Abstract
Background:
Randomized clinical trials have shown the 9-valent human papillomavirus (HPV) vaccine to be highly effective against types 31/33/45/52/58 compared with the 4-valent. Evidence on the added health and economic benefit of the 9-valent is required for policy decisions. We compare population-level effectiveness and cost-effectiveness of 9- and 4-valent HPV vaccination in the United States.
Methods:
We used a multitype individual-based transmission-dynamic model of HPV infection and disease (anogenital warts and cervical, anogenital, and oropharyngeal cancers), 3% discount rate, and societal perspective. The model was calibrated to sexual behavior and epidemiologic data from the United States. In our base-case, we assumed 95% vaccine-type efficacy, lifelong protection, and a cost/dose of $145 and $158 for the 4- and 9-valent vaccine, respectively. Predictions are presented using the mean (80% uncertainty interval [UI] = 10th−90th percentiles) of simulations.
Results:
Under base-case assumptions, the 4-valent gender-neutral vaccination program is estimated to cost $5500(80% UI = 2400–9400) and $7300 (80% UI = 4300−11 000)/quality-adjusted life-year (QALY) gained with and without cross-protection, respectively. Switching to a 9-valent gender-neutral program is estimated to be cost-saving irrespective of cross-protection assumptions. Finally, the incremental cost/QALY gained of switching to a 9-valent gender-neutral program (vs 9-valent girls/4-valent boys) is estimated to be $140 200 (80% UI = 4200−>1 million) and $31 100 (80% UI = 2100−>1 million) with and without cross-protection, respectively. Results are robust to assumptions about HPV natural history, screening methods, duration of protection, and healthcare costs.
Conclusions:
Switching to a 9-valent gender-neutral HPV vaccination program is likely to be cost-saving if the additional cost/dose of the 9-valent is less than $13. Giving females the 9-valent vaccine provides the majority of benefits of a gender-neutral strategy.
In the United States, the 4-valent human papillomavirus (HPV) vaccine has been recommended for females (age 11–26 years) and males (age 11–21 years) since 2006 and 2011, respectively (1,2). The Advisory Committee on Immunization Practices (ACIP) recommendations were based on the burden of HPV-related diseases, vaccine safety and efficacy, and the predicted impact and cost-effectiveness of vaccination. The 4-valent vaccine targets types HPV6/11/16/18, responsible for the majority of HPV-related disease burden (HPV16/18: 52% of high-grade cervical lesions, 70%−75% of cervical cancers, and 43%−80% of other HPV-related cancers; HPV6/11: 85%−90% of anogenital warts) (3–9). Large international randomized controlled clinical trials have shown the 4-valent vaccine to be safe and highly effective against persistent vaccine-type HPV infection and precancerous lesions (vaccine efficacy = 93%−100%) (10,11). Before the introduction of HPV vaccination in the United States, each year an estimated 50 million women underwent Pap testing, 3.5 to 5.0 million had positive tests requiring follow-up, and 12 000 were diagnosed with cervical cancer (1,12,13). In addition, there were approximately 17 500 new HPV-positive cancers of the oropharynx, anus, vulva, vagina, and penis (13) and 355 000 anogenital warts cases diagnosed annually in men and women (12). Prevention and treatment of HPV-attributable diseases resulted in more than $8 billion in direct costs annually (12). Mathematical models have predicted that 4-valent HPV vaccination would substantially reduce this burden and be highly cost-effective (14–20).
On December 10, 2014, the 9-valent HPV vaccine was approved by the US Food and Drug Administration (21). In addition to the types included in the 4-valent vaccine, the 9-valent includes HPV31/33/45/52/58, which cause an estimated 28%−31%, 15%−20%, and 5%−20% of high-grade cervical lesions, cervical cancers, and other HPV-related cancers, respectively (3–8). Recent results from large randomized clinical trials have shown that, compared with the 4-valent vaccine, the 9-valent prevented over 95% of persistent HPV31/33/45/52/58 infections and associated cervical, vulvar, and vaginal disease of any grade (22). The 9-valent also generated immune responses to HPV6/11/16/18 that were noninferior to those generated by the 4-valent (23). Finally, the immune response to the 9-valent types was noninferior in young adolescent females and males compared with females in the efficacy trial (24).
On February 26th, 2015, the ACIP recommended the use of the 9-valent for females and males. In addition to safety and efficacy, a determining factor in the policy recommendations about substituting the 9-valent for the 4-valent vaccine was the trade-off between the additional health benefits and healthcare cost saved by 9-valent vaccination vs its additional price (25). To address this issue, we used transmission-dynamic modeling to evaluate the incremental population-level effectiveness and cost-effectiveness of switching from the 4- to the 9-valent vaccine in the United States.
Methods
Vaccination Scenarios Investigated and Study Design
Using HPV-ADVISE, a multitype individual-based transmission-dynamic model of HPV infection and disease, we examined four HPV vaccination scenarios: 0) no vaccination, 1) current 4-valent gender-neutral (females/males) HPV vaccination program, 2) switching to a 9-valent vaccine for females but maintaining the 4-valent for males, or 3) switching to a 9-valent gender-neutral vaccination program (Supplementary Figure 1, available online). For scenarios 1–3, we used observed age- and gender-specific three-dose HPV vaccine uptake rates in the United States from 2007 to 2013 (26) and assumed these rates remained constant at 2013 levels from 2014 onwards. In 2013, the average three-dose vaccination coverage in girls and boys aged 13 to 17 years was 38% and 14%, respectively (26). Under base-case assumptions, our model predicts that vaccination coverage among those aged 13 to 17 years will reach 46% and 25% in 2017 among girls and boys, respectively (with 62% and 38% coverage by 17 years of age). We performed sensitivity analysis on future vaccination coverage, given its uncertainty (see Supplementary Figure 2, available online, for vaccination coverage scenarios and fit to observed data). All changes to the current HPV vaccination strategy were modeled to occur at the beginning of 2015.
For model predictions of population-level effectiveness, the primary outcome was the relative reduction in HPV-attributable disease after HPV vaccination (scenarios 1–3) compared with no vaccination. In addition, we calculated the number needed to vaccinate to prevent one cervical intraepithelial neoplasia grade 2 or 3 (CIN2/3) episode, cervical cancer, HPV-attributable cancer, or death. For the economic analysis, we performed cost-utility analysis (cost/quality-adjusted life-year [QALY] gained) from the societal perspective, including only direct medical costs. We discounted costs and benefits at 3% per year. All costs were inflated to $US 2010 using the medical care component of the US consumer price index (27). All outcomes were modeled over a 70-year time horizon to capture both short- and long-term benefits of HPV vaccination.
Model Structure
HPV-ADVISE was originally developed to inform HPV vaccination policy in Canada (28–30). The model was adapted and recalibrated to represent the United States. The model contains six fully integrated components: 1) sociodemographic characteristics, 2) sexual behavior and HPV transmission, 3) natural history of HPV-attributable diseases, 4) QALYs and healthcare costs, 5) screening and treatment, and 6) vaccination (see http://www.marc-brisson.net/HPVadvise-US.pdf for an in-depth description of US HPV-ADVISE).
The simulated US population is open and stable (ie, age-specific mortality rates balanced by the birth rate). Individuals enter the population prior to sexual debut (9 years of age) and are assigned three different characteristics to represent heterogeneity in the risk of HPV infection and/or disease: 1) gender, 2) level of sexual activity (4 levels: low to high), and 3) cervical screening behavior (5 levels: frequently to never screened). Risk of HPV infection is gender- and age–specific and depends on sexual behavior and on HPV type-specific biologic parameters (eg, probability of transmission, duration of infectiousness, and natural immunity). Eighteen HPV types, including vaccine types, are modeled independently. The HPV-related diseases modeled are anogenital warts and cervical, vulvar, vaginal, anal, penile, and oropharyngeal cancers (see Supplementary Figure 3, available online, for flow diagrams). QALYs and medical costs are attributed to clinical outcomes over time (see Supplementary Table 1, available online).
HPV-ADVISE reproduces different screening algorithms at the individual level by simulating each woman’s screening history. Screening behavior parameters were estimated using US population-based data (31). Screening algorithms and treatment were based on 2012 guidelines (32). In their simplified form, the guidelines for routine screening stated that: 1) those aged 21 to 29 years should have a cytology test every three years, and 2) those aged 30 to 65 years should have the choice between cytology every three years or cytology and HPV DNA cotesting every five years. Cytology-based screening was chosen as our base-case. However, we examined cytology and HPV DNA cotesting for women aged 30 to 65 years in the sensitivity analysis. Finally, the model assumes that HPV vaccines are prophylactic and have no therapeutic effects.
Model Parameters
Sexual behavior and natural history parameter values were estimated using previously developed calibration methodology (see http://www.marc-brisson.net/HPVadvise-US.pdf for details). Briefly, we identified multiple parameter sets that simultaneously fit 776 US sexual behavior, HPV epidemiology, and screening data target points, taken from the literature and population-based datasets (Supplementary Table 2, available online) (4,8,31,33–40). For model predictions, we selected the 50 best-fitting parameter sets using Least Squares. Multiple parameter sets were used to account for parameter uncertainty. Of particular relevance for the added benefit of 9-valent vaccination, we capture uncertainty in HPV type-specific progression and regression rates towards cervical cancer, and thus uncertainty in HPV type-specific positivity in CIN2/3, and cervical and noncervical cancers. For the economic parameters, we used previously published data on healthcare resources use, direct medical costs and QALY-weights parameter values (Supplementary Table 1, available online) (12,16,19,37,41–49).
In the base-case, the cost of the 4- and 9-valent vaccines is $145 and $158 per dose (including administration fees), respectively (50). Vaccine-type efficacy and duration of protection is 95% and lifelong, respectively. Given the uncertainty around 4-valent efficacy against the additional five nonvaccine types and its likely importance on the incremental impact of the 9-valent, we presented all base-case results with and without cross-protection for the 4-valent. In cross-protection scenarios, vaccine efficacy against HPV-31/33/45/52/58 is 46/29/8/18/6% based on a meta-analysis (51) and duration of protection is assumed to be lifelong (Supplementary Table 3, available online).
Sensitivity Analysis
Uncertainty on natural history parameters is represented in model predictions as the mean, 10th, and 90th percentiles of simulation results using the 50 parameter sets identified through calibration (referred to as the 80% uncertainty intervals [80% UI]). We performed univariate sensitivity analysis on key vaccination characteristics (coverage, efficacy, and duration), screening assumptions, and economic parameters.
Results
Population-Level Effectiveness
Current 4-Valent HPV Program
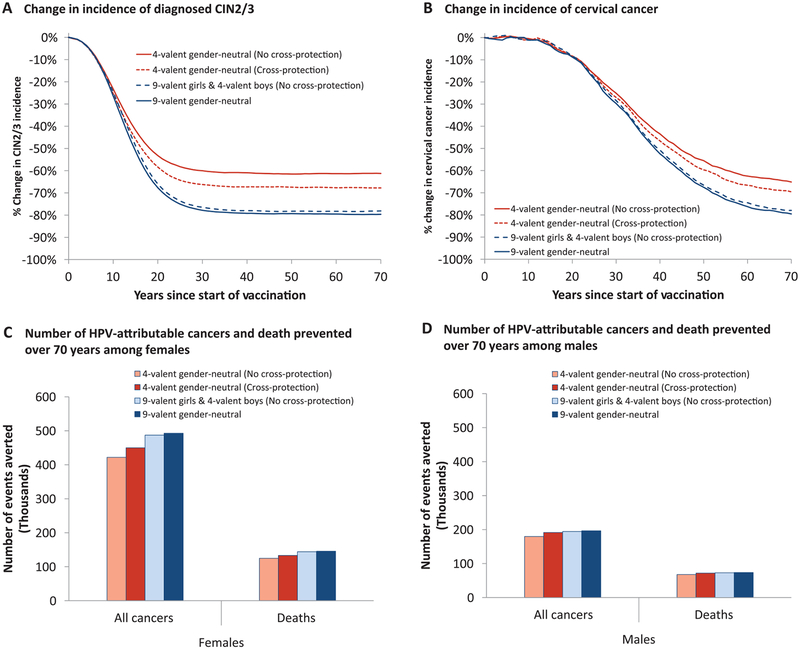
Assuming no cross-protection, the current US 4-valent gender-neutral HPV vaccination program is predicted to reduce the incidence of CIN2/3 and cervical cancer by 61 (80% UI = 57–66) and 65 (80% UI = 60–69) percentage points, respectively, after 70 years (Figure 1, A and B; Supplementary Table 4, available online). In addition, the vaccination program would also lead to 76 (80% UI = 74–76) and 80 (80% UI = 75–87) percentage point reductions in the incidence of other HPV-attributable cancers and anogenital warts in women and men after 70 years (Supplementary Figure 4 and Supplementary Table 4, available online). In total, 4-valent vaccination is predicted to prevent 602 000 HPV-attributable cancers (422 000 and 180 000 among women and men, respectively) and 193 000 associated deaths (125 000 and 68 000 among women and men, respectively) in the United States over the program’s first 70 years (Figure 1, C and D). Incidence of CIN2/3 and HPV-attributable cancers would be further decreased by 4 to 7 percentage points if the 4-valent provides cross-protection (Figure 1, A–D; Supplementary Table 4, available online).
Figure 1.
Estimated population-level impact of 4- and 9-valent vaccination strategies in the United States. Estimated percentage change following vaccination in the incidence of (A) diagnosed cervical intraepithelial neoplasia grade 2 and 3 (CIN2/3) and (B) cervical cancer. C–D) Number of human papillomavirus (HPV)-attributable cancers and deaths averted in females and males over the first 70 years of 4- or 9-valent vaccination programs. Base-case: vaccine duration = lifetime, vaccine-type efficacy = 95%, cross-protective vaccine efficacy presented in Supplementary Table 3 (available online), 4-valent cost per dose = $145, 9-valent cost per dose = $158. Predictions: mean estimate generated by the 50 best-fitting parameter sets. Mean prevaccination incidence rate of diagnosed CIN2/3 = 123 per 100 000 women-years, and cervical cancer = 8 per 100 000 women-years. HPV-attributable cancers: cervix, oropharynx, anus, vulva, vagina, and penis. CIN2/3 = cervical intraepithelial neoplasia of grade 2 or 3.
Switching to a 9-Valent Program
Assuming no 4-valent cross-protection, switching females only to the 9-valent is estimated to further reduce the incidence of CIN2/3 and cervical cancer by 17 (80% UI = 12–21) and 13 (80% UI = 9–18) percentage points, respectively, after 70 years (Figure 1, A and B; Supplementary Table 4, available online). Such a switch would produce small additional reductions in other HPV-attributable cancers but have no incremental impact on the incidence of anogential warts (Supplementary Figure 4 and Supplementary Table 4, available online). In total, switching females only to the 9-valent is predicted to prevent an additional 88 000 HPV-attributable cancers (71 000 and 17 000 among women and men, respectively) and 27 000 associated deaths (21 000 and 6 000 among women and men, respectively) over the first 70 years of the program (Figure 1, C and D). These incremental benefits are 35% to 60% lower when the 4-valent is assumed to provide cross-protection (Figure 1, A–D; Supplementary Table 4, available online). Finally, the incremental benefits of switching to a 9-valent gender-neutral program (vs 9-valent females/4-valent males) are estimated to be minimal (Figure 1, A and B; Supplementary Table 4, available online).
Number Needed to Vaccinate
Over the first 70 years of the HPV vaccination program, the predicted number needed to vaccinate (NNV) with the 4-valent to prevent one CIN2/3 episode, HPV-attributable cancer, and death is 23 (80% UI = 17–30), 251 (80% UI = 242–262) and 784 (80% UI = 750–821), respectively (Table 1, assuming no 4-valent cross-protection). If females only are switched to the 9-valent, one additional CIN2/3 episode, HPV-attributable cancer and death is predicted to be prevented for every 51 (80% UI = 37–81), 1086 (80% UI = 860–1556), and 4538 (80% UI = 3400–7837) females vaccinated with the 9-valent, respectively. The NNV increases more than six-fold when examining the incremental benefit of switching to a 9-valent gender-neutral program rather than a 9-valent females/4-valent males program (Table 1).
Table 1.
Number needed to vaccinate and cost-effectiveness
| Vaccination scenarios | Comparison scenarios | Mean (80% UI) | ||||||
|---|---|---|---|---|---|---|---|---|
| Number needed to vaccinate* | Incremental cost-effectiveness* | |||||||
| CIN2/3 | Cervical cancer | All cancers† | Deaths | Change in costs ($ million) | Change in QALYs gained (1000 QALY) | ICER ($/QALY gained) | ||
| No cross-protection for 4-valent | ||||||||
| (1) 4-valent gender-neutral | 1 vs no vaccination | 23 (17 to 30) | 505 (473 to 550) | 251 (242 to 262) | 784 (750 to 821) | 7493 (4802 to 10 791) | 1032 (960 to 1102) | 7300 (4300 to 11 000) |
| (2) 9-valent girls 4-valent boys | 2 vs l | 51 (37 to 81) | 1580 (1154 to 2677) | 1086 (860 to 1556) | 4538 (3400 to 7837) | −1993 (−3368 to −648) | 134 (51 to 202) | CS (CS to CS) |
| (3) 9-valent gender-neutral | 3 vs 2 | 376 (249 to 632) | 11124 (2782 to >1 million) | 6527 (2382 to >1 million) | 32 074 (6877 to >1 million) | 436 (54 to 849) | 14 (0 to 82) | 31 100 (2100 to >1 million) |
| 3 vs 1 | 76 (56 to 112) | 2335 (1777 to 3139) | 1583 (1307 to 1928) | 6708 (4940 to 10 550) | −1558 (−3022 to −107) | 148 (85 to 212) | CS (CS to CS) | |
| With cross-protection for 4-valent | ||||||||
| (1) 4-valent gender-neutral | 1 vs no vaccination | 21 (15 to 27) | 472 (429 to 507) | 236 (223 to 246) | 737 (702 to 779) | 6072 (2757 to 9670) | 1097 (1013 to 1191) | 5500 (2400 to 9400) |
| (2) 9-valent girls 4-valent boys | 2 vs 1 | 78 (58 to 110) | 2283 (1729 to 3012) | 1755 (1364 to 2190) | 6753 (4991 to 9796) | −906 (−1652 to −79) | 92 (43 to 128) | CS (CS to CS) |
| (3) 9-valent gender-neutral | 3 vs 2 | 503 (305 to 1509) | 30 792 (4508 to >1 million) | 15 029 (3811 to >1 million) | 76 234 (9266 to >1 million) | 583 (244 to 872) | 4 (0 to 63) | 140 200 (4200 to >1 million) |
| 3 vs l | 115 (87 to 153) | 3509 (1777 to 3139) | 2632 (2030 to 3856) | 10 291 (7111 to 15 139) | −322 (−1114 to 587) | 96 (39 to 162) | CS (CS to 11100) | |
Number needed to vaccinate (NNV) are undiscounted and incremental cost-effectiveness ratio are discounted at 3% annually. Base-case: vaccine-type efficacy = 95%; cross-protective vaccine efficacy presented in Supplementary Table 3 (available online); duration = lifelong; 4-valent cost/dose = $145; 9-valent cost/dose = $158. Predictions: mean estimate generated by the 50 best fitting parameter sets. Each parameter set run 20 times. Uncertainty intervals: 10th and 90th percentiles of model results based on the 50 best-fitting parameter sets, reflects uncertainty in the natural history parameters. NNV = (number of individuals vaccinated with the 9-valent)÷(additional events prevented by vaccinating with 9-valent). CIN2/3 = cervical intraepithelial neoplasia grade 2 and 3; CS = cost saving; ICER = incremental cost-effectiveness ratio; QALY = quality-adjusted life-year; UI = uncertainty interval.
Includes cervical cancer and all other human papillomavirus–attributable cancers.
Cost-Effectiveness Analysis
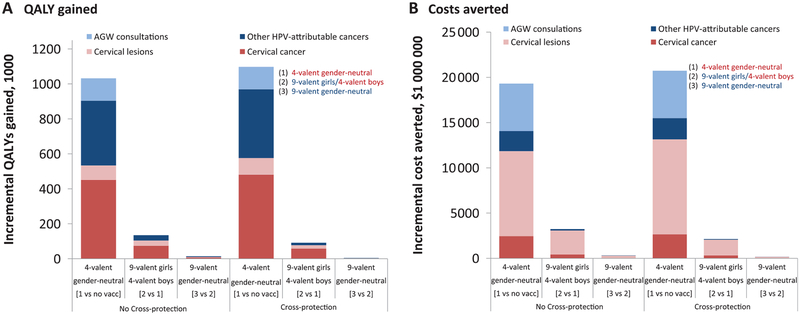
The US 4-valent HPV vaccination program is predicted to produce 1 032 000 (80% UI = 960 000–1 102 000) discounted QALY gains and $19 305 (80% UI = 16 016−21 990) million in direct medical costs averted over 70 years (Figure 2, no 4-valent cross-protection). Switching females only to the 9-valent is estimated to result in 134 000 (80% UI = 51 000–202 000) incremental QALYs gained and $3225 (80% UI = 1863–4591) million in direct medical costs averted over 70 years. Vaccinating boys with the 9-valent instead of the 4-valent produces very small QALY gains and medical costs averted. The incremental QALY gains and medical costs averted from 9-valent vaccination are mainly because of increased prevention of cervical cancer and reduction in the costs of treating and managing cervical lesions, respectively (Figure 2).
Figure 2.
Incremental (A) quality-adjusted life-years (QALYs) gained and (B) medical costs averted of 4- and 9-valent vaccination strategies in the United States (discounted at 3% over 70 years). Base-case: vaccine duration = lifetime, vaccine-type efficacy = 95%, cross-protective vaccine efficacy presented in Supplementary Table 3 (available online). Predictions: mean estimate generated by the 50 best-fitting parameter sets. AGW = anogenital warts; HPV = human papillomavirus; QALY = quality-adjusted life-year; vacc = vaccination.
Assuming no cross-protection, the 4-valent gender-neutral vaccination program is estimated to cost $7300 (80% UI = 4300−11 000)/QALY-gained (Table 1). Switching females only to the 9-valent is estimated to be cost-saving, while the incremental cost-effectiveness ratio of switching to a 9-valent gender-neutral program (vs females-only 9-valent) is estimated to be $31 100 (80% UI = 2100−>1 million)/QALY gained (Table 1). Assuming 4-valent cross-protection has very little impact on the estimated cost-effectiveness ratios of 4-valent gender-neutral ($5500 (80% UI = 2400–9400)/QALY-gained) and 9-valent female/4-valent male (cost-saving) vaccination strategies but increases the incremental cost-effectiveness ratio of the 9-valent gender-neutral vaccination strategy to $140 200 (80% UI = 4200−>1 million)/QALY-gained (Table 1).
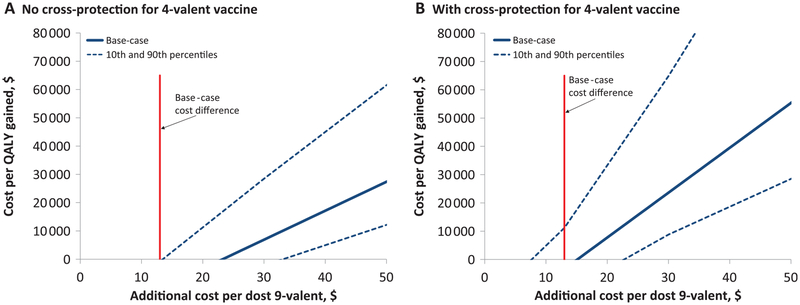
Results were relatively insensitive to assumptions about HPV natural history, vaccination coverage, duration of protection (lifelong or 20 years), healthcare costs, burden of disease, outcomes examined (from all HPV-related outcomes to cervical lesions only), time horizon of analysis (70 or 30 years) and current screening guidelines (cytology screening only or cytology and HPV DNA co-testing) (Table 2). Under all sensitivity analysis scenarios investigated, 9-valent gender-neutral vaccination was either cost-saving or highly cost-effective compared with 4-valent gender-neutral vaccination. The only scenarios where the 9-valent was not cost-saving was when assuming 4-valent cross-protection and 1) assuming minimum healthcare costs, 2) assuming high vaccination coverage, 3) including only cervical cancer or cervical lesion outcomes, 4) using a 30 year time horizon, or 5) assuming only a small proportion of CIN2/3 and cervical cancers are associated with HPV31/33/45/52/58 (Table 2). Finally, results were very sensitive to variation in the additional cost of the 9-valent compared with the 4-valent vaccine (Figure 3).
Table 2.
Sensitivity analysis - incremental cost-effectiveness ($ per QALY-gained)
| Mean (80% UI) | ||||
|---|---|---|---|---|
| Scenarios | 4-valent gender-neutral vs no vaccination | 9-valent gender-neutral vs 4-valent gender-neutral | ||
| No cross-protection | ||||
| Base-case | 7300 | (4300–11 000) | CS | (CS–CS) |
| Vaccination characteristics | ||||
| Duration of protection = 20 y | 9100 | (5700–13 200) | CS | (CS–CS) |
| Vaccination coverage* | ||||
| High-coverage scenario | 13 000 | (10 200–17 300) | CS | (CS–4100) |
| Low-coverage scenario | 4000 | (1200–7300) | CS | (CS–CS) |
| Cervical screening | ||||
| Cytology/HPV DNA co-testing† | N/A | CS | (CS–CS) | |
| Disease outcomes | ||||
| Disease outcomes included | ||||
| Cervical cancer only | 28 000 | (20 800–39 800) | CS | (CS–200) |
| Cervical lesions only | 212 500 | (108 200–556 900) | CS | (CS–32 000) |
| Low HPV31/33/45/52/58 positivity‡ | ||||
| Cervical cancers | 8800 | (7300–10 400) | CS | (CS–1800) |
| CIN2/3 | 7000 | (4900–10 000) | CS | (CS–3000) |
| Health economic parameters | ||||
| Minimum healthcare costs§ | 14 900 | (12 400–17 800) | CS | (CS–9700) |
| Minimum burden of disease§ | 9900 | (6500–14 400) | CS | (CS–CS) |
| Time horizon | ||||
| 30 y | 30 600 | (21 700–42 500) | CS | (CS→1 million) |
| With cross-protection | ||||
| Base-case | 5500 | (2400–9400) | CS | (CS–11 100) |
| Vaccination characteristics | ||||
| Duration of protection | ||||
| 9- & 4-valent= 20 y | 7300 | (3600–11 200) | CS | (CS–12 700) |
| Crossprotection = 20 y | 5700 | (2600–9600) | CS | (CS–8500) |
| Vaccination coverage* | ||||
| High-coverage scenario | 10 800 | (7600–14 900) | 4600 | (CS–22 100) |
| Low-coverage scenario | 2500 | (CS–6200) | CS | (CS–3900) |
| Cervical screening | ||||
| Cytology/HPV DNA co-testing† | N/A | CS | (CS–11 800) | |
| Disease outcomes | ||||
| Disease outcomes included | ||||
| Cervical cancer only | 23 700 | (16 000–33 600) | 1100 | (CS–19 500) |
| Cervical lesions only | 172 500 | (100 500–458 500) | 3500 | (CS–93 300) |
| Low HPV31/33/45/52/58 positivity‡ | ||||
| Cervical cancers | 7300 | (6100–9300) | 3500 | (CS–19 900) |
| CIN2/3 | 6100 | (3800–8700) | 6000 | (100–20 600) |
| Health economic parameters | ||||
| Minimum healthcare costs§ | 13 200 | (10 400–16 200) | 6600 | (1000–21 800) |
| Minimum burden of disease§ | 7800 | (4300–12 400) | CS | (CS–14 400) |
| Time horizon | ||||
| 30 y | 26 800 | (18 100–39 100) | 7600 | (CS→1 million) |
High vaccination coverage scenario: We use 1 dose US estimates and assume vaccine protection after 1 dose (Supplementary Figure 1, available online); low vaccination coverage scenario: We assume that the 3 dose coverage remains constant at 2012 levels from 2013 onwards (2012 was the lowest estimated uptake rate between 2010–2013).
Co-testing: 21- to 29-year-olds have a cytology test every 3 years, and 30- to 65-year-olds have cytology/HPV DNA co-testing every 5 years.
The 12 parameter sets out of 50, which produce the lowest HPV31/33/45/52/58 positivity in CIN2/3 (≤27% positive) and cervical cancers (≤23% positive).
Minimum: All cancer costs or QALY-lost parameters are set at the minimum value identified from the US literature (Supplementary Table 1, available online). Base-case: vaccine-type efficacy = 95%; cross-protective vaccine efficacy presented in Supplementary Table 3 (available online); duration = lifelong; 4-valent cost/dose = $145; 9-valent cost/dose = $158. CIN2/3 = cervical intraepithelial neoplasia grade 2 and 3; CS = cost saving; ICER = incremental cost-effectiveness ratio; NNV = number needed to vaccinate; QALY = quality-adjusted life-year; UI = uncertainty interval.
Figure 3.
Sensitivity analysis. Cost per dose of the 9-valent vaccine (gender-neutral vaccination) compared with the 4-valent. A) No cross-protection for 4-valent vaccine. B) With cross-protection for 4-valent vaccine. Note: The maximum additional cost per dose for the 9-valent vaccine to remain cost-saving compared with the 4-valent is the value at which the incremental cost-effectiveness ratio is equal to $0. Base-case: vaccine duration = lifetime, vaccine-type efficacy = 95%, cross-protective vaccine efficacy presented in Supplementary Table 3 (available online), 4-valent cost per dose = $145, 9-valent cost per dose = $158. Predictions: mean, and 10th and 90th percentile of model results based on the 50 best-fitting parameter sets (20 runs per parameter set). The 10th and 90th percentiles reflect the uncertainty in the natural history parameters. QALY = quality-adjusted life-year.
Discussion
In this transmission-dynamic modeling study, we predict that substituting the 9-valent vaccine for the 4-valent is likely to be cost-saving in the US More specifically, switching to 9-valent HPV vaccination would lead to substantial reductions in cervical lesions and cancers, and the associated healthcare cost-savings in treatment/management of cervical lesions would be greater than the additional costs of the 9-valent program. Vaccinating girls with the 9-valent provides the majority of cost-savings and QALYs-gained of a 9-valent gender-neutral program.
Four key issues must be considered when using our results for decision-making, the additional price of the 9-valent vaccine, shifting HPV vaccination paradigm towards two rather than three doses, future changes to cervical screening, and feasibility of vaccinating girls and boys with different HPV vaccines. Firstly, the conclusions are highly dependent on the price of the 9-valent vaccine, which has yet to be decided. We used the hypothetical vaccine prices presented by the manufacturer at an international conference (50). Increases in the additional price per dose of the 9-valent vaccine beyond $13 could change our predictions and make the switch to 9-valent vaccination less cost-effective than the current 4-valent program (Figure 3). Secondly, recent trials and post-vaccination surveillance data suggest that two doses of the 2- or 4-valent HPV vaccines may be as protective as three doses in pre-adolescent girls (52–54). Based on these results and cost-effectiveness modeling (55,56), the World Health Organization’s Strategic Advisory Group of Experts on Immunization has recommended a two-dose schedule for girls aged 9–14 years (57), and many countries have recently switched to two-dose vaccine schedules (eg, Switzerland, the Netherlands, Mexico, the United Kingdom and Canada). Although trials are under way (58), there is no evidence on 9-valent two-dose vaccine efficacy. Policy-makers may have to decide whether to switch to a three dose 9-valent vaccine, or switch to two dose schedules with the 2- or 4-valent vaccines. Using HPV-ADVISE, we estimated that the cost per QALY-gained of a 3-dose 9-valent gender-neutral vaccination program compared with a 2-dose 4-valent gender-neutral vaccination program is $38 900 (80% UI = $20 300–80 500), when assuming both strategies have equal vaccine-type efficacy (95%) and duration of protection (lifelong), and 2-dose 4-valent vaccination does not provide cross protection (results not shown). Hence, the 9-valent is highly likely to be cost-effective compared with 2-dose 4-valent vaccination. Thirdly, we predict that switching to 9-valent is cost-saving under current screening guidelines (either with cytology-based screening or cytology and HPV DNA co-testing) (32). However, to alter our conclusions, future changes in screening would have to substantially reduce the cost of treating/managing CIN2/3 lesions (which is the key driver of the additional cost-savings from 9-valent vaccination). Finally, we presented both 9-valent girls/4-valent boys and 9-valent gender-neutral vaccination scenarios to isolate the incremental cost and benefits of vaccinating girls and boys with the 9-valent vaccine. However, vaccinating girls and boys with different HPV vaccines would raise both logistical/feasibility issues and acceptability/equity concerns.
Although the United States has similar sexual behavior (59,60) and HPV epidemiology (15,67−70) to other high income countries (HIC), generalization of results should be made with care. The key reason for the cost-saving results of the 9-valent in the United States is the large reduction in the costs of treating/managing HPV31/33/45/52/58 associated cervical lesions. This is highlighted by the fact that the 9-valent remains cost-saving when only including the health gains and costs saved from the added prevention of HPV-31/33/45/52/58 associated cervical lesions (Table 2). Given that healthcare costs are lower in other HIC (eg, European countries, Canada, Australia), the price differential between the 9- and 4-valent vaccines will have to be smaller in order for 9-valent vaccination to be cost-saving. In a previous exploratory cost-effectiveness analysis for Canada (30), we found that, although not cost-saving, 9-valent vaccination would likely be cost-effective. Finally, our results should not be extrapolated to resource-poor settings because of major differences in screening, healthcare costs and HPV epidemiology.
Four main limitations should be considered when interpreting our results. Firstly, we assumed equal 9-valent vaccine efficacy for girls and boys based on immunogenicity bridging data (24). However, we predict that vaccinating boys with the 9-valent has very little incremental benefits, thus assumptions about vaccine efficacy among boys is unlikely to impact our overall conclusions. Secondly, the duration of 4- and 9-valent vaccine efficacy and future HPV vaccination coverage remain unknown. In our base-case, we assumed that duration of protection would be lifelong and coverage would plateau among both girls and boys based on current vaccine uptake rates. However, our results clearly show that our findings are insensitive to vaccine duration and that even when vaccination coverage is high, switching to a 9-valent gender-neutral vaccination remained very cost-effective. Thirdly, we did not examine the cost-effectiveness of the 9-valent vaccine relative to the 2-valent, as the 4-valent is used in the United States and most HIC. Finally, we did not examine scenarios where the 9-valent vaccination strategy would allow more aggressive changes in screening, further reducing future costs. However, even without this potential additional impact, switching to 9-valent vaccination was estimated to be cost-saving.
Our mathematical model and analyses have several strengths. First, our multi-type HPV transmission-dynamic model includes sexual behavior, health seeking behavior (vacci-nation and screening), type-specific HPV transmission, screening performance in detection of underlying health states, and natural history of infection and disease. By doing so, our model can predict herd effects and reproduce 1) progression/clearance through different clinical cytological classifications (eg, CIN1 to CIN3), and 2) the course of underlying HPV infection progression/clearance to CIN3 based on infection duration and HPV-type (61). Second, the model was calibrated to highly-stratified data on sexual behavior, natural history and cervical cancer screening from the United States Predictions were performed using the 50 best fitting parameter sets to represent uncertainty in the natural history of HPV infection/disease.
To our knowledge, this is the first published economic evaluation of 9-valent HPV vaccination in the United States From the societal perspective, switching from a 4-valent to 9-valent gender-neutral HPV vaccination program is predicted to be cost-saving if the additional cost/dose of the 9-valent is less than $13, irrespective of key sources of uncertainty (duration of protection, burden of disease, and use of cytology or co-testing as primary screening). When vaccination coverage is assumed to be high, the switch to a 9-valent vaccination is predicted to be very cost-effective, though it may not be cost-saving. The findings were driven by the incremental health benefits and medical costs averted from preventing cervical lesions and cervical cancer associated with HPV-types 31/33/45/52/58.
Supplementary Material
Funding
This work was supported by a contract from the Centers for Disease Control and Prevention (OOOHCVJD-2012–47104) and the Canada Research Chairs program (support for MB).
Footnotes
Notes
The funders had no role in the design and conduct of the study; the collection, management, analysis, or interpretation of the data; or the preparation, review, or approval of the manuscript.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
We are indebted to Compute Canada for providing us with the power necessary to run the simulations.
MB designed the study, drafted the article, had full access to all of the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis. JFL, HWC, MD, TM, MCB, and LEM commented on the study design and model structure. MB and MCB designed the first version of HPV-ADVISE. MB and JFL programmed the economic components of the model, as well as the natural history of HPV-related cancers other than cervical. JFL and TM programmed the United States screening algorithms under the supervision of MB. MB and JFL performed the analysis. HWC and LEM provided United States-specific data necessary for the analysis. All authors contributed to the interpretation of results, critically revised the manuscript for important intellectual content, and approved the final version submitted for publication. Finally, Dr. Nicolas Van de Velde programmed many core components of HPV-ADVISE (ie, HPV transmission and natural history of cervical cancer) and helped design the initial model.
In the past three years, MB received an unrestricted grant from Merck Frosst related to Zoster burden of illness (no grants ongoing). MD consulted for GSK (Zoster vaccine). JFL, HWC, TM, MCB, and LEM have no conflicts of interest to declare.
References
- 1.Centers for Disease Control and Prevention. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2007;56 (No. RR-2). [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Recommendations on the use of quadrivalent human papillomavirus vaccine in males-Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60(50):1705–1708. [PubMed] [Google Scholar]
- 3.Clifford GM, Smith JS, Aguado T, et al. Comparison of HPV type distribution in high-grade cervical lesions and cervical cancer: a meta-analysis. Br J Cancer. 2003;89(1):101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hariri S, Unger ER, Schafer S, et al. HPV type attribution in high grade cervical lesions: assessing the potential benefits of vaccines in a population-based evaluation in the United States. Cancer Epidemiol Biomarkers Prev. 2015;24(2):393–399. [DOI] [PubMed] [Google Scholar]
- 5.Munoz N, Bosch FX, de Sanjose S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348(6):518–527. [DOI] [PubMed] [Google Scholar]
- 6.Serrano B, Alemany L, Tous S, et al. Potential impact of a nine-valent vaccine in human papillomavirus related cervical disease. Infect Agent Cancer. 2012;7(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Vuyst H, Clifford GM, Nascimento MC, et al. Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: a meta-analysis. Int J Cancer. 2009;124(7):1626–1636. [DOI] [PubMed] [Google Scholar]
- 8.Saraiya M, Unger E, Thompson T, et al. US assessment of HPV types in cancers: implications for current and 9-valent HPV vaccines. J Natl Cancer Inst. 2015;107(6):XXX-XXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garland SM, Steben M, Sings HL, et al. Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J Infect Dis. 2009;199(6):805–814. [DOI] [PubMed] [Google Scholar]
- 10.Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356(19):1928–1943. [DOI] [PubMed] [Google Scholar]
- 11.Future II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356(19):1915–1927. [DOI] [PubMed] [Google Scholar]
- 12.Chesson HW, Ekwueme DU, Saraiya M, et al. Estimates of the annual direct medical costs of the prevention and treatment of disease associated with human papillomavirus in the United States. Vaccine. 2012;30(42):6016–6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jemal A, Simard EP, Dorell C, et al. Annual Report to the Nation on the Status of Cancer, 1975–2009, Featuring the Burden and Trends in Human Papilloma-virus (HPV)-Associated Cancers and HPV Vaccination Coverage Levels. J Natl Cancer Inst. 2013;105(3):175–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chesson HW, Ekwueme DU, Saraiya M, et al. The cost-effectiveness of male HPV vaccination in the United States. Vaccine. 2011;29(46):8443–8450. [DOI] [PubMed] [Google Scholar]
- 15.Chesson HW, Ekwueme DU, Saraiya M, et al. Cost-effectiveness of human papillomavirus vaccination in the United States. Emerg Infect Dis. 2008;14(2):244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JJ, Goldie SJ. Health and economic implications of HPV vaccination in the United States. N Engl J Med. 2008;359(8):821–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JJ, Goldie SJ. Cost effectiveness analysis of including boys in a human papillomavirus vaccination programme in the United States. BMJ. 2009;339:b3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elbasha EH, Dasbach EJ. Impact of vaccinating boys and men against HPV in the United States. Vaccine. 2010;28(42):6858–6867. [DOI] [PubMed] [Google Scholar]
- 19.Elbasha EH, Dasbach EJ, Insinga RP. Model for assessing human papillomavirus vaccination strategies. Emerg Infect Dis. 2007;13(1):28–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jit M, Brisson M, Portnoy A, et al. Cost-effectiveness of female human papillomavirus vaccination in 179 countries: a PRIME modelling study. Lancet. Global Health 2014;2:e406–e414. [DOI] [PubMed] [Google Scholar]
- 21.Merck. FDA Accepts for Review Merck’s Biologics License Application for V503, Investigational 9-valent Human Papillomavirus Vaccine. 2014. http://www.mercknewsroom.com/news-release/research-and-development-news/fda-accepts-review-mercks-biologics-license-application-v. Accessed November 2014.
- 22.Joura EA, Giuliano AR, Iversen OE, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8):711–723. [DOI] [PubMed] [Google Scholar]
- 23.Van Damme P, Vesikari T, Brodszki N, et al. Immunogenicity and safety of a novel 9-valent vaccine in girls 9–15 years of age compared to the quadriva-lent vaccine. Abstract presented at the Eurogin 2013 International Congress. Florence, November 3–6, 2013. [Google Scholar]
- 24.Olsson SE, Van Damme P, Herrera T, et al. Immunogenicity and safety of a novel 9-valent HPV L1 virus-like particle vaccine in boys and girls 9–15 years old, comparison to women 16–26 years old. Abstract presented at the 29th International Papillomavirus Conference and Public Health & Clinical Workshops. Seattle, August 21–25, 2014. [Google Scholar]
- 25.Advisory Committee on Immunization Practices (ACIP). Meeting of the Advisory Committee on Immunization Practices (ACIP) - Summary Report. Atlanta, Centers for Disease Control and prevention (CDC), October 29–30, 2014. [Google Scholar]
- 26.Stokley S, Jeyarajah J, Yankey D, et al. Human papillomavirus vaccination coverage among adolescents, 2007–2013, and postlicensure vaccine safety monitoring, 2006–2014-United States. MMWR Morb Mortal Wkly Rep. 2014;63(29):620–624. [PMC free article] [PubMed] [Google Scholar]
- 27.Bureau of Labor Statistics, Labor USDo. Consumer Price Index. Item Medical Care. Series CUUR0000SAM. http://data.bls.gov/timeseries/CUUR0000SAM?include_graphs=false&output_type=column&years_option=all_years. Accessed November 2013.
- 28.Van de Velde N, Boily MC, Drolet M, et al. Population-level impact of the bivalent, quadrivalent, and nonavalent human papillomavirus vaccines: a model-based analysis. J Natl Cancer Inst. 2012;104(22):1712–1723. [DOI] [PubMed] [Google Scholar]
- 29.Brisson M, Laprise JF, Drolet M, et al. Comparative cost-effectiveness of the quadrivalent and bivalent human papillomavirus vaccines: a transmission-dynamic modeling study. Vaccine. 2013;31(37):3863–3871. [DOI] [PubMed] [Google Scholar]
- 30.Drolet M, Laprise JF, Boily MC, et al. Potential cost-effectiveness of the nonava-lent human papillomavirus (HPV) vaccine. Int J Cancer. 2014;134(9):2264–2268. [DOI] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention (CDC). Cervical Cancer Screening Among Women Aged 18–30 Years — United States, 2000–2010. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6151a2.htm. Accessed July 2014.
- 32.Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. J Low Genit Tract Dis. 2012;16(3):175–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention. National Survey of Family Growth (NSFG 2006–2010). http://www.cdc.gov/nchs/nsfg.htm. Accessed November 2013.
- 34.Haderxhanaj LT, Leichliter JS, Aral SO, et al. Sex in a lifetime: Sexual behaviors in the United States by lifetime number of sex partners, 2006–2010. Sex Transm Dis. 2014;41(6):345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey (NHANES). http://www.cdc.gov/nchs/nhanes.htm. Accessed November 2013.
- 36.Wheeler CM, Hunt WC, Cuzick J, et al. A population-based study of human papillomavirus genotype prevalence in the United States: baseline measures prior to mass human papillomavirus vaccination. Int J Cancer. 2013;132(1):198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoy T, Singhal PK, Willey VJ, et al. Assessing incidence and economic burden of genital warts with data from a US commercially insured population. Curr Med Res Opin. 2009;25(10):2343–2351. [DOI] [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention, National Cancer Institute. Centers for Disease Control and Prevention’s National Program of Cancer Registries and National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program.
- 39.Insinga RP, Glass AG, Rush BB. Diagnoses and outcomes in cervical cancer screening: a population-based study. Am J Obstet Gynecol. 2004;191(1):105–113. [DOI] [PubMed] [Google Scholar]
- 40.Watson M, Saraiya M, Benard V, et al. Burden of cervical cancer in the United States, 1998–2003. Cancer. 2008;113(10 Suppl):2855–2864. [DOI] [PubMed] [Google Scholar]
- 41.Camenga DR, Dunne EF, Desai MM, et al. Incidence of genital warts in adolescents and young adults in an integrated health care delivery system in the United States before human papillomavirus vaccine recommendations. Sex Transm Dis. 2013;40(7):534–538. [DOI] [PubMed] [Google Scholar]
- 42.Drolet M, Brisson M, Maunsell E, et al. The impact of anogenital warts on health-related quality of life: a 6-month prospective study. Sex Transm Dis. 2011;38(10):949–956. [DOI] [PubMed] [Google Scholar]
- 43.Woodhall S, Ramsey T, Cai C, et al. Estimation of the impact of genital warts on health-related quality of life. Sex Transm Infect. 2008;84(3):161–166. [DOI] [PubMed] [Google Scholar]
- 44.Drolet M, Brisson M, Maunsell E, et al. The psychosocial impact of an abnormal cervical smear result. Psychooncology. 2012;21(10):1071–1081. [DOI] [PubMed] [Google Scholar]
- 45.Insinga RP, Dasbach EJ, Myers ER. The health and economic burden of genital warts in a set of private health plans in the United States. Clin Infect Dis. 2003;36(11):1397–1403. [DOI] [PubMed] [Google Scholar]
- 46.Hu D, Goldie S. The economic burden of noncervical human papillomavirus disease in the United States. Am J Obstet Gynecol. 2008;198(5):500e1–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schabert VF, Ye X, Insinga RP, et al. Five-year routine cervical cancer screening rates and intervals in a US health plan. Curr Med Res Opin. 2008;24(9):2429–2435. [DOI] [PubMed] [Google Scholar]
- 48.Henk HJ, Insinga RP, Singhal PK, et al. Incidence and costs of cervical intraepithelial neoplasia in a US commercially insured population. J Low Genit Tract Dis. 2010;14(1):29–36. [DOI] [PubMed] [Google Scholar]
- 49.Insinga RP, Glass AG, Rush BB. The health care costs of cervical human papillomavirus-related disease. Am J Obstet Gynecol. 2004;191(1):114–120. [DOI] [PubMed] [Google Scholar]
- 50.Weiss T, Pillsbury M, Dasbach EJ. Potential health and economic impact of the 9-valent HPV vaccine in the United States. Abstract presented at the 29th International Papillomavirus Conference and Public Health & Clinical Workshops. Seattle, United Stated, August 21–25 2014. [Google Scholar]
- 51.Malagon T, Drolet M, Boily MC, et al. Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(10):781–789. [DOI] [PubMed] [Google Scholar]
- 52.Kreimer AR, Rodriguez AC, Hildesheim A, et al. Proof-of-principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 vaccine. J Natl Cancer Inst. 2011;103(19):1444–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dobson SR, McNeil S, Dionne M, et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA. 2013;309(17):1793–1802. [DOI] [PubMed] [Google Scholar]
- 54.Herweijer E, Leval A, Ploner A, et al. Association of varying number of doses of quadrivalent human papillomavirus vaccine with incidence of condyloma. JAMA. 2014;311(6):597–603. [DOI] [PubMed] [Google Scholar]
- 55.Laprise JF, Drolet M, Boily MC, et al. Comparing the cost-effectiveness of twoand three-dose schedules of human papillomavirus vaccination: a transmission-dynamic modelling study. Vaccine. 2014;32(44):5845–5853. [DOI] [PubMed] [Google Scholar]
- 56.Jit M, Brisson M, Laprise J, et al. Comparison of two dose and three dose human papillomavirus vaccine schedules: cost effectiveness analysis based on transmission model. BMJ. 2015;350:g7584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.World Health Organization. Summary of the SAGE April 2014 meeting. http://www.who.int/immunization/sage/meetings/2014/april/report_summary_april_2014/en/. Accessed November 2014.
- 58.National Institute of Health. A Phase III Study of a 2-dose Regimen of a Multivalent Human Papillomavirus (HPV) Vaccine (V503), Administered to 9 to 14 Year-olds and Compared to Young Women, 16 to 26 Years Old (V503–010). http://clinicaltrials.gov/show/NCT01984697. Accessed November 2014.
- 59.Statistics Canada. Canadian Community Health Survey (CCHS- Cycle 3.1). 2005 (January 2011). www.statcan.gc.ca. Accessed November 2014.
- 60.Wellings K, Collumbien M, Slaymaker E, et al. Sexual behaviour in context: a global perspective. Lancet. 2006;368(9548):1706–1728. [DOI] [PubMed] [Google Scholar]
- 61.Campos NG, Burger EA, Sy S, et al. An updated natural history model of cervical cancer: derivation of model parameters. Am J Epidemiol. 2014;180(5):545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.