Abstract
Background
A clear understanding of the epidemiology of malaria and dengue co-infection is essential for informed decisions on appropriate control strategies for dengue and malaria. This systematic review synthesized evidence on the relationship of malaria and dengue co-infection and related it to alterations in platelet, hemoglobin, hematocrit, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) levels when compared to malaria mono-infection.
Methods
A systematic review in accordance with PRISMA guidelines was conducted. All published articles available in PubMed and Web of Science (ISI) databases before October 21, 2017 were recruited. All epidemiological studies except case reports on the prevalence or incidence of malaria and dengue co-infection among patients visiting hospitals with febrile illness were included. Studies that involved conference abstracts, protocols, systematic reviews, only mono-dengue or mono-malaria infections, and only animal or in vitro studies were excluded after screening the titles, abstracts, and body texts. Studies were additionally excluded after full text review when they lacked epidemiologic data on malaria and dengue co-infection. Two reviewers independently screened, reviewed, and assessed all the studies. Cochrane Q (Chi-square) and Moran’s I2 were used to assess heterogeneity, and the funnel plot was used to examine publication bias. The summary odds ratio (OR) and 95% confidence intervals (CI) were estimated using a fixed-effects model. Thirteen cross-sectional and two retrospective studies were eligible to be included in the systematic review and meta-analysis.
Results
Out of the 2269 citations screened, 15 articles were eligible to be included in the systematic review and meta-analysis. The 15 studies involved 13,798 (10,373 cases with malaria and 3425 with dengue) patients in 9 countries. Thirteen studies compared the incidence and odds of Plasmodium sp. infection, five studies compared the odds of mean platelet, three studies compared Plasmodium parasite density, and four studies compared the odds of hemoglobin, hematocrit, AST, and ALT levels among co-infected groups and single-malaria-infected groups.
Conclusions
This study showed that dengue and malaria co-infection was associated with decreased odds of malaria infection, malaria parasitemia, AST, and ALT levels when compared to malaria mono-infection. However, malaria and dengue co-infection was associated with increased odds of platelet and hemoglobin levels when compared to malaria mono-infection.
Electronic supplementary material
The online version of this article (10.1186/s12889-019-7488-4) contains supplementary material, which is available to authorized users.
Keywords: Hematological parameters, Malaria prevalence, Dengue prevalence, Dual infection
Author summary
A clear understanding of the epidemiology of malaria and dengue co-infection is essential for informed decisions on appropriate control strategies for both malaria and dengue. In this systematic review and meta-analysis, prevalence/incidence of malaria and dengue infection was related to differences in parasite density, hemoglobin, hematocrit, platelet count, and liver enzymes; AST and ALT among patients were synthesized. All published articles available in PubMed and Web of Sciences (ISI) before October 21, 2017 were searched. We found thirteen cross-sectional and two retrospective studies eligible to be included in the systematic review and meta-analysis. A summarized analysis of the study findings showed that dengue and malaria co-infection was associated with decreased odds of malaria infection, malaria parasitemia, AST, and ALT levels when compared to malaria mono-infection. However, malaria and dengue co-infection was associated with increased odds of platelet and hemoglobin levels when compared to malaria mono-infection.
Background
Malaria and dengue are common in tropical and sub-tropical areas of the world, causing a high rate of morbidity and mortality especially among children [1, 2]. In 2015, about 212 million people were infected with malaria and 429,000 were estimated to have died globally due to malaria infection [1]. Additionally, more than 390 million people required preventive treatment for dengue and close to 96 million manifested clinical symptoms associated with severe dengue annually [2]. Plasmodium sp. can infect humans and manifest a wide range of signs and symptoms ranging from asymptomatic malaria to severe malaria [3]. Cerebral malaria, hypoglycemia, pulmonary edema, bleeding, acidosis, severe anemia, and acute renal failure were the major complications of severe malaria, which may result in death if no prompt or effective treatments are administered [3]. However, people living in endemic areas of malaria usually show asymptomatic or some non-specific symptoms such as fever, fatigue, chills, and malaise [4]. In the endemic areas of P. falciparum malaria, children up to 5 years of age had more common cases than older children and adults. This might be due to older children and adults receiving partial immunity from the infection [4, 5]. As mosquitoes are usually present in a tropical country, the co-infection of both malaria and dengue is evident and can cause acute febrile illness among patients. Atypical lymphocytosis, hemoconcentration, and thrombocytopenia are specific markers of dengue infection, which help differentiate the diagnosis of dengue infection from malaria infection [6–8].
A clear understanding of the epidemiology of malaria during dengue co-infection is essential for informed decisions on appropriate control strategies for dengue and malaria. In addition, we do not know the severity of co-infections when compared to single infections. The outcomes of co-infections are distinct among studies, especially in the selection criteria and diagnostic methods used in each study. Hence, the aim of this study was to perform a systematic review and meta-analysis to quantify the odds of Plasmodium infection, parasite density, and malaria-related alterations in hemoglobin, hematocrit, platelet, AST, and ALT levels among co-infected patients and mono-infected patients.
Methods
Search methods for identification of studies
The protocol for this systematic review and meta-analysis was conducted following the PRISMA guidelines (Checklist S1) [9], and the previous study reported the relationship of S. haematobium or S. mansoni and P. falciparum malaria infection [10]. Two authors (MK and KU) independently conducted a search in PubMed and Web of Science (ISI) databases using the keywords: “Plasmodium” OR “malaria” in combination with “dengue” (Additional file 1: Table S2) for articles published before October 21, 2017. The search was limited to human cases and to articles in the English language. Duplicates, abstracts, and titles were excluded from this study. A total of 2269 papers were screened for eligibility criteria, and 45 papers were chosen for full text evaluation. The discrepancies of choosing papers in this review were judged by a third reviewer. However, there was a very low degree of discrepancy between the two authors in this report.
Eligibility criteria
All epidemiological studies which reported prevalence or incidence of Plasmodium sp. infection and dengue infection were included. Unpublished studies, case studies, conference abstracts, protocols, systematic reviews, and studies that involved only animal or in vitro studies were excluded after screening the titles and abstracts. Studies were additionally excluded following full text review if they lacked epidemiologic data on Plasmodium and dengue co-infection.
Outcome measures
The primary outcome was prevalence/incidence of Plasmodium and dengue co-infection. Malaria was defined as microscopic confirmation of the Plasmodium parasite in blood without signs or symptoms of severe malaria. The secondary outcomes included parasite density, hemoglobin, hematocrit, platelet, AST, and ALT levels.
Data extraction and management
Information about the authors, study area, study design, sample size enrolled, age range, prevalence of malaria and dengue co-infection, diagnosis techniques, and the main findings on prevalence/incidence of Plasmodium infection related to parasite density, hematocrit, platelet, AST, and ALT levels were abstracted and entered into an Excel sheet.
Assessment of reporting biases
Quality and risk of bias of the studies was evaluated using the Effective Public Health Practice Project [11]. The quality of the studies was assessed on the basis of selection of the study participants, study design, confounder, blinding, data collection methods, withdrawals, and drop-outs comparability.
Data synthesis
Heterogeneity was assessed using Cochrane Q (Chi-square) and Moran’s I2 (Inconsistency) using RevMan 5 software (Version 5, London, UK) [12]. Publication bias was evaluated using a funnel plot [13]. Odds ratio and mean differences along with the 95% confidence intervals were used as effect measures. The 95% CI for mean differences in parasite density, hemoglobin, hematocrit, platelet, AST, and ALT levels among those co-infected with dengue and those uninfected with dengue for the studies by Magalhaes et al. [14] and Mendonça et al. [15] were estimated using the mean and standard deviations values. The mean and standard deviations of hemoglobin, hematocrit, platelet, AST, and ALT levels for Mendonça et al. [15] and Assir et al. [16] were estimated from the median and interquartile range based on the formula suggested by Higgins et al. [17]. A fixed-effects model was used to estimate the summary Mantel-Haenszel odds ratio of malaria infection among patients infected with dengue and those uninfected with dengue.
Results
Search results and study characteristics
A total of 2811 citations were identified from PubMed (n = 1382) and Web of Science (n = 1429) databases, of which 542 articles were found to be duplicates. Of the 2269 articles screened, there were 1202 articles excluded after reading the titles and abstracts due to irrelevant records. Of the 1067 articles screened thereafter, 577 articles were excluded due to a lack of full text. Of the 490 full text articles reviewed, 443 were excluded. Of the 47 full text articles reviewed, 32 articles were excluded due to a lack of information on co-infection. A total of 15 articles were considered for the systematic review and meta-analysis (Fig. 1). The characteristics of the 15 studies with 13,798 subjects (10,373 cases with malaria and 3425 with dengue) included in this review were summarized in Table 1. Thirteen studies were cross-sectional and two studies were retrospective.
Fig. 1.

PRISMA diagram. Flow chart for study selection
Table 1.
Characteristics of the included studies
| Reference | Study area (years of the survey) |
Age range | Plasmodium sp. | The magnitude of outcome in those co-infected compared to those with only malaria | Case enrolled | Case of co-infection | Case of malaria alone | Case of dengue alone | Diagnostic Techniques |
|---|---|---|---|---|---|---|---|---|---|
| Assir et al., 2014 [16] | Pakistan (2012) | 12–90 | P.falciparum and P.vivax |
1. Co-infection rate = 1.99% 2. Hemoglobin = similar 3. Hematocrit = similar 4. Platelet count = similar |
52 | 17 | 18 | 5 |
Malaria: Microscopy Dengue: ELISA NS1,IgM, RT-PCR |
| Baba et al., 2013 [18] | Nigeria (2008) | < 1- > 80 y | P. falciparum | 1. Co-infection rate 7.17% | 310 | 18 | 31 | 175 |
Malaria: Microscopy Dengue: Plaque reduction neutralization test (PRNT) |
| Barua and Gill, 2016 [19] | India (2014) | > 12 y | P.falciparum and P.vivax |
1. Co-infection rate 10.25% 2. Hemoglobin = higher 3. Hematocrit = similar 4. Platelet count = lower 5. AST = higher 6. ALT = higher |
156 | 16 | 55 | 85 |
Malaria: Microscopy Dengue: IgM, NS1 |
| Carme et al., 2009 [20] | French Guiana (2004–2005) | NA | P.falciparum and P.vivax | 1. Co-infection rate 1% | 1723 | 17 | 376 | 221 |
Malaria: Microscopy Dengue: IgM serology, culture, RT-PCR |
| Chipwaza et al., 2014 [21] | Tanzania (2013) | 2–13 | NA | 1. Co-infection rate 8.5% | 364 | 31 | 52 | 45 |
Malaria: Microscopy Dengue: ELISA |
| Epelboin et al., 2012 [22] | French Guiana (2004–2010) | 6 m-83 y | P.falciparum and P.vivax |
1. Parasitemia = similar 2. Hemoglobin = similar 3. Hematocrit = lower 4. Platelet count = lower 5. AST = similar 6. ALT = similar |
Not given | 104 | 208 | 104 |
Malaria: Microscopy Dengue: Cell-culture virus isolation, RT-PCR, NS1, IgM, IgM, IgM + IgA |
| Hati et al., 2012 [23] | India (2005–2010) | NA | P.falciparum and P.vivax | 1. Co-infection rate 1.5% | 2971 | 46 | 194 | 559 |
Malaria: Microscopy Dengue: ELISA |
| Magalhaes et al., 2014 [14] | Brazil (2009–2011) | 0- > 60 y | P.falciparum and P.vivax |
1. Co-infection rate = 3.16% 2. Parasitemia = similar 3. Hematocrit = higher 4. Platelet count = lower 5. AST = higher 6. ALT = higher |
1578 | 44 | 176 | 584 |
Malaria: PCR. Dengue: IgM, NS1, RT-PCR |
| Mendonça et al., 2015 [15] | Brazil (2009–2013) | IQR20.8–52.25 y | P. vivax |
1. Parasitemia = similar 2. Hemoglobin = similar 3. Hematocrit = similar 4. Platelet count = similar 5. AST = lower 6. ALT = higher |
Not given | 30 | 52 | 30 |
Malaria: Microscopy, PCR Dengue: RT-PCR |
| Mohapatra et al., 2012 [24] | India (2011) | NA | P.falciparum and P.vivax |
1. Co-infection rate = 6% 2. Parasitemia = lower 3. Hemoglobin = higher 4. Platelet count = lower 5. AST = lower 6. ALT = lower |
469 | 27 | 102 | 340 |
Malaria: Microscopy Dengue: RDT (NS1, IgM) |
| Mueller et al., 2014 [25] | Cambodia (2008–2010) | 7–49 y | P.falciparum and P.vivax | 1. Co-infection rate = 3.24% | 1475 | 27 | 727 | 53 |
Malaria: RDT, Nested-PCR Dengue: RT-PCR |
| Rao et al., 2016 [26] | India (2013) | < 1- > 15 y | P.falciparum and P.vivax | 1. Co-infection rate = 1% | 1980 | 22 | 229 | 723 |
Malaria: Microscopy, RDT. Dengue: Dengue NS1, ELISA, RT-PCR |
| Swoboda et al., 2014 [27] | Bangladesh (2007–2010) | ≥8 y | P.falciparum and P.vivax | 1. Co-infection rate = 0.76% | 659 | 5 | 362 | 35 |
Malaria: Microscopy, RDT. Dengue: ELISA IgM |
| Sow et al., 2016 [28] | Senegal (2009–2013) | > 1 y | P. falciparum | 1. Co-infection rate = 0.01% | 13,845 | 1 | 7386 | 2 |
Malaria: Microscopy, RDT Dengue: RT-PCR |
| Zaki and Shanbag, 2010 [29] | India (2005) | 1 m-12 y | P.falciparum and P.vivax | 1. Co-infection rate = 0.33% | 602 | 2 | 33 | 79 |
Malaria: Microscopy Dengue: ELISA |
Thirteen studies can be used to compare the odds of Plasmodium sp. infection. These studies included: Assir et al. [16], Baba et al. [18], Barua and Gill. 2016 [19], Carme et al. [20], Chipwaza et al. [21], Hati et al. [23], Magalhaes et al. [14], Mohapatra et al. [24], Mueller et al. [25], Rao et al. [26], Swoboda et al. [27], Sow et al. [28], Zaki and Shanbag 2010. [29].
Five studies had data related to platelet count [14–16, 19, 24]. Three studies had data related to Plasmodium parasitemia [14, 15, 24]. Four studies had data related to hemoglobin, hematocrit, AST, and ALT levels [14, 15, 19, 24]. A study by Epelboin et al. [22] reported the incidence of those parameters in terms of percentages (without mean or median); therefore, this study was excluded from the meta-analysis.
Prevalence of dengue and malaria infection
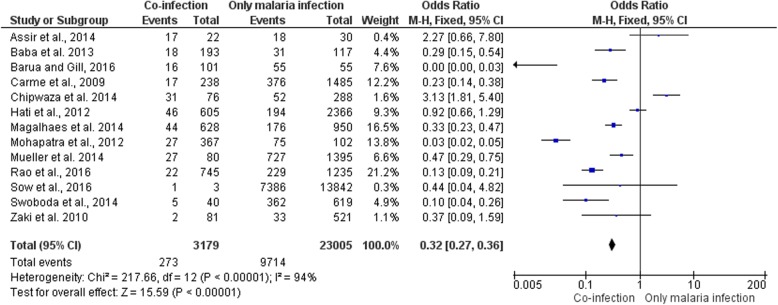
Thirteen studies examined the relationship of dengue infection with the odds of Plasmodium infection. Based on the meta-analysis shown in Fig. 2, cross-sectional studies in Nigeria [18], India [19, 24, 26], French Guiana [20], Brazil [14], Cambodia [25], and Bangladesh [27] showed significantly lower odds of co-infection when compared to those uninfected with dengue (OR: 0.29; 95% CI = 0.15, 0.54). A cross-sectional studies in Pakistan [16], Senegal [28], and India [23, 29] showed no significant odds of co-infection when compared to those uninfected with dengue (OR: 2.27; 95% CI = 0.66, 7.80). However, a study in Tanzania [21] showed significantly higher odds of co-infection when compared to those uninfected with dengue (OR: 3.13; 95% CI = 1.81, 5.40). The overall estimates based on thirteen studies showed significantly lower odds of Plasmodium infection among patients infected with dengue than those uninfected with dengue (summary OR: 0.32; 95% CI: 0.27, 0.36; I2: 94%) [14, 16, 18–21, 23, 25–29].
Fig. 2.
Forest plot showing the difference in the prevalence of malaria and dengue co-infection and those of malaria mono-infection
Dengue infection and Plasmodium parasite density
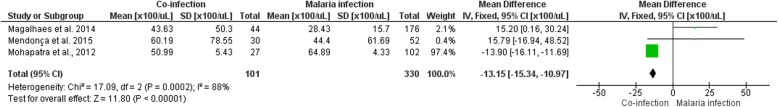
Out of the fifteen studies included in this systematic review, three studies reported data in regard to the percent of the parasitemia of Plasmodium sp. Among these three studies, a study in the Brazilian Amazon reported co-infection with higher levels of parasitemia when compared to those uninfected with dengue (mean 4363 vs 2843 parasites/mm3) [14]. Another study in the Brazilian Amazon also showed a higher parasitemia level in patients with co-infection when compared to those uninfected with dengue [15]. However, a study in India [24] showed a lower parasitemia level in patients with co-infection when compared to those uninfected with dengue (mean 5098.8 vs 6489.4 parasites/mm3). A summary analysis based on these three studies showed significantly lower odds of Plasmodium parasitemia in patients co-infected with dengue as compared to those uninfected with dengue (summary mean difference = − 13.15; 95% CI = − 15.34, − 10.97; I2 = 88%) (Fig. 3) [14, 15, 24].
Fig. 3.
Forest plot showing the difference in parasitemia level of malaria and dengue co-infection and those of malaria mono-infection
A study in French Guiana showed a lack of data in the mean or median Plasmodium parasitemia in infected patients; however, the proportion of patients with low parasitemia (proportion = 19.2%) was higher when compared to those uninfected with dengue (proportion = 11.5%), but the data was not statistically significant (p = 0.08) [22].
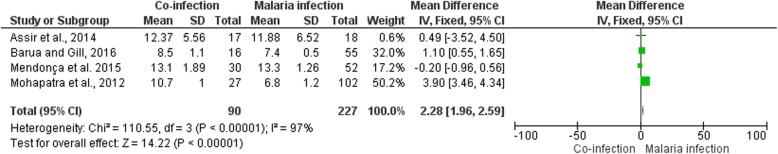
Status of co-infection and hemoglobin level
Four studies reported data in regard to the hemoglobin level of co-infection with dengue and Plasmodium. A study by Assir et al. reported no difference in the hemoglobin level between patients with co-infection and those uninfected with dengue (median 13.0 vs 12.5 g/dL, P value = 0.09) [16]. A study by Barua and Gill [19] reported a significantly higher hemoglobin level in patients with co-infection than those uninfected with dengue (mean 8.5 vs 7.4 g/dL, P value < 0.001). A study by Mendonça et al. reported no significant difference in the hemoglobin level between patients with co-infection and those uninfected with dengue (median 13.0 vs 13.2 g/dL, P value = 0.48) [15]. Another study by Mohapatra et al. reported a significantly higher hemoglobin level in patients with co-infection than those uninfected with dengue (mean 10.7 vs 6.8 IU/L, P value = 0.001) [24].
A summary analysis based on these four studies showed no significant differences in the odds of hemoglobin level between patients co-infected with dengue and those uninfected with dengue (summary mean difference = − 0.43; 95% CI = − 1.39, 0.53; I2 = 22%) (Fig. 4) [15, 16, 19, 24]. A study in French Guiana showed that the proportion of co-infected patients with low hemoglobin (< 12 g/dl) (proportion = 35.6%) was not significantly different when compared to those uninfected with dengue (proportion = 20.7%) (p = 0.05) [22].
Fig. 4.
Forest plot showing the difference in hemoglobin level of malaria and dengue co-infection and those of malaria mono-infection
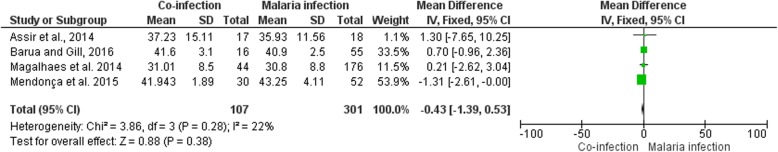
Status of co-infection and hematocrit level
Out of the fifteen studies included in this review, four studies reported data in regard to the hematocrit level of co-infection and that of Plasmodium sp. infection only. However, there was no difference found in the hematocrit level of patients with co-infection when compared to those uninfected with dengue (Magalhaes et al. mean 31.01 vs 30.8%, P value = 0.473; Mendonça et al. median 42.05 vs 43.35%, P value = 0.373; Assir et al. median 39.3 vs 36.0%, P value = 0.69; Barua and Gill mean 41.6% vs 40.9%) [14, 15]. A summary analysis based on these four studies also showed no significant difference in the odds of hematocrit level between patients co-infected and those uninfected with dengue (summary mean difference = − 0.43; 95% CI = − 1.39, 0.53; I2 = 22%) (Fig. 5). A study in French Guiana showed a lack of data in the mean or median hematocrit level in infected patients; however, the proportion of co-infected patients with low hematocrit (< 36%) (proportion = 54.3%) was significantly higher when compared to those uninfected (proportion = 23.6%) with dengue (p = 0.002) [22].
Fig. 5.
Forest plot showing the difference in hematocrit level of malaria and dengue co-infection and those of malaria mono-infection
Status of co-infection and platelet level
Out of the fifteen studies included in this review, five studies reported data in regard to the platelet level of co-infection and that of Plasmodium sp. infection only. A study by Assir et al. [16] in Pakistan reported no difference in platelets between patients with co-infection and those uninfected with dengue (median 54,000 vs 46,000/mm3, P value = 0.35). A study by Barua and Gill reported a significantly lower platelet level in those with co-infection when compared to those uninfected with dengue (mean 47,587 vs 76,422/mm3, P value < 0.001) [19].
A study by Magalhaes et al. in the Brazilian Amazon reported no difference in platelets between patients with co-infection and those uninfected with dengue (mean 69,772 vs 115,114/mm3, P value = 0.055) [14]. A study by Mendonça et al. in the Brazilian Amazon also reported no significant difference between patients with co-infection and those uninfected with dengue (median 87,500 vs 102,000/mm3, P value = 0.108) [15]. Another study by Mohapatra et al. reported no significant difference between patients with co-infection and those uninfected with dengue (median 58,230 vs 145,000/mm3, P value = 0.001) [24].
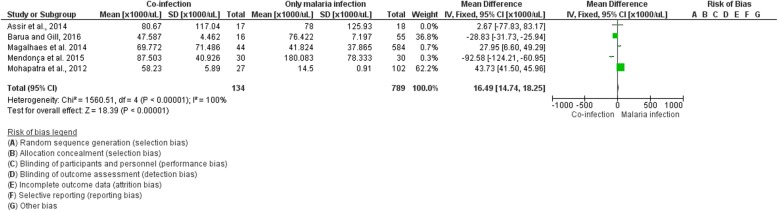
A summary analysis based on these five studies showed significantly higher odds of platelets among patients co-infected with dengue as compared to those uninfected with dengue (summary mean difference = 16.49; 95% CI = 14.74, 18.25; I2 = 100%) (Fig. 6). A study in French Guiana showed a lack of data in the mean or median platelet level in infected patients; however, the proportion of co-infected patients with deep thrombocytopenia (< 50 g/L) (proportion = 23%) was significantly higher when compared to those uninfected (proportion = 6%) with dengue (p < 0.001) [22].
Fig. 6.
Forest plot showing the difference in platelet level of malaria and dengue co-infection and those of malaria mono-infection
Status of co-infection and AST level
Four studies reported data in regard to the AST level of co-infection with dengue and Plasmodium. A study by Barua and Gill in India reported a significantly higher AST level in patients with co-infection when compared to those uninfected with dengue (mean 116.3 vs 96.6 IU/L, P value < 0.001) [19].
A study by Magalhaes et al. in the Brazilian Amazon reported no difference in the AST level between patients with co-infection and those uninfected with dengue (mean 90.9 vs 73.1 IU/L, P value = 0.263) (Fig. 7) [14]. However, another study by Mendonça et al. in the Brazilian Amazon reported a significantly higher AST level in patients with co-infection than those uninfected with dengue (median 47 vs 67.5 IU/L, P value = 0.005) [15]. A study by Mohapatra et al. reported a significantly lower AST level in patients with co-infection than those uninfected with dengue (mean 34 vs 51.7 IU/L, P value = 0.001) [24].
Fig. 7.
Forest plot showing the difference in AST level of malaria and dengue co-infection and those of malaria mono-infection
A summary analysis based on these four studies showed significantly lower odds of AST level in patients co-infected with dengue when compared to those uninfected with dengue (summary mean difference = − 1.6; 95% CI = − 14.24, − 8.96; I2 = 97%) (Fig. 6) [14, 15, 19, 24]. A study in French Guiana showed a lack of data in the mean or median AST level in infected patients; however, the proportion of co-infected patients with high AST (> 2 folds) (proportion = 10.2%) was not significantly different when compared to those uninfected with dengue (proportion = 16%) (p = 0.2) [22].
Status of co-infection and ALT level
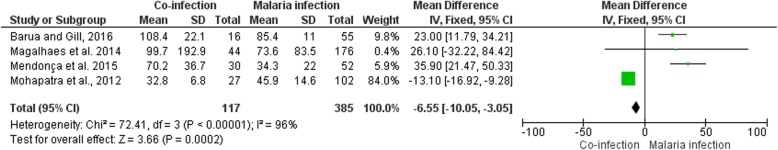
Four studies reported data in regard to the ALT level of co-infection with dengue and Plasmodium. A study by Barua and Gill in India reported a significantly higher ALT level in patients with co-infection when compared to those uninfected with dengue (mean 108.4 vs 85.4 IU/L, P value = 0.328) [19]. A study by Magalhaes et al. in the Brazilian Amazon reported no difference in the ALT level between patients with co-infection and those uninfected with dengue (mean 90.7 vs 73.6 IU/L, P value < 0.001) [14]. However, another study by Mendonça et al. in the Brazilian Amazon reported a significantly higher ALT level in patients with co-infection than those uninfected with dengue (median 69 vs 33 IU/L, P value < 0.0001) [15]. A study by Mohapatra et al. reported a significantly lower ALT level in patients with co-infection than those uninfected with dengue (mean 32.8 vs 45.9 IU/L, P value = 0.001) [24].
A summary analysis based on these four studies showed significantly lower odds of ALT level in patients co-infected with dengue than those uninfected with dengue (summary mean difference = − 6.55; 95% CI = − 10.05, − 3.05; I2 = 96%) (Fig. 8) [14, 15, 19, 24]. A study in French Guiana showed that the proportion of co-infected patients with high ALT (> 2 folds) (proportion = 13%) was not significantly different when compared to those uninfected with dengue (proportion = 41%) (p = 0.16) [22].
Fig. 8.
Forest plot showing the difference in ALT level of malaria and dengue co-infection and those of malaria mono-infection
Quality of the studies
Selection bias, study design, confounders, blinding, data collection methods, withdrawals, and dropouts according to the Effective Public Health Practice Project were summarized in Table 2 [11]. The majority of the studies showed strong quality in confounder and data collection methods. Most studies in this review were moderate in terms of selection bias. However, low quality in study design (cross-sectional study) and blinding were found in most of the studies. The overall rating based on the six criteria showed that none of the studies were of strong quality. Ten studies were of moderate quality and five studies were of weak quality. However, none of these studies were excluded from this review. The funnel plot showed that there was no publication bias detected in the meta-analysis (Fig. 9).
Table 2.
Assessment of the quality of the studies included in the review based on Effective Public Health Practice Project: Quality assessment tool for quantitative studies
| No. | Author, Year | Selection Bias | Study Design | Confounders | Blinding | Data collection method | Withdrawals and Drop-Out | Final Rating |
|---|---|---|---|---|---|---|---|---|
| 1 | Assir et al., 2014 [16] | 3 | 3 | 1 | 3 | 1 | NA | 3 |
| 2 | Baba et al., 2013 [18] | 2 | 3 | 3 | 3 | 2 | NA | 3 |
| 3 | Barua and Gill, 2016 [19] | 2 | 3 | 1 | 3 | 1 | NA | 2 |
| 4 | Carme et al., 2009 [20] | 2 | 3 | 1 | 3 | 2 | NA | 2 |
| 5 | Chipwaza et al., 2014 [21] | 2 | 3 | 3 | 3 | 2 | NA | 3 |
| 6 | Epelboin et al., 2012 [22] | 2 | 3 | 1 | 2 | 1 | NA | 2 |
| 7 | Hati et al., 2012 [23] | 3 | 3 | 2 | 3 | 2 | NA | 3 |
| 8 | Magalhaes et al., 2014 [14] | 2 | 3 | 1 | 2 | 1 | NA | 2 |
| 9 | Mendonça et al., 2015 [15] | 2 | 3 | 1 | 2 | 1 | NA | 2 |
| 10 | Mohapatra et al., 2012 [24] | 2 | 3 | 2 | 3 | 1 | NA | 2 |
| 11 | Mueller et al., 2014 [25] | 2 | 3 | 3 | 2 | 1 | NA | 2 |
| 12 | Rao et a., 2016 [26] | 2 | 3 | 3 | 3 | 1 | NA | 2 |
| 13 | Sow et al., 2016 [28] | 2 | 3 | 2 | 3 | 1 | NA | 2 |
| 14 | Swoboda et al., 2014 [27] | 3 | 3 | 3 | 3 | 2 | NA | 3 |
| 15 | Zaki SA and Shanbag P, 2010 [29] | 2 | 3 | 3 | 3 | 3 | NA | 3 |
Fig. 9.
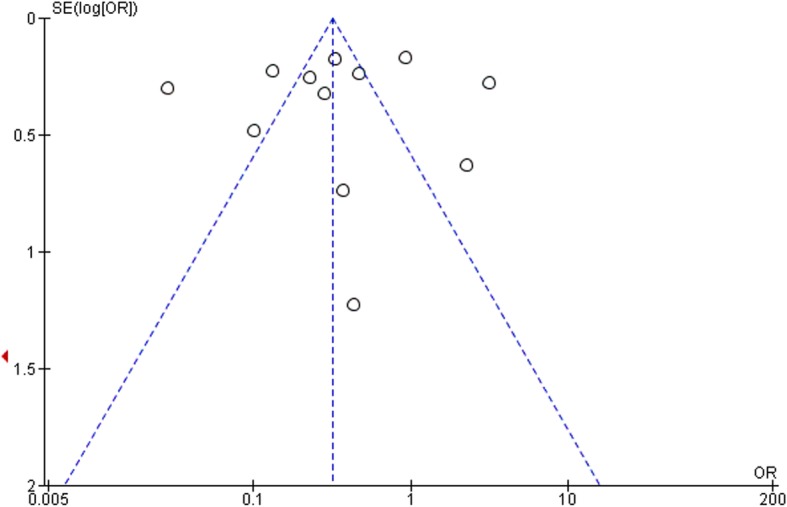
The funnel plot showed that there was no publication bias detected in the meta-analysis
Discussion
In the present systematic review of 13 studies based on 12,546 patients infected with malaria and/or dengue, a summary meta-analysis of these 13 studies confirmed decreased odds of co-infected patients as compared to those uninfected with dengue. The finding of a higher prevalence of Plasmodium co-infection with dengue could be due to both diseases sharing the same endemic regions [14]. In those areas (especially in rural, semi-urban, and urban areas), the vector Anopheles and Aedes are present throughout the year. Geographical overlap of both diseases exists for the 3.2 and 3.9 billion people who live in an endemic area for malaria and dengue, respectively [30, 31]. Therefore, the co-infection of both agents in a patient could not be ignored by physicians [26]. The co-infection of malaria and dengue had been reported in the Brazilian Amazon, Nigeria, India, French Guiana, and Tanzania [14, 18, 20, 21, 26, 32]. Infection by these two pathogens may share similar and non-specific clinical signs and symptoms – such as fever, headache, body ache, and fatigue – which may result in the difficulty of identifying one pathogen from the other [33]. A study indicated that the prevalence of co-infection was estimated among hospitalized patients but not in the community [14]. Another study in French Guiana reported that co-infection was due to the high rate of the population’s mobility to malaria endemic areas [20]. One study reported that co-infection frequency was higher especially during September to November [23]. Moreover, another study reported on an asymptomatic malaria infection with a low parasitemia course co-infection in an individual patient [34].
In regard to the immunity of individual patients, a previous study showed that co-infection has been associated with a strong activation of acute phase response, such as Interleukin 6 (IL-6), Tumor necrosis factor-α (TNF-α), and IL1-β and also Th1 cytokines (IFN-γ and IL-12). However, the lower levels of inflammation in the co-infected group were similar to DENV mono-infected subjects [33]. A co-infection exhibited positive TNF, IL-6, Interferon gamma (IFN-γ), IL-7, C-C Motif Chemokine Ligand 4 (CCL4), and IL-10 which was not observed in malaria mono-infection [15]. The co-infection may be caused by the DENV infection reactivating the hypnozoites of P. vivax in the liver, which were asymptomatic for months or years [33]. Previous studies that involved P. falciparum co-infection have reported fatalities [8, 35–38]. However, the frequency of severe clinical symptoms occurs in P. vivax co-infection [14, 22]. Those severe clinical symptoms may be caused by the activation of acute phase response including IL-6, TNF-α, IL1-β, IFN-γ, and IL-12 [33]. A previous study also showed that co-infection resulted in similar days of fever as compared to single malaria infection, which should therefore raise the suspicion of malaria co-infection [14].
The results from our meta-analysis found that co-infected patients exhibited lower malaria parasitemia than those with malaria single infection. This was in accordance with a previous study in French Guiana [22]. A previous study indicated that low parasitemia is a good predictive marker for less severe symptoms in co-infected patients [24]. The good outcome was because the concurrence of dengue and malaria led to patients seeking out medical treatment earlier (2.2 ± 0.4 days) than those with single malaria infection (5.5 ± 0.9 days), resulting in early diagnosis and treatment with antimalarial drugs [7, 36]. This was the frequency found in P. vivax co-infection [39–41].
Several studies reported on co-infection cases with severe anemia single malaria infection [14, 16, 22, 42]. Based on this meta-analysis, the hemoglobin level of co-infected patients were significantly higher than those with malaria single infection. This was in contrast to previous studies that indicated low hemoglobin in patients with co-infection [16, 42]. Malaria infection causes destruction of red blood cells followed by hemolysis and anemia [43]. Considering the clinical outcome, co-infection resulted in a lower rate of jaundice than those with dengue single infection [16].
Hematocrit is a marker used to diagnose dengue infection. Severe dengue results in hemoconcentration (the basal hematocrit > 20%), which is the result of increased vascular permeability and plasma leakage in endothelial [44]. Several studies reported no significant change in hematocrit among patients with co-infection when compared to those with malaria single infection [14–16, 19]. For malaria infection cases, low hematocrit was due to anemia, a common complication in both P. falciparum and P. vivax malaria [45]. However, this meta-analysis showed no significant odds in hematocrit level among those two groups of patients.
This meta-analysis found that co-infected patients had higher odds of platelet count as compared to malaria infected patients. This indicated that co-infected patients had a higher platelet level than malaria infected patients. Some studies indicated that co-infected patients also exhibited lower platelet count or thrombocytopenia than those with malaria single infection [15, 19, 22]. In regard to this meta-analysis, a significantly lower platelet level among co-infected patients was found in two studies [15, 19]. However, two other studies reported that malaria single infection exhibited thrombocytopenia more frequently than co-infection [14, 24]. For the clinical outcome of thrombocytopenia, co-infection had a higher chance of bleeding when compared to malaria single infection (OR 12.5, 95% CI: 4.7–33.3, P value = 0.001) [14].
AST is found in highest concentrations in the heart and also found in the liver, whereas ALT is found mainly in the liver [46]. Elevated AST and ALT can be seen in any type of liver cell injury [47]. Currently, the difference between liver enzymes (AST and ALT) and the clinical outcome of co-infection and malaria single infection is not well established. This meta-analysis found significantly lower levels of AST and ALT in co-infection when compared to malaria single infection. This indicated less liver injuries in the co-infected group. Liver injury was prominent in dengue single infection but not in malaria infection. A previous study showed that liver injuries and bleeding can lead to fulminant liver failure in dengue infection [48]. However, previous studies reported that hepatomegaly was very frequent in the co-infected group [14, 19]. Moreover, higher AST and ALT levels were associated with jaundice and hepatomegaly [14, 19]. Nevertheless, a report in French Guiana showed no differences in AST/ALT and parasitemia levels between co-infection and malaria single infection [22].
In terms of mortality, co-infection can lead to an increased mortality rate when compared to malaria single infection (6.3% compared to 5.5%) [19]. However, a study by Mohapatra et al. found a lower rate of mortality in co-infection as compared to malaria single infection [24]. The clinical outcome of co-infection was more similar to dengue single infection than malaria single infection. Therefore, the physician must be aware of co-infections in malaria cases with inadequate treatment response as well as screening for malaria parasite in patients with dengue [24].
The interaction of dengue and malaria in co-infections is unknown but the multiple infections may lead to a failure in treatment [49]. The underlying conditions in co-infections are rhabdomyolysis and sickle cell disease, which result from TNF-α and RBC sequestration in skeletal muscle, increased blood viscosity, and toxins from the parasite together with lactic acidosis [35]. A study found that co-infection had a predominance of Immunoglobulin M (IgM) antibody [22]. Another study indicated that malaria infection might be triggered by dengue infection, especially in the P. vivax infection [50].
This study had limitations. First, there was a high level of bias in the study design of the enrolled studies that were reviewed. Second, there was a high level of heterogeneity among the studies examining co-infection as compared to malaria single infection (Moran’s I2: 94%). Third, we lacked the access to some full text papers because our university does not subscribe to some publishers which reported on the co-infection of both agents.
Since malaria and dengue frequently co-exist in the same geographical areas, there are some public health implications. In addition, the clinical outcomes of co-infection were more like dengue mono-infection than malaria mono-infection. Therefore, healthcare workers including physicians, medical technicians, and nurses need to collaborate with each other in order to solve the difficulty of differentiating between both diseases in similar areas. Using clinical outcomes such as fever with typical paroxysm, cerebral malaria, renal failure, and multi-organ failure might rule out patients with co-infection. On the other hand, using bleeding signs might indicate patients with co-infection. Moreover, screening for malaria parasite in patients with dengue infection might help to diagnose patients suspected with co-infection [24].
Conclusion
In conclusion, the study findings showed that dengue and malaria co-infection was associated with decreased odds of malaria infection, malaria parasitemia, AST, and ALT levels when compared to malaria mono-infection. However, malaria and dengue co-infection was associated with increased odds of platelet and hemoglobin levels when compared to malaria mono-infection.
Additional file
Table S2. Search details for the PubMed. (DOCX 13 kb)
Acknowledgements
The authors would like to thank all published research that contributed to the data for this study. The authors are also grateful to Mr. David C. Chang for editing the grammar of this manuscript.
Abbreviations
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- CCL4
C-C Motif Chemokine Ligand 4
- CI
Confidence intervals
- DENV
Dengue virus
- IFN-γ
Interferon gamma
- IgM
Immunoglobulin M
- IL-6
Interleukin 6
- OR
Odds ratio
- TNF-α
Tumor necrosis factor-α
Authors’ contributions
MK and KU participated in the study design, data analysis, and writing of the paper. All authors read and approved the final paper.
Funding
This research was partially supported by the new strategic research (P2P) project, Walailak University, Thailand. The funders had a role in the collection, analysis, and interpretation of the data.
Availability of data and materials
The datasets used during the current study are available from the corresponding author based on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Manas Kotepui, Phone: +66954392469, Email: manas.ko@wu.ac.th.
Kwuntida Uthaisar Kotepui, Email: kwuntida.ut@wu.ac.th.
References
- 1.WHO . World malaria report 2016. Geneva: World Health Organization; 2017. [Google Scholar]
- 2.WHO . Dengue control. Geneva: World Health Organization; 2017. [Google Scholar]
- 3.Severe falciparum malaria. World Health Organization, Communicable Diseases Cluster . Transactions of the Royal Society of Tropical Medicine and Hygiene. 2000. pp. S1–90. [PubMed] [Google Scholar]
- 4.Chen I, Clarke SE, Gosling R, Hamainza B, Killeen G, Magill A, et al. “Asymptomatic” malaria: a chronic and debilitating infection that should be treated. PLoS Med. 2016;13(1):e1001942. doi: 10.1371/journal.pmed.1001942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartoloni A, Zammarchi L. Clinical aspects of uncomplicated and severe malaria. Mediterr J Hematol Infect Dis. 2012;4(1):e2012026. doi: 10.4084/mjhid.2012.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deresinski S. Concurrent plasmodium vivax malaria and dengue. Emerg Infect Dis. 2006;12(11):1802. doi: 10.3201/eid1211.060341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaushik RM, Varma A, Kaushik R, Gaur KJ. Concurrent dengue and malaria due to Plasmodium falciparum and P. vivax. Trans R Soc Trop Med Hyg. 2007;101(10):1048–1050. doi: 10.1016/j.trstmh.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 8.Charrel RN, Brouqui P, Foucault C, de Lamballerie X. Concurrent dengue and malaria. Emerg Infect Dis. 2005;11(7):1153–1154. doi: 10.3201/eid1107.041352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Degarege A, Degarege D, Veledar E, Erko B, Nacher M, Beck-Sague CM, et al. Plasmodium falciparum infection status among children with Schistosoma in sub-Saharan Africa: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2016;10(12):e0005193. doi: 10.1371/journal.pntd.0005193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quality assessment tool for quantitative studies 1998. Available from: http://www.ephpp.ca/index.html. Accessed 12 Jan 2017.
- 12.Comunity C. RevMan 5 2017. Available from: http://community.cochrane.org/tools/review-production-tools/revman-5. Accessed 12 Jan 2017.
- 13.Hayashino Y, Noguchi Y, Fukui T. Systematic evaluation and comparison of statistical tests for publication bias. J Epidemiol. 2005;15(6):235–243. doi: 10.2188/jea.15.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magalhaes BM, Siqueira AM, Alexandre MA, Souza MS, Gimaque JB, Bastos MS, et al. P. Vivax malaria and dengue fever co-infection: a cross-sectional study in the Brazilian Amazon. PLoS Negl Trop Dis. 2014;8(10):e3239. doi: 10.1371/journal.pntd.0003239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mendonca VR, Andrade BB, Souza LC, Magalhaes BM, Mourao MP, Lacerda MV, et al. Unravelling the patterns of host immune responses in Plasmodium vivax malaria and dengue co-infection. Malar J. 2015;14:315. doi: 10.1186/s12936-015-0835-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Assir MZ, Masood MA, Ahmad HI. Concurrent dengue and malaria infection in Lahore, Pakistan during the 2012 dengue outbreak. Int J Infect Dis. 2014;18:41–46. doi: 10.1016/j.ijid.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. 2008. [Google Scholar]
- 18.Baba M, Logue CH, Oderinde B, Abdulmaleek H, Williams J, Lewis J, et al. Evidence of arbovirus co-infection in suspected febrile malaria and typhoid patients in Nigeria. J Infect Dev Ctries. 2013;7(1):51–59. doi: 10.3855/jidc.2411. [DOI] [PubMed] [Google Scholar]
- 19.Barua A, Gill NA. Comparative study of concurrent dengue and malaria infection with their Monoinfection in a teaching Hospital in Mumbai. J Assoc Physicians India. 2016;64(8):49–52. [PubMed] [Google Scholar]
- 20.Carme B, Matheus S, Donutil G, Raulin O, Nacher M, Morvan J. Concurrent dengue and malaria in Cayenne hospital, French Guiana. Emerg Infect Dis. 2009;15(4):668–671. doi: 10.3201/eid1504.080891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chipwaza B, Mugasa JP, Selemani M, Amuri M, Mosha F, Ngatunga SD, et al. Dengue and chikungunya fever among viral diseases in outpatient febrile children in Kilosa district hospital, Tanzania. PLoS Negl Trop Dis. 2014;8(11):e3335. doi: 10.1371/journal.pntd.0003335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Epelboin L, Hanf M, Dussart P, Ouar-Epelboin S, Djossou F, Nacher M, et al. Is dengue and malaria co-infection more severe than single infections? A retrospective matched-pair study in French Guiana. Malar J. 2012;11:142. doi: 10.1186/1475-2875-11-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hati AK, Bhattacharjee I, Mukherjee H, Bandyopadhayay B, Bandyopadhyay D, De R, et al. Concurrent dengue and malaria in an area in Kolkata. Asian Pac J Trop Med. 2012;5(4):315–317. doi: 10.1016/S1995-7645(12)60046-7. [DOI] [PubMed] [Google Scholar]
- 24.Mohapatra MK, Patra P, Agrawala R. Manifestation and outcome of concurrent malaria and dengue infection. J Vector Borne Dis. 2012;49(4):262–265. [PubMed] [Google Scholar]
- 25.Mueller TC, Siv S, Khim N, Kim S, Fleischmann E, Ariey F, et al. Acute undifferentiated febrile illness in rural Cambodia: a 3-year prospective observational study. PLoS One. 2014;9(4):e95868. doi: 10.1371/journal.pone.0095868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rao MR, Padhy RN, Das MK. Prevalence of dengue viral and malaria parasitic co-infections in an epidemic district, Angul of Odisha, India: an eco-epidemiological and cross-sectional study for the prospective aspects of public health. J Infect Public Health. 2016;9(4):421–428. doi: 10.1016/j.jiph.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 27.Swoboda P, Fuehrer HP, Ley B, Starzengruber P, Ley-Thriemer K, Jung M, et al. Evidence of a major reservoir of non-malarial febrile diseases in malaria-endemic regions of Bangladesh. Am J Trop Med Hyg. 2014;90(2):377–382. doi: 10.4269/ajtmh.13-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sow A, Loucoubar C, Diallo D, Faye O, Ndiaye Y, Senghor CS, et al. Concurrent malaria and arbovirus infections in Kedougou, southeastern Senegal. Malar J. 2016;15:47. doi: 10.1186/s12936-016-1100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zaki SA, Shanbag P. Clinical manifestations of dengue and leptospirosis in children in Mumbai: an observational study. Infection. 2010;38(4):285–291. doi: 10.1007/s15010-010-0030-3. [DOI] [PubMed] [Google Scholar]
- 30.WHO . Fact Sheet: World Malaria Day 2016. Geneva: World Health Organization; 2016. [Google Scholar]
- 31.WHO . Dengue and severe dengue. Geneva: World Health Organization; 2017. [Google Scholar]
- 32.Santana Vdos S, Lavezzo LC, Mondini A, Terzian AC, Bronzoni RV, Rossit AR, et al. Concurrent dengue and malaria in the Amazon region. Rev Soc Bras Med Trop. 2010;43(5):508–511. doi: 10.1590/S0037-86822010000500007. [DOI] [PubMed] [Google Scholar]
- 33.Halsey ES, Baldeviano GC, Edgel KA, Vilcarromero S, Sihuincha M, Lescano AG. Symptoms and immune markers in Plasmodium/dengue virus co-infection compared with mono-infection with either in Peru. PLoS Negl Trop Dis. 2016;10(4):e0004646. doi: 10.1371/journal.pntd.0004646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alves FP, Durlacher RR, Menezes MJ, Krieger H, Silva LH, Camargo EP. High prevalence of asymptomatic Plasmodium vivax and Plasmodium falciparum infections in native Amazonian populations. Am J Trop Med Hyg. 2002;66(6):641–648. doi: 10.4269/ajtmh.2002.66.641. [DOI] [PubMed] [Google Scholar]
- 35.Yong KP, Tan BH, Low CY. Severe falciparum malaria with dengue coinfection complicated by rhabdomyolysis and acute kidney injury: an unusual case with myoglobinemia, myoglobinuria but normal serum creatine kinase. BMC Infect Dis. 2012;12:364. doi: 10.1186/1471-2334-12-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ward DI. A case of fatal Plasmodium falciparum malaria complicated by acute dengue fever in East Timor. Am J Trop Med Hyg. 2006;75(1):182–185. doi: 10.4269/ajtmh.2006.75.182. [DOI] [PubMed] [Google Scholar]
- 37.Ali N, Nadeem A, Anwar M, Tariq WU, Chotani RA. Dengue fever in malaria endemic areas. J Coll Physicians Surg Pak. 2006;16(5):340–342. [PubMed] [Google Scholar]
- 38.Alam A, Dm M. A case of cerebral malaria and dengue concurrent infection. Asian Pac J Trop Biomed. 2013;3(5):416–417. doi: 10.1016/S2221-1691(13)60087-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mueller I, Widmer S, Michel D, Maraga S, McNamara DT, Kiniboro B, et al. High sensitivity detection of Plasmodium species reveals positive correlations between infections of different species, shifts in age distribution and reduced local variation in Papua New Guinea. Malar J. 2009;8:41. doi: 10.1186/1475-2875-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steenkeste N, Rogers WO, Okell L, Jeanne I, Incardona S, Duval L, et al. Sub-microscopic malaria cases and mixed malaria infection in a remote area of high malaria endemicity in Rattanakiri province, Cambodia: implication for malaria elimination. Malar J. 2010;9:108. doi: 10.1186/1475-2875-9-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katsuragawa TH, Gil LH, Tada MS, de Almeida e Silva A, Costa JD, Araujo Mda S, et al. The dynamics of transmission and spatial distribution of malaria in riverside areas of Porto Velho, Rondonia, in the Amazon region of Brazil. PLoS One. 2010;5(2):e9245. doi: 10.1371/journal.pone.0009245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abbasi A, Butt N, Sheikh QH, Bhutto AR, Munir SM, Ahmed SM. Clinical features, diagnostic techniques and management of dual dengue and malaria infection. J Coll Physicians Surg Pak. 2009;19(1):25–29. [PubMed] [Google Scholar]
- 43.Haldar K., Mohandas N. Malaria, erythrocytic infection, and anemia. Hematology. 2009;2009(1):87–93. doi: 10.1182/asheducation-2009.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.WHO . Clinical diagnosis. Geneva: World Health Organization; 1997. [Google Scholar]
- 45.Douglas NM, Anstey NM, Buffet PA, Poespoprodjo JR, Yeo TW, White NJ, et al. The anaemia of Plasmodium vivax malaria. Malar J. 2012;11:135. doi: 10.1186/1475-2875-11-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mauro PRB, Wouter W. In: ER CAB, David EB, editors. Tietz text book of clinical chemistry and molecular diagnostics. 4th ed. St. Louis: Elsevier; 2006. p. 604–16.
- 47.Thapa BR, Walia A. Liver function tests and their interpretation. Indian J Pediatr. 2007;74(7):663–671. doi: 10.1007/s12098-007-0118-7. [DOI] [PubMed] [Google Scholar]
- 48.Seneviratne SL, Malavige GN, de Silva HJ. Pathogenesis of liver involvement during dengue viral infections. Trans R Soc Trop Med Hyg. 2006;100(7):608–614. doi: 10.1016/j.trstmh.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 49.Singhsilarak T, Phongtananant S, Jenjittikul M, Watt G, Tangpakdee N, Popak N, et al. Possible acute coinfections in Thai malaria patients. Southeast Asian J Trop Med Public Health. 2006;37(1):1–4. [PubMed] [Google Scholar]
- 50.Hanf M, Stephani A, Basurko C, Nacher M, Carme B. Determination of the Plasmodium vivax relapse pattern in Camopi, French Guiana. Malar J. 2009;8:278. doi: 10.1186/1475-2875-8-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S2. Search details for the PubMed. (DOCX 13 kb)
Data Availability Statement
The datasets used during the current study are available from the corresponding author based on reasonable request.