Neurocysticercosis (NCC) is a major cause of neurologic disease and epilepsy worldwide. Clinical manifestations of NCC are primarily due to the inflammatory response against degenerating cysts of the Taenia solium tapeworm, which occurs when cysts lose their ability to evade host immune responses. Patients with subarachnoid or severe parenchymal NCC may also develop chronic inflammation requiring long-term immunosuppression.2
Corticosteroids are first-line anti-inflammatory agents in NCC; however, prolonged treatment is associated with multiorgan toxicity. While recent guidelines recommend methotrexate (MTX) as a steroid-sparing agent (SSA), when to initiate SSAs and alternatives to MTX in the case of failure or intolerance remain unspecified.1 Here, we describe 3 patients followed at a single hospital for NCC-associated inflammation requiring SSAs.
Patient 1
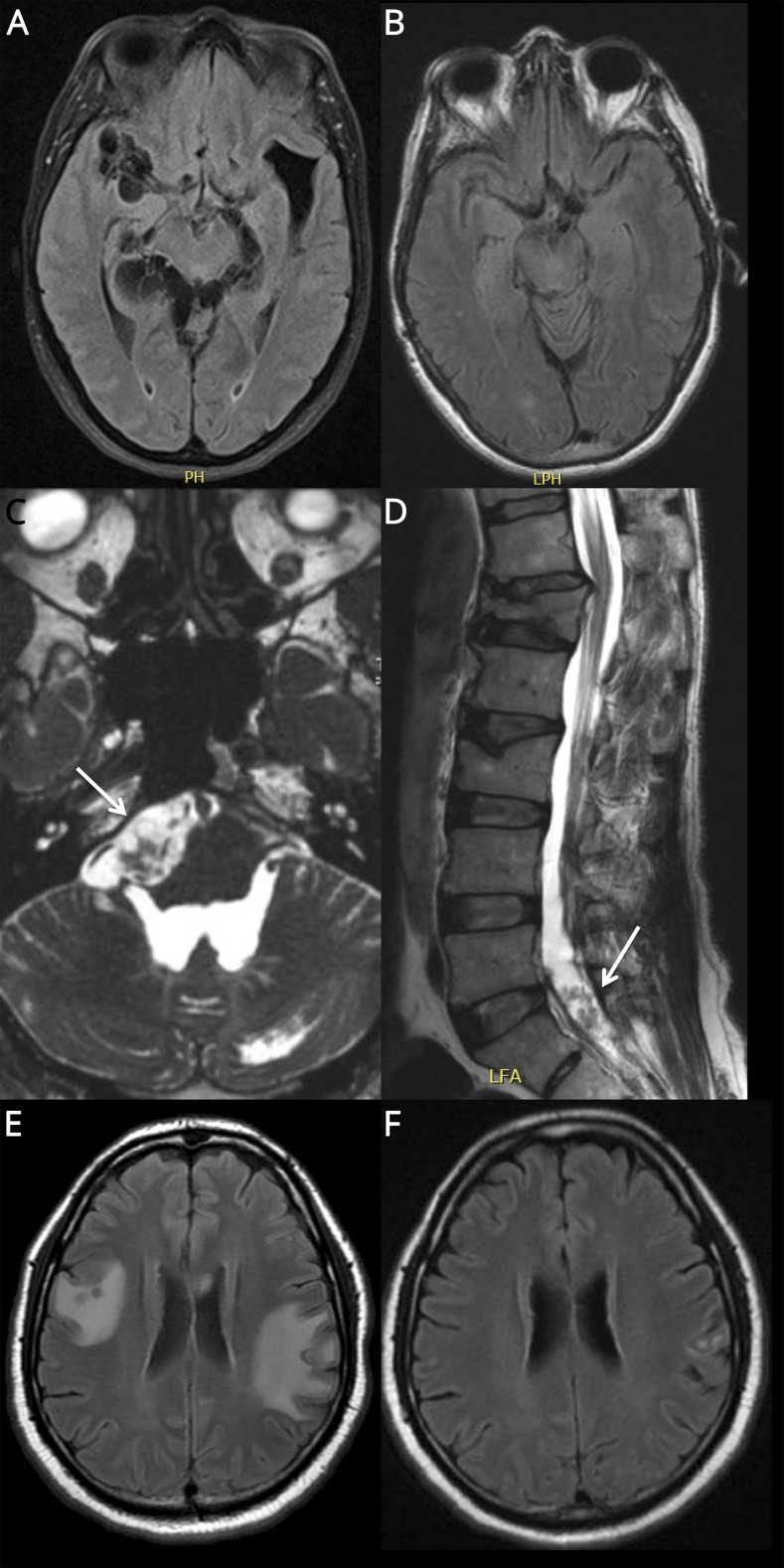
A 64-year-old Cape Verdean woman presented with headaches, seizures, and multiple subarachnoid cysts on brain MRI (figure 1A) with a positive CSF cysticercal antigen. She had communicating hydrocephalus requiring a 3rd ventriculostomy and subsequent ventriculoperitoneal (VP) shunt and was treated with albendazole and prednisone 20–60 mg daily for 24 months because of persistent headaches and subarachnoid cysts on MRI. She experienced a clinical and radiographic relapse following prednisone taper over 18 weeks. CSF analysis showed a negative cysticercal antigen. Weekly MTX 7.5 mg was tried but resulted in ulcerative stomatitis and leukopenia. Prednisone 60 mg daily was resumed without antiparasitic therapy and was tapered over 10 months with resolution of her symptoms and MRI findings (figure 1B).
Figure. MRI findings in complicated NCC.
Patient 1: T2‐weighted FLAIR sequences on MRI brain show (A) subarachnoid cysts in the basilar, perimesencephalic, quadrigeminal, and perisylvian cisterns prior to treatment and (B) resolution of cystic lesions after treatment with corticosteroids. Patient 2: Highly T2-weighted fast imaging employing steady-state acquisition sequences on MRI brain and T2-weighted sequences on MRI spine show cystic lesions of (C) prepontine and (D) sacral cisterns. Patient 3: T2-weighted FLAIR sequences on MRI brain show (A) extensive perilesional edema in spite of MTX and corticosteroid therapy and (B) marked improvement in perilesional edema following the addition of adalimumab. FLAIR = fluid‐attenuated inversion recovery; NCC = neurocysticercosis.
Patient 2
A 48-year-old Guatemalan man presented with headaches, falls, and multiple cysts in the prepontine and lumbar cisterns (figure 1, C and D) and 4th ventricle. He underwent removal of the intraventricular cyst and placement of a VP shunt and was treated with albendazole and dexamethasone 4–6 mg daily for 6 months because of persistent leptomeningeal enhancement, headaches, and a 6th cranial nerve palsy. He ran out of medication and returned 1 month later with vertigo. He resumed dexamethasone for 6 additional months, complicated by lumbar vertebral fracture. Because of persistent inflammation identified on his brain MRI, he was transitioned to MTX 7.5 mg weekly with clinical stability for 5 months.
Patient 3
A 43-year-old Cape Verdean man presented with headache, aphasia, and parenchymal cysts with surrounding edema identified on brain MRI. He was treated with a 14-day course of praziquantel, albendazole, and dexamethasone 6 mg daily followed by a dexamethasone taper. Two months later, he returned with headaches, seizures, and new perilesional edema. He was re-treated with albendazole and praziquantel for 14 days with prednisone 50 mg daily, complicated by avascular necrosis of the hip. Because of the ongoing perilesional edema on high-dose prednisone, he initiated MTX with a maximum dose of 20 mg weekly. Perilesional edema persisted on MTX and high-dose prednisone (figure 1E), and he was started on adalimumab 50 mg every 14 days with clinical and radiographic resolution (figure 1F). MTX and prednisone were tapered over 16 weeks without recurrence. His seizures and headaches returned during a 6-week interruption in adalimumab treatment and resolved with re-initiation.
Discussion
While use of anti-inflammatory agents is common practice in NCC treatment, limited data exist on the transition from corticosteroids to SSAs. Here, we describe 3 cases of persistent NCC-associated inflammation that highlight both the efficacy and complications of SSA use in NCC.
Treatment of chronic perilesional edema with corticosteroids can be associated with rebound inflammation in the setting of corticosteroid taper.3 Two prior case series have described the successful use of MTX in controlling perilesional edema and facilitating corticosteroid discontinuation,2,4 supported by the clinical course of patient 2. By contrast, patients 1 and 3 highlight challenges posed by MTX toxicity or failure.
Tumor necrosis factor alpha (TNF-α) is an important modulator of inflammation, and elevated levels of CSF TNF-α have been found in patients with subarachnoid NCC.5 In a pig model of parenchymal NCC, TNF-α expression was increased following antiparasitic therapy. In the same model, pretreatment with etanercept, a TNF-α inhibitor, led to reduced TNF-α levels after antiparasitic therapy, suggesting that TNF-α may be an appropriate target in persistent NCC-associated inflammation.6 Patient 3 in our series showed dramatic improvement on adalimumab following MTX and corticosteroid failure. To our knowledge, there is only one published case series of TNF-α use in human patients with NCC.7 Our findings reinforce that TNF-α inhibitors are effective in treating chronic inflammation in NCC.
Though limited by the small number of patients, our series emphasizes the need for further studies on the use of SSAs in the treatment of NCC-related inflammation. Future guidelines should consider TNF-α inhibitors as part of the repertoire of anti-inflammatory therapies in NCC.
Appendix. Authors
Study funding
No targeted funding reported.
Disclosure
P. Anand, S. Mukerji, J. Thon, S. Gunaratne, T. Cho, and N. Venna report no disclosures. Go to Neurology.org/NN for full disclosures.
References
- 1.White AC, Coyle CM, Rajshekhar V, et al. Diagnosis and treatment of neurocysticercosis: 2017 clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Am J Trop Med Hyg 2018;66:1159–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keiser PB, Nash TE. Prolonged perilesional edema after treatment of parenchymal neurocysticercosis: methotrexate as a corticosteroid sparing agent. Clin Infect Dis 2003;36:e122–e126. [DOI] [PubMed] [Google Scholar]
- 3.Mejia R, Nash TE. Corticosteroid withdrawal precipitates perilesional edema around calcified Taenia solium cysts. Am J Trop Med Hyg 2013;89:919–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitre E, Talaat KR, Sperling MR, Nash TE. Methotrexate as a corticosteroid- sparing agent in complicated neurocysticercosis. Clin Infect Dis 2007;44:549–553. [DOI] [PubMed] [Google Scholar]
- 5.Aguilar-Rebolledo F, Cedillo-Rivera R, Llaguno-Violante P, Torres-López J, Muñoz-Hernandez O, Enciso-Moreno JA. Interleukin levels in cerebrospinal fluid from children with neurocysticercosis. Am J Trop Med Hyg 2001;64:35–40. [DOI] [PubMed] [Google Scholar]
- 6.Mahanty S, Orrego MA, Cangalaya C, et al. TNF- blockade suppresses pericystic inflammation following anthelmintic treatment in porcine neurocysticercosis. PLoS Negl Trop Dis 2017;11:e0006059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nash TE, Ware JAM, Coyle CM, Mahanty S. Etanercept to control inflammation in the treatment of complicated neurocysticercosis. Am J Trop Med Hyg 2019;100:609–616. [DOI] [PMC free article] [PubMed] [Google Scholar]