Abstract
Background
Hereditary transthyretin amyloidosis (hATTR) is associated with significant morbidity and mortality. Early diagnosis and treatment are essential to improve patient's outcome. Carpal tunnel syndrome (CTS) is a common complication of hATTR amyloidosis. However, because CTS is also common in the general population, we wanted to assess whether CTS, when associated with systemic manifestations, could help direct physicians to screen for TTR gene mutation and early diagnosis.
Methods
We reviewed the charts and interviewed the patients with hATTR seen between 2017 and 2018. We noted the details of CTS diagnosis, treatment, and other systemic features of the disease.
Results
Seventeen of the 23 patients studied had CTS. CTS was the first manifestation of the disease in 10 of 17 patients. On average, CTS symptoms occurred 10.4 years before their diagnosis of hATTR amyloidosis. In 6 of 10 patients with CTS, the following systemic symptoms were present as the first manifestation: erectile dysfunction, dysautonomia, polyneuropathy, exercise intolerance, and gastrointestinal and ocular symptoms.
Conclusion
CTS occurs in most patients with hATTR amyloidosis and frequently precedes the hATTR diagnosis. Most patients with CTS preceding hATTR diagnosis have systemic features. Recognizing systemic features at the time of CTS presentation may help in early diagnosis of hATTR amyloidosis.
Patients with hereditary transthyretin amyloidosis (hATTR) present with nonspecific symptoms that are usually attributed to more common disorders.1 This creates a challenge for a correct and early diagnosis.2 For example, carpal tunnel syndrome (CTS) may be the first manifestation of hATTR amyloidosis. Therefore, hATTR amyloidosis might be suspected in some patients with CTS, and this could potentially lead to an earlier diagnosis and treatment.3 However, CTS is common in the general population, and it would be impossible and of low yield to screen all patients with CTS for TTR mutations.4–6 On the other hand, the presence of other nonspecific symptoms at the time of CTS diagnosis or treatment may help select patients in whom testing for TTR mutation is reasonable. To investigate the concept of CTS with other associated symptoms at the time of diagnosis or treatment of CTS, we interviewed patients with hATTR amyloidosis seen over a course of 2 years and studied specifically the time of onset of CTS regarding hATTR diagnosis as well as additional symptoms at the time of diagnosis of CTS.
Methods
Standard protocol approvals, registrations, and patient consents
The Oregon Health & Science University (OHSU) IRB approved the study. We reviewed all 31 charts of patients with TTR mutations seen in our neuromuscular clinic between 2017 and 2018. We also interviewed the patients during their clinic visit and noted the details regarding the patients' demographics, whether or not they had CTS, and the timing of CTS relative to hATTR diagnosis. We also noted the type of TTR mutation, delay in diagnosis, associated systemic symptoms with CTS, organs involved during the course of the disease, other systemic features of the disease, treatment received for CTS and amyloidosis, as well as symptom resolution, recurrence, or progression after CTS release. We considered positive systemic features regardless of whether those were present at the time of CTS symptoms onset or at the time of CTS release because it was easier for patients to recall details this way. In most cases, we did not have nerve conduction studies to review and those were not included in the analysis. This was a descriptive study, and no formal statistics were performed.
Data availability
All of the data recorded during this study have been included in this article.
Results
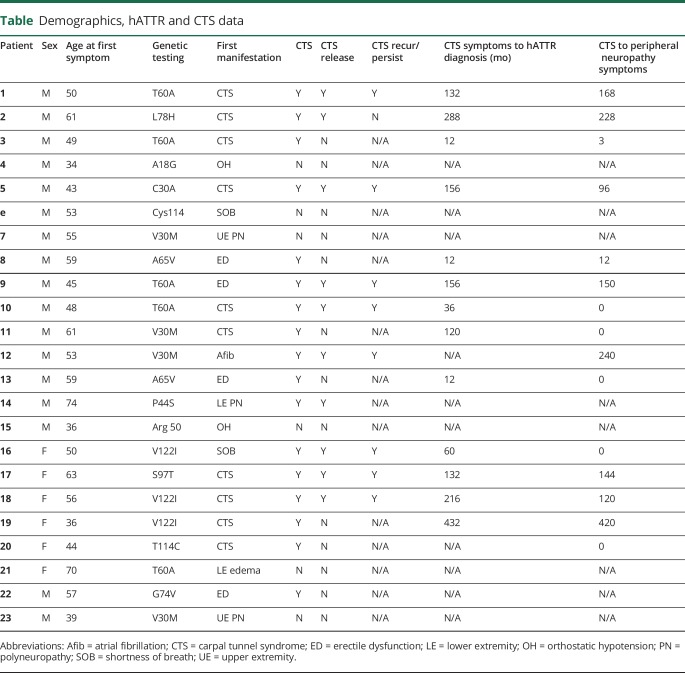
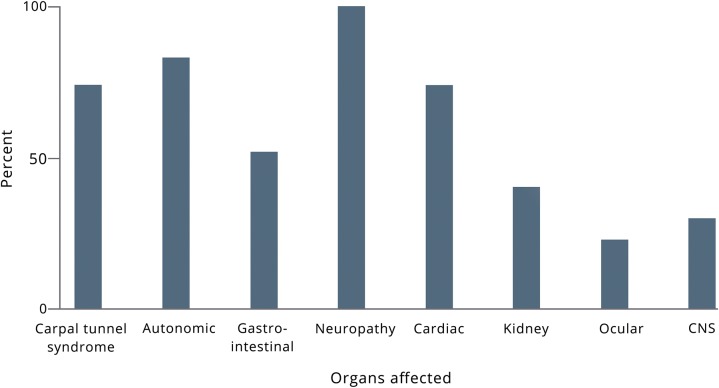
Eight patients carried pathogenic TTR mutations but were asymptomatic, and we excluded them from the analysis. We interviewed 23 patients. The median age at first symptom was 53 years old (range 34–74 years), and the median age at diagnosis of hATTR amyloidosis was 61 years old (range 30–77 years). There was a delay in the diagnosis for all patients, who did not have a family history (14 patients), and for 4 of 10 patients with a known family history. The median delay in diagnosis for those 17 patients was 72 months (range 12–432). We summarized patients' characteristics in the table, table e-1 (links.lww.com/CPJ/A95), and the figure.
Table.
Demographics, hATTR and CTS data
Figure. Organs affected at time of interview.
CNS = central nervous system involvement.
In the 23 patients interviewed, 17 patients had a diagnosis of CTS. CTS was bilateral in all cases. In 10 of those patients, CTS symptoms occurred before the diagnosis of hATTR amyloidosis and before the development of any systemic symptoms. In the remaining 7 patients, CTS symptoms occurred after other systemic manifestations of amyloidosis had already developed. The median age at which patients started having symptoms attributed to CTS was 48.5 years (range 36–63). The median time between CTS symptoms and hATTR amyloidosis diagnosis was 11 years (range 0–36 years). The median time between CTS symptoms and systemic symptoms was 7 years (range 0–35 years). Three patients had upper extremity onset of their neuropathy, which was not felt to be related to median nerve entrapment at the wrist but rather directly to amyloid polyneuropathy. Five patients had T60A mutation, 3 had V30M mutations, and 3 had V122I. The TTR mutations in the remaining 12 patients varied (table).
Six of the 10 patients diagnosed with CTS before hATTR amyloidosis diagnosis underwent CTS release. Only 1 patient experienced persistent postsurgical relief from his CTS symptoms. In the remaining patients, symptoms in the upper extremities either persisted (1), resolved partially (1), or recurred after a symptoms-free period (3). In the latter group, symptoms recurred after 8 years in 2 patients and after 1 year in 1 patient. In comparison, in the 7 patients who had a diagnosis of CTS after other systemic manifestations of amyloidosis, 4 had CTS release. Of those 4 patients who underwent CTS release, 1 patient experienced persistent symptom relief, 2 patients had transient relief, and 1 patient noted no improvement of symptoms.
In 6 of 10 patients with CTS diagnosis before hATTR amyloidosis, at least 1 of the following associated systemic symptoms was present at the time of CTS release: orthostatic hypotension, diarrhea and constipation, erectile dysfunction (ED), exercise intolerance, and burning pain in the feet. Exercise intolerance reflects cardiac involvement in our patients. Interestingly, ED was very common and occurred in 12 of 16 male patients. ED was the first symptom in 4 of them, and occurred at age 45 in 1 patient and late 50s in the other 3 patients. CTS symptoms preceded the neuropathy in 10 of 23 patients. Median time from CTS to neuropathy in those patients was 147 months (range 3–420 months).
Discussion
In this study, we found that CTS occurred not only in two-thirds of patients with neuropathy related to hATTR amyloidosis but also preceded hATTR amyloidosis diagnosis by a decade. Can this alone help diagnose patients with hATTR amyloidosis earlier? Not likely. CTS is common in the general population: up to 6% of the general population will develop CTS at some point.4,5 Furthermore, a third of Charcot-Marie-Tooth (CMT) disease patients have CTS, and equally about a third of patients with diabetic neuropathies also have CTS.4,5 Hence, screening all patients with CTS for TTR mutations would have a very low yield. In this study, we showed that many hATTR amyloidosis patients have other systemic symptoms around the same time of CTS onset or release. This finding could narrow the patient population with CTS to be considered for TTR mutation screening. Unfortunately, these systemic symptoms (GI disturbance, exercise intolerance, ED, burning pain in the feet, etc.) are also nonspecific. However, combination of systemic symptoms without a common alternative explanation and CTS should raise suspicion for amyloidosis. For example, we found that many men have ED at the time of CTS release and some had ED several years before the onset of CTS symptoms. The combination of ED and CTS, in the absence of diabetes, for example, should raise concern about amyloidosis.
Early diagnosis of hATTR amyloidosis remains difficult, especially in the absence of family history and when patients with CTS do not have any other associated symptoms. In addition, some patients with hATTR may have had CTS due to another reason because CTS is a common disorder. However, we did observe a higher incidence of CTS in our cohort compared with the general population or what is reported in patients with diabetes or CMT. This finding suggests that in most patients in our cohort who developed CTS, the CTS was related to amyloidosis. In TTR amyloidosis, deposition of amyloid in the flexor retinaculum would cause compression of the median nerve in the carpal tunnel.6–8
Our study has limitations. First, it is biased because patients were evaluated by a neurologist and were referred to neurology only if they had a neuropathy, which means that we did not study patients with cardiac amyloidosis without neuropathy. Second, there is a recall bias because patients were interviewed many years after the onset of symptoms. Third, it is difficult to tell which of our patients truly had CTS vs an upper extremity neuropathy as part of their amyloid polyneuropathy.9–11 We did note 3 patients with upper extremity onset of their neuropathy and those were not considered CTS. In addition, we chose to analyze the group of patients who had the diagnosis of CTS before the neuropathy. Furthermore, most patients did report improvement after the surgeries, and as such, one could assume that in most cases, the diagnosis of CTS was correct. We could not always interpret the nerve conduction studies and electromyography ourselves because most patients were referred to us and their previous studies were not always available. However, the limitations of this study do not affect clinical practice because our main message from this study remains the same regardless of whether the diagnosis of CTS was actually correct.
In summary, we found that CTS is common in hATTR amyloidosis. The CTS frequently preceded the systemic manifestation and diagnosis of hATTR amyloidosis by several years. Most patients with CTS preceding hATTR diagnosis have other systemic features (such as orthostatism, diarrhea and constipation, ED, exercise intolerance, and burning pain in the feet) at the time of CTS release. Recognizing systemic features at the time of CTS release may help early diagnosis of hATTR amyloidosis. Early diagnosis is particularly important in an era of new advances in hATTR neuropathy treatment, which are more effective when given earlier in the disease process.
Appendix. Authors
Study funding
No targeted funding reported.
Disclosure
C. Karam has received consulting honorarium from Acceleron, Akcea, Alnylam, Alexion, Biogen, CSL Behring, Biogen, and Genzyme. D. Dimitrova reports no disclosures. M. Christ reports no disclosures. S.B. Heitner has received consulting honorarium from Alnylam, Akcea, Ionis, and Eidos. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Cortese A, Vegezzi E, Lozza A, et al. Diagnostic challenges in hereditary transthyretin amyloidosis with polyneuropathy: avoiding misdiagnosis of a treatable hereditary neuropathy. J Neurol Neurosurg Psychiatry 2017;88:457–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papoutsidakis N, Miller EJ, Rodonski A, Jacoby D. Time course of common clinical manifestations in patients with transthyretin cardiac amyloidosis: delay from symptom onset to diagnosis. J Card Fail 2018;24:131–133. [DOI] [PubMed] [Google Scholar]
- 3.Tojo K, Tsuchiya-Suzuki A, Sekijima Y, Morita H, Sumita N, Ikeda SI. Upper limb neuropathy such as carpal tunnel syndrome as an initial manifestation of ATTR Val30Met familial amyloid polyneuropathy. Amyloid 2010;17:32–35. [DOI] [PubMed] [Google Scholar]
- 4.Panosyan FB, Kirk CA, Marking D, et al. Carpal tunnel syndrome in inherited neuropathies: a retrospective survey. Muscle Nerve 2018;57:388–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perkins BA, Olaleye D, Bril V. Carpal tunnel syndrome in patients with diabetic polyneuropathy. Diabetes Care 2002;25:565–569. [DOI] [PubMed] [Google Scholar]
- 6.Kyle RA, Eilers SG, Linscheid RL, Gaffey TA. Amyloid localized to tenosynovium at carpal tunnel release: natural history of 124 cases. Am J Clin Pathol 1989;91:393–397. [DOI] [PubMed] [Google Scholar]
- 7.Samões R, Taipa R, Valdrez K, et al. Amyloid detection in the transverse carpal ligament of patients with hereditary ATTR V30M amyloidosis and carpal tunnel syndrome. Amyloid 2017;24:73–77. [DOI] [PubMed] [Google Scholar]
- 8.Nakagawa M, Sekijima Y, Yazaki M, et al. Carpal tunnel syndrome: a common initial symptom of systemic wild-type ATTR (ATTRwt) amyloidosis. Amyloid 2016;23:58–63. [DOI] [PubMed] [Google Scholar]
- 9.Mariani LL, Lozeron P, Théaudin M, et al. French Familial Amyloid Polyneuropathies Network (CORNAMYL) Study Group. Genotype-phenotype correlation and course of transthyretin familial amyloid polyneuropathies in France. Ann Neurol 2015;78:901–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koike H, Tanaka F, Hashimoto R, et al. Natural history of transthyretin Val30Met familial amyloid polyneuropathy: analysis of late-onset cases from non-endemic areas. J Neurol Neurosurg Psychiatry 2012;83:152–158. [DOI] [PubMed] [Google Scholar]
- 11.Koike H, Kawagashira Y, Iijima M, et al. Electrophysiological features of late-onset transthyretin Met30 familial amyloid polyneuropathy unrelated to endemic foci. J Neurol 2008;255:1526–1533. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All of the data recorded during this study have been included in this article.