. Curr Opin Plant Biol. 2008;11:171–179. doi: 10.1016/j.pbi.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Sadasivam S, Manickam A. Biochemical methods. 2. New Delhi: New Age International Limited; 1996. [Google Scholar]
- Sairam RK, Deshmukh PS, Shukla DS. Tolerance of drought and temperature stress in relation to increased antioxidant enzyme activity in wheat. J Agron Crop Sci. 1997;178:171–178. doi: 10.1111/j.1439-037X.1997.tb00486.x. [DOI] [Google Scholar]
- Shani U, Dudley LM. Field studies of crop response to water and salt stress. Soil Sci Soc Am J. 2001;65:1522–1528. doi: 10.2136/sssaj2001.6551522x. [DOI] [Google Scholar]
- Shao HB, Guo QJ, Chu LY, Zhao XN, Su ZL, Hu YC, Cheng JF. Understanding molecular mechanism of higher plant plasticity under abiotic stress. Colloids Surf B Biointerfaces. 2007;54:37–45. doi: 10.1016/j.colsurfb.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Siddique MRB, Hamid A, Islam MS. Drought stress effects on water relations of wheat. Bot Bull Acad Sin. 2001;41:35–39. [Google Scholar]
- Smart RE, Bingham GE. Rapid estimates of relative water content. Plant Physiol. 1974;53:258–260. doi: 10.1104/pp.53.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanhill G. Water use efficiency. Adv Agron. 1987;39:53–85. doi: 10.1016/S0065-2113(08)60465-4. [DOI] [Google Scholar]
- Surendar KK, Devi DD, Ravi I, Jeyakumar P, Velayudham K. Effect of water deficit on relationship between yield and physiological attributes of banana cultivars and hybrids. Afr J Plant Sci. 2013;7:374–383. doi: 10.5897/AJPS2013.1032. [DOI] [Google Scholar]
- Szabados L, Savouré A. Proline: a multifunctional amino acid. Trends Plant Sci. 2010;15:89–97. doi: 10.1016/j.tplants.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Tanner CB, Sinclair TR. Efficient water use in crop production. Madison, Wisconsin: American Society of Agronomy, Crop Science Society of America and Soil Science Society of America; 1983. [Google Scholar]
- Varga B, Varga-László E, Bencze S, Balla K, Veisz O. Water use of winter cereals under well-watered and drought-stressed conditions. Plant Soil Environ. 2013;59:150–155. doi: 10.17221/658/2012-PSE. [DOI] [Google Scholar]
- Waraich EA, Ahmad R, Ali A, Ullah S. Irrigation and nitrogen effects on grain development and yield in wheat (Triticum aestivum L.) Pak J Bot. 2007;39:1663–1672. [Google Scholar]