Abstract
Context:
Viscosupplementation is widely used for management of knee osteoarthritis. Many formulations of hyaluronic acid (HA) are available, ranging from a single injection to a series of up to 5 injections per treatment.
Objective:
To compare efficacy between single and multiple HA injection formulations.
Data Sources:
MEDLINE, EMBASE, Cochrane, Web of Science, Scopus databases were all searched.
Study Selection:
Full-text prospective randomized and nonrandomized controlled human trials, cohort studies, and cost-effectiveness evaluations in the English language comparing different injection regimens of viscosupplementation were included.
Study Design:
Systematic review.
Level of Evidence:
Level 1.
Data Extraction:
Data were collected using the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. Four databases were searched by a librarian and the principal investigator, identifying 6196 articles for screening.
Results:
Eleven studies met the inclusion criteria. Of the studies comparing single- with multiple-injection formulations of HA for treatment of knee osteoarthritis, there was no consistent difference in patient-reported outcomes. Furthermore, 5-injection formulations do not appear to be superior to 3-injection formulations.
Conclusion:
There are limited head-to-head trials comparing viscosupplementation formulations that differ based on number of injections, and in particular, there is a paucity of trials evaluating single-injection formulations. Based on the currently available data, there appears to be similar efficacy with the possibility for greater cost-effectiveness and less patient inconvenience with single-injection formulations.
Keywords: hyaluronic acid, viscosupplementation, knee osteoarthritis
Osteoarthritis (OA) is the most common disease affecting synovial joints and a leading cause of morbidity affecting more than 30 million US adults.10 Knee OA contributes significantly to the financial burden of health care in this country, with an estimated average discounted lifetime cost per person of $140,300.17,22,25 Thus, there has been a justifiable focus on continued development of cost-effective nonoperative treatments of knee OA that have the potential to manage symptoms, improve function, and delay the need for joint replacement.
Viscosupplementation, also referred to as hyaluronic acid (HA), was approved by the Food and Drug Administration for treatment of knee OA in 1997 and designed to address the observed decrease in HA molecular weight and concentration of synovial fluid in the setting of OA.29 Native HA is a glycosaminoglycan synthesized by type-B synoviocytes, fibrobasts, and chondrocytes consisting of repeating units of disaccharide N-acetylglucosamine and sodium glucoronate with rheologic properties ranging between viscous and elastic behaviors.4,29 In vivo studies have demonstrated that viscosupplementation has the ability to contribute to normalization of synovial fluid properties, suppression of cartilage degeneration, and protection of joint surfaces.19,29 Clinical studies have shown its association with alleviation of pain, stiffness, and functional impairments, with the greatest improvement in younger patients with mild to moderate knee OA.8,16
Intra-articular HA injections are associated with a longer duration of symptomatic relief and less potential for cumulative harm than corticosteroid injections.7 There are currently 12 FDA-approved viscosupplementation products available in the United States (Table 1).29 The products vary in their origin, elastoviscous properties, and dosing schedules.5 The early formulations were composed of 3- or 5-injection dosing regimens; however, Synvisc-One became FDA approved in 2009 as the first single-injection option. Though there have been comparative studies on high versus low molecular weight preparations, we have a particular interest in the comparative effectiveness of the multiple- versus single-injection preparations.18,20,24,28,29 We hypothesize that the single-injection regimens (Durolane, Gel-One, Monovisc, Synvisc-One) provide a similar efficacy and have a reduced burden to both patient and provider in the way of cost, time, and potential for adverse events as compared with the 3- or 5-injection series (Euflexxa, Hyalgan, Orthovisc, Supartz FX, Synvisc, Gelsyn-3, Genvisc 850).
Table 1.
List of FDA-approved viscosupplementation formulations
| Brand | Medication | Dosing Regimen | Drug Cost per Injection, a $ | Drug Cost per Treatment, $ | Total Cost (Drug + Procedure) per Treatment, b $ |
|---|---|---|---|---|---|
| Durolane | Hyaluronic acid 60 mg per 3 mL | Single injection | N/A | N/A | N/A |
| Euflexxa | Sodium hyaluronate 20 mg per 2 mL | 3 weekly injections | 141.79 | 425.37 | 569.01 |
| Gel-One | Hyaluronate 30 mg per 3 mL | Single injection | 537.36 | 537.36 | 585.25 |
| Gelsyn-3 | 16.8 mg per 2 mL | 3 weekly injections | N/A | N/A | N/A |
| Genvisc 850 | Sodium hyaluronate 25 mg per 2.5 mL | 5 weekly injections | N/A | N/A | N/A |
| Hyalgan | Sodium hyaluronate 20 mg per 2 mL | 3-5 weekly injections | 85.30 | 255.90 (3) 426.5 (5) |
399.54 (3) 665.90 (5) |
| Hymovis | Hyaluronan 24 mg per 3 mL | 2 weekly injections | N/A | N/A | N/A |
| Monovisc | Hyaluronan 88 mg per 4 mL | Single injection | 779.35 | 779.35 | 827.23 |
| Orthovisc | Hyaluronan 30 mg per 2 mL | 3 weekly injections | 147.79 | 443.37 | 587.01 |
| Supartz FX | 25 mg per 2.5 mL | 3 weekly injections | 85.30 | 255.90 | 399.54 |
| Synvisc | Hylan G-F 20 16 mg per 2.25 mL | 3 weekly injections | 189.22 | 567.66 | 711.30 |
| Synvisc-One | Hylan G-F 20 48 mg per 6 mL | Single injection | 567.66 | 567.66 | 615.54 |
FDA, Food and Drug Administration; N/A, not applicable.
According to Centers for Medicare & Medicaid Services October 2018 Average Sales Price Pricing File (updated September 12, 2018).
Accounting for $47.88 charge for Current Procedural Terminology code 20610, “Drain/inj joint/bursa w/o us” performed in a national payment amount for a procedure performed in a facility according to Centers for Medicare & Medicaid Services 2018 Physician Fee Schedule Search.
Methods
Search Strategy
This study was constructed using the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.21 Comprehensive literature searches investigating HA injections for the treatment of knee OA were developed and performed by a medical librarian, on consultation with the principal investigator.
All searches were run on August 3, 2017, via Ovid MEDLINE (In-Process & Other Non-Indexed Citations and Ovid MEDLINE 1946 to Present); Ovid EMBASE (1974 to present); Web of Science (Core Collection); and The Cochrane Library (Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, Cochrane Methodology Register, and Technology Assessments). Search terms included all subject headings and/or keywords associated with “Hyaluronic Acid,” “Viscosupplements,” “Viscosupplementation,” “Intra-articular Injections,” “Knee Joint,” “Knee Osteoarthritis,” and “Osteoarthritis.” Returned articles were limited to human-only studies. There were no language, publication date, or article-type restrictions implemented at this stage. The full Ovid MEDLINE Search strategy is available in Appendix 1 (available in the online version of this article).
Study Selection
Citations were imported into Covidence, an evidence synthesis software product. After excluding duplicates, 2 researchers independently screened the title and abstract of 2890 citations. Discrepancies were resolved by an independent third investigator. All citations were reviewed against predefined inclusion criteria, which included randomized controlled trials, cohort studies, controlled trials, case-control studies, and case series in the English language involving human cohorts at least 18 years of age with OA receiving viscosupplementation.
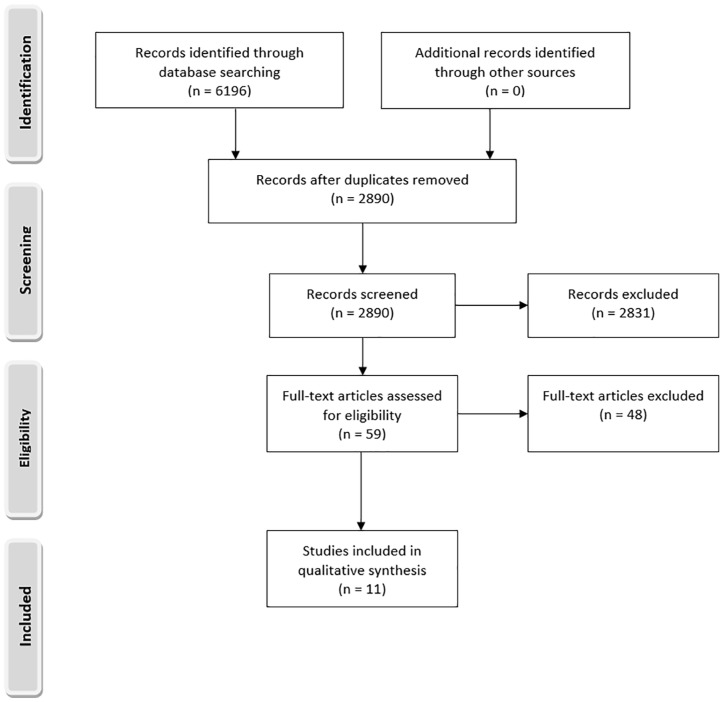
Full-text review followed the initial title and abstract screening phase. Articles selected for final inclusion followed the same process utilized above during title and abstract screening. Overall, a total of 11 articles1,3,9,11,13,15,18,20,24,26,28 met inclusion criteria (Appendix Table A1 available online). See the full PRISMA flow diagram in Figure 1.
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram.
Results
Single Versus Multiple Injections
Four prospective randomized studies1,9,13,15 specifically evaluated single-injection formulations versus 3- or 5-injection series in a head to head manner.
Carrabba et al9 in 1992 evaluated 100 patients’ Lequesne’s Index of Severity for Osteoarthritis of the Knee (ISOAK) and visual analogue scale (VAS) scores at intervals up to 60 days. The patients were divided into 5 groups: placebo group, arthrocentesis-alone group, and 3 sodium hyaluronate 20 mg/2 mL (Hyalgan) groups (given once weekly for 1 week, 3 weeks, and 5 weeks). ISOAK scoring accounts for pain, maximum distance walking, and ease of completing activities of daily living. Though there was a statistically significant 23.2% improvement in ISOAK score at 60 days in favor of the 5 injections versus the single injection (9.9% improvement, P < 0.0051), there was no difference between 3 injections and 5 injections. Limitations of this study included a relatively small sample size, presence of an effusion as an inclusion criterion, loss of 80% of patients to follow-up after 4 months, and lack of volume adjustment thereby delivering a lower molecular weight than the other available FDA-approved single-injection formulations.
In 2009, Conrozier et al13 published a pilot prospective multicenter, open label, randomized trial in France and Germany that also evaluated variable volumes of hylan G-F 20 (Synvisc) over a period of 6 months. A total of 120 patients with unilateral OA were randomized into 5 groups including a single 6-mL injection group and a single 4-mL injection group. The remainder of the groups received a combination of volumes given either 2 or 3 weeks apart. Using a primary outcome of VAS pain at 24 weeks, the single 6-mL injection was found to be as efficacious and well tolerated as 2 mL weekly for 3 consecutive weeks. Additionally, there was a retreatment algorithm within this study for those patients who had worsening VAS by at least 15 mm at week 24, and the 6-mL single-injection group had the lowest number of retreated patients. Though this study had a relatively small sample size and was not double-blinded, it used an appropriate FDA-approved volume of a single-injection formulation and demonstrated similar outcomes in pain and function when compared with the multiple injection series.
Al-Omran and Azam1 in 2014 published an additional prospective double-blinded randomized study comparing hylan G-F 20 (Synvisc, 3 injections), hyaluronic acid 60 mg/3 ml (Durolane, single injection), and hyaluronic acid 40 mg/2 mL (Osteonil, 3 or 5 injections). A total of 227 patients were blinded and randomized to 1 of 3 groups as above. Baseline Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores were compared at intervals up to 6 months and demonstrated hylan G-F 20 (Synvisc) to be most effective, showing statistically significant improvement over both HA 40 mg/2 mL (Osteonil) (mean difference 7.68, P < 0.001) and HA 60 mg/3 mL (Durolane) (mean difference 15.61, P < 0.001). HA 40 mg/2 mL (Osteonil) was also found to be superior to HA 60 mg/3 mL (Durolane) (mean difference 7.93, P < 0.001).
Finally, Estades-Rubio et al15 performed a prospective randomized cost analysis study in 2017 evaluating HA 60 mg/3 mL (Durolane) (single injection), versus sodium hyaluronate 25 mg/2.5 mL (Supartz FX) (5 injections) in 54 patients with unilateral OA. Though the sample size was small and patients were not followed beyond 24 weeks, they had improved WOMAC scores, less need for additional analgesia (P = 0.006), and there was a lower cost of HA 60 mg/3 mL (Durolane) single-injection formulation compared with the 5-injection formulation (152 vs 167 euros).
Three Versus 5 Injections
Though there is less evidence in the literature related to the evaluation of single-injection preparations, there have been several studies comparing 3 injections with 5 injections in an effort to examine the role of molecular weight.
A prospective open label randomized trial conducted by Lee et al20 in 2006 compared sodium hyaluronate 20 mg/2 mL (Hyruan Plus) with a high molecular weight of 3000 kDa given once weekly for 3 weeks, to sodium hyaluronate 20 mg/2 mL (Hyal/Hyalgan) with a low molecular weight of 750 kDa given once weekly for 5 weeks in 146 patients. No significant difference was found between groups in weightbearing VAS, WOMAC, or adverse events. This was an open label trial prohibiting the advantage of double-blinding, however, allowing for the FDA-recommended dosing regimens to be used.
In 2007, Stitik et al28 published a single-blinded parallel design study in 60 patients comparing 3 versus 5 injections as well as 3 injections versus 3 injections plus a home exercise program (HEP). All groups had mean symptomatic improvement that was clinically and statistically significant. The 3 weekly injections plus HEP group had faster onset and greater peak effect as well as longer duration of relief. This study was limited by its small sample size, the 13.3% of participants that discontinued prematurely in the first 6 months of the study, and the 53.3% of participants that discontinued within the first 12 months. However, there was not a significant difference between the regimens when the HEP was removed.
In 2008, Raman et al24 compared a 3-week series of hylan G-F 20 (Synvisc) to a 5-week series of sodium hyaluronate 20 mg/2 mL (Hylagan) in 392 patients with primary unilateral knee OA. Difference in VAS at 6 months was greater in those who received the hylan G-F 20 (Synvisc), and the effects lasted for 12 months when compared with the 5-dose regimen that resulted in lower overall VAS change and 6-month duration of effectiveness. Of note, there was a compliance difference between the groups (99.4% in the Hylan G-F 20 [Synvisc] group vs 92.2% in the sodium hyaluronate [Hyalgan] group), likely related to the increased number of injections required in the 5-injection cohort.
Atay et al3 performed a similar study of Hylan G-F 20 (Synvisc) 3-dose weekly injection series compared with the sodium hyaluronate 20 mg/2 mL (Hyalgan) 5-dose weekly injection series and a control group (saline) in 45 patients 1 year from arthroscopic debridement. There was no difference in WOMAC scores between the 3 groups at 6 months after the debridement; however, pre- and posttreatment scores improved equally in both the hylan G-F 20 (Synvisc) and sodium hyaluronate 20 mg/2 mL (Hyalgan) groups compared with the controls.
Chou et al11 compared 3 injections of hylan G-F 20 (Synvisc) in 1 knee with 5 injections of sodium hyaluronate 25 mg/2.5 mL (Supartz FX) in the contralateral knee in 37 patients in 2009 and demonstrated time-dependent score improvement in all patients, but improved VAS scores before week 20 and better cost-effectiveness in hylan G-F 20 (Synvisc). This study was limited by its small sample size and nonrandomized design. In addition, the majority of patients chose sodium hyaluronate 25 mg/2.5 mL (Supartz FX) for the more painful knee, as it was the better established option in Taiwan at that time.
Finally, in 2016, Gigis et al18 compared high- and low-molecular-weight HA in 80 patients with unilateral knee OA via VAS and WOMAC scores at intervals up to 1 year. Again, both groups had significant improvements in pain, stiffness, and function starting at 5 weeks into the treatment that lasted for about 1 year, and there was no significant difference between the groups.
Two Versus 3 Injections
In 1994, Scale et al26 evaluated hylan G-F 20 (Synvisc) over a 6-month period administered as 2 separate injections given 2 weeks apart and as 3 separate injections 1 week apart. Both dosing frequencies resulted in better pain scores and overall evaluation of treatment at 12 weeks compared with placebo, and the 3-week dosing regimen was initially superior to the 2-week regimen with similar results at 6 months. This was an industry-sponsored study with 80 participants.
Discussion
Knee OA is the most common cause of disability in the United States, with costs nearing $128 billion dollars a year.14,27 Physical inactivity related to disease processes, such as OA, can lead to a number of noncommunicable diseases such as heart disease, stroke, diabetes, breast and colon cancer, hypertension, obesity, and mental health disorders.31 Additionally, according to the World Health Organization, physical inactivity is the fourth leading cause of death worldwide.
The continued rise in the mean age of the active patient population has placed a greater emphasis on the need for treatment options that allow patients to maintain an active lifestyle. Between 2010 and 2012, 52.5 million adults in the United States aged 18 years and older (22.7% of the total adult population) were diagnosed with some form of arthritis.6 Currently, the United States—when compared with other developed countries around the world—spends the most amount of money on health care and has the poorest outcomes.23 As health care evolves from the traditional fee-for-service structure, value-based models that emphasize improved quality of care at lower costs become more beneficial for the health of populations.
This systematic review found no consistent difference in efficacy of viscosupplementation formulations based on the number of injections when compared head to head. Many of the studies were limited by small samples sizes, nonblinding, lack of a control group, and additional confounding variables such as differences in molecular weights of the injections being compared and concurrent OA treatments. A recent systematic review and meta-analysis compared the pooled effect of single versus multiple injections of HA versus saline injections.12 This review found improved outcomes at 3 and 6 months from studies involving 2 to 4 injections compared with 5 injections or more. There were few studies of single-injection formulations, thereby limiting the analysis. However, studies of 5 or more injections reported higher adverse events compared with single or 2- to 4-injection formulations. Our current review identified only studies that performed a head-to-head comparison of a varying number of HA injections. Although there was an insufficient number of these studies and too much heterogeneity to perform a meta-analysis, through this systematic review we found no optimal number of injections that consistently demonstrated improved effectiveness. Further high-quality research on viscosupplementation formulations is needed to refine nonoperative treatment of knee OA.
When counseling patients with knee OA on the available treatment options, physicians should discuss not only the debated effectiveness of viscosupplementation but also the various considerations for single- versus multiple-injection formulations. This includes the cost of the medication, procedure fee(s), discomfort and adverse events associated with each injection, travel expenses to appointments, and time away from normal life activities.2,7,30 Although not all pricing data are currently available for the FDA-approved HA injections, the most conservative estimates for the overall treatment costs range from about $400 to more than $800 (Table 1). This does not take into consideration the indirect costs of multiple treatments, such as transportation, parking, and time away from work. Additionally, scheduling patients for multiple injections when fewer injections may provide similar relief can also result in decreased access and increased wait times for other patients needing care.
In the current health care environment in the United States where medical spending continues to increase,23 physicians are being encouraged to be conscious of costs associated with ordering tests and prescribing treatments. Since at this time, there does not appear to be a specific HA preparation or dosing regimen that is clearly most effective, physicians should be aware of the direct costs of the available options and also consider the various indirect costs and effect on patient access. The results of this review should encourage physicians to evaluate their treatment practices to ensure that the safest and most cost-effective approach is recommended to their patients.
Supplemental Material
Supplemental material, SPH861545_Supplemental_Material_CLN for Comparative Effectiveness of Alternative Dosing Regimens of Hyaluronic Acid Injections for Knee Osteoarthritis: A Systematic Review by Kathryn McElheny, Brett Toresdahl, Daphne Ling, Keith Mages and Irfan Asif in Sports Health: A Multidisciplinary Approach
Footnotes
The following authors declared potential conflicts of interest: I.A. is an associate editor for Sports Health.
References
- 1. Al-Omran A, Azam Q. Efficacy of viscosupplementation in knee osteoarthritis: a clinical trial of three agents. Bahrain Med Bull. 2014;36(3):1. [Google Scholar]
- 2. American Academy of Orthopaedic Surgeons. Treatment of osteoarthritis of the knee: evidence-based guideline. 2nd ed. 2013. https://www.aaos.org/research/guidelines/treatmentofosteoarthritisofthekneeguideline.pdf. Accessed Ocotber 31, 2018. [DOI] [PubMed]
- 3. Atay T, Aslan A, Baydar M, Ceylan B, Baykal B, Kirdemir V. The efficacy of low- and high-molecular-weight hyaluronic acid applications after arthroscopic debridement in patients with osteoarthritis of the knee. Acta Orthop Traumatol Turc. 2008;42:228-233. [DOI] [PubMed] [Google Scholar]
- 4. Bagga H, Burkhardt D, Sambrook P, March L. Longterm effects of intraarticular hyaluronan on synovial fluid in osteoarthritis of the knee. J Rheumatol. 2006;33:946-950. [PubMed] [Google Scholar]
- 5. Balazs EA. Viscosupplementation for treatment of osteoarthritis: from initial discovery to current status and results. Surg Technol Int. 2004;12:278-289. [PubMed] [Google Scholar]
- 6. Barbour KE, Helmick CG, Boring M, Brady TJ. Vital signs: prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation—United States, 2013-2015. MMWR Morb Mortal Wkly Rep. 2017;66:246-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;2:CD005321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bhandari M, Bannuru RR, Babins EM, et al. Intra-articular hyaluronic acid in the treatment of knee osteoarthritis: a Canadian evidence-based perspective. Ther Adv Musculoskelet Dis. 2017;9:231-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carrabba M, Paresce E, Angelini M, et al. Efficacy and safety of different dose-schedules of intraarticular injections of hyaluronic-acid in painful and hydarthrodial osteoarthritis of the knee—results of a prospective randomized, double-blind, placebo or arthrocentesis controlled-study. Br J Rheumatol. 1992;31:127. [Google Scholar]
- 10. Centers for Disease Control and Prevention. Arthritis: National Statistics 2018. https://www.cdc.gov/arthritis/data_statistics/national-statistics.html. Accessed October 31, 2018.
- 11. Chou CW, Lue KH, Lee HS, Lin RC, Lu KH. Hylan G-F 20 has better pain relief and cost-effectiveness than sodium hyaluronate in treating early osteoarthritic knees in Taiwan. J Formos Med Assoc. 2009;108:663-672. [DOI] [PubMed] [Google Scholar]
- 12. Concoff A, Sancheti P, Niazi F, Shaw P, Rosen J. The efficacy of multiple versus single hyaluronic acid injections: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2017;18:542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Conrozier T, Jerosch J, Beks P, et al. Prospective, multi-centre, randomised evaluation of the safety and efficacy of five dosing regimens of viscosupplementation with hylan G-F 20 in patients with symptomatic tibio-femoral osteoarthritis: a pilot study. Arch Orthop Trauma Surg. 2009;129:417-423. [DOI] [PubMed] [Google Scholar]
- 14. Department of Health and Human Services. Human cells, tissues, and cellular and tissue-based products. Title 21 Code of Federal Regulations, Pt. 1271 2018. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?cfrpart=1271&showfr=1. Accessed October 31, 2018.
- 15. Estades-Rubio F, Reyes-Martin A, Morales-Marcos V, et al. Knee viscosupplementation: cost-effectiveness analysis between stabilized hyaluronic acid in a single injection versus five injections of standard hyaluronic acid. Int J Mol Sci. 2017;18:e658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Evaniew N, Hanson B, Winemaker M. Viscosupplementation for knee osteoarthritis: current evidence and recommendations. J Long Term Eff Med Implants. 2013;23:151-159. [DOI] [PubMed] [Google Scholar]
- 17. Felson DT. The epidemiology of knee osteoarthritis: results from the Framingham Osteoarthritis Study. Semin Arthritis Rheum. 1990;20(3)(suppl 1):42-50. [DOI] [PubMed] [Google Scholar]
- 18. Gigis I, Fotiadis E, Nenopoulos A, Tsitas K, Hatzokos I. Comparison of two different molecular weight intra-articular injections of hyaluronic acid for the treatment of knee osteoarthritis. Hippokratia. 2016;20:26-31. [PMC free article] [PubMed] [Google Scholar]
- 19. Karatay S, Kiziltunc A, Yildirim K, Karanfil RC, Senel K. Effects of different hyaluronic acid products on synovial fluid levels of intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in knee osteoarthritis. Ann Clin Lab Sci. 2004;34:330-335. [PubMed] [Google Scholar]
- 20. Lee PB, Kim YC, Lim YJ, et al. Comparison between high and low molecular weight hyaluronates in knee osteoarthritis patients: open-label, randomized, multicentre clinical trial. J Int Med Res. 2006;34:77-87. [DOI] [PubMed] [Google Scholar]
- 21. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:B2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Losina E, Paltiel AD, Weinstein AM, et al. Lifetime medical costs of knee osteoarthritis management in the United States: impact of extending indications for total knee arthroplasty. Arthritis Care Res (Hoboken). 2015;67:203-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA. 2018;319:1024-1039. [DOI] [PubMed] [Google Scholar]
- 24. Raman R, Dutta A, Day N, Sharma H, Shaw C, Johnson G. Efficacy of Hylan G-F 20 and sodium hyaluronate in the treatment of osteoarthritis of the knee—a prospective randomized clinical trial. Knee. 2008;15:318-324. [DOI] [PubMed] [Google Scholar]
- 25. Rosen J, Sancheti P, Fierlinger A, Niazi F, Johal H, Bedi A. Cost-effectiveness of different forms of intra-articular injections for the treatment of osteoarthritis of the knee. Adv Ther. 2016;33:998-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Scale D, Wobig M, Wolpert W. Viscosupplementation of osteoarthritic knees with hylan—a treatment schedule study. Curr Ther Res Clin Exp. 1994;55:220-232. [Google Scholar]
- 27. Showery JE, Kusnezov NA, Dunn JC, Bader JO, Belmont PJ, Jr, Waterman BR. The rising incidence of degenerative and posttraumatic osteoarthritis of the knee in the United States military. J Arthroplasty. 2016;31:2108-2114. [DOI] [PubMed] [Google Scholar]
- 28. Stitik TP, Blacksin MF, Stiskal DM, et al. Efficacy and safety of hyaluronan treatment in combination therapy with home exercise for knee osteoarthritis pain. Arch Phys Med Rehabil. 2007;88:135-141. [DOI] [PubMed] [Google Scholar]
- 29. Stitik TP, Issac SM, Modi S, Nasir S, Kulinets I. Effectiveness of 3 weekly injections compared with 5 weekly injections of intra-articular sodium hyaluronate on pain relief of knee osteoarthritis or 3 weekly injections of other hyaluronan products: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2017;98:1042-1050. [DOI] [PubMed] [Google Scholar]
- 30. Trojian TH, Concoff AL, Joy SM, Hatzenbuehler JR, Saulsberry WJ, Coleman CI. AMSSM scientific statement concerning viscosupplementation injections for knee osteoarthritis: importance for individual patient outcomes. Clin J Sport Med. 2016;26:1-11. [DOI] [PubMed] [Google Scholar]
- 31. Warburton DE, Charlesworth S, Ivey A, Nettlefold L, Bredin SS. A systematic review of the evidence for Canada’s Physical Activity Guidelines for Adults. Int J Behav Nutr Phys Act. 2010;7:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, SPH861545_Supplemental_Material_CLN for Comparative Effectiveness of Alternative Dosing Regimens of Hyaluronic Acid Injections for Knee Osteoarthritis: A Systematic Review by Kathryn McElheny, Brett Toresdahl, Daphne Ling, Keith Mages and Irfan Asif in Sports Health: A Multidisciplinary Approach