Space flight presents a set of physiological challenges to the space explorer which result from the absence of gravity (or in the case of planetary exploration, partial gravity), radiation exposure, isolation and a prolonged period in a confined environment,2 distance from Earth, the environment of the destination, and numerous other factors. In the case of many organ systems, these challenges can result in significant adaptive changes that, while not necessarily pathological in terms of deal- ing with the new environment, may be deleterious upon return to a normal Earth gravity (1g) environment. By way of example, after space flight, there are cardiovascular problems, such as or- thostatic intolerance,3 and weight-bearing bones4 and muscles5 show significant dysfunction.
This narrative review discusses the greatest challenges to the lungs of future planetary explorers. It provides a synthesis of evidence from published literature in combination with knowl- edge gained from the author’s experience and active research into the effects of space flight on the human lung.
The lung is potentially vulnerable to the effects of partial grav- ity both by the nature of its intrinsic structure and because it presents a large surface area to the environment. The delicate structure of the lung means that it deforms under its own weight, resulting in significant differences in alveolar volume6 and ventilation7 between the top and bottom of the upright lung. Furthermore, the low perfusion pressures within the pulmonary circulation mean that blood flow is affected by hydrostatic gra- dients within the vasculature.8 As a consequence, the heteroge- neity of ventilation–perfusion ratio (V/Q) (the principal cause of arterial hypoxaemia) on Earth is markedly different between the top and bottom of the lung.9 Removing these gravitational effects would be expected to significantly alter lung function in partial or zero gravity.
Extravehicular activity (EVA), or spacewalk, presents a direct challenge to the lung through the low pressure environment of modern spacesuits. Further, in the context of space exploration beyond low Earth orbit, be it the moon, a near-Earth object (aster- oid or comet), or Mars, surface activities bring with them the risk of exposure to inhaled particulate matter, as do incidents within a spacecraft itself, such as the fire that occurred on the Russian space station Mir in 1997. Radiation exposure, especially in combination with high inspired oxygen (O2) levels may also be im- portant,10 but there are no published studies in humans to date.
Low gravity and lung function
Early space flight
The earliest measurements of pulmonary function in space flight date back to the early 1970s during the Skylab series of flights. The principal measurement was that of vital capacity. Data from an 84-day period in zero gravity on Skylab 4 showed about 10% reduction in vital capacity compared with before and after flight in the three crew members.11 However, because of the physical structure of the Skylab space station (it was built in the fuel tank of a rocket upper stage), the absolute cabin pressure was only 258 mmHg, and in order to avoid severe hypoxia, the fraction of inspired oxygen (Fio2) was 0.70 (70%). Ground cham- ber tests with a comparable atmosphere also showed a similar reduction in vital capacity,12 likely through the development of some atelectasis.
Short duration space flight
It was not until 1991, when the Spacelab Life Sciences-1 (SLS-1) mission flew, that measurements could be made in a normoxic, normobaric environment (ie, 21% O2, < 0.5% carbon dioxide [CO2], cabin pressure 760 mmHg, and < 50% relative humidity). Those measurements showed that while vital capacity was reduced by about 10% after 24 hours in zero gravity compared with that measured standing in 1g,13 after 4–9 days in zero gravity, vital capacity had returned to its pre-flight value. The interpretation was that, early in flight, the headward translocation of circulat- ing blood into the thorax occupied space that was normally part of the vital capacity, but that after a downward adjustment of circulating blood volume, vital capacity returned to that present on the ground. Functional residual capacity in zero gravity was seen to be intermediate to that in the upright and supine position in 1g in keeping with the forces on the abdominal contents and diaphragm.13 Residual volume was reduced somewhat (~ 18%), likely due to more uniform emptying of the lungs.13
SLS-1 and subsequent flights included studies of numerous aspects of lung function (Box 1). As expected from the delicate structure of the lung, there were marked reductions in the het- erogeneity of both ventilation18 and perfusion,27 as inferred from variants of single breath washout tests. One of the more important results was that while both ventilation and perfu- sion became much more uniform in zero gravity, the matching between them (V/Q) did not become more uniform, remaining comparable to that seen on the ground.28 While this may seem counterintuitive, it points to the intrinsic spatial interdepen- dence of ventilation and perfusion: anatomical heterogeneity in one pathway (airways or vasculature) is likely matched in the other by the parallel nature of the airway and pulmonary arte- rial tree40 (Box 2). As such, from a gas exchange efficiency stand- point, the normal lung behaves much the same in zero gravity as in 1g, a result that serves to refute a previous anecdotal report of significant gas exchange impairment in-flight aboard Mir.41
1. Lung function measurements performed in zero gravity.
| Topic | Key results | References |
|---|---|---|
| Lung volumes | Vital capacity unaltered after an initial reduction | 13 |
| Functional residual capacity intermediate between standing and supine positions | ||
| Residual volume reduced | ||
| Forced spirometry | Modest changes possibly consistent with increased lung water | 14,15 |
| Cardiac output | Increased initially with subsequent decline | 16,17 |
| Diffusing capacity | Sustained increase | 16 |
| Increases in both components (membrane diffusing capacity and pulmonary capillary blood volume) | ||
| Heterogeneity of ventilation | Reduced in vital capacity breaths | 18,19-22 |
| Minimal changes in tidal volume breaths | ||
| Ventilation heterogeneity in lung periphery | Altered in sustained but not transient zero gravity | 23-26 |
| Heterogeneity of perfusion | Reduced but not absent | 27 |
| Gas exchange and ventilation–perfusion matching | Largely unchanged in zero gravity | 28,29 |
| Control of ventilation | Reduction in hypoxic but not hypercapnic ventilatory responses | 30,31 |
| Sleep disordered breathing | Evidence for a reduction in zero gravity | 32,33 |
| Heart rate variability | Unaltered respiratory sinus arrhythmia in-flight, reduced post-flight | 34 |
| Chest wall mechanics | Increase in abdominal contribution to breathing | 33,35 |
| Extravehicular activity | No pulmonary disruption | 36 |
| Long duration zero gravity | Minimal sustained changes post-flight | 37,38 |
The reader is referred to the primary articles (cited in the table) and to a more extensive review39 for details of these studies.
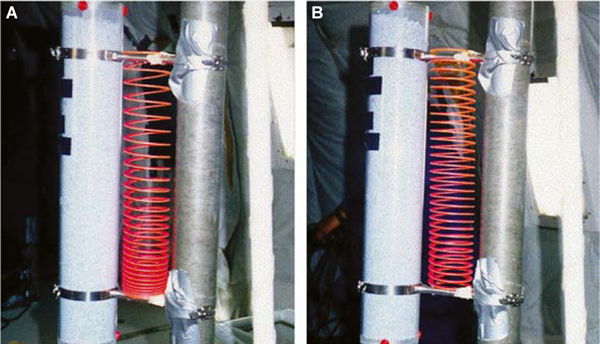
2. A slinky spring provides a useful analogy for the mechanical effects of the removal of gravity on the human lung.
Panel A shows the spring under the effects of gravity. The spring is deformed by its own weight so that coils at the top are farther apart and those at the bottom are closer together. The space between the coils can be thought of as a surrogate for regional lung volume, with the more dependent part of the lung having a lower regional lung volume than the non-dependent. Regional ventilation can be thought of as the change in the separation of the coils as the spring is stretched. As the lower part of the spring is less expanded than the upper part, it is more able to respond to a stretch, and so ventilation to the dependent lung is greater than to the non-dependent lung. Perfusion can be thought of as blood flowing through the spring itself. Because the coils are closer together at the bottom of the lung, regional perfusion is greater in the dependent lung than in the non-dependent lung. Panel B shows the same spring in zero gravity. The coils are now uniformly separated, and both ventilation and perfusion are more uniform than under gravity. However, because the deformation of the spring affects both ventilation and perfusion, the matching between the two is not greatly altered, mimicking the minimal change seen in ventilation–perfusion ratio matching in space flight.
Other important aspects relating to gas exchange were clearly altered by zero gravity, but not in a deleterious manner. While not strictly a pulmonary variable, cardiac output is important because the lung receives virtually 100% of the right heart out- put. Early in flight there was a marked rise (~ 30%) in cardiac output compared with upright position in 1g, rising to about the cardiac output levels of subjects who were acutely supine in 1g.16 However, after a few days, as circulating blood volume was ad- justed downwards, reducing venous return, cardiac output fell to values nearer to that seen pre-flight. That of course sets the scene for a marked reduction in cardiac output upon return to 1g, a major contributor to post-flight orthostatic intolerance im- mediately after landing.
Diffusing capacity of the lung (measured using a single breath carbon monoxide technique) rose by about 28% and remained elevated for the duration of exposure to zero gravity. This variation was due to simultaneous and sustained increases in both the amount of blood in the pulmonary capillaries and the membrane surface area available for gas exchange.16 This was interpreted to be the result of the abolition of the hydrostatic gra- dients in the pulmonary vasculature, with the result that, unlike the situation in 1g where the uppermost part of the lung is rela- tively poorly perfused,42 the entire pulmonary vasculature was fully recruited and participated in gas exchange.
It had long been speculated that zero gravity would result in an increase in transudation of fluid through the pulmonary capillaries, with the possible consequence of the development of pulmonary oedema. The observed increase in the diffusing capacity for carbon monoxide argues against significant alveolar oedema, as does the absence of an increase in lung tissue volume.44 However, single breath washout tests using trace amounts of helium and sulfur hexafluoride, which probe the lung structure near the acinar entrance45 (the entrance to an independent gas exchange unit of the lung), suggested the possible development of pulmonary interstitial oedema.23 The effect was not seen in the transient zero gravity of parabolic flight (~ 25 seconds),24 even though the study included one of the space flight subjects. Thus, the effect was not simply one of lung distortion brought about by the removal of gravity, but rather, it was caused by something that took longer than 25 seconds to develop. It remains unclear whether these studies indicate some reduced margin of safety for the development of pulmonary oedema that might impair gas exchange in zero gravity.
Long duration space flight
All of the results that came from the shuttle era pertain to rela- tively short exposure to zero gravity, with the longest exposure being 17 days. To expand that exposure duration, a limited number of studies were performed in the first few years of the International Space Station (ISS), with exposure times of 130–196 days. Much more extensive measurements were made on multi- ple occasions before flight and following return to 1g. Similar to that seen in the shuttle flights, heterogeneity of V/Q was largely unchanged, gas exchange was preserved and, overall, the lung appeared to be able to function perfectly well in long duration zero gravity.38 Vital capacity was unchanged over the course of the flight, and respiratory muscle strength was preserved throughout the flight.37
The pre-flight versus post-flight comparison of over ten key variables showed that there were changes in a few variables in the first week back in 1g after about 6 months in zero gravity.38 Interestingly, the speculated possible marker of interstitial oedema was still present. However, by one week after about 6 months in zero gravity, there were no significant changes that persisted in any of these variables. This result is important in the context of future long duration exposure to zero gravity that will be part of any exploration class mission (a mission beyond low Earth orbit). While these exposure times are still much less than those that might be experienced in missions such as a visit to Mars, in which the duration would likely be about 2 years, the rapid recovery to pre-flight conditions suggests that, provided the zero gravity exposure is under normoxic and normobaric conditions, the lung can be expected to function well and to not suffer deleterious consequences.
Decompression
Space flight and, in particular, exploration class missions bring with them significant operational challenges that extend beyond just being in partial gravity, including EVA. Mission models for a 6-month stay on the moon call for surface activities (including EVA) 5 days a week. All current EVA suits operate at a very low absolute pressure (220–290 mmHg; 100% O2) in order to maintain ad- equate mobility, and the engineering challenges of a high pressure suit are sufficiently great that this low pressure design will likely persist. As such, there is a significant risk of decompression illness in going from the 760 mmHg and about 79% nitrogen (N2) at-mosphere inside the ISS to the suit,46 as N2 dissolves in the blood and comes out of solution, forming bub- bles. In order to avoid decompression illness, there is an extensive and time-consuming denitrogenation protocol undertaken as the preparatory steps to an EVA. However, despite this, ground studies in hy- pobaric chambers have shown about 50% incidence of venous gas emboli, and about 24% incidence of clinical decompression illness,47 although there are no recorded cases of decompression illness associated with EVA. Venous gas emboli are filtered by the pulmonary vasculature, and studies of saturation divers after surfacing have found increased hetero- geneity of V/Q in the lung.48
As part of the research on the ISS, a study was per- formed to address whether there was disruption to gas exchange in the lung following EVA. Such a dis- ruption would be expected if the denitrogenation protocol was ineffective at preventing venous gas em- boli. The experiment measured the heterogeneity of pulmonary perfusion and of V/Q before and on the day following EVA.49 The result showed that there were no persisting effects of the EVA on the following day, which is opera- tionally important as it shows that current EVA denitrogenation protocols serve to protect the lung to an adequate degree.
The fact that the post-EVA measurements were performed on the day following EVA raises an important point for future exploration class missions. The delay in the post-EVA mea- surements was driven by the long (~ 4 hours) denitrogenation protocol required before EVA, making the work day so long that the measurements had to be postponed to the following day. In the context of a mission model with five EVAs a week, a 4-hour denitrogenation protocol is a huge mission overhead. However, this can be reduced by appropriate choice of the hab- itat (living quarters) atmosphere. The core target here is to reduce the N2 partial pressure in the habitat, all while avoiding excessive hypoxia (which would result from the reduction in absolute pressure) and avoiding excessive fire risk from rais- ing the oxygen fraction too high in order to minimise hypoxia. A compromise habitat atmosphere of about 392 mmHg abso- lute pressure, Fio2 0.32, with an equivalent altitude in terms of the inspired partial pressure of O2 (Pio2) of about 6500 ft (~ 2000 m) offers a pre-EVA denitrogenation time of much less than one hour.50 How the lung will respond to this non- standard atmosphere is unknown; however, the Fio2 0.32 is much less than the Fio2 0.70 used in Skylab, and is a much smaller stress in terms of the development of atelectasis that was thought to be the genesis of the vital capacity changes seen on Skylab.
Inhaled aerosols in low gravity
The Apollo series of lunar landings showed clearly that, although surface exploration occurs within the confines of a sealed spacesuit, exposure to surface dust remains an issue. The dust sticks to the surface of the suit, is difficult to remove and is tracked into the cabin interior of the spacecraft. Upon doffing of the suit, the crew are inevitably exposed to the dust, and the nuisance it imposed was a universal complaint in every crew debrief of each Apollo landing mission (Box 3). Furthermore, the dust itself contains a significant fine fraction in the respirable range, with about 10% being less than 10 μm in diameter.51 Since in 1g particles between about 8 μm and 0.5 μm in diameter deposit primarily by gravitational sedimen- tation, aerosol transport and deposition in partial gravity are likely to be significantly altered. In terms of the risks associ- ated with exposure to this dust, the amount and site of deposi- tion is important, as is the nature of the dust itself in terms of potential toxicity.52 The crews of the Apollo landing missions have not presented evidence of dust-associated disease (eg, silicosis), but the population is small and the exposure times quite limited, with the longest surface stay being only about 72 hours.
3. Lunar exploration (and similarly, Mars exploration) results in significant exposure to surface dust.
(A) The surface dust on the moon is extremely fine-grained, as evidenced by the detail seen in the boot print. (B) Cloud of fine dust particles surrounding the astronaut while on the lunar surface. (C) The dust sticks to the outside of the extravehicular activity suits. (D) Eugene Cernan (1934–2017), commander of Apollo 17, inside the lunar module on the lunar surface after doffing his spacesuit. Note the dust on his face and clothing. There was a noticeable smell upon removing the helmet of the suit, described as similar to that of a gun being fired (https://science.nasa.gov/science-news/science-at-nasa/2006/30jan_smellofmoondust). All Apollo crews who landed on the moon commented on the problems of dust. Photo credits: National Aeronautics and Space Administration (NASA) — public domain.

Deposition
Unlike the studies discussed above, all of the information on aerosol transport and deposition in the lung has been derived from studies performed in transient zero or partial gravity in parabolic flights aboard research aircraft. This platform provides short periods of zero gravity (20–25 seconds) and slightly longer periods of partial gravity (~ 35 seconds in lunar gravity).53
The first studies of total deposition54 examined the deposition of 2 μm particles in 1g in zero gravity and in the periods of hypergravity afforded as part of parabolic flight.53 They showed a predictable lin- ear relationship in deposition as a function of gravity, consistent with gravitational sedimentation being the dominant mechanism. Subsequent studies55 showed an unexpectedly high deposition of 1 μm particles in zero gravity,56 suggesting another mechanism that was serving to raise deposition. A theoretical and in vitro study57 provided a possible explanation by point- ing out that because of the complicated nature of the flow streamlines within the airway tree, streamlines that were initially widely separated could be brought into close apposition with each other, resulting in a very short mixing distance, enhancing deposition. The effect was termed “stretch and fold”, based on the repeated stretching and folding of a sheet of pastry that serves to bring normally widely separated points into close proximity. Attempts to verify this experi- mentally58 suggested that the mechanism occurred within a single breath, leading to the deposition of particles of about 1 μm in size being higher than would be expected — an important point in terms of the risks this might pose in space exploration.52
Clearance
Another important factor in the context of potential tox- icity is how long the deposited particles remain in the lungs before being removed by their clearance mecha- nisms. Much of this depends on where in the airway tree the deposition occurs. The more central airways are lined with a ciliated epithelium that works to effec- tively transport deposited material mouthward, where it is ultimately eliminated. As such, residence time of particles in the ciliated airways is only in the order of hours to days.59 However, for particles that penetrate further into the airway tree, beyond the ciliated region, the residence time is vastly longer, with a timescale of months.60 Studies showed that in lunar gravity the amount of a 1 μm aerosol that deposited was reduced compared with that in 1g, but did so at a more peripheral site of deposition.61 In essence, gravity serves to protect the lung periphery by causing deposition to occur in the ciliated airways. In the absence of gravity, those particles that would otherwise have deposited by gravitational sedimentation remain in suspension and are able to be carried deeper into the airway tree, where they eventually deposit. These studies are consistent with a study that used about 1 μm ferric oxide particles administered during the zero gravity portion of parabolic flight to spontaneously breath- ing rats. The deposition was subsequently measured in the ex- cised and fixed lungs using magnetic resonance imaging.62 Taken together, these studies suggest that the relative deposition of these small particles occurs more peripherally in partial gravity than in 1g. The implication is that the residence time of those deposited particles would therefore be higher when the deposition occurred in partial gravity, but this has never been directly measured.
Toxicity
Taken overall, these studies serve to suggest that deposition of inhaled aerosol in zero or partial gravity will be reduced; how- ever, for the fine size fraction (~ 1 μm), deposition will likely occur in more peripheral locations in the airway tree. As a direct consequence, it is likely that the residence time of these depos- ited particles will be longer, which may raise their toxicological potential. How great such an effect is will depend on the na- ture of the particles.52 In the case of lunar dust, there is a sig- nificant concern that the lack of any atmosphere means that the dust generated by meteoric bombardment over millennia will remain highly reactive and will thus have a high toxicologi- cal potential. In the terrestrial environment, freshly fractured crystalline silica is considered a major inhalation hazard, and the concern is that the unweathered lunar dust may be similar. While toxicological studies of lunar dust show it to be of only modest toxicological potential,63 there have been no studies of unweathered lunar dust. Samples that have been studied have all been exposed to oxygen and humidity, likely reducing their surface chemical activity. By comparison, Martian dust has been exposed to an atmosphere (albeit a rather thin atmosphere), al- though there remain other concerns, such as the presence of perchlorate in Martian dust.64 Little is known about other ex- traterrestrial dust sources (comets and asteroids). Answering these concerns will require either on-surface studies or sample return missions with appropriate sample handling.
Conclusion
Studies show that while there are changes in lung function in partial or zero gravity, the lung continues to function well in this novel environment. There is limited evidence to suggest that the water content of the lung, presumably in the form of interstitial oedema, may be slightly elevated in zero gravity. This does not seem to produce any impairment in gas exchange, and while it might be hypothesised that this reduces the safety margin of the lung to an insult, there is no direct evidence to support or refute this concept.
From the standpoint of space flight operations, EVA presents a potentially large challenge because of the change in absolute pressure going from the cabin environment to that of the suit. However, the available evidence suggests that the current de- nitrogenation protocols in the ISS are effective at avoiding neg- ative consequences for the lung. Appropriate engineering of the atmosphere of an exploration habitat can greatly reduce the risk of a significant decompression stress that would have the poten- tial to affect the lung, and the necessary increase in Fio2 (0.32 at a reduced absolute pressure) can probably be well tolerated by the lung.
But perhaps the biggest challenge to the lung in the realm of exploration (including activities on celestial bodies) is the most prosaic: dust. The Apollo experience showed that exposure to dust was unavoidable and, despite a diligent application of en- gineering to minimise this risk, dust exposure seems almost inevitable. The nature of extraterrestrial dust will depend on where the exploration occurs, and it seems likely that the moon (and perhaps near-Earth objects) will present the largest chal- lenge because they lack an atmosphere that has the potential to chemically passivate the dust. What is universal is that partial gravity environments will serve to alter the location and amount of deposited aerosol and quite likely increase the residence time of deposited particles in the lung. Combined with the potential of a reactive dust species, this may prove to be the largest routine challenge to the lung in future space exploration.
Summary.
Space flight presents a set of physiological challenges to the space explorer which result from the absence of gravity (or in the case of planetary exploration, partial gravity), radiation exposure, isolation and a prolonged period in a confined environment, distance from Earth, the need to venture outside in the hostile environ- ment of the destination, and numerous other factors.
Gravity affects regional lung function, and the human lung shows considerable alteration in function in low gravity; however, this alteration does not result in deleterious changes that compromise lung function upon return to Earth.
The decompression stress associated with extravehicular activity, or spacewalk, does not appear to compromise lung function, and future habitat (living quarter) designs can be engineered to mini- mise this stress.
Dust exposure is a significant health hazard in occupational settings such as mining, and exposure to extraterrestrial dust is an almost inevitable consequence of planetary exploration. The com- bination of altered pulmonary deposition of extraterrestrial dust and the potential for the dust to be highly toxic likely makes dust exposure the greatest threat to the lung in planetary exploration.
Acknowledgements:
Many of the studies were funded by the National Aeronautics and Space Administration (NASA) and the National Space Biomedical Research Institute under various contracts and grants. G Kim Prisk is currently funded by the National Institutes of Health under R01 HL119263.
Footnotes
Competing interests: No relevant disclosures.
Provenance: Commissioned; externally peer reviewed.
References
- 1.Chancellor JC, Blue RS, Cengel KA, et al. Limitations in predicting the space radiation health risk for exploration astronauts. NPJ Microgravity 2018; 4: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yi B, Matzel S, Feuerecker M, et al. The impact of chronic stress burden of 520-d isolation and confinement on the physiological response to subsequent acute stress challenge. Behav Brain Res 2015; 281: 111–115. [DOI] [PubMed] [Google Scholar]
- 3.Perhonen MA, Franco F, Lane LD, et al. Cardiac atrophy after bed rest and spaceflight. J Appl Physiol 2001; 91: 645–653. [DOI] [PubMed] [Google Scholar]
- 4.Lang T, Van Loon JJWA, Bloomfield S, et al. Towards human exploration of space: the THESEUS review series on muscle and bone research priorities. NPJ Microgravity 2017; 3: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Widrick JJ, Knuth ST, Norenberg KM, et al. Effect of a 17 day spaceflight on contractile properties of human soleus muscle fibres. J Physiol 1999; 516: 915–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glazier JB, Hughes JM, Maloney JE, West JB. Vertical gradient of alveolar size in lungs of dogs frozen intact. J Appl Physiol 1967; 23: 694–705. [DOI] [PubMed] [Google Scholar]
- 7.Bryan AC, Milic-Emili J, Pengelly D. Effect of gravity on the distribution of pulmonary ventilation. J Appl Physiol 1966; 21: 778–784. [DOI] [PubMed] [Google Scholar]
- 8.West JB, Dollery CT, Naimark A. Distribution of blood flow in isolated lung: relation to vascular and alveolar pressures. J Appl Physiol 1964; 19: 713–724. [DOI] [PubMed] [Google Scholar]
- 9.West JB. Pulmonary gas flow and gas exchange In: West JB, editor. Respiratory physiology:people and ideas. New York: Oxford Press, 1966:140–196. [Google Scholar]
- 10.Christofidou-Solomidou M, Pietrofesa RA, Arguiri E, et al. Space radiation-associated lung injury in a murine model. Am J Physiol Lung Cell Mol Physiol 2015; 308: L416–L428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sawin CF, Nicogossian AE, Rummel JA, Michel EL. Pulmonary function evaluation during the Skylab and Apollo-Soyuz missions. Aviat Space Environ Med 1976; 47: 168–172. [PubMed] [Google Scholar]
- 12.Robertson WG, McRae GL. Study of man during a 56-day exposure to an oxygen-helium atmosphere at 258 mm Hg total Pressure. VII. Respiratory function. Aerosp Med 1966; 37:453–456. [PubMed] [Google Scholar]
- 13.Elliott AR, Prisk GK, Guy HJ, West JB. Lung volumes during sustained microgravity on spacelab SLS-1. J Appl Physiol 1994; 77:2005–2014. [DOI] [PubMed] [Google Scholar]
- 14.Guy HJ, Prisk GK, Elliott AR, West JB. Maximum expiratory flow-volume curves during short periods of microgravity. J Appl Physiol 1991; 70:2587–2596. [DOI] [PubMed] [Google Scholar]
- 15.Elliott AR, Prisk GK, Guy HJ, et al. Forced expirations and maximum expiratory flow- volume curves during sustained microgravity on SLS-1. J Appl Physiol 1996; 81: 33–43. [DOI] [PubMed] [Google Scholar]
- 16.Prisk GK, Guy HJ, Elliott AR, et al. Pulmonary diffusing capacity, capillary blood volume, and cardiac output during sustained microgravity. J Appl Physiol 1993; 75: 15–26. [DOI] [PubMed] [Google Scholar]
- 17.Prisk GK, Fine JM, Elliott AR, West JB. Effect of 6 degrees head-down tilt on cardiopulmonary function: comparison with microgravity. Aviat Space Environ Med 2002; 73: 8–16. [PubMed] [Google Scholar]
- 18.Guy HJ, Prisk GK, Elliott AR, et al. Inhomogeneity of pulmonary ventilation during sustained microgravity as determined by single-breath washouts. J Appl Physiol 1994; 76: 1719–1729. [DOI] [PubMed] [Google Scholar]
- 19.Prisk GK, Guy HJB, Elliott AR, et al. Ventilatory inhomogeneity determined from multiple- breath washouts during sustained microgravity on Spacelab SLS-1. J Appl Physiol 1995; 78:597–607. [DOI] [PubMed] [Google Scholar]
- 20.Verbanck S, Linnarsson D, Prisk GK, Paiva M. Specific ventilation distribution in microgravity. J Appl Physiol 1996; 80: 1458–1465. [DOI] [PubMed] [Google Scholar]
- 21.Prisk GK, Elliott AR, Guy HJ, et al. Multiple- breath washin of helium and sulfur hexafluoride in sustained microgravity. J Appl Physiol 1998;84: 244–252. [DOI] [PubMed] [Google Scholar]
- 22.Dutrieue B, Verbanck S, Darquenne C, Prisk GK. Airway closure in microgravity. Respir Physiol Neurobiol 2005; 148: 97–111. [DOI] [PubMed] [Google Scholar]
- 23.Prisk GK, Lauzon AM, Verbanck S, et al. Anomalous behavior of helium and sulfur hexafluoride during single-breath tests in sustained microgravity. J Appl Physiol 1996; 80:1126–1132. [DOI] [PubMed] [Google Scholar]
- 24.Lauzon AM, Prisk GK, Elliott AR, et al. Paradoxical helium and sulfur hexafluoride single-breath washouts in short-term vs. sustained microgravity. J Appl Physiol 1997; 82:859–865. [DOI] [PubMed] [Google Scholar]
- 25.Dutrieue B, Lauzon AM, Verbanck S, et al. Helium and sulfur hexafluoride bolus washin in short-term microgravity. J Appl Physiol 1999; 86:1594–1602. [DOI] [PubMed] [Google Scholar]
- 26.Dutrieue B, Paiva M, Verbanck S, et al. Tidal volume single breath washin of SF6 and CH4 in transient microgravity. J Appl Physiol 2003; 94:75–82. [DOI] [PubMed] [Google Scholar]
- 27.Prisk GK, Guy HJB, Elliott AR, West JB. Inhomogeneity of pulmonary perfusion during sustained microgravity on SLS-1. J Appl Physiol 1994; 76: 1730–1738. [DOI] [PubMed] [Google Scholar]
- 28.Prisk GK, Elliott AR, Guy HJ, et al. Pulmonary gas exchange and its determinants during sustained microgravity on Spacelabs SLS-1 and SLS-2. J Appl Physiol 1995; 79: 1290–1298. [DOI] [PubMed] [Google Scholar]
- 29.Lauzon AM, Elliott AR, Paiva M, et al. Cardiogenic oscillation phase relationships during single- breath tests performed in microgravity. J Appl Physiol 1998; 84: 661–668. [DOI] [PubMed] [Google Scholar]
- 30.Elliott AR, Prisk GK, Schöllman C, Hoffman U. Hypercapnic ventilatory response in humans before, during, and after 23 days of low level CO2 exposure. Aviat Space Environ Med 1998; 69:391–396. [PubMed] [Google Scholar]
- 31.Prisk GK, Elliott AR, West JB. Sustained microgravity reduces the human ventilatory response to hypoxia but not hypercapnia. J Appl Physiol 2000; 88: 1421–1430. [DOI] [PubMed] [Google Scholar]
- 32.Dijk DJ, Neri DF, Wyatt JK, et al. Sleep, performance, circadian rhythms, and light-dark cycles during two space shuttle flights. Am J Physiol Regul Integr Comp Physiol 2001; 281: R1647–R1664. [DOI] [PubMed] [Google Scholar]
- 33.Sá RC, Prisk GK, Paiva M. Microgravity alters respiratory abdominal and rib cage motion during sleep. J Appl Physiol 2009; 107: 1406–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Migeotte PF, Prisk GK, Paiva M. Microgravity alters respiratory sinus arrhythmia and short- term heart rate variability in humans. Am J Physiol Heart Circ Physiol 2003; 284: H1195–H2006. [DOI] [PubMed] [Google Scholar]
- 35.Wantier M, Estenne M, Verbanck S, et al. Chest wall mechanics in sustained microgravity. J Appl Physiol 1998; 84: 2060–2065. [DOI] [PubMed] [Google Scholar]
- 36.Prisk GK, Fine JM, Cooper TK, West JB. Pulmonary gas exchange is not impaired 24-hours following extra-vehicular activity. J Appl Physiol 2005; 99:2233–2238. [DOI] [PubMed] [Google Scholar]
- 37.Prisk GK, Fine JM, Cooper TK, West JB. Vital capacity, respiratory muscle strength, and pulmonary gas exchange during long-duration exposure to microgravity. J Appl Physiol 2006; 101:439–447. [DOI] [PubMed] [Google Scholar]
- 38.Prisk G, Fine J, Cooper T, West J. Lung function is unchanged in the 1 G environment following 6-months exposure to microgravity. Eur J Appl Physiol 2008; 103: 617–623. [DOI] [PubMed] [Google Scholar]
- 39.Prisk GK. Microgravity and the respiratory system. Eur Respir J 2014; 43: 1459–1471. [DOI] [PubMed] [Google Scholar]
- 40.Weibel ER. Morphometry of the human lung. New York: Academic Press, 1963. [Google Scholar]
- 41.Haase H, Baranov VM, Asyamolova NM, et al. First results of Po2 examinations in the capillary blood of cosmonauts during a long-term flight in the space station “Mir”. International Astronautical Congress and International Astronautical Federation; 1990. Proceedings of the 41st Congress of the International Astronautical Federation; Dresden (Germany), 6–12 October 1990. Paris, France: International Astronautical Federation; pp. 1–4. [Google Scholar]
- 42.West JB, Dollery CT. Distribution of blood flow and ventilation-perfusion ratio in the lung, measured with radioactive CO2. J Appl Physiol 1960; 15: 405–410. [DOI] [PubMed] [Google Scholar]
- 43.Permutt S Pulmonary circulation and the distribution of blood and gas in the lungs Physiology in the space environment. Washington: National Academy of Science, National Research Council 1485B; 1967. pp. 38–56. [Google Scholar]
- 44.Verbanck S, Larsson H, Linnarsson D, et al. Pulmonary tissue volume, cardiac output, and diffusing capacity in sustained microgravity. J Appl Physiol 1997; 83: 810–816. [DOI] [PubMed] [Google Scholar]
- 45.Van Muylem A, Antoine M, Yernault JC, et al. Inert gas single-breath washout after heart-lung transplantation. Am J Respir Crit Care Med 1995;152: 947–952. [DOI] [PubMed] [Google Scholar]
- 46.Conkin J, Klein JS, Acock KE. Description of 103 cases of hypobaric sickness from NASA- sponsored research (1982–1999). Houston, TX: Johnson Space Center; 2003. Report No. NASA/ TM-2003–212052. https://ston.jsc.nasa.gov/collections/TRS/_techrep/TM-2003-212052.pdf (viewed July 2019). [Google Scholar]
- 47.Waligora JM, Horrigan D, Conkin J, Hadley AT. Verification of an altitude decompression sickness prevention protocol for shuttle operations utilizing a 10.2-psi pressure stage. NASA Technical Memorandum 58259. Houston, TX: NASA, 1984; pp. 1–44. https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/19840020323.pdf (viewed July 2019). [Google Scholar]
- 48.Cotes JE. Lung function assessment and application in medicine; 5th ed Boston: Oxford Blackwell Scientific Publications, 1993: 251–262. [Google Scholar]
- 49.Prisk GK, Fine JM, Cooper TK, West JB. Pulmonary gas exchange is not impaired 24 h af ter extravehicular activity. J Appl Physiol 2005; 99:2233–2238. [DOI] [PubMed] [Google Scholar]
- 50.NASA Exploration Atmospheres Working Group. Recommendations for exploration spacecraft internal atmospheres: the final report of the NASA Exploration Atmospheres Working Group. NASA; 2010. Contract No. NASA/TP-2010–216134. https://ston.jsc.nasa.gov/collections/trs/_techrep/TP-2010-216134.pdf (viewed July 2019). [Google Scholar]
- 51.Graf JC. Lunar soils grain size catalog. Report No. NASA-RP-1265. NASA, 1993. https://ntrs.nasa. gov/archive/nasa/casi.ntrs.nasa.gov/19930012474.pdf (viewed July 2019). [Google Scholar]
- 52.Linnarsson D, Carpenter J, Fubini B, et al. Toxicity of lunar dust. Planet Space Sci 2012; 74: 57–71. [Google Scholar]
- 53.Karmali F, Shelhamer M. The dynamics of parabolic flight: flight characteristics and passenger percepts. Acta Astronaut 2008; 63:594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoffman RA, Billingham J. Effect of altered G levels on deposition of particulates in the human respiratory tract. J Appl Physiol 1975; 38: 955–960. [DOI] [PubMed] [Google Scholar]
- 55.Darquenne C, West JB, Prisk GK. Deposition and dispersion of 1-μm aerosol boluses in the human lung: effect of micro- and hypergravity. J Appl Physiol 1998; 85: 1252–1259. [DOI] [PubMed] [Google Scholar]
- 56.Darquenne C, Paiva M, West JB. Prisk GK. Effect of microgravity and hypergravity on deposition of 0.5- to 3-μm-diameter aerosol in the human lung. J Appl Physiol 1997; 83: 2029–2036. [DOI] [PubMed] [Google Scholar]
- 57.Butler JP, Tsuda A. Effect of convective stretching and folding on aerosol mixing deep in the lung, assessed by approximate entropy. J Appl Physiol 1998; 83: 800–809. [DOI] [PubMed] [Google Scholar]
- 58.Darquenne C, Prisk GK. Effect of small flow reversals on aerosol mixing in the alveolar region of the human lung. J Appl Physiol 2004; 97: 2083–2089. [DOI] [PubMed] [Google Scholar]
- 59.Bennett WD, Chapman WF, Lay JC, Gerrity TR. Pulmonary clearance of inhaled particles 24 to 48 hours post deposition: effect of beta-adrenergic stimulation. J Aerosol Med 1993; 6: 53–62. [Google Scholar]
- 60.Moller W, Häussinger K, Winkler-Heil R, et al. Mucociliary and long-term particle clearance in the airways of healthy nonsmoker subjects. J Appl Physiol. 2004; 97: 2200–2206. [DOI] [PubMed] [Google Scholar]
- 61.Darquenne C, Prisk G. Deposition of inhaled particles in the human lung is more peripheral in lunar than in normal gravity. Eur J Appl Physiol 2008; 103: 687–695. [DOI] [PubMed] [Google Scholar]
- 62.Darquenne C, Borja MG, Oakes JM, et al. Increase in relative deposition of fine particles in the rat lung periphery in the absence of gravity. J Appl Physiol 2014; 117: 880–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lam CW, Scully RR, Zhang Y, et al. Toxicity of lunar dust assessed in inhalation-exposed rats. Inhal Toxicol 2013; 25: 661–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Levine JS, Winterhalter D, Kerschmann RL. Dust in the atmosphere of mars and its impact on human exploration. Cambridge: Cambridge Scholars Publishing, 2018. [Google Scholar]


