Abstract
Background:
Men who have sex with men (MSM) who have a current or recent history of rectal Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (GC) infection are at greater risk for HIV than MSM with no history of rectal infection. Screening and treating MSM for rectal CT/GC infection may help reduce any increased biological susceptibility to HIV infection.
Methods:
We used 2 versions of a Markov state-transition model to examine the impact and cost-effectiveness of screening MSM for rectal CT/GC infection in San Francisco: a static version that included only the benefits to those screened and a dynamic version that accounted for population-level impacts of screening. HIV prevention through reduced susceptibility to HIV was the only potential benefit of rectal CT/GC screening that we included in our analysis. Parameter values were based on San Francisco program data and the literature.
Results:
In the base case, the cost per quality-adjusted life year gained through screening MSM for rectal CT/GC infection was $16,300 in the static version of the model. In the dynamic model, the cost per quality-adjusted life year gained was less than $0, meaning that rectal screening was cost-saving. The impact of rectal CT/GC infection on the risk of HIV acquisition was the most influential model parameter.
Conclusions:
Although more information is needed regarding the impact of rectal CT/GC screening on HIV incidence, rectal CT/GC screening of MSM can potentially be a cost-effective, scalable intervention targeted to at-risk MSM in certain urban settings such as San Francisco.
Men who have sex with men (MSM) who have a current or recent history of rectal Chlamydia trachomatis (CT) and/ or Neisseria gonorrhoeae (GC) infection are at greater risk for HIV than MSM with no history of rectal infection.1–3 This increased risk may be attributable to biological factors, behavioral factors, or both.1–3 Screening and treating MSM for rectal CT and GC infection may help reduce any increased biological susceptibility to HIV infection and identify men at increased risk for HIV infection.1–3
The burden of rectal sexually transmitted diseases (STDs) among MSM has been well documented.4–10 However, most infections are asymptomatic, suggesting that routine screening is needed to identify and treat rectal STDs.6,10 Although the Centers for Disease Control and Prevention recommends rectal screening for CT and GC infection among men who have had receptive anal intercourse in the past year,11 rectal screening rates are limited. For example, most MSM with HIV in urban areas are not screened for rectal CT or GC infection in any given year, although screening rates can vary from clinic to clinic.12 San Francisco is one example of a setting with relatively high rectal screening rates because the San Francisco Department of Health (SFDPH) has supported extragenital testing in a variety of clinical sites. The purpose of this study was to estimate the impact and cost-effectiveness of screening for rectal CT and GC infection among MSM in San Francisco, using estimates of current rectal screening coverage rates.
MATERIALS AND METHODS
The Model
We used a Markov state-transition model to examine the potential impact of screening MSM for rectal CT and GC infection. The model consists of 4 mutually exclusive health states based on HIV infection status and rectal CT/GC infection status (Fig. 1), where CT/GC denotes CT and/or GC infection. Transition from one state to another occurs when rectal CT/GC infection is acquired, when rectal CT/GC infection is cleared, or when HIV is acquired. The incidence rate of rectal CT/GC infection is λSTDΩSTD and among those without and with HIV, respectively, where λSTD and are the incidence rates of rectal GC infection at the onset of the screening program among those without and with HIV, respectively, and ΩSTD is an adjustment factor to account for changes in the prevalence of CT/GC in sex partners over time as a result of the screening program. The clearance rate (r) of rectal CT/GC infection is a function of 2 factors: duration of infection and screening rates. The HIV incidence rate among those without rectal CT/GC infection is λHIVΩHIV, where λHIV is the HIV incidence at the onset of the screening program and ΩHIV is an adjustment factor to account for changes in the prevalence of HIV in sex partners over time. The rate of HIV incidence among those with rectal CT/GC infection was assumed to be ϴ times that of those without rectal CT/GC infection. The population of MSM was assumed to be 65,000, with an initial HIV prevalence of 25%.13 Initially, 1511 of the 48,750 MSM without HIV and 1576 of the 16,250 MSM with HIV were assumed to have rectal CT/GC infection. We performed all calculations using Excel 2007 (Microsoft Corporation, Redmond, WA). A more detailed description of the model is provided in a supplemental appendix available from the lead author upon request (http://links.lww.com/OLQ/A58).
Figure 1.
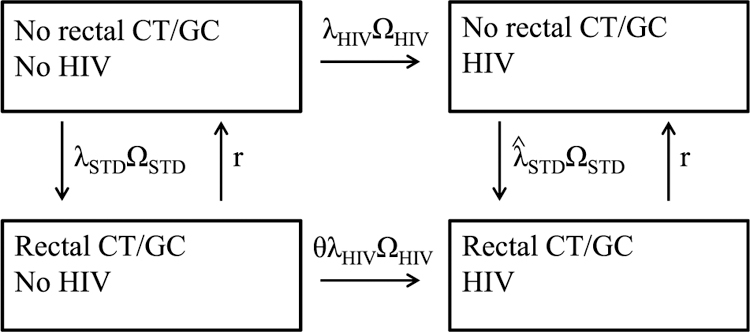
Illustration of the model. The 4 health states are mutually exclusive, and transition occurs when rectal CT/GC infection is acquired, when rectal CT/GC infection is cleared, or when HIV is acquired. The HIV incidence rate among those without rectal CT/GC infection is λHIVΩHIV, where λHIV is the HIV incidence at the onset of the screening program and ΩHIV is an adjustment factor to account for changes in the prevalence of HIV in sex partners over time. The rate of HIV incidence among those with rectal CT/GC infection is assumed to be ϴ times that of those without rectal CT/GC infection. The incidence rate of rectal CT/GC infection is λSTDΩSTD and among those without and with HIV, respectively, where λSTD and are the incidence rates of rectal CT/GC infection at the onset of the screening program among those without and with HIV, respectively, and ΩSTD is an adjustment factor to account for changes in the prevalence of CT and GC in sex partners over time as a result of the screening program. The clearance rate of rectal CT/GC infection (r) is a function of 2 factors: duration of infection and screening rates.
Parameter Values
Parameter values and costs applied in the model were based on the literature and on data from SFDPH, as listed in Table 1. Data from SFDPH were used to estimate annual incidence rates of rectal CT/GC infection in MSM with and without HIV (0.129 and 0.041, respectively, as described in Table 1). HIV incidence among MSM without rectal CT/GC infection was based on HIV incidence rates reported by SFDPH and additional assumptions (as described in Table 1).13 The relative risk ϴ of acquiring HIV among those with rectal CT/GC infection was 1.9 (range, 1.2–2.6), based on HIV incidence reported in a retrospective cohort analysis of HIV-uninfected MSM diagnosed as having rectal CT/GC infection,1 along with the calculation of HIV incidence among MSM without rectal CT/GC (Table 1).
TABLE 1.
Parameter Values Used in a Model to Estimate the Impact and Cost-Effectiveness of Screening MSM for Rectal Chlamydial and Gonococcal Infection to Prevent HIV
| Parameter | Base Case | Lower Bound | Upper Bound | Source |
|---|---|---|---|---|
| Annual incidence rate of rectal CT/GC, MSM without HIV (λSTD) | 0.041 | 0.026 | 0.062 | Calculated* |
| Annual incidence rate of rectal CT/GC, MSM with HIV | 0.129 | 0.084 | 0.160 | Calculated* |
| Annual HIV incidence rate among those without rectal CT/GC (λHIV) | 0.0124 | 0.0112 | 0.0134 | 13† |
| Relative risk of acquiring HIV among those with rectal CT/GC (ϴ) | 1.9 | 1.2 | 2.6 | 1,13‡ |
| Duration of CT/GC infection in the absence of screening (d), years | 0.75 | 0.5 | 1 | 14,15 |
| Lifetime number of QALYs lost per HIV case | 6.95 | 4.85 | 9.05 | 19 |
| Lifetime cost per case of HIV | $314,000 | $236,000 | $391,000 | 20,21 |
| Cost of rectal CT/GC screening | $41 | $25 | $56 | 18,22 |
| Cost of rectal CT/GC treatment | $50 | $43 | $58 | 18,23 |
| Annual rate of rectal CT/GC screening among MSM without HIV | 0.37 | 0.15 | 0.65 | Assumed§ |
| Annual rate of rectal CT/GC screening among MSM with HIV | 0.58 | 0.25 | 1.00 | Assumed§ |
All costs are in 2011 US dollars. For more information, see supplemental appendix, http://links.lww.com/OLQ/A58.
Incidence rates were approximated as prevalence rates divided by duration. Prevalence rates of 3.1% and 9.7% were estimated for MSM without and with HIV, based on 462 and 676 cases of rectal CT/GC detected, respectively, among approximately 15,131 and 6984 MSM screened, respectively. These estimated case numbers for rectal CT/GC reflect the assumption that rectal CT/GC cases for which HIV status was unknown were distributed in the same proportion as the known cases (SFDPH, unpublished data). The annual rates of screening shown in the bottom rows of the table correspond to annual probabilities of screening of 31% and 44% for MSM without and with HIV, respectively. Assuming 48,810 MSM without HIVand 15,873 MSM with HIV,13 there would be approximately 15,131 MSM without HIV (31% of 48,810) and 6984 MSM with HIV (44% of 15,873) screened each year. In the model, the number of MSM was rounded to 65,000, and HIV prevalence was rounded to 25%.
HIV incidence among MSM without rectal CT/GC was based on HIV incidence rates reported by SFDPH as follows. Assuming that (1) rectal CT/GC prevalence among MSM without HIV is 3.1%, (2) HIV incidence among MSM with rectal CT/GC is 2.36% (calculated as the weighted average among men with 0, 1, and 2 prior rectal infections in the previous 2 years),1 and (3) HIV incidence among MSM overall is 1.27%, as reported in the 2010 SFDPH HIV/AIDS Annual Report,13 HIV incidence among those without rectal GC can be estimated as [0.0127 – (0.031 * 0.0236)]/(1 – 0.031) = 0.0124.
The relative risk of acquiring HIV among those with rectal CT/GC was 1.9 (calculated as 0.0236 divided by 0.0124) in the base case, with a lower bound of 1.2 (0.0149/0.0124) and an upper bound of 2.6 (0.0326/0.0124). For the lower and upper bounds, the numerators (0.0149 and 0.0326) are from the 95% confidence interval of HIV incidence among those with rectal CT/GC from the 2010 study by Bernstein et al.1
Based on STOP AIDS Behavioral Risk Assessments, San Francisco 2010 (personal communication, Jennifer Hecht to Kyle Bernstein, February 15, 2012), and adjusted for potential overreporting, as described in the supplemental appendix, http://links.lww.com/OLQ/A58.
CT/GC infection denotes CT and/or GC infection; CT/GC screening denotes screening for both CT and GC infections.
Duration of rectal CT/GC infection in the absence of screening was assumed to be 9 months (range, 6 months–1 year).14,15 The annual rates of rectal CT/GC screening was assumed to be 0.37 (range, 0.15–0.65) in MSM without HIV and 0.58 (range, 0.25–1) in MSM with HIV (Table 1).
The San Francisco City Clinic uses Gen-Probe APTIMA Combo 2 for testing rectal specimens,1 for which sensitivities and specificities of 92.3% and 98.7%, respectively, have been reported for rectal GC (93.5% and 97.7%, respectively, for rectal CT).16 For simplicity, we assumed 100% sensitivity and specificity of screening. Treatment has been documented in more than 95% of those with rectal CT/GC in San Francisco, with a median time to treatment of 2 days (SFDPH, unpublished data). Given this high rate of treatment, we assumed for simplicity that all rectal CT/GC infections detected through screening would be treated successfully immediately upon detection.
We estimated 2 model versions: a static version and a dynamic version. The static version included benefits of rectal CT/GC screening only to those who are screened, whereas the dynamic version included benefits of rectal CT/GC screening to those who are screened, their partners, their partners’ partners, and so on. In the dynamic version, the adjustment factor (ΩSTD) was used to account for changes in the prevalence of CT/GC infection in sex partners over time as a result of rectal CT/GC screening. The adjustment factor in week t + 1 was calculated based on the ratio of rectal CT/GC prevalence in week t in the scenario of rectal screening to rectal CT/GC prevalence in week t in the scenario of no-rectal screening, raised to the power of 0.5. The ratio was raised to the power of 0.5 because rectal CT/GC screening was assumed to have less of an impact on genital CT/GC prevalence than on rectal CT/GC prevalence, similar to the way in which female-only STD screening might have less of an impact on male STD prevalence than female STD prevalence.17 The adjustment factor (ΩHIV) for changes in the prevalence of HIV in sex partners was calculated in an analogous manner as the adjustment factor for CT/GC (ΩSTD). The adjustment factors (ΩHIV and ΩSTD) were varied only in the dynamic version of the model; in the static model, both values were set equal to 1 and held constant.
The number of quality-adjusted life years (QALYs) lost per HIV infection, the direct costs for testing and treatment for CT/GC infection, and the direct lifetime medical cost per case of HIV were drawn from the literature.18–23 All costs were updated to 2011 US dollars using the medical care component of the consumer price index (www.bls.gov/cpi/data.htm).
Cost-Effectiveness
The study question we addressed is as follows: what is the cost-effectiveness of current screening and treatment for rectal CT/GC infection among MSM in San Francisco compared with a strategy of no screening? HIV prevention was the only benefit of rectal CT/GC screening that we assessed; we did not include other health and economic benefits of treating rectal CT/GC infection. Costs and benefits were assessed from the health system perspective; we did not include nonmedical costs such as patient time and transportation costs or indirect costs (lost productivity) of HIV or rectal STDs. All future costs and benefits were discounted at 3% annually. We applied a 10-year time frame and a lifetime analytic horizon; that is, we included all program costs over the 10-year time frame (STD screening and treatment costs) as well as the lifetime costs and lifetime number of QALYs lost for the HIV cases that occurred over the 10-year time frame.
The cost-effectiveness of screening was expressed in terms of cost per QALY gained and calculated as the incremental cost of CT/GC screening divided by the incremental number of QALYs gained by CT/GC screening. The incremental cost of CT/GC screening was calculated as the total costs of CT/GC screening (cost of screening for both CT and GC; cost of treating CT, GC, or both; and HIV costs) minus the HIV costs in the scenario of no-CT/GC screening. The incremental number of QALYs gained by CT/GC screening was calculated as the lifetime number of QALYs lost due to HIV in the scenario of no-CT/GC screening minus the lifetime number of QALYs lost due to HIV in the screening scenario.
Sensitivity Analysis
In sensitivity analyses, we varied the parameter values according to the ranges described in Table 1 to see how the estimated cost per QALY gained by rectal CT/GC screening would change. We first conducted 1-way sensitivity analyses, calculating the cost per QALY gained by rectal CT/GC screening when varying one parameter at a time from its lower bound to its upper bound value while holding all other parameters at their base case values. The parameters varied in the 1-way sensitivity analyses were as follows: the incidence of rectal CT/GC infection (λSTD and ), HIV incidence among those without rectal CT/GC infection (λHIV), the relative risk of HIV among those with rectal CT/GC infection (ϴ), the duration of CT/GC infection (d), the lifetime cost and number of QALYs lost per HIV case, the cost of rectal CT/GC screening and treatment, and the rate of rectal CT/GC screening. We then conducted probabilistic sensitivity analyses in which all of these parameter values (except the rate of CT/GC of screening) were varied simultaneously, assuming a uniform distribution for each parameter between its lower and upper bound values. Finally, we conducted a threshold analysis to determine what values of ϴ (the relative risk of acquiring HIV among those with rectal CT/GC infection) would result in cost-effectiveness ratios of $25,000, $50,000, and $100,000 per QALY gained by rectal CT/GC screening.
RESULTS
In the base case, the cost per QALY gained by screening MSM for rectal CT/GC infection was $16,300 in the static version of the model and less than $0 in the dynamic version of the model (Table 2). Thus, when taking into account dynamic reductions in CT/GC prevalence among MSM over time as a result of rectal CT/GC screening, the cost per QALY gained was less than $0, meaning that rectal screening is cost-saving. In 1-way sensitivity analyses, the estimated cost per QALY gained by screening ranged from less than $0 to $227,800 in the static version of the model and from less than $0 to $98,400 in the dynamic version of the model (Table 3). The 2 most influential parameters were the relative risk of acquiring HIV among those with rectal CT/GC infection and the duration of rectal CT/GC infection. In the 1-way sensitivity analyses, the cost per QALY gained did not exceed $50,000 in the static version of the model or $5000 in the dynamic version of the model, except when varying the relative risk of HIV or the duration of rectal CT/GC infection.
TABLE 2.
Estimated Impact, Cost, and Cost Per QALY Gained by Screening MSM for Rectal Chlamydial and Gonococcal Infection*
| Model Result | No Screening | Screening, Static Version of Model† | Screening, Dynamic Version of Model‡ |
|---|---|---|---|
| Prevalence of rectal CT/GC in MSM after 10 y | 4.9% | 3.7% | 2.8% |
| Percent reduction in rectal CT/GC due to screening | 0.0% | 25.4% | 43.1% |
| No. HIV cases averted by screening | 0.0 | 24.4 | 46.2 |
| No. QALYs lost due to HIV | 35,099 | 34,929 | 34,778 |
| Costs of screening and treatment for rectal CT/GC | $0 | $10,417,600 | $10,324,800 |
| HIV treatment costs | $1,585,764,500 | $1,578,102,800 | $1,571,266,700 |
| Total costs | $1,585,764,500 | $1,588,520,400 | $1,581,591,500 |
| Incremental cost (compared to no screening) | — | $2,755,900 | –$4,173,000 |
| Incremental number of QALYs gained (compared with no screening) | — | 169.6 | 320.9 |
| Cost-effectiveness ratio (incremental cost per QALY gained) | — | $16,300 | <$0 (cost-saving) |
The cost-effectiveness ratio shows the incremental cost per QALY gained by rectal CT/GC screening of MSM compared with no screening.
All costs are in 2011 US dollars, and cost estimates are rounded to the nearest $100.
Results from the static version of model include benefits of screening and treatment only to those who are screened (i.e., “indirect effects” of screening are not included).
Results from the dynamic version of model include benefits of screening and treatment to those screened, their partners, their partners’ partners, and so on (i.e., indirect effects of screening are included).
TABLE 3.
One-Way Sensitivity Analyses: Incremental Cost Per QALY Gained Screening MSM for Rectal Chlamydial and Gonococcal Infection When Varying One Parameter Value at a Time
| Parameter Varied | Range of Cost Per QALY Estimates When Varying Parameter From Its Lower to Upper Bound Value ($ per QALY) |
|
|---|---|---|
| Static Version of the Model | Dynamic Version of the Model | |
| No parameters varied (base case) | 16,300 | <0 |
| Incidence of rectal CT/GC, MSM without HIV (λSTD) | <0–44,700 | <0–2,300 |
| Incidence of rectal CT/GC, MSM with HIV | 15,700–16,600 | <0 |
| HIV incidence among those without rectal CT/GC (λHIV) | 12,400–21,800 | <0 |
| Relative risk of HIV among those with rectal CT/GC (ϴ) | 0–227,800 | <0–98,400 |
| Duration of CT/GC infection (d) | 0–73,200 | <0–13,500 |
| Lifetime no. QALYs lost per HIV case | 12,500–23,300 | <0 |
| Lifetime cost per case of HIV | 5200–27,500 | <0 |
| Cost of rectal CT/GC screening | <0–37,700 | <0 |
| Cost of rectal CT/GC treatment | 15,900–16,700 | <0 |
| Annual rate of rectal CT/GC screening (per year) | 10,900–24,400 | <0 |
Cost per QALY estimates are reported in 2011 US dollars and are rounded to the nearest $100. For λSTD, λHIV, ϴ, d, and the lifetime cost per case of HIV and number of QALYS lost per case of HIV, applying the lower bound value resulted in less favorable cost-per-QALY estimates than applying the upper bound value. For all other parameters, applying the lower bound value generally resulted in more favorable cost per QALY estimates than applying the upper bound value.
In the probabilistic sensitivity analyses, the cost per QALY gained by rectal CT/GC screening ranged from less than $0 to $403,100 in 90% of the simulations using the static version of the model and from less than $0 to $120,100 in 90% of the simulations using the dynamic version of the model (Table 4). In the static version of the model, the cost per QALY was less than $0 (cost-saving) in 31% of the simulations and was less than $100,000 in 75% of the simulations. In the dynamic version of the model, the cost per QALY was less than $0 (cost-saving) in 59% of the simulations and was less than $100,000 in 94% of the simulations.
TABLE 4.
Probabilistic Sensitivity Analyses: Incremental Cost Per QALY Gained Screening MSM for Rectal Chlamydial and Gonococcal Infection When Varying Numerous Model Parameter Values Simultaneously
| Static Version of the Model | Dynamic Version of the Model | |
|---|---|---|
| Cost per QALY (base case) | $16,300 | <$0 |
| Cost per QALY (5th percentile across the 3000 simulations) | <$0 | <$0 |
| Cost per QALY (95th percentile across the 3000 simulations) | $403,100 | $120,100 |
| Percent of simulations in which the cost per QALY was <$0 | 31 | 59 |
| Percent of simulations in which the cost per QALY was <$25,000 | 48 | 76 |
| Percent of simulations in which the cost per QALY was <$50,000 | 60 | 85 |
| Percent of simulations in which the cost per QALY was <$100,000 | 75 | 94 |
Cost per QALY estimates are reported in 2011 US dollars and are rounded to the nearest $100.
In threshold analyses of the relative risk of acquiring HIV among those with rectal CT/GC infection (not shown), cost per QALY estimates of $25,000, $50,000, and $100,000 were obtained when the relative risk was set to 1.80, 1.60, and 1.40, respectively, in the static version of the model and when the relative risk was set to 1.43, 1.33, and 1.20, respectively, in the dynamic version of the model.
DISCUSSION
Our results suggest that screening MSM for rectal CT/GC infection can be a cost-effective intervention to reduce HIV infection, particularly when taking into account the population-level reductions in CT/GC prevalence in MSM over time as a result of screening and treatment. In many scenarios we examined, screening MSM for rectal CT/GC infection was cost-saving, meaning that the discounted costs of screening and treatment were less than the discounted cost of averted HIV infections. Although there is no official cost-per-QALY cutoff to determine whether public health interventions in the United States are cost-effective, a threshold of $50,000 per QALY is often cited (a QALY can be thought of as 1 year of life in perfect health).24,25 In our sensitivity analyses, the cost per QALY gained by rectal screening was less than $50,000 in 60% of the simulations when using the static version of the model and in 85% of the simulations when using the dynamic version of the model. Our base-case estimate of the cost per QALY gained by screening MSM for rectal CT/GC ($16,300 in the static version of the model) is consistent with estimates of the cost per QALY gained by screening women and high-risk heterosexual men for urogenital CT infection26,27 and compares favorably with many clinical preventive services and public health interventions.28
The degree to which rectal CT/GC increases susceptibility to HIV is the most important input in our model. The cost per QALY gained by screening MSM for rectal CT/GC was less than $25,000 in the dynamic version of our model when the relative risk of acquiring HIV among MSM with rectal CT/GC infection (compared with MSM without rectal CT/GC infection) was at least 1.43. Thus, if rectal CT/GC infection increases susceptibility to HIV by at least 43%, then screening MSM for rectal CT/GC infection would be a highly cost-effective strategy for HIV prevention, according to our dynamic model results. Such an increase in susceptibility seems plausible, given that a case-control study found an odds ratio of 4.73 (95% confidence interval, 1.75–12.76) for HIV seroconversion among MSM with rectal gonorrhea compared with MSM controls.2 Furthermore, among MSM with a current rectal CT/GC infection, HIV seroconversion was 8 times more likely among MSM with 2 prior CT/GC infections than among MSM without a prior CT/GC infection.1 In addition, there is a substantial overall body of evidence suggesting that STDs can facilitate the acquisition and transmission of HIV.29–34 However, it is difficult to determine how much of the increased risk of HIV in those with rectal CT/GC infection is attributable to biological factors and how much is attributable to other factors such as sexual behaviors and sex partner characteristics.3,33,34 Future research is needed to help determine more precisely the causal role of rectal CT/GC infection on HIV acquisition.1,3
Our study is subject to several important limitations. First, data are limited regarding the 2 most important parameters in our analysis: the relative risk of acquiring HIV among those with rectal CT/GC infection (vs. those without) and the duration of rectal CT/GC infection. To address this limitation, we conducted sensitivity analyses to show how the results can change when these key assumptions are varied. Second, we did not account specifically for repeat rectal CT/GC infections. Evidence suggests that the risk of HIV seroconversion is higher for MSM with 1 and 2 repeat rectal CT/GC infections than for MSM with a single rectal CT/GC infection.1 Third, we used a relatively simple approach to estimate the impact of a rectal CT/GC screening program. Given that the cost per QALY gained by rectal CT/GC screening was notably lower in the dynamic version of our model than in the static version, future studies could use a more complex dynamic transmission model to examine the potential impact of screening in more detail. Fourth, we made several simplifying assumptions such as 100% sensitivity and specificity of screening and that all rectal CT/GC infections detected would be treated successfully immediately upon detection. Although these optimistic assumptions can result in cost-effectiveness estimates that are unduly favorable to rectal screening, our analysis was otherwise conservative in that HIV prevention through reduced susceptibility to HIV was the only potential benefit of rectal CT/GC screening that we included. For example, we did not consider the possibility that rectal CT/GC screening in MSM with HIV could reduce the probability of HIV transmission to their sex partners. Similarly, we focused solely on biological impacts of rectal CT/GC infection on HIV acquisition, although it is possible that rectal CT/GC screening could reduce the risk of HIV acquisition and transmission through behavioral impacts, as well. Rectal CT/GC screening could be markedly more cost-effective than we estimated, had we included all of these potential benefits of screening. Fifth, we assumed that our model inputs would be constant over time. For example, we did not allow for the possibility that increased use of antiretroviral therapy might affect the average cost per case of HIV, the average impact of HIV on length and quality of life, and the HIV incidence rate. Finally, our model used parameter values specific to MSM in San Francisco, and our results might not be generalizable to other areas.
Despite limitations, our model offers a useful approximation of the health impact and cost-effectiveness of rectal CT/GC screening in San Francisco to reduce future HIV acquisition. If rectal CT/GC infection does indeed increase susceptibility to HIV, rectal CT/GC screening of MSM could be a cost-effective tool to prevent HIV and might even pay for itself in terms of averted HIV treatment costs. The Centers for Disease Control and Prevention has called for a high-impact HIV prevention approach, defined as the use of combinations of “scientifically proven, cost-effective, and scalable interventions targeted to the right populations in the right geographic areas.”35 Although more information is needed regarding the impact of rectal CT/GC screening on the risk of HIV acquisition, our analysis shows that rectal CT/GC screening of MSM can potentially be a cost-effective, scalable intervention targeted to at-risk MSM in certain urban settings such as San Francisco.
Supplementary Material
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (http://www.stdjournal.com).
REFERENCES
- 1.Bernstein KT, Marcus JL, Nieri G, et al. Rectal gonorrhea and chlamydia reinfection is associated with increased risk of HIV seroconversion. J Acquir Immune Defic Syndr 2010; 53:537–543. [DOI] [PubMed] [Google Scholar]
- 2.Craib KJ, Meddings DR, Strathdee SA, et al. Rectal gonorrhoea as an independent risk factor for HIV infection in a cohort of homosexual men. Genitourin Med 1995; 71:150–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zetola NM, Bernstein KT, Wong E, et al. Exploring the relationship between sexually transmitted diseases and HIV acquisition by using different study designs. J Acquir Immune Defic Syndr 2009; 50:546–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Annan NT, Sullivan AK, Nori A, et al. Rectal chlamydia—a reservoir of undiagnosed infection in men who have sex with men. Sex Transm Infect 2009; 85:176–179. [DOI] [PubMed] [Google Scholar]
- 5.Gunn RA, O’Brien CJ, Lee MA, et al. Gonorrhea screening among men who have sex with men: value of multiple anatomic site testing, San Diego, California, 1997–2003. Sex Transm Dis 2008; 35:845–848. [DOI] [PubMed] [Google Scholar]
- 6.Kent CK, Chaw JK, Wong W, et al. Prevalence of rectal, urethral, and pharyngeal chlamydia and gonorrhea detected in 2 clinical settings among men who have sex with men: San Francisco, California, 2003. Clin Infect Dis 2005; 41:67–74. [DOI] [PubMed] [Google Scholar]
- 7.Marcus JL, Bernstein KT, Kohn RP, et al. Infections missed by urethral-only screening for chlamydia or gonorrhea detection among men who have sex with men. Sex Transm Dis 2011; 38:922–924. [DOI] [PubMed] [Google Scholar]
- 8.Marcus JL, Bernstein KT, Stephens SC, et al. Sentinel surveillance of rectal chlamydia and gonorrhea among males—San Francisco, 2005–2008. Sex Transm Dis 2010; 37:59–61. [DOI] [PubMed] [Google Scholar]
- 9.Mimiaga MJ, Mayer KH, Reisner SL, et al. Asymptomatic gonorrhea and chlamydial infections detected by nucleic acid amplification tests among Boston area men who have sex with men. Sex Transm Dis 2008; 35:495–498. [DOI] [PubMed] [Google Scholar]
- 10.Young H, Manavi K, McMillan A. Evaluation of ligase chain reaction for the non-cultural detection of rectal and pharyngeal gonorrhoea in men who have sex with men. Sex Transm Infect 2003; 79:484–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Workowski KA, Berman S. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep 2010; 59:1–110. [PubMed] [Google Scholar]
- 12.Hoover KW, Butler M, Workowski K, et al. STD screening of HIV-infected MSM in HIV clinics. Sex Transm Dis 2010; 37:771–776. [DOI] [PubMed] [Google Scholar]
- 13.HIV Epidemiology Section, San Francisco Department of Public Health. HIV/AIDS Epidemiology Annual Report San Francisco, CA: San Francisco Department of Public Health, 2011. [Google Scholar]
- 14.Althaus CL, Heijne JCM, Low N. Towards more robust estimates of the transmissibility of Chlamydia trachomatis. Sex Transm Dis 2012; 39:402–404. [DOI] [PubMed] [Google Scholar]
- 15.Althaus CL, Heijne JCM, Roellin A, et al. Transmission dynamics of Chlamydia trachomatis affect the impact of screening programmes. Epidemics 2010; 2:123–131. [DOI] [PubMed] [Google Scholar]
- 16.Schachter J, Moncada J, Liska S, et al. Nucleic acid amplification tests in the diagnosis of chlamydial and gonococcal infections of the oropharynx and rectum in men who have sex with men. Sex Transm Dis 2008; 35:637–642. [DOI] [PubMed] [Google Scholar]
- 17.Turner KME, Adams EJ, LaMontagne DS, et al. Modelling the effectiveness of chlamydia screening in England. Sex Transm Infect 2006; 82:496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Begley CE, McGill L, Smith PB. The incremental cost of screening, diagnosis, and treatment of gonorrhea and chlamydia in a family planning clinic. Sex Transm Dis 1989; 16:63–67. [DOI] [PubMed] [Google Scholar]
- 19.Desai K, Sansom SL, Ackers ML, et al. Modeling the impact of HIV chemoprophylaxis strategies among men who have sex with men in the United States: HIV infections prevented and cost-effectiveness. AIDS 2008; 22:1829–1839. [DOI] [PubMed] [Google Scholar]
- 20.Hutchinson AB, Farnham PG, Dean HD, et al. The economic burden of HIV in the United States in the era of highly active antiretroviral therapy: Evidence of continuing racial and ethnic differences. J Acquir Immune Defic Syndr 2006; 43:451–457. [DOI] [PubMed] [Google Scholar]
- 21.Schackman BR, Gebo KA, Walensky RP, et al. The lifetime cost of current human immunodeficiency virus care in the United States. Med Care 2006; 44:990–997. [DOI] [PubMed] [Google Scholar]
- 22.Steece R Determination of laboratory costs: Or what does it cost to run a nucleic acid amplification test (NAAT). Presented at: 2008 American Society for Microbiology 108th General Meeting; 2008; Boston. [Google Scholar]
- 23.Thompson Healthcare. 2008 Red Book: Pharmacy’s Fundamental Reference Montvale, NJ: Thompson Healthcare, 2008. [Google Scholar]
- 24.Grosse SD. Assessing cost-effectiveness in healthcare: history of the $50,000 per QALY threshold. Expert Rev Pharmacoecon Outcomes Res 2008; 8:165–178. [DOI] [PubMed] [Google Scholar]
- 25.Tengs TO. Cost-effectiveness versus cost–utility analysis of interventions for cancer: Does adjusting for health-related quality of life really matter? Value Health 2004; 7:70–78. [DOI] [PubMed] [Google Scholar]
- 26.Hu D, Hook EW 3rd, Goldie SJ. Screening for Chlamydia trachomatis in women 15 to 29 years of age: A cost-effectiveness analysis. Ann Intern Med 2004; 141:501–513. [DOI] [PubMed] [Google Scholar]
- 27.Gift TL, Gaydos CA, Kent CK, et al. The program cost and cost-effectiveness of screening men for chlamydia to prevent pelvic inflammatory disease in women. Sex Transm Dis 2008; 35:S66–S75. [DOI] [PubMed] [Google Scholar]
- 28.Maciosek MV, Edwards NM, Coffield AB, et al. Priorities among effective clinical preventive services: Results of a systematic review and analysis. Am J Prev Med 2006; 31:52–61. [DOI] [PubMed] [Google Scholar]
- 29.Cohen CR, Plummer FA, Mugo N, et al. Increased interleukin-10 in the endocervical secretions of women with non-ulcerative sexually transmitted diseases: A mechanism for enhanced HIV-1 transmission? AIDS 1999; 13:327–332. [DOI] [PubMed] [Google Scholar]
- 30.Cohen MS, Hoffman IF, Royce RA, et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: Implications for prevention of sexual transmission of HIV-1. Lancet 1997; 349:1868–1873. [DOI] [PubMed] [Google Scholar]
- 31.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: The contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect 1999; 75:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanson J, Posner S, Hassig S, et al. Assessment of sexually transmitted diseases as risk factors for HIV seroconversion in a New Orleans sexually transmitted disease clinic, 1990–1998. Ann Epidemiol 2005; 15:13–20. [DOI] [PubMed] [Google Scholar]
- 33.Rottingen JA, Cameron DW, Garnett GP. A systematic review of the epidemiologic interactions between classic sexually transmitted diseases and HIV—How much really is known? Sex Transm Dis 2001; 28:579–597. [DOI] [PubMed] [Google Scholar]
- 34.Ward H, Ronn M. Contribution of sexually transmitted infections to the sexual transmission of HIV. Curr Opin HIV AIDS 2010; 5:305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention. High-Impact HIV Prevention. CDC’s Approach to Reducing HIV Infections in the United States Atlanta, GA: CDC, 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


