Abstract
Background
We investigated patterns in the time from recombinant tissue-type plasminogen activator (rt-PA) treatment to symptomatic intracranial hemorrhage (sICH) onset in acute ischemic stroke.
Methods
We retrospectively reviewed all admitted “stroke code” patients from 2003 to 2017 at the University of California San Diego Medical Center from a prospective stroke registry. We selected patients that received IV rt-PA within 4.5 hours after onset/last known well and had sICH prehospital discharge. sICH diagnosis was made by prospective review. Endovascular-treated patients were excluded, given the variability of practice. sICH was prospectively defined as any new radiographic (CT/MRI) hemorrhage after rt-PA treatment and any worsened neurologic examination. Time to sICH was the time from rt-PA administration start to documented STAT head CT order time with the first evidence of new hemorrhage. Charts were reviewed for examination time metrics, demographics, clinical history, and neuroimaging.
Results
sICH was identified in 28 rt-PA-only treated patients. The mean time to sICH was 18.28 hours (range 2.4–34 hours). Median time to sICH was 18.25 hours. sICH was correlated with increased age (p = 0.02) and increased NIH Stroke Scale (p = 0.01).
Conclusions
Our findings suggest that rt-PA patients have the highest risk of post rt-PA sICH within the first 24 hours after treatment. This supports monitoring of rt-PA-treated patients in specialized settings such as neuro-intensive care units or stroke units. Our findings suggest that the probability of sICH is low 36 hours post rt-PA. Future larger studies are warranted to identify the patterns of bleeding after rt-PA administration.
Recombinant tissue-type plasminogen activator (rt-PA) remains the only Food and Drug Administration-approved thrombolytic medication and first-line treatment for acute ischemic stroke.1 The most severe risk with treatment is symptomatic intracranial hemorrhage (sICH), which occurs in 6.4% of patients.2 Because of this risk, the American Heart/American Stroke Association (AHA/ASA) and the American Academy of Neurology (AAN) recommend that post rt-PA patients be monitored in units such as neuro-intensive care units or stroke units with stroke expert clinicians who can rapidly identify neurologic changes.3 Most patients with intensive care unit (ICU) needs develop them before the rt-PA infusion is finished, suggesting that rt-PA patients may require less than 24 hours high resource monitoring.4 There are limited data on the patterns of timing of sICH post-treatment. The purpose of this study was to investigate patterns in the time from rt-PA treatment to sICH onset in acute ischemic stroke.
After tissue plasminogen activator (tPA) administration, patients are routinely monitored in an ICU or stroke unit for bleeding complications. Antiplatelet treatments and pharmacologic venous thrombosis prophylaxis are withheld until at least 24 hours after tPA administration.3 Follow-up brain imaging is obtained routinely and at other times acquired for unrelated indications. Asymptomatic ICH is occasionally observed on such imaging, although this generally provides only nonspecific information on the time at which the hemorrhage occurred.5
Factors such as edema or mass effect4 have been identified as potential predictors of hemorrhagic transformation after tPA administration; however, it remains unclear how long after administration of tPA do symptomatic hemorrhages occur. Although hemorrhage may be incidentally noted on follow-up imaging after thrombolytic administration, the absence of symptoms to alert the clinician does not allow for identification of the precise time of hemorrhage. tPA in humans have been found to have circulating plasma half-lives of 3–8 minutes, although this time appears to be prolonged on the surface of the fibrin-rich thrombus.6 This might suggest that symptomatic hemorrhages might be more likely to occur soon after the drug is given, although this has not been definitively borne out of clinical experience at this time. In this study, we investigated patterns in the time from rt-PA treatment to sICH onset in acute ischemic stroke.
Methods
We retrospectively reviewed all admitted patients from our institutional review board-approved stroke registry between June 2003 and June 2017. Patients were included in the analysis if they received rt-PA within 4.5 hours after onset/last known well and were diagnosed with sICH within 36 hours of treatment. This operational definition was based on the NIH rt-PA study. Endovascular-treated patients were excluded, given the variability of practice. sICH was prospectively defined as any new radiographic hemorrhage on CT or MRI after rt-PA treatment accompanied by any worsened neurologic examination within 36 hours of treatment. The time to sICH was defined as the time from rt-PA administration bolus to documented head CT order time with the first evidence of new hemorrhage. This allowed for standardization of times in this retrospective review. Patient demographics, clinical history, neuroimaging, and outcomes were extracted from the chart.
Demographic and baseline characteristics were assessed for frequencies. Continuous variables were assessed for mean, median, maximum, and minimum values as appropriate. Correlations between variables and sICH were performed using Spearman rho. Forward logistic regression was performed with variables significantly correlated with sICH. Because this was an exploratory analysis, no adjustments for multiple comparisons were made and a p value of ≤0.05 was considered statistically significant. All analyses are presented unadjusted. Statistical analyses were performed with SPSS version 24.
Standard protocol approvals, registrations, and patient consents
This retrospective study used an IRB-approved protocol approved by the University of California San Diego Medical Center ethical committee standard.
Data availability
Anonymized data and statistical analysis are available on request.
Results
A total of 933 patients received rt-PA in the study period. Of those treated, 26 sICH cases were identified (institutional sICH rate of 3%). Baseline demographics of sICH patients are shown in table 1. The mean, range, and median hours to sICH were 18.3, 2.4–34, and 18.25 hours, respectively. The median last known well (proxy for onset) to rt-PA was 58 minutes. The median last well known to sICH time was 21.5 hours. Figure demonstrates the pattern of timing of sICH after rt-PA treatment. Of the sICH cohort, there was an equal distribution of sex and ethnic representation consistent with our tertiary region. Most patients were >75 years of age (73%). sICH occurred in the anterior circulation 73% of the time, consistent with the baseline lesion. Regarding timing patterns, sICH occurred in 11% of patients in <12 hours (n = 3), 76% within 24 hours (n = 20), and 13% within 36 hours (n = 3). Table 2 shows the functional outcomes on discharge and severity of NIH changes with head CT. Of note, 34% (n = 9) of patients had a significant clinical change (defined as NIH change of >4) at the time of repeat CT head, with 30% (n = 8) deceased and 46% (n = 12) requiring skilled nursing facility on discharge. Two patients were excluded as they were >36 hours, with one case involving therapeutic hypothermia and the other with severe sepsis.
Table 1.
Demographics of sICH after rt-PA
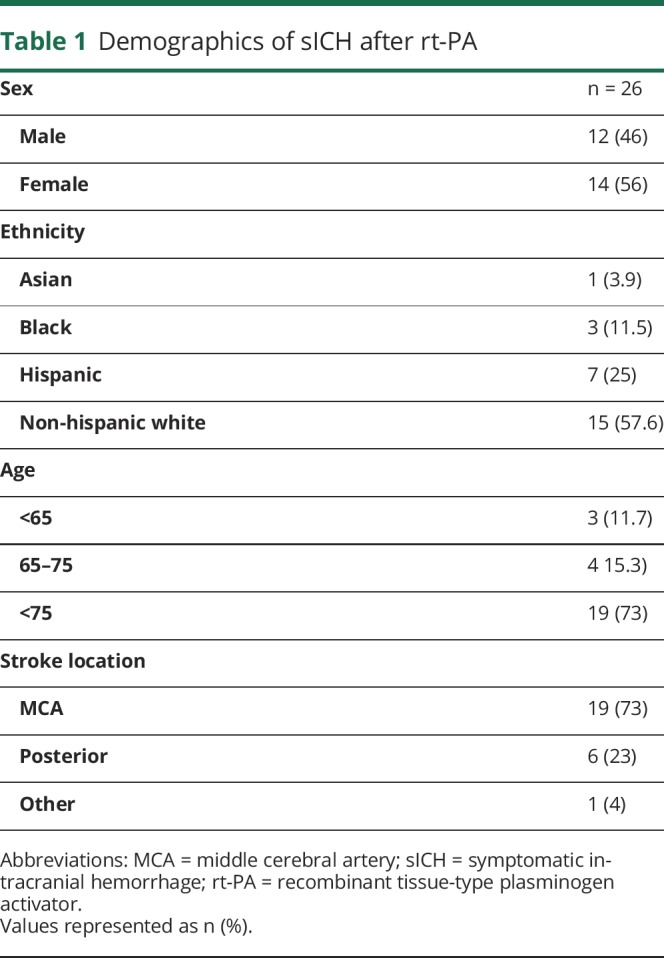
Figure. Timing of symptomatic intracranial hemorrhage (sICH) after recombinant tissue-type plasminogen activator (rt-PA).
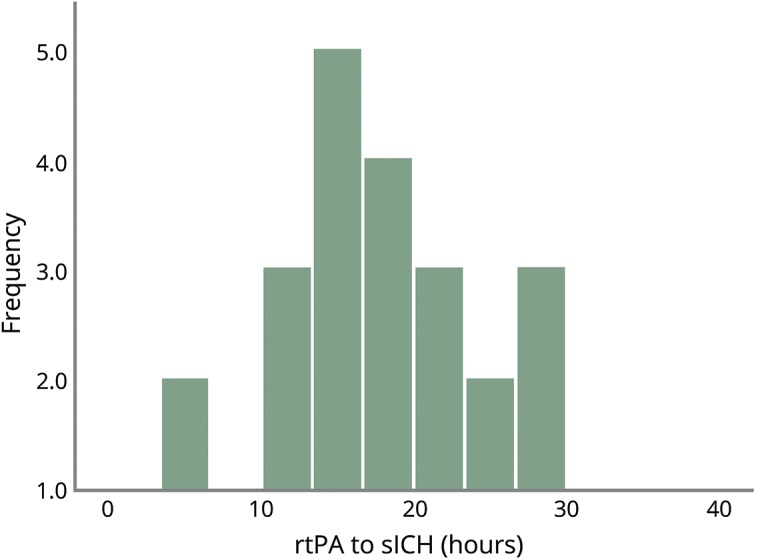
Table 2.
Outcomes and severity of clinical change of sICH after rt-PA
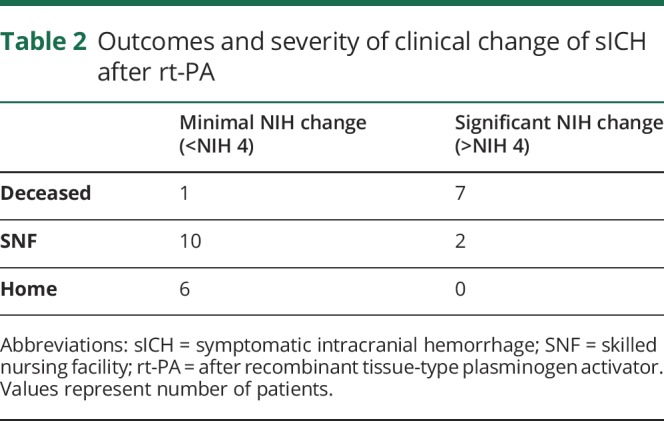
Multivariable logistic regression demonstrated that sICH was not correlated with time from rt-PA treatment (p = 0.71). Timing of sICH after rt-PA treatment was not significantly correlated with onset to treatment time (r = 0.16, p = 0.45). sICH was correlated with increasing age (p = 0.02) and increased NIH Stroke Scale (p = 0.01).
Discussion
Our findings suggest that rt-PA patients have the highest risk of post rt-PA sICH within the first 24 hours after treatment. This study supports monitoring of rt-PA-treated patients in specialized settings (neuro-ICU, stroke units) and it is consistent with current AAN and AHA/ASA guidelines.3
Only 11% of sICH occurred within the first 12 hours. This contrasts with recently published data, which showed that 80% of sICH occurred in the first 12 hours.6 This difference could be because of our smaller sample size, the exclusion of thrombectomy, or differences in regional rt-PA and interventional practices. Of note, the previously conducted study showed that thrombectomy with rt-PA increases the risk of sICH, although its effect on time intervals was not investigated.6 Our rt-PA isolated data set may underestimate this predisposition. Thus, there may be an underlying mechanistic aspect or confounding factor in our individual cases yet to be delineated.
Our study shows that sICH appears to be observed in a delayed fashion. This is interesting given that the half-life of rt-PA is less than 10 minutes, with a peak effect in the range of 1–4 hours.6 Of note, these findings of delayed sICH are consistent with multiple previous studies, which suggest that the probability of sICH is low after 36 hours post rt-PA.2,7 There was no statistical relationship between time intervals and sICH, because the idea of sICH time clusters has to be proven yet. This may require very large data sets to examine. Close monitoring by expert stroke clinicians allows for detection of acute neurologic deterioration, which improves the utility of repeat brain imaging and changes active management of the patient.2 Contrasting studies that support differing sICH timing speak to the need for further delineation of factors leading to earlier bleeding and may support utilizing step-down units in lieu of ICU resources.
Thrombectomy cases were excluded from our analysis given the variance of practice but also to represent the substantial proportion of patients that do not have large vessel occlusions. Previous studies have advocated for extended monitoring with these patients.2,7
The correlation of the NIHSS with sICH reflects multiple previous studies with high NIHSS likely being a surrogate for large infarct core size.6 Age above 80 years has also been shown to be an independent factor for sICH, although the mechanism of this is still unknown.8 Furthermore, we did not find a relationship between last known well to rt-PA time—a proxy for duration of ischemic penumbra severity—and sICH timing.
This may in part be from the relatively homogenous distribution of rt-PA times at our academic center. Our rt-PA sICH rate of 3% was lower than the overall populations for similar tertiary centers and the NINDS rt-PA study. This may represent underlying differences in the patient demographics, disease severity, or more clinical experience within the 20 years that rt-PA has been approved for treatment of ischemic stroke.
Finally, we used a relatively liberal definition for sICH to include any given clinical change associated with radiographic hemorrhage. Nevertheless, 40% of sICH patients had a significant neurologic change (typically herniation syndrome) at the time of repeat head CT, further supporting the clinical relevance of these findings. Furthermore, 76% of patients either required skilled nursing facility or were deceased on discharge; the baseline critical nature of these patients may in itself warrant extended monitoring.
There are limitations to this study. First, the retrospective nature makes the analysis dependent on the integrity of the medical record. This is a single institution study across 2 different hospital systems that may not be generalizable. The data are collected prospectively by vascular neurology fellows and are adjudicated by academic vascular neurology specialists. This makes the best data quality possible. Second, the inclusion of only IV rt-PA cases results in a smaller patient population. This was chosen to minimize the confounding factors associated with the varied interventional practices in the study timeline.
Overall, this exploratory study suggests that most sICH occurs within the first 24 hours. Furthermore, this risk is likely reduced outside of 36 hours, and most patients would benefit from extended intensive monitoring. Future larger studies are warranted to identify risk factors and patterns of sICH after rt-PA administration. This may aid in allocating interventions to reduce sICH risk, resources, and expertise in the most critical period for rt-PA-treated patients.
Appendix. Authors
Study funding
This study was in part funded by National Institutes of Health SPOTRIAS grant P50N5044148 and StrokeNet grant 5U10NS086535.
Disclosure
D. M. Meyer serves on a speakers' bureau for Portola. The remaining authors report no disclosures relevant to the manuscript. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
TAKE-HOME POINTS
→ sICH risk after rt-PA appears to be highest in the first 24 hours with a low probability at 36 hours.
→ sICH was not correlated with the time from rt-PA treatment or last known well to rt-PA time.
→ This study supports the monitoring of patients in specialized neurologic units and the need for larger studies.
References
- 1.National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581–1587. [DOI] [PubMed] [Google Scholar]
- 2.Yaghi S, Willey JZ, Cucchiara B, et al. Treatment and outcome of hemorrhagic transformation after intravenous alteplase in acute ischemic stroke: a scientific statement for healthcare professionals from the American Heart association/American stroke association. Stroke 2017;48:e343–e361. [DOI] [PubMed] [Google Scholar]
- 3.Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart association/American stroke association. Stroke 2018;49:e46–e110. [DOI] [PubMed] [Google Scholar]
- 4.Faigle R, Sharrief A, Marsh EB, Llinas RH, Urrutia VC. Predictors of critical care needs after IV thrombolysis for acute ischemic stroke. PLoS One 2014;9:e88652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.George AJ, Boehme AK, Dunn CR, et al. Trimming the fat in acute ischemic stroke: an assessment of 24-h CT scans in tPA patients. Int J Stroke 2015;10:37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang A, Llinas EJ, Chen K, et al. Shorter intensive care unit stays? The majority of post-intravenous tPA (Tissue-Type plasminogen activator) symptomatic hemorrhages occur within 12 hours of treatment. Stroke 2018;49:1521–1524. [DOI] [PubMed] [Google Scholar]
- 7.Strbian D, Sairanen T, Meretoja A, et al. Patient outcomes from symptomatic intracerebral hemorrhage after stroke thrombolysis. Neurology 2011;77:341–348. [DOI] [PubMed] [Google Scholar]
- 8.Zhou C, Zhu L, Wu J, et al. Tissue plasminogen activator (tPA) for ischemic stroke in patients aged >/=80 years. Acta Neurol Scand 2013;128:e17–18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data and statistical analysis are available on request.


