Abstract
Objective:
To estimate new human immunodeficiency virus (HIV) diagnosis rates among HIV negative men who have sex with men (MSM) who are repeatedly tested for HIV in sexually transmitted disease (STD) clinics and assess the impact of demographic and disease-specific characteristics that are associated with higher HIV diagnosis rates.
Study Design:
Retrospective analysis using 2010 to 2013 data from the STD Surveillance Network (SSuN), a sentinel surveillance system comprised of health departments in 12 cities conducting sentinel surveillance in 40 STD clinics. We analyzed data from all MSM repeatedly (≥2 times) tested for HIV, with an initial negative HIV test required for staggered cohort entry. Follow-up time was accrued from the date of the first negative HIV test to the most recent negative test or the first positive HIV test. The STD diagnoses during the follow-up period were reviewed. We estimated HIV diagnoses rates (number of HIV diagnoses/total number of person-years [PY] at risk) by demographic and clinical characteristics with 95% confidence intervals (CI) using an inverse variance weighted random effects model, adjusting for heterogeneity between SSuN jurisdictions.
Results:
Overall, 640 HIV diagnoses occurred among 14,824 individuals and 20,951.6 PYof observation, for an adjusted incidence of HIV diagnosis of 3.0 per 100 PY (95% CI, 2.6–3.4). Rates varied across race/ethnicity groups with the highest rate among Blacks (4.7/100 PY; 95% CI, 4.1–5.3) followed by Hispanics, whites, and persons of other races/ethnicities. Men who have sex with men having a diagnosis of primary or secondary (P&S) syphilis on or after the first negative HIV test had a higher new HIV diagnosis rate (7.2/100 PY; 95% CI, 5.8–9.0) compared with MSM who did not have a P&S syphilis diagnosis (2.8/100 PY; 95% CI, 2.6–3.1). Men who have sex with men who tested positive for rectal gonorrhea (6.3/100 PY; 95% CI, 5.7–6.9) or rectal chlamydia (5.6/100 PY; 95% CI, 4.6–6.6) had higher rates of new HIV diagnosis when compared to those with negative test results.
Conclusions:
Men who have sex with men attending SSuN STD clinics have high rates of new HIV diagnoses, particularly those with a previous diagnosis of P&S syphilis, rectal chlamydia, and/or gonorrhea. Sexually transmitted disease clinics continue to be important clinical setting for diagnosing HIV among MSM populations.
The high risk of human immunodeficiency virus (HIV) infection among gay, bisexual, and other men who have sex with men (MSM) (collectively referred to as MSM) in the United States remains a major public health crisis. Men who have sex with men make up a small proportion (~2%) of the US population.1 Although the vast majority of MSM do not have HIV, 70% of diagnosed HIV infections were attributed to male-to-male sexual contact from 2010 to 2014.2 The US Centers for Disease Control and Prevention (CDC) currently recommends that individuals at increased risk for HIV infection, including sexually active MSM, undergo HIV testing at least annually.
Yet despite efforts to promote routine HIV testing and decades of research dedicated to the prevention of HIV infection, there remains a substantial amount of new HIV infections annually.
Sexually transmitted disease (STD) clinics have historically been an important health care setting for clients who may not otherwise have access to health care services but are at high risk for HIV infection and unaware of their HIV status. The patients who seek treatment for STDs, including those attending STD clinics, should be screened routinely for HIV during each visit for a new complaint, regardless of whether the patient is known or suspected to have specific behavioral risks for HIV infection.3 Additionally, STD clinics are relevant to men who may be less likely to seek preventive care and have a usual source of care compared with women.4–6 As such, these can be critical settings to estimate the incidence of new HIV diagnoses among MSM, with the ultimate goal of informing prevention strategies. Comorbid infections with bacterial STDs may serve as risk markers for incident HIV infection, including previous bacterial STDs, as have been documented in multiple studies.7–11 However, many of these published studies focused on a single jurisdiction, and a few were constrained by small sample sizes, limiting the ability to report estimates stratified by certain patient characteristics. This study retrospectively reviews the data collected on all MSM patients attending a network of US STD clinics with the goal of (1) documenting new HIV diagnosis rates among previously HIV uninfected individuals who are repeatedly tested for HIV and (2) assessing the impact of demographic and disease-specific characteristics that may be associated with higher HIV diagnosis rates. Understanding these factors associated with a new HIV diagnosis will further enhance current understanding and development of more effective HIV prevention interventions for at-risk populations.
METHODS
We examined data from the STD Surveillance Network (SSuN), a sentinel surveillance system that includes 40 STD clinics within 12 US cities (Birmingham, AL (1 clinic); Baltimore, MD (2 clinics); Los Angeles, California (12 clinics); Hartford and New Haven, Connecticut (2 clinics); Denver, Colorado (1 clinic); Chicago, Illinois (5 clinics); New Orleans, Louisiana (3 clinics); New York City, New York (8 clinics); Philadelphia, Pennsylvania (2 clinics); San Francisco, California (1 clinic); Richmond, Virginia (2 clinics); Seattle, Washington (1 clinic)). All STD clinics participating in SSuN routinely offer voluntary HIV counseling and testing services and collect data on patient demographics, HIV test results, STD diagnoses, and sex of sex partner information as part of routine clinical care.
We included all MSM with at least 2 HIV tests in SSuN STD clinics between January 1, 2010, and June 30, 2013, and whose initial HIV test was negative. Men who have sex with men were defined as males who identified as gay or bisexual and/or who reported at any clinic visit ever having sex with 1 or more male partner(s). A STD diagnosis, defined as a positive laboratory test result for Neisseria gonorrhoeae or Chlamydia trachomatis, or a diagnosis of primary or secondary (P&S) syphilis based on clinical examination, history, and laboratory results, was recorded if it occurred on the patient’s initial visit where they tested negative for HIV or during any subsequent visits of their follow-up period. The HIV testing was performed with either rapid HIV antibody test and/or enzyme immunoassay, with positive results confirmed with Western blot.
Person-years (PY) at risk were calculated from the date of the first negative HIV test to the date of the last HIV-negative test or to the first HIV-positive test divided by 365.25 days. The follow-up period for MSM that remained HIV-negative included the time between their first and last negative HIV tests. The follow-up period for MSM who were diagnosed with HIV included the time between the first negative and the first positive HIV test. We calculated jurisdiction-adjusted new HIV diagnosis rates using a 2-stage meta-analysis model to account for the substantial diversity in patients across the SSuN jurisdictions. First, HIV diagnosis rates per 100 PY were calculated as number of events of HIV diagnoses divided by PY at risk for a single jurisdiction, multiplied by 100. Then, using the jurisdiction-specific estimates, we estimated the overall rate with 95% confidence intervals (CI) using an inverse variance weighted random effects model, adjusting for heterogeneity between SSuN jurisdictions. We used this method to estimate new HIV diagnosis rates by the following: age group categories, race/ethnicity, SSuN jurisdiction, diagnosed chlamydia or gonorrhea (among those tested for chlamydia and/or gonorrhea in STD clinics, and stratified by anatomic site of infection), and diagnosed P&S syphilis during the follow-up period. Because time to HIV diagnosis may be impacted by testing frequency, which may be more frequent for MSM diagnosed with an STD, we estimated the mean number of clinic visits and testing visits by STD history. Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
Although the objectives of the present analysis did not focus on other individuals attending SSuN STD clinics, we did examine HIV diagnoses rates among women and men who have sex with women (MSW) between January 1, 2010, and June 30, 2013, for comparison purposes only. The MSW were defined as men who only reported female sex partners or who identified as heterosexual and who did not report any male sex partners. Similar methods were used to assess new HIV diagnoses as described above.
Data from participating jurisdictions were collected as part of CDC sentinel surveillance activities exempt from CDC human subjects review; SSuN protocols were approved by the Office of Management and Budget (control 0920–0842 and 0920–1072) and did not include personal identifiers on individual patients.
RESULTS
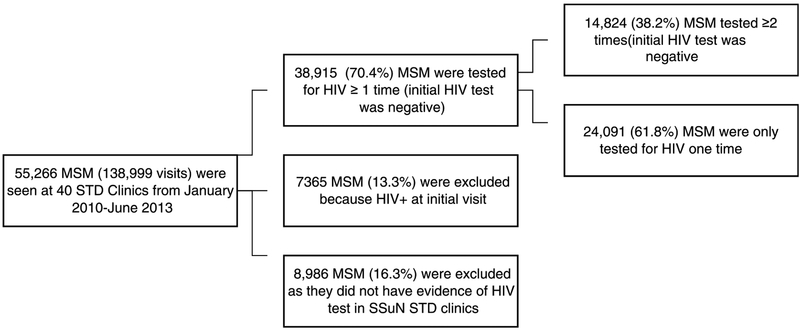
Between January 1, 2010, and June 30, 2013, there were 138,999 patient visits made by 55,266 unique MSM across the 40 STD clinics (Fig. 1). Among the MSM tested for HIV at least once (n = 38,915), 14,824 had a visit with a negative HIV test result followed by at least 1 other visit where an HIV test was performed. This group of 14,824 MSM, described in Table 1, represents 26.9% of all 55,266 MSM seen in SSuN STD clinics and is the focus of all subsequent analyses. These 14,824 MSM were similar to the MSM who were tested for HIV only once (n = 24,091) with regard to age, but the group of 14,824 MSM were marginally more likely to be black (24% vs 20%, P > .05) and/or Hispanic (27% vs 23%; P > .05). Differences in the number of MSM (n = 14,824) varied across jurisdictions, with the majority (56%) of MSM seen in STD clinics in New York City and San Francisco. The median age of the included MSM was 29.0 years (interquartile range [IQR], 25–37 years).
Figure 1.
Selection of the study population.
TABLE 1.
Patient Characteristics and Rates of HIV Diagnosis Among 14,824 HIV-Negative MSM Attending SSuN STD Clinics, 2010–2013
| Characteristics | Total, N (%) | HIV Diagnoses/PY | Adjusted HIV Diagnosis Per 100 PY (95% CI) |
|---|---|---|---|
| Overall | 14,824 (100) | 640/20,951.6 | 3.0 (2.6–3.4) |
| Age, y | |||
| ≤ 19 | 364 (2.5) | 23/334.4 | 6.8 (5.3–8.3) |
| 20–24 | 3162 (21.3) | 216/3959.4 | 5.0 (4.3–5.7) |
| 25–29 | 3965 (26.5) | 181/5640.8 | 2.9 (2.5–3.3) |
| 30–39 | 4230 (28.5) | 143/6246.1 | 2.0 (1.7–2.3) |
| 40–49 | 2006 (13.5) | 54/3053.5 | 1.5 (1.2–1.8) |
| ≥ 50 | 1097 (7.4) | 23/1717.8 | 1.2 (0.9–1.5) |
| Race/ethnicity | |||
| White | 5554 (37.5) | 154/7387.9 | 1.8 (1.5,2.1) |
| Black | 3639 (24.6) | 271/5470.1 | 4.7 (4.1–5.3) |
| Hispanic | 3961 (26.6) | 173/5844.3 | 2.8 (2.5–3.1) |
| Other/unknown | 1670 (11.3) | 42/2249.3 | 1.9 (1.4–2.4) |
| Jurisdiction* | |||
| Birmingham, AL | 414 (2.8) | 15/603.7 | 2.5 (1.5–4.1) |
| Baltimore, MD | 421 (2.8) | 43/593.0 | 7.3 (5.4–9.8) |
| Los Angeles County, CA | 1441 (9.7) | 43/2051.5 | 2.1 (1.6–2.8) |
| Chicago, IL | 412 (2.8) | 8/509.8 | 1.6 (0.8–3.1) |
| Denver, CO | 849 (5.7) | 25/1169.1 | 2.1 (1.6–2.8) |
| Hartford and New Haven, CT | 107 (0.7) | 1/140.1 | 0.7 (0.1–5.1) |
| New Orleans, LA | 252 (1.7) | 26/316.9 | 8.2 (5.6–12.1) |
| New York City, NY | 5689 (38.4) | 295/8470.1 | 3.5 (3.1–3.9) |
| Philadelphia, PA | 543 (3.9) | 36/813.8 | 4.4 (3.2–6.1) |
| San Francisco, CA | 2554 (17.2) | 64/3389.9 | 1.9 (1.5–2.4) |
| Richmond, VA | 160 (1.1) | 15/193.7 | 7.7 (4.7–12.8) |
| Seattle, WA | 1961 (13.2) | 69/2700.1 | 2.6 (2.8–3.3) |
| STD history† | |||
| P&S syphilis | 699 (4.7) | 80/1107.6 | 7.2 (6.0–8.4) |
| Gonorrhea (any site) | 3686 (24.9) | 271/5663.2 | 4.0 (3.5–4.5) |
| Urogenital gonorrhea | 2253 (15.7) | 169/3466.3 | 3.7 (3.1–4.3) |
| Rectal gonorrhea | 1262 (8.5) | 129/1943.8 | 6.3 (5.7–6.9) |
| Pharyngeal gonorrhea | 1395 (9.4) | 90/2188.8 | 4.5 (3.7–5.3) |
| Chlamydia (any) | 3521 (23.8) | 238/5567.0 | 2.8 (2.2–3.4) |
| Urogenital chlamydia | 2016 (13.6) | 91/3259.6 | 2.2 (1.7–2.7) |
| Rectal chlamydia | 1728 (11.7) | 164/2711.0 | 5.6 (4.6–6.6) |
| Pharyngeal chlamydia | 228 (1.5) | 17/384.6 | 4.1 (2.7–5.5) |
| No STD diagnoses‡ | 8766 (59.1) | 251/11736.4 | 2.0 (1.7–2.3) |
Individual jurisdiction-specific rates are not adjusted.
This includes only persons with positive laboratory test results for Neisseria gonorrhoeae or Chlamydia trachomatis, or a diagnosis of P&S syphilis on/or after initial negative HIV testing
Defined as absence of a positive laboratory test for chlamydia or gonorrhea or a clinical diagnosis of primary or secondary syphilis in SSuN clinics.
Among 14,824 MSM who were tested for HIV at least twice (median, 3 HIV tests; IQR, 2–4 HIV tests), 640 (4.3%) subsequently tested positive for HIV (Table 1). These 14,824 MSM contributed 20,951.6 PY of follow-up time. After adjusting for SSuN jurisdictions, the overall new HIV diagnosis rate was 3.0 per 100 PY (95% CI, 2.6–3.4). For those MSM who were diagnosed with HIV, the median time between the first HIV-negative and first HIV-positive test was 1.2 years (IQR, 231 days to 1.9 years). For the patients who remained HIV-negative, the median follow-up time was 1.3 years (IQR, 230 days to 2.1 years). New HIV diagnosis rates varied by jurisdiction and was lowest in New Haven/Hartford (0.7/100 PY; 95% CI 0.1–5.1) and highest in New Orleans (8.2/100 PY; 95% CI, 5.6–12.1).
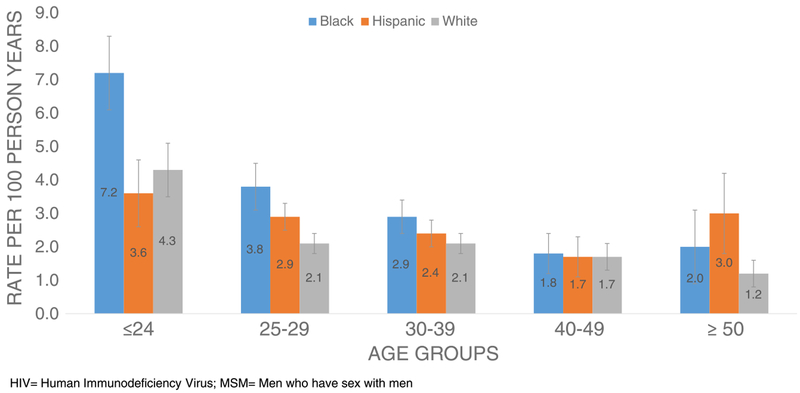
Rates of new HIV diagnoses among MSM varied across the race/ethnicity groups with the highest rate among blacks (4.7/100 PY; 95% CI, 4.1–5.3) followed by Hispanics, whites, and persons of other races and ethnicities (Table 1). New HIV diagnosis rates declined as age increased; the diagnosis rate among MSM aged 19 years or younger was over 4 times that among MSM older than 50 years. When stratified by race, new HIV diagnosis rates were significantly higher among black MSM younger than 25 years (7.2/100 PY; 95% CI, 6.1–8.3) compared with their white (4.3/100 PY; 95% CI: 3.5–5.1) and Hispanic counterparts (3.6/100 PY; 95% CI, 2.6–4.6) (Fig. 2).
Figure 2.
Estimated rates of HIV diagnosis among MSM (n = 14,824) by race/ethnicity and age, 2010–2013.
The mean number of clinic visits during the observation period for the MSM without STDs and with STDs diagnosed were 5.3 and 6.8 (P < 0.05), respectively. The mean number of HIV testing visits during the observation period for the MSM without STDs and with STDs diagnosed were 2.7 and 3.3 (P < 0.05), respectively. A statistically significantly higher HIV diagnosis rate was observed among MSM with a STD diagnosis (4.1; 95% CI, 3.6–4.6) compared with those MSM not diagnosed with STD (2.0; 95% CI, 1.7–2.3).
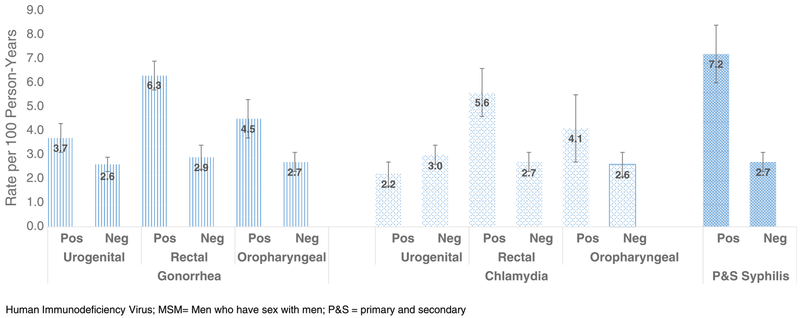
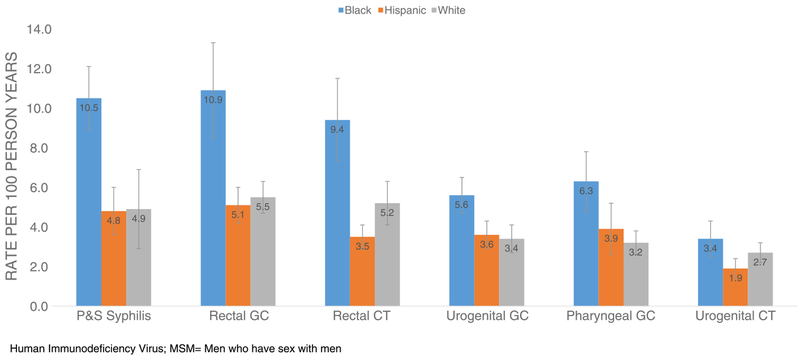
We estimated the adjusted rates of new HIV diagnosis by specific STD diagnosis during the follow-up period (Table 1). Overall, MSM who had a diagnosis of P&S syphilis on or after the first negative HIV test had a higher new HIV diagnosis rate(7.2/100 PY; 95% CI, 6.0–8.4) compared with MSM who did not have a P&S syphilis diagnosis (2.7/100 PY; 95% CI, 2.3–3.1). Similarly, MSM who tested positive for gonorrhea (4.0/100 PY; 95% CI, 3.5–4.5) or chlamydia (2.8/100 PY; 95% CI, 2.2–3.4) had higher rates of new HIV diagnoses when compared with those with negative test results. Compared with MSM not diagnosed with rectal chlamydia or gonorrhea, MSM with 1 or more episodes of rectal chlamydia (5.6/100 PY; 95% CI, 4.6–6.6) or rectal gonorrhea (6.3/100 PY; 95% CI, 5.7–6.9) had higher rates of HIV diagnosis (Fig. 3). For MSM with P&S syphilis, the rate of HIV diagnosis for black MSM was 10.5 per 100 PY (95% CI, 8.9–11.6) (Fig. 4). This was significantly different from that of whites but not Hispanics. Additionally, blacks had significantly higher rates of HIV diagnoses if they were diagnosed with either rectal gonorrhea and/or chlamydia when compared with their Hispanic and white counterparts.
Figure 3.
Estimated rates of HIV diagnosis among MSM (n = 14,824) tested and positive for gonorrhea or chlamydia by anatomic site and P&S syphilis, 2010–2013.
Figure 4.
Estimated rates of HIV diagnosis among MSM (n = 14,824) by anatomic site of STD diagnosis and race/ethnicity, 2010–2013.
When HIV diagnosis rates among MSM were compared with heterosexual men and women, markedly higher MSM rates were observed in every SSuN jurisdiction. Sixty-nine new HIV diagnoses were identified in MSW with an adjusted HIV diagnosis rate of 0.12/100 PY (95% CI, 0.10–0.14) (range, 0.1–0.4 by SSuN jurisdiction). Sixty-six new HIV diagnoses were identified in women for an adjusted HIV diagnosis rate of 0.12/100 PY (CI 0.1–0.14) (range, 0.05–0.4 by SSuN jurisdiction).
DISCUSSION
Men who have sex with men are disproportionately affected by HIV, and STD clinics continue to be important clinical settings that provide HIV testing services to a large number of MSM. Among MSM tested at least twice for HIV in this network of STD clinics, we report an overall incidence of HIV diagnosis of 3.0 per 100 PY, with even greater rates observed among MSM diagnosed with rectal gonorrhea, rectal chlamydia, or P&S syphilis. Previous studies conducted in STD clinics have shown comparable HIV incidence rates with higher observed rates in patients with bacterial STDs,11–16 echoing the need to target HIV prevention interventions to these key affected populations of men.
Our estimates corroborate racial/ethnic and age disparities in HIV diagnosis rates that have been documented elsewhere by other investigators.1,2,17–20 In our analysis, the observed rate among black MSM (4.7 per 100 PY) was almost twice that of other race/ethnicities and young black MSM (≤24 years of age) had the highest rate (7.2 per 100 PY). The HIV diagnosis rates among blacks with P&S syphilis, rectal gonorrhea, and rectal chlamydia were notably higher than HIV diagnosis rates among other MSM without STD diagnoses. Several economic and sociocultural factors proposed to explain racial and ethnic disparities in risk for HIV include unemployment, poverty, lack of health insurance, issues with literacy and low education rates, barriers to testing, and higher HIV prevalence among some sexual networks.21–23 A recent National HIV Behavioral Surveillance analysis by Wejnert et al24 examined a 2008 to 2014 surveillance data from MSM from 20 cities and noted that black MSM have the highest HIV prevalence and the lowest awareness of their HIV infection, which was especially true for young black MSM. Young MSM of minority races and ethnicities could be targeted with prevention efforts that address their complex social needs as well as the structural barriers that increase their HIV risk, including targeted messaging about frequent HIV testing and expanding access to a variety of testing venues.
Given that an STD diagnosis may indicate HIV-related sexual risk behavior, targeting prevention efforts to MSM with a bacterial STD may identify those at greatest risk for HIV infection. However, of the 640 MSM identified with incident HIV diagnosis in our analysis, approximately 60% had a STD diagnosis in the SSuN STD clinics during the follow-up period. Concentrating prevention efforts on only those at the very highest risk, such as those who were diagnosed with P&S syphilis or rectal gonorrhea and/or chlamydia, will likely reach only a subset of the MSM who will ultimately acquire HIV infection. Also, although MSM who were not diagnosed with chlamydia, gonorrhea, or P&S syphilis in the SSuN STD clinics had a lower rate of HIV diagnosis of 2.0 per 100 PY, their rate of HIV diagnoses is consistently orders of magnitude higher than among women and heterosexual men. This indicates the presence of continued high-risk sexual behavior and signals the potential for STD and HIV acquisition.
These results have important limitations. First, MSM in this analysis included those attending STD clinics, and the results may not be generalizable to other populations of MSM. However, the high risk for HIV diagnoses among STD clinic patients suggests that this population may serve as a sentinel population for emerging disease trends and is an important population with which to intervene. Second, approximately 16% (Fig. 1) of the total MSM presenting for STD services at SSuN clinics were excluded because they did not have evidence of an HIV test in SSuN STD clinics, and of those who were tested for HIV, approximately 62% were tested only once. Thus, our inclusion criteria could have impacted our findings if MSM not tested for HIV in these clinics were at differential risk for HIV. If most MSM not tested for HIV in these clinics were previously positive, there would be no influence in our HIV diagnosis rates. However, if most patients are negative, it is difficult to say without more information about these patients because the bias could be in either direction. Third, patients may have had testing at an outside clinic, suggesting that our measures of new HIV diagnoses and incident STDs are minimum estimates. Fourth, our associations by demographic characteristics or STDs of interest could be impacted by other unmeasured factors. Finally, this analysis encompassed data from 2010 to 2013, and some rates or patterns in HIV acquisition may have changed since then.
Despite these limitations, our findings have important public health implications. First, these kinds of analyses are difficult to perform in smaller single jurisdictions because of small sample size. Hence, the strength of our analysis is the inclusion of multiple jurisdictions. In doing so, we are able to pool the data to estimate robust new HIV diagnosis rates. Although the majority of new HIV diagnoses in our analysis were concentrated in heavily populated jurisdictions, rates of new diagnoses among MSM in jurisdictions with much smaller MSM populations were among the highest noted. These findings are likely the result of predominately testing only those at the highest of risk presenting to STD clinics in the different jurisdictions, providing useful information for HIV prevention and care services. These data build on previous studies that some populations of MSM continue to have disproportionately higher rates of new HIV diagnoses, including young black MSM and those with a previous diagnosis of P&S syphilis, rectal chlamydia, and/or gonorrhea. However, equally important, this work demonstrates that men who are HIV uninfected and seeking STD services also are at high risk for HIV infection regardless of demographic or STD diagnoses.
Footnotes
Conflict of Interest and Sources of Funding: None declared.
REFERENCES
- 1.Purcell DW, Johnson CH, Lansky A, et al. Estimating the population size of men who have sex with men in the United States to obtain HIV and syphilis rates. Open AIDS J 2012; 6(Supp 1:M6):98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. HIV Surveillance Report, 2015. vol 27 Available at: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html Published November 2016. Accessed November 13, 2017. [Google Scholar]
- 3.Centers for Disease Control and Prevention. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006; 55(No. RR-14). [PubMed] [Google Scholar]
- 4.Hoover KW, Parsell BW, Leichliter JS, et al. Continuing need for sexually transmitted disease clinics after the affordable care act. Am J Public Health 2015; 105(S5):S690–S695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirzinger WK, Cohen RA, Gindi RM. Health care access and utilization among young adults aged 19–25: Early release of estimates from the National Health Interview Survey, January–September 2011. National Center for Health Statistics. May 2012. Available at: http://www.cdc.gov/nchs/nhis/releases.htm. Accessed August 16, 2017.
- 6.Viera AJ, Thorpe JM, Garrett JM. Effects of sex, age, and visits on receipt of preventive healthcare services: A secondary analysis of national data. BMC Health Serv Res 2006; 6: doi: 10.1186/1472-6963-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernstein KT, Marcus JL, Nieri G, et al. Rectal gonorrhea and chlamydia reinfection is associated with increased risk of HIV seroconversion. J Acquir Immune Defic Syndr 2010; 53:537–543. [DOI] [PubMed] [Google Scholar]
- 8.Taylor MM, Newman DR, Gonzalez J, et al. HIV status and viral loads among men testing positive for rectal gonorrhoea and chlamydia, Maricopa County, Arizona, USA, 2011–2013. HIV Med 2015; 16:249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchacz K, Klausner JD, Kerndt PR, et al. HIV incidence among men diagnosed with early syphilis in Atlanta, San Francisco, and Los Angeles, 2004 to 2005. J Acquir Immune Defic Syndr 2008; 47:234–240. [PubMed] [Google Scholar]
- 10.Pathela P, Braunstein SL, Blank S, et al. HIV incidence among men with and those without sexually transmitted rectal infections: Estimates from matching against an HIV case registry. Clin Infect Dis 2013; 57: 1203–1209. [DOI] [PubMed] [Google Scholar]
- 11.Mehta SD, Ghanem KG, Rompalo AM, et al. HIV seroconversion among public sexually transmitted disease clinic patients: Analysis of risks to facilitate early identification. J Acquir Immune Defic Syndr 2006; 42:116–122. [DOI] [PubMed] [Google Scholar]
- 12.Weinstock H, Sweeney S, Satten GA, et al. HIV seroincidence and risk factors among patients repeatedly tested for HIV attending sexually transmitted disease clinics in the United States, 1991 to 1996. J Acquir Immune Defic Syndr 1998; 19:506–512. [DOI] [PubMed] [Google Scholar]
- 13.Dukers NH, Spaargaren J, Geskus RB, et al. HIV incidence on the increase among homosexual men attending an Amsterdam sexually transmitted disease clinic: Using a novel approach for detecting recent infections. AIDS 2002; 16:F19–F24. [DOI] [PubMed] [Google Scholar]
- 14.Menza TW, Hugher JP, Celum CL, et al. Prediction of HIV acquisition among men who have sex with men. Sex Transm Dis 2009; 36:547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pathela P, Jamison K, Braunstein SL, et al. Incidence and predictors of HIV infection among men who have sex with men attending public sexually transmitted disease clinics, New York City, 2007–2012. AIDS Behav 2016; 21:1444–1451. [DOI] [PubMed] [Google Scholar]
- 16.Schwarcz SK, Kellogg TA, McFarland W, et al. Characterization of sexually transmitted disease clinic patients with recent human immunodeficiency virus infection. J Infec Dis 2002; 186:1019–1022. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. HIV Surveillance Report, 2014; vol. 26 November 2015. Available at: http://www.cdc.gov/hiv/library/reports/surveillance/. Accessed August 16, 2017. [Google Scholar]
- 18.Beer L, Oster AM, Mattson CL, et al. Disparities in HIV transmission risk among HIV-infected black and white men who have sex with men United States, 2009. AIDS 2014; 28:105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Millett GA, Peterson JL, Wolitski RJ, et al. Greater risk for HIV infection of black men who have sex with men: A critical literature review. Am J Public Health 2006; 96:1007–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pathela P, Braunstein SL, Blank S, et al. The high risk of an HIV diagnosis following a diagnosis of syphilis: A population-level analysis of New York City men. Clin Infect Dis 2015; 61:281–287. [DOI] [PubMed] [Google Scholar]
- 21.Millett GA, Peterson JL, Flores SA, et al. Comparisons of disparities and risks of HIV infection in black and other men who have sex with men in Canada, UK, and USA: A meta-analysis. Lancet 2012; 380: 341–348. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan PS, Rosenberg ES, Sanchez TH, et al. Explaining racial disparities in HIV incidence in black and white men who have sex with men in Atlanta, GA: A prospective observational cohort study. Ann Epidemiol 2015; 25:445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hernandez-Romieu AC, Sullivan PS, Rothenberg R, et al. Heterogeneity of HIV prevalence among the sexual networks of black and white men who have sex with men in Atlanta: Illuminating a mechanism for increased HIV risk for young black men who have sex with men. Sex Transm Dis 2015; 42:505–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wejnert C, Hess KL, Rose CL, et al. Age-specific race and ethnicity disparities in HIV infection and awareness among men who have sex with men—20 US cities, 2008–2014. J Infect Dis 2016; 213:776–783. [DOI] [PMC free article] [PubMed] [Google Scholar]