Abstract
Background
Cytokines were correlated with survival and disease progression in acute myeloid leukemia (AML). We aimed to evaluate the multivariate effect of TNF‐α rs361525, rs1800750, rs1800629, IL‐10 rs1800896, rs1800872, IL‐6 rs1800795, TGF‐β1 rs1800470, IFN‐γ rs2430561 single nucleotide polymorphisms (SNPs) on AML risk, the multivariate effect of SNPs on overall survival (OS) in AML and the association between the investigated SNPs and prognostic factors in AML.
Methods
All SNPs were genotyped in 226 adult AML cases and 406 healthy individuals. AML patients were investigated for FLT3 (ITD, D835), DNMT3A (R882), and NPM1 type A mutations.
Results
Univariate analysis revealed that age above 65 years had a negative influence on survival (P < .001). The presence of the rs1800750 variant genotype (P = .005) or FLT3‐ITD mutation (P = .009) in a cytogenetic high‐risk group (P = .003) negatively influenced OS. A negative association was observed between Eastern Cooperative Oncologic Group Scale status > 2, lactate dehydrogenase (LDH) level, platelet (PLT) count <40 000 cells/mm3, and OS. Multivariate Cox regression analysis showed that the presence of the rs1800750 variant genotype was a risk factor for death (P = .007), and that blast percentage, LDH level (≥600 IU/L), and cytogenetic high‐risk were independent significant predictors for death in AML (P = .04, corrected HR = 1.20; P = .022, corrected HR = 1.24; P = .021, corrected HR = 1.34, respectively).
Conclusions
Age above 65 years, PLT count, TNF‐α rs1800750 variant genotype, blast percentage, LDH level, and cytogenetic high‐risk may be used as independent risk factors to assess AML mortality.
Keywords: acute myeloid leukemia, cytokine, gene polymorphisms, mortality, prognostic factors
We aimed to evaluate the multivariate effect of TNF‐α rs361525, rs1800750, rs1800629, IL‐10 rs1800896, rs1800872, IL‐6 rs1800795, TGF‐β1 rs1800470, IFN‐γ rs2430561 single nucleotide polymorphisms (SNPs) on AML risk, the multivariate effect of SNPs on overall survival (OS) in AML and the association between the investigated SNPs and prognostic factors in AML. A negative association was observed between Eastern Cooperative Oncologic Group Scale status >2, lactate dehydrogenase (LDH) level, platelet (PLT) count <40 000 cells/mm3, and OS. Ages above 65 years, PLT count, TNF‐α rs1800750 variant genotype, blast percentage, LDH level, and cytogenetic high‐risk may be used as independent risk factors to assess AML mortality.

1. INTRODUCTION
Acute myeloid leukemia (AML) is a complex and dynamic human malignancy characterized by multiple somatically‐acquired driver mutations, poor prognosis, and short survival, less than 20% of adult AML patients surviving 5 years after diagnosis.1, 2
Cytokines (interleukins [ILs], growth factors, interferons, etc) play an important role in regulating the inflammatory response, and chronic inflammation and are involved in cancer development.3, 4 Chronic inflammation is associated with the release of various mediators (pro‐inflammatory and oncogenic ones), such as reactive nitrogen oxygen species, inflammatory cytokines (IL‐1β, IL‐2, IL‐6, and tumor necrosis factor alpha [TNF‐α]), growth factors, and chemokines.4
Based on their inflammatory activity, cytokines are divided into pro‐inflammatory (IL‐6, IL‐17, IL‐18, TNF‐α, interferon gamma [IFN‐γ], etc) 5 and anti‐inflammatory (Il‐4, IL‐10, IL‐13, etc) ones, whereas several cytokines have a dual role (IL‐10, IL‐22, TGF‐β1).6 Interleukin‐10, an anti‐inflammatory cytokine, has dual functions, being both immune‐suppressive (tumor‐inhibiting) and immune‐stimulating (tumor‐promoting), and may, therefore, influence tumor susceptibility and development. Transforming growth factor beta (TGF‐β) regulates normal hematopoiesis, which is frequently disrupted in hematologic malignancies. The most common alteration in hematologic malignancies is the development of resistance to TGF‐β homeostatic functions such as proliferation, differentiation, and apoptosis.7
Aberrant cytokine production was hypothesized to play a substantial role in the pathogenesis of cancer and several hematological malignancies, including myeloproliferative neoplasms (MPN) and AML.8
Disturbances in proinflammatory (IL‐1, IL‐2, IL‐6, IL‐8, IL‐12, TNF‐α, and IFN‐γ) and anti‐inflammatory (IL‐4, IL‐10, etc) cytokines, as well as different growth factors, confirm the existence of an inflammatory reaction associated with MPNs that may trigger disorder initiation and lead to the development of myelofibrosis.9
A recent study revealed that inflammatory cytokines play a critical role in the expansion of leukemic cells and AML progression, demonstrating the promoting effect and functional relevance of the aberrant production of IL‐1 cytokines in the pathobiology of AML.10
Cytokine plasma levels have been correlated with survival, event‐free survival, and disease progression in AML cases.8
Single nucleotide polymorphisms (SNPs) have been found in cytokine genes, suggesting that certain alleles could lead to variations in cytokine production capacity.11, 12 Therefore, it could be hypothesized that cytokine gene variants may influence gene expression and plasma levels and could, therefore, be associated with the pathogenesis of hematological malignancies.
The association between the IFN‐γ +874T>A (rs2430561) polymorphism and the risk of chronic myeloid leukemia (CML)13 or chronic lymphocytic leukemia (CLL) was previously evaluated.14 A recent meta‐analysis suggested that the IFN‐γ +874T>A polymorphism contributes to CML and CLL susceptibility.15 Currently, studies investigating patients with AML and cytokine polymorphisms involvement are infrequent.
In a previous study, no association was found between TGF‐β1 rs1800470 polymorphism and leukemia.16 In contrast, Nursal et al showed that variants of TNF‐α 238G>A rs361525, IL‐10 (−1082G>A rs1800896, −819C>T rs1800871, −592C>A rs1800872), and TGF‐β1 (codon 25) genes may have a significant association with AML etiopathogenesis.17 However, no association was observed between TNF‐α −308G>A, IL‐10 (−592T>G, −819T>C, −1082T>C), IFN‐γ +874T>A and TGF‐β1 (codons 10 and 25) polymorphisms and the risk of CML.13
Carcinogenesis is affected by the action of several genes, in which an isolated SNP plays only a small role. Therefore, it is necessary to evaluate more than one SNP in the same study to take into account the polygenic model of inherited cancer susceptibility.18
To the best of the authors' knowledge, there are no published studies describing the possible association between cytokine polymorphisms and biological parameters, overall survival (OS), and AML prognostic impact.
The objectives of this study were to evaluate: (a) the multivariate effect of eight SNPs [TNF‐α 238G>A (rs361525); 376G>A (rs1800750); 308G>A (rs1800629)], IL‐10 −1082T>C (rs1800896); −592T>G (rs1800872), IL‐6 174C>G (rs1800795), TGF‐β1 869C>T (rs1800470), IFN‐γ +874T>A (rs2430561)] on AML risk; (b) the multivariate effect of the eight SNPs on OS in AML patients; (c) the association between studied polymorphisms and clinical prognostic factors in AML patients.
2. MATERIAL AND METHODS
2.1. Patients and controls
The AML cases were comprised of 226 adults from the Central and North‐Eastern regions of Romania diagnosed with AML at the Hematology Clinics from Targu Mures and the Hematology Department of The Oncology Institute “Prof. Dr Ion Chiricuta” in Cluj‐Napoca, Romania.
For comparison, a control group of 406 healthy Romanian non‐related individuals from the same geographic region was included in the study. These healthy individuals had no history of malignancy and had been initially referred to the Emergency County Hospital from Targu Mures for the investigation of anemia or leukocytosis.
All participants signed written informed consent. The study was approved (No. 67 from 14 April 2017) by the Board of the Ethical Committee of the University of Medicine and Pharmacy of Targu Mures and was carried out in conformity with the principles of the Helsinki Declaration.
According to the World Health Organization (WHO) 2016 classification,19 the AML cases were categorized as follows: 67 AML cases with recurrent genetic abnormalities (29.6), 32 AML cases with myelodysplasia‐related changes (14.2%), no AML cases with myeloid sarcoma (0%), no AML cases with Down syndrome‐related myeloid proliferations (0%), four AML cases with therapy‐related myeloid neoplasms (1.8%), and 123 AML cases not otherwise specified (54.4%).
2.2. Genotyping
Quick‐gDNA MiniPrep kits (Zymo Research), Wizard Genomic DNA Purification kits (Promega), and PureLink Genomic DNA Mini Kits (ThermoFisher Scientific) were used for DNA isolation from fresh whole blood samples collected in Ethylenediaminetetraacetic acid (EDTA) tubes.
TaqMan technology was used for SNP genotyping. Pre‐designed TaqMan SNP Genotyping assays for TNF‐α rs361525 and rs1800750, and IL‐10 rs1800896 and rs1800872, were used on a 7500 Fast Dx Real‐time PCR System.
TNF‐α rs1800629, IL‐6 rs1800795, TGF‐β1 rs1800470, and IFN‐γ rs2430561 were analyzed using the ARMS‐PCR method.20
FLT3 (ITD, D835), DNMT3A (R882), and NPM1 type A (c.863_864insTCTG) mutations were analyzed as previously reported.21, 22, 23, 24 Fragment analysis of FLT3 ITD and NPM1 type A mutation was performed in all cases and allow us to establish the ratio of mutated to normal alleles. High resolution melting (in‐house method) was used to re‐genotype 10% of the AML cases for FLT3 and DNMT3A mutations. CastPCR was used for NPM1 c.863_864insTCTG mutation analysis, using ThermoFisher assay numbers Hs00000953_mu and Hs00001029_rf.
The Ensembl genome browser (release version 93) was used to designate wild‐type and variant alleles for the investigated SNPs.25
2.3. Statistical analysis
Continuous variables with a Gaussian distribution were presented using statistics as the mean ± standard deviation (SD), whereas deviations from the normal probability law were described by the median and interquartile range (Q1; Q3). The distributions of the nominal variables were presented as absolute frequencies with percentages. Binomial logistic regression was used to test and quantify the impact of genetic and clinical factors on AML risk. Odds ratios with a 95% confidence interval (95% CI) were estimated.
The distribution of OS time was presented using medians with their 95% CI for the studied groups. In order to quantify the multivariate effects of all studied SNPs on OS, we used Cox regression, the full model tested consisting in eight SNPs (rs361525, rs1800750, rs1800629, rs1800896, rs1800872, rs1800795, rs1800470, and rs2430561 SNPs), four somatic mutations (FLT3‐ITD, FLT3 D835, NPM1, DNMT3A R882), and 12 demographic and clinical determinants (age group, gender, platelet count [PLT], blasts [%], hematocrit, hemoglobin and lactate dehydrogenase [LDH] level, Eastern Cooperative Oncologic Group Scale (ECOG) performance status, cytogenetic risk with two dummy variables, white blood cell [WBC] count, AML type).
Due to the large set of possible predictors for OS in AML patients (eight SNPs, four somatic mutations, and 12 demographic and clinical determinants) and an EPV (number events per predictor) equal to 7 (EPV = 7), we used the Minimizing approximated Information Criterion (MIC) as a new sparse estimation method to select a set of predictors for OS in AML patients.26, 27 Those predictors of the full model that did not significantly contribute to the OS were excluded and a reduced model obtained by the MIC estimation method was presented.
The sample size of patients used for the Cox regression analysis was 204 cases with 170 events. The MIC estimation method retained seven out of 24 candidate predictors for OS in AML patients. The effect size of selected predictors on OS in the reduced model was described by corrected HR determined from the shrinkage estimators of regression coefficients and adjusted HR determined from classical estimators.
Cox proportional hazards regression analysis with parameters estimated via the MIC method was performed using the “coxphMIC” package in the R statistical computing environment.
For the haplotype analysis of IL‐10 (rs1800896 and rs1800872) and TNF‐α (rs361525, rs1800629, and rs1800750) SNPs, we used the Haplotype Analysis28 free software.
3. RESULTS
3.1. Description of AML and control groups
Demographic data, as well as clinical and biological features of our AML patients, are presented in Table 1. Most AML cases were included in the intermediate cytogenetic risk group, as ECOG performance status grade 3 was observed in about 40% of investigated patients and most of them received a high dose of chemotherapy.
Table 1.
Characteristics of AML patients
| Variables | Number (%) |
|---|---|
| Age | |
| ≤20 y | 5 (2.2) |
| 21‐40 y | 47 (20.8) |
| 41‐60 y | 79 (35.0) |
| 60‐90 y | 95 (42.0) |
| Gender | |
| Female | 113 (50.0) |
| Men | 113 (50.0) |
| WBC (cells/mm3) | 11 270.0 [31 00.0; 38 700.0]a |
| <50 000 | 183 (81.0) |
| ≥50 000 | 43 (19.0) |
| PLT (cells/mm3) | 36 500 [11 000.0; 77 000.0]a |
| <40 000 | 119 (52.7) |
| ≥40 000 | 107 (47.3) |
| Hgb (g/dL) | 8.73 [7.8; 10.2]a |
| <10 | 166 (73.5) |
| ≥10 | 60 (26.5) |
| LDH level (IU/L) | 688.5 [461.0; 1132.0]a |
| <600 | 96 (42.5) |
| ≥600 | 130 (57.5) |
| Blasts (in bone marrow, %) | 60.0 [40.0; 78.0]a |
| <70 | 138 (61.1) |
| ≥70 | 88 (38.9) |
| Cytogenetic risk | |
| Low risk (favorable prognostic) | 24 (10.6) |
| Intermediate | 123 (54.4) |
| High risk (adverse) | 57 (25.3) |
| Not available (NA) | 22 (9.7) |
| ECOG performance status | |
| 0 | 3 (1.3) |
| 1 | 36 (15.9) |
| 2 | 37 (16.4) |
| 3 | 91 (40.3) |
| 4 | 59 (26.1) |
| ECOG performance status | |
| ≤1 | 39 |
| ≥2 | 187 |
| AML subtype | |
| de novo AML | 182 (81.5) |
| Secondary AML | 40 (17.7) |
| Therapy related AML | 4 (1.8) |
| FLT3 status (ITD+D835) | |
| Absent | 182 (81.4) |
| Present | 42 (18.6) |
| FLT3‐ITD status | |
| Absent | 191 (84.5) |
| Present | 35 (15.5) |
| FLT3 D835 status | |
| Absent | 214 (94.7) |
| Present | 12 (5.3) |
| DNMT3A R882 status | |
| Wild‐type | 200 (88.5) |
| Mutant | 26 (11.5) |
| NPM1 c.863_864insTCTG status | |
| Wild‐type | 189 (83.6) |
| Mutant | 37 (16.4) |
| Treatment | |
| HD | 119 (52.7) |
| LD | 91 (40.3) |
| HD + HSCT | 16 (7.0) |
Abbreviations: AML, acute myeloid leukemia; ECOG, Eastern Cooperative Oncologic Group Scale; HD, high dose of chemotherapy; HSCT, hematopoietic stem cell transplantation; LD, low dose of chemotherapy; LDH, lactate dehydrogenase; PLT, platelet; WBC, white blood cell.
Median and interquartile range (percentile 25%; percentile 75%).
The mean age ± SD of the AML groups was 54.44 ± 16.78 years (range 19‐87 years old), whereas the mean age ± SD for the controls was 56.01 ± 15.567 years (range 20‐85 years old). The control group included 213 females (52.5%) and 193 males (47.5%), whereas the AML group was made up of 113 (50%) females and 113 (50%) males. Age and gender distributions were similar in both the AML and controls (P ≥ .05). Seventy‐four AML patients (74/226, 32.7%) were ≥65 years of age.
3.2. Investigated SNPs and AML risk
The genotype distribution for all investigated TGF‐β1, IFN‐γ, TNF‐α, IL‐6, and IL‐10 cytokine SNPs in AML patients and controls are presented in Table 2. None of the IFN‐γ rs2430561, IL‐10 (rs1800872, rs1800896) and TNF‐α (rs361525, rs1800629, rs1800750), genotypes demonstrated deviation from the Hardy‐Weinberg equilibrium (HWE) in either the AML cases or controls. TGF‐β1 rs1800470 and IL‐6 rs1800795 genotypes were not consistent with HWE (P < .05) in AML patients but were in HWE on controls. The TGF‐β1 rs1800470 heterozygous genotype was associated with AML development risk and IFN‐γ rs2430561 heterozygous genotype was a protective factor for AML (OR = .63, 95% CI: 0.42‐0.94, P = .024), while the other studied SNPs were not associated with AML risk. Regarding allele distribution, no differences were observed between the investigated groups except for the variant A allele of TNF‐α rs1800750 (P = .002).
Table 2.
Genotype of the investigated cytokine gene polymorphisms in AML patients and healthy controls
| SNP | Control group (n1 = 406) | AML group (n2 = 226) | COR (95% CI) | P a | AOR (95% CI) | P b |
|---|---|---|---|---|---|---|
| TGF‐β1 rs1800470 | ||||||
| Additive model | n (%) c | n (%) d | ||||
| GG | 50 (12.3) | 16 (7.1) | Ref. | Ref. | ||
| GA | 165 (40.6) | 135 (59.7) | 2.56 (1.39‐4.69) | .002 | 2.53 (1.38‐4.65) | .003 |
| AA | 191 (47.0) | 75 (33.2) | 1.23 (0.66‐2.29) | .520 | 1.20 (0.65‐2.26) | .550 |
| Dominant model | ||||||
| AA | 50 (12.3) | 16 (7.1) | Ref. | Ref. | ||
| GA + AA | 356 (87.7) | 210 (92.9) | 1.84 (1.02‐3.32) | .042 | 1.82 (1.01‐3.29) | .046 |
| IFN‐γ rs2430561 | ||||||
| Additive model | ||||||
| TT | 67 (16.5) | 54 (23.9) | Ref. | Ref. | ||
| TA | 215 (53.0) | 105 (46.5) | 0.61 (0.40‐0.93) | .022 | 0.61 (0.40‐0.94) | .024 |
| AA | 124 (30.5) | 67 (29.6) | 0.67 (0.42‐1.07) | .092 | 0.67 (0.42‐1.07) | .092 |
| Dominant model | ||||||
| TT | 67 (16.5) | 54 (23.9) | Ref. | Ref. | ||
| TA + AA | 339 (83.5) | 172 (76.1) | 0.63 (0.42‐0.94) | .024 | 0.63 (0.42‐0.95) | .026 |
| TNF‐α rs361525 | ||||||
| Additive model | ||||||
| GG | 389 (95.8) | 211 (93.4) | Ref. | Ref. | ||
| GA | 17 (4.2) | 15 (6.6) | 1.63 (0.77‐3.32) | .182 | 1.09 (0.79‐1.52) | .207 |
| TNF‐α rs1800629 | ||||||
| Additive model | ||||||
| GG | 271 (66.7) | 148 (65.5) | Ref. | Ref. | Ref. | |
| GA | 127 (31.3) | 73 (32.3) | 1.05 (0.74‐1.50) | .775 | 1.05 (0.74‐1.49) | .778 |
| AA | 8 (2.0) | 5 (2.2) | 1.14 (0.37‐3.56) | .816 | 1.14 (0.38‐3.74) | .756 |
| Dominant model | ||||||
| GG | 271 (66.7) | 148 (65.5) | Ref. | Ref. | ||
| GA + AA | 135 (33.3) | 78 (34.5) | 1.06 (0.75‐1.49) | .748 | 1.06 (0.75‐1.50) | .739 |
| TNF‐α rs1800750 | ||||||
| GG | 402 (100.0) | 220 (97.3) | Ref. | .002 | Ref. | Ref. |
| GA | 0 (0.0) | 6 (2.7) | ND | ND | ND | |
| IL‐6 rs1800795 | ||||||
| CC | 68 (16.7) | 32 (14.2) | Ref. | Ref. | ||
| CG | 188 (46.3) | 127 (56.2) | 1.44 (0.89‐2.31) | .137 | 1.43 (0.89‐2.30) | .143 |
| GG | 150 (36.9) | 67 (29.6) | 0.95 (0.57‐1.58) | .841 | 0.93 (0.56‐1.55) | .782 |
| CG + GG | 338 (83.3) | 194 (85.8) | 1.22 (0.77‐1.92) | .393 | 1.21 (0.79‐1.52) | .417 |
| IL‐10 rs1800872 | ||||||
| GG | 222 (54.7) | 117 (51.8) | Ref. | Ref. | Ref. | |
| GT | 158 (38.9) | 99 (43.8) | 1.19 (0.85‐1.67) | .314 | 1.21 (0.86‐1.70) | .271 |
| TT | 26 (6.4) | 10 (4.4) | 0.73 (0.34‐1.57) | .418 | 0.73 (0.34‐1.56) | .410 |
| GT + TT | 184 (45.3) | 109 (48.2) | 1.12 (0.81‐1.56) | .482 | 1.14 (0.82‐1.58) | .435 |
| IL‐10 rs1800896 | ||||||
| TT | 144 (35.5) | 74 (32.7) | Ref. | Ref. | Ref. | |
| TC | 188 (46.3) | 109 (48.2) | 1.13 (0.78‐1.63) | .519 | 1.12 (0.77‐1.61) | .560 |
| CC | 74 (18.2) | 43 (19.0) | 1.13 (0.71‐1.81) | .607 | 1.11 (0.69‐1.77) | .677 |
| TC + CC | 262 (64.5) | 152 (67.3) | 1.13 (0.80‐1.59) | .490 | 1.11 (0.79‐1.57) | .545 |
Abbreviations: AOR, adjusted odd ratio for age and gender; CI, confidence interval; COR, crude odd ratio.
P‐value obtained from Chi‐square test, Fisher's Exact test or univariate binomial logistic regression.
P‐value obtained from multivariable logistic model.
Percentages were calculated for genotypes relative to the number of controls.
Percentages were calculated for genotypes relative to the number of cases.
Bold values denoted statistically significant results (P < 0.05).
We analyzed whether there were any differences between the investigated groups in the presence of at least two variant genotypes and observed that the presence of four variant genotypes from the investigated SNPs were associated with AML risk with a tendency towards statistical significance (P = .059, OR = 1.45, 95% CI: 0.99‐2.13).
3.3. Bivariate associations between studied SNPs and clinical factors
The clinical features of AML patients according to the presence of wild‐type and variant genotypes for each cytokine polymorphism are presented in Table 3. No significant associations were observed between investigated SNPs and age when we divided our cases into three groups (≤40 years, 41‐60 years, ≥61 years). A significant difference in frequencies was found only in the case of IL‐10 rs1800872 variant genotype in patients <40 years vs patients ≥40 years of age (P = .03), with a higher frequency of the variant genotype in elderly patients. There was an association between age ≥65 years and IL‐10 rs1800896 (P = .019).
Table 3.
Patients' characteristics according to the TGF‐β1 rs1800470, IFN‐γ rs2430561, TNF‐α rs361525, rs1800629, rs1800750, IL‐10 rs1800896, rs1800872, IL‐6 rs1800795 genotypes
| TGF‐β1 rs1800470 | IFN‐γ rs2430561 | TNF‐α rs361525 | TNF‐α rs1800629 | TNF‐α rs1800750 | IL‐6 rs1800795 | IL‐10 rs1800872 | IL‐10 rs1800896 | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GG | GA + AA | P‐value | TT | TA + AA | P‐value | GG | GA + AA | P‐value | GG | GA + AA | P‐value | GG | GA + AA | P‐value | CC | CG + GG | P‐value | GG | GT + TT | P‐value | TT | TC + CC | P‐value | |||
| Age groups | ||||||||||||||||||||||||||
| 19‐40 y | 3 | 49 | .208 | 12 | 40 | .369 | 47 | 5 | .199 | 35 | 17 | .821 | 50 | 2 | .438 | 9 | 43 | .623 | 21 | 31 | .101 | 18 | 34 | .108 | ||
| 41‐60 y | 3 | 46 | 23 | 56 | 72 | 7 | 53 | 26 | 76 | 3 | 9 | 70 | 47 | 32 | 19 | 60 | ||||||||||
| 61‐90 y | 10 | 85 | 19 | 76 | 92 | 3 | 60 | 35 | 94 | 1 | 14 | 81 | 49 | 46 | 37 | 58 | ||||||||||
| Gender | ||||||||||||||||||||||||||
| Female | 12 | 101 | .038 | 30 | 83 | .349 | 106 | 7 | .789 | 76 | 37 | .576 | 109 | 4 | .683 | 17 | 96 | .849 | 57 | 56 | .690 | 33 | 80 | .257 | ||
| Male | 4 | 109 | 24 | 89 | 105 | 8 | 72 | 41 | 111 | 2 | 15 | 98 | 60 | 53 | 41 | 72 | ||||||||||
| WBC (cells/mm3) | ||||||||||||||||||||||||||
| <50 000 | 13 | 170 | .977 | 41 | 142 | .279 | 172 | 11 | .453 | 113 | 72 | .015 | 180 | 3 | .085 | 25 | 158 | .658 | 93 | 90 | .555 | 61 | 122 | .697 | ||
| ≥50 000 | 3 | 40 | 13 | 10 | 39 | 4 | 35 | 8 | 40 | 3 | 7 | 36 | 24 | 19 | 13 | 30 | ||||||||||
| PLT (cells/mm3) | ||||||||||||||||||||||||||
| <40 000 | 9 | 110 | .765 | 28 | 91 | .892 | 108 | 11 | .097 | 78 | 41 | .984 | 114 | 5 | .216 | 16 | 103 | .745 | 62 | 57 | .916 | 39 | 80 | .992 | ||
| ≥40 000 | 7 | 100 | 26 | 81 | 103 | 4 | 70 | 37 | 106 | 1 | 16 | 91 | 55 | 52 | 35 | 72 | ||||||||||
| Hgb (g/dL) | ||||||||||||||||||||||||||
| <10 | 13 | 153 | .646 | 39 | 127 | .815 | 153 | 13 | .23 | 114 | 52 | .094 | 161 | 5 | 1.00 | 25 | 141 | .518 | 89 | 77 | .356 | 53 | 113 | .664 | ||
| ≥10 | 3 | 57 | 15 | 45 | 58 | 2 | 34 | 26 | 59 | 1 | 7 | 53 | 28 | 32 | 21 | 39 | ||||||||||
| LDH level (IU/L) | ||||||||||||||||||||||||||
| <600 | 6 | 90 | .676 | 20 | 76 | .354 | 92 | 4 | .20 | 53 | 43 | .005 | 95 | 1 | .244 | 10 | 86 | .165 | 47 | 49 | .467 | 30 | 66 | .681 | ||
| ≥600 | 10 | 120 | 34 | 96 | 119 | 11 | 95 | 35 | 125 | 5 | 22 | 108 | 70 | 60 | 44 | 86 | ||||||||||
| Blasts (in bone marrow, %) | ||||||||||||||||||||||||||
| <70 | 12 | 126 | .236 | 29 | 109 | .204 | 129 | 9 | .930 | 89 | 49 | .694 | 134 | 4 | 1.00 | 19 | 119 | .833 | 73 | 65 | .671 | 39 | 99 | .072 | ||
| ≥70 | 4 | 84 | 25 | 63 | 82 | 6 | 59 | 29 | 86 | 2 | 13 | 75 | 44 | 44 | 35 | 53 | ||||||||||
| AML subtype | ||||||||||||||||||||||||||
| de novo | 13 | 169 | .75 | 44 | 138 | .75 | 168 | 14 | .483 | 123 | 59 | .071 | 176 | 6 | .636 | 28 | 154 | .03 | 93 | 89 | .897 | 63 | 199 | .517 | ||
| sAML | 3 | 37 | 10 | 30 | 39 | 1 | 21 | 19 | 40 | 0 | 2 | 38 | 22 | 18 | 10 | 30 | ||||||||||
| tAML | 0 | 4 | 0 | 4 | 4 | 0 | 4 | 0 | 4 | 0 | 2 | 2 | 2 | 2 | 1 | 3 | ||||||||||
| Cytogenetic risk | ||||||||||||||||||||||||||
| Low‐risk | 1 | 23 | .153 | 7 | 17 | .649 | 22 | 2 | .121 | 10 | 14 | .083 | 24 | 0 | .184 | 5 | 19 | .441 | 12 | 12 | .409 | 7 | 17 | .007 | ||
| Intermediate | 13 | 110 | 32 | 91 | 117 | 6 | 84 | 39 | 121 | 2 | 17 | 106 | 59 | 64 | 43 | 80 | ||||||||||
| High‐risk | 1 | 56 | 11 | 46 | 54 | 3 | 39 | 18 | 55 | 2 | 9 | 48 | 35 | 22 | 11 | 46 | ||||||||||
| NA | 1 | 21 | 4 | 18 | 18 | 4 | 15 | 7 | 20 | 2 | 1 | 21 | 11 | 11 | 13 | 9 | ||||||||||
| ECOG status | ||||||||||||||||||||||||||
| ≤1 | 4 | 35 | .153 | 10 | 29 | .778 | 36 | 3 | .728 | 20 | 19 | .044 | 37 | 2 | .277 | 3 | 36 | .203 | 19 | 20 | .675 | 13 | 26 | .931 | ||
| ≥2 | 12 | 175 | 44 | 143 | 175 | 12 | 128 | 59 | 183 | 4 | 29 | 158 | 98 | 89 | 61 | 126 | ||||||||||
Abbreviations: sAML, secondary AML; tAML, therapy related AML; NA, not available.
Bold values denoted statistically significant results (P < 0.05).
A higher frequency in females was observed for TGF‐β1 rs1800470 variant genotype (Table 3). We observed an association between WBC and TNF‐α rs1800629, the variant genotype being higher in patients with WBC <50 000/mm3 than in patients with WBC >50 000/mm3 (P = .015).
Furthermore, a higher frequency of the TNF‐α rs1800629 variant genotype was found in patients with LDH <600 IU/L (P = .005; OR = 0.45; 95% CI: 0.26‐0.80).
No associations were observed between blast percentage and investigated SNPs, but there was a tendency towards statistical significance between blasts <70% and IL‐10 rs1800896 (P = .072).
Regarding AML subtypes, we found an association between the IL‐6 rs1800795 variant genotype (P = .03) and a higher frequency of variant genotypes between the AML de novo cases, with a trend towards statistical significance (P = .071) in the case of TNF‐α rs1800629 SNP.
Regarding the relationship between cytogenetic risk groups and cytokine gene polymorphisms, there was a significant association in the case of IL‐10 rs1800896 variant genotype (P = .007) and a trend towards statistical significance (P = .083) in case of TNF‐α rs1800629 for high cytogenetic risk.
We observed an association between each ECOG performance status grade and the variant genotypes of IFN‐γ rs2430561 (P = .043), TNF‐α rs1800750 (P = .005) while TNF‐α rs1800629 variant genotype was associated with ECOG grade ≥2 (P = .044).
Regarding the association between somatic mutations and investigated cytokines SNPs, the presence of TNF‐α rs1800629 variant genotype was associated with FLT3‐ITD (P = .049), FLT3 (ITD+D835) (P = .048), and DNMT3A somatic mutations (P = .008), whereas the FLT3‐D835 or NPM1 type A mutations showed no association (P > .05).
We also tested the association between somatic mutations and combinations of 3, 4, 5, or 6 variant genotypes in the studied SNPs, but only the presence of five variant genotypes with the FLT3‐ITD mutation (P = .081) and six variant genotypes with the NPM1 mutation (P = .081) showed a trend towards statistical significance.
There was not found significant associations between studied SNPs and type of treatment (P > .05) on all AML cases (n = 226).
3.4. The individual impact of studied SNPs, demographic and clinical factors on OS time
There was an association between AML patients' age and survival (P < .001). Patients under 65 had a better survival than patients over 65 [median survival time, 95% CI: 9.0 (7.4‐10.6) vs 3.0 (2.1‐3.9)], whereas gender and AML subtype showed no difference (P = .523). On the other hand, there was a significant difference in survival curves distributions (P < .001) between low, intermediate, and high cytogenetic risk groups [median survival time, 95% CI: 12.0 (6.3‐17.7); 7.0 (5.1‐8.9), and 3.0 (1.4‐4.6)].
We observed a better outcome in AML patients with an ECOG performance status ≤1 compared to those with a status ≥2 (P = .001, median survival time, 95% CI: 12.0 (6.9‐17.1) vs 6.0 (4.4‐7.6)). Survival time showed a significant difference (P < .001) in AML patients treated with high‐dose (HD) chemotherapy (median survival time, 95% CI: 9.0 [6.9‐11.0]), low‐dose (LD) chemotherapy (median survival time, 95% CI: 4.0 [3.3‐4.7]), and HD chemotherapy + hematopoietic stem cell transplantation (HSTC) (median survival time, 95% CI: 12.0 [9.1‐14.9]).
Patients with WBC >50 000/mm3 at diagnosis had a median survival time of 7 months vs 5 months for patients with WBC <50 000/mm3 at diagnosis (P = 0.053).
Kaplan‐Meier analysis showed that AML patients with PLT >40 000/mm3 at diagnosis had an increased rate of survival compared to those with lower PLT levels (Log‐Rank test, P = .05). When we analyzed the univariate impact of LDH level on OS, we observed that LDH levels above 600 IU/L were significantly associated with a shorter OS (P = .003, median survival time, 95% CI: 5.0 [3.7‐6.3] vs 10.0 [6.8‐13.2)].
Patients with the FLT3‐ITD mutation had a significantly shorter OS (P = .004) than those with FLT3‐ITD negative status (median survival time, 95% CI: 2.0 [0.3‐3.7] vs 7.0 [5.2‐8.8]). The Kaplan‐Meier curves depicted in Figure 1 showed the percentage of survival in the AML cases with FLT3‐ITD positive and negative status. Moreover, in AML patients with FLT3 mutation, the presence of the D835 mutation did not affect OS (P = .689). When we analyzed FLT3 (ITD+D835) positive status, we observed a trend towards statistical significance for this association (P = .078) with survival. No association was found between NPM1 or DNMT3A status, hemoglobin, hematocrit levels, and bone marrow blast percentage at diagnosis and OS (P > .05).
Figure 1.
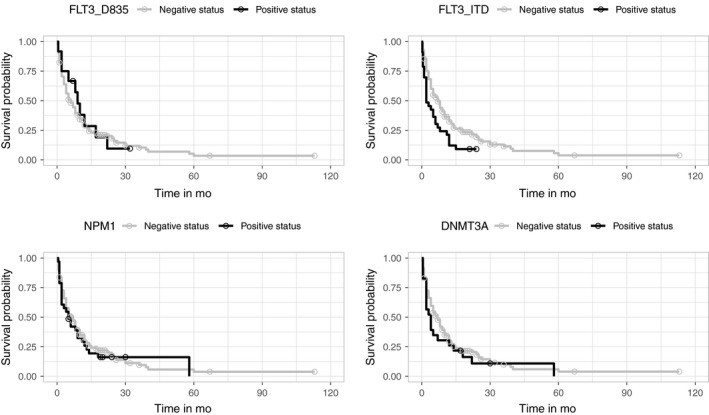
The Kaplan‐Meier curves for FLT3‐D835, FLT3‐ITD, NPM1 and DNMT3A of all patients included in survival analysis. Circles marks indicated censored cases
The TNF‐α (rs361525, rs1800750, and rs1800629), IL‐10 (rs1800896 and rs1800872), IL‐6 (rs1800795), TGF‐β1 (rs1800470), and IFN‐γ (rs2430561) variant genotypes were not associated with patients' OS (P > .05), and neither were the combined variant genotypes (presence of >3 variant genotypes). Kaplan‐Meier curves were plotted to show the crude survival rates for the eight SNPs. (Figures 2 and 3).
Figure 2.
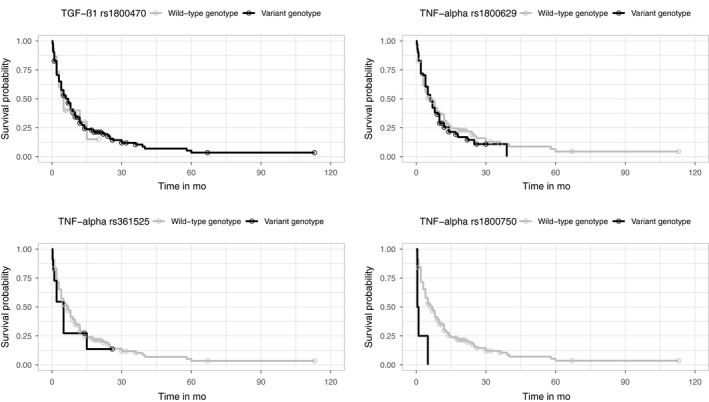
The Kaplan‐Meier curves for TGF‐β1 rs1800470, TNF‐α rs361525, TNF‐α rs1800629 and TNF‐α rs1800750 of all patients included in survival analysis. Circles marks indicated censored cases
Figure 3.
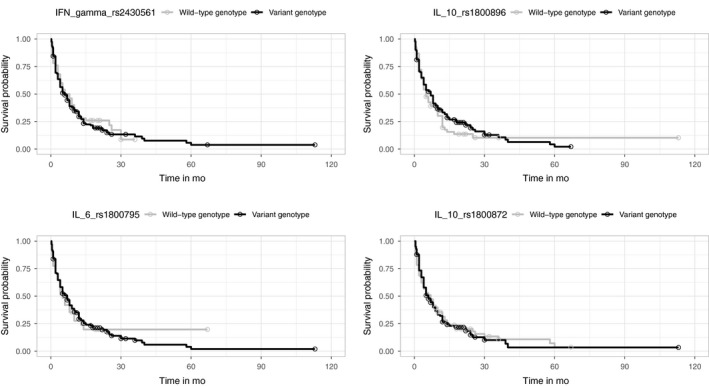
The Kaplan‐Meier curves for IFN‐γ rs2430561, IL‐6 rs1800795, IL‐10 rs1800896 and IL‐10 rs1800872 of all patients included in survival analysis. Circles marks indicated censored cases
3.5. The size effect of studied SNPs and clinical factors on mortality
The effects of the studied SNPs on OS in AML patients were assessed by Cox regression analysis (univariable and multivariable). Patients lacking cytogenetic risk group data were not included in this analysis, therefore only 204 AML cases were investigated, with death occurring in 170 of these patients.
The results of the Cox proportional hazards regression regarding the effects of the studied cytokine SNPs on mortality in AML patients are presented in Table 4.
Table 4.
The results of the Cox proportional hazards regression: unadjusted and adjusted effects of the studied SNP on overall survival in AML patients
| Factors | Levels/genotypes | Events (%) | Univariable analysis | Multivariable analysis‐reduced model | |||
|---|---|---|---|---|---|---|---|
| HRa (95% CI) | P‐value | HRb (95% CI) | Correctedc HR (95% CI) | P‐value | |||
| Gender | F | 79 (80.6) | Reference | .293 | NA | NA | NA |
| M | 91 (85.8) | 1.18 (0.87‐1.59) | |||||
| Age | <65 y | 109 (79.0) | Reference | <.001 | Reference | Reference | <.001 |
| ≥65 y | 61 (92.4) | 2.15 (1.55‐2.99) | 2.76 (1.96‐3.89) | 1.61 (1.37‐1.89) | |||
| TGF‐β1 rs1800470 | Wild‐type genotype | 11 (73.3) | Reference | .857 | NA | NA | NA |
| variant | 159 (84.1) | 0.95 (0.51‐1.75) | |||||
| IFN‐γ rs2430561 | Wild‐type genotype | 41 (82.0) | Reference | .705 | NA | NA | NA |
| variant | 129 (83.8) | 1.07 (0.75‐1.53) | |||||
| TNF‐α rs361525 | Wild‐type genotype | 161 (83.4) | Reference | .546 | NA | NA | NA |
| variant | 9 (81.8) | 1.23 (0.63‐2.41) | |||||
| TNF‐α rs1800629 | Wild‐type genotype | 111 (83.5) | Reference | .500 | Reference | Reference | .070 |
| variant | 59 (83.1) | 1.12 (0.81‐1.54) | 1.39 (0.99‐1.93) | 1.17 (1.00‐1.38) | |||
| TNF‐α rs1800750 | Wild‐type genotype | 166 (83.0) | Reference | .005 | Reference | Reference | .007 |
| variant | 4 (100.0) | 4.22 (1.54‐11.52) | 5.84 (2.07‐16.45) | 1.27 (1.10‐1.47) | |||
| IL‐6 rs1800795 | Wild‐type genotype | 24 (77.4) | Reference | .941 | NA | NA | NA |
| variant | 146 (84.4) | 0.98 (0.64‐1.52) | |||||
| IL‐10 rs1800872 | Wild‐type genotype | 88 (83.0) | Reference | .885 | NA | NA | NA |
| variant | 82 (83.7) | 1.02 (0.76‐1.38) | |||||
| IL‐10 rs1800896 | Wild‐type genotype | 52 (85.2) | Reference | .385 | NA | NA | NA |
| variant | 118(82.5) | 0.87 (0.62‐1.20) | |||||
| FLT3‐ITD status | Absent | 140 (81.9) | Reference | .009 | NA | NA | NA |
| Present | 30 (90.9) | 1.70 (1.14‐2.54) | |||||
| FLT3 D835 status | Absent | 160 (83.3) | Reference | .739 | NA | NA | NA |
| Present | 10 (83.3) | 0.90 (0.47‐1.70) | |||||
| NPM1 status | Absent | 142 (83.0) | Reference | .618 | NA | NA | NA |
| Present | 28 (84.8) | 1.11 (0.74‐1.66) | |||||
| DNMT3A R882 status | Absent | 149 (82.3) | Reference | .368 | NA | NA | NA |
| Present | 21 (91.3) | 1.23 (0.78‐1.95) | |||||
| Hgb level | <10 | 127 (82.5) | Reference | .217 | NA | NA | NA |
| ≥10 | 43 (86.0) | 0.80 (0.57‐1.14) | |||||
| Htc | >35 | 11 (100.0) | Reference | .851 | NA | NA | NA |
| ≤35 | 159 (82.4) | 0.94 (0.51‐1.74) | |||||
| PLT | <40 000 | 93 (84.5) | 1.40 (1.03‐1.89) | .039 | 1.39 (0.99‐1.85) | 1.17 (1.00‐1.38) | .075 |
| ≥40 000 | 77 (81.9) | Reference | Reference | Reference | |||
| Blasts (%) | <70 | 98 (79.0) | Reference | .325 | Reference | Reference | .042 |
| >70 | 72 (90.0) | 1.17 (0.86‐1.58) | 1.46 (1.06‐2.03) | 1.20 (1.03‐1.41) | |||
| LDH level (IU/L) | <600 | 68 (78.2) | Reference | .008 | Reference | Reference | .022 |
| ≥600 | 102 (87.2) | 1.52 (1.11‐2.06) | 1.54 (1.10‐2.16) | 1.24 (1.05‐1.47) | |||
| ECOG status | ≤1 | 18 (56.3) | Reference | .010 | NA | NA | NA |
| ≥2 | 152 (88.4) | 1.90 (1.16‐3.10) | |||||
| Cytogenetic risk (1) | Low‐risk | 17 (70.8) | Reference | .422 | NA | NA | NA |
| Intermediate | 98 (79.7) | 1.24 (0.74‐2.07) | |||||
| Cytogenetic risk (2) | Low‐risk | 17 (70.8) | Reference | .003 | Reference | Reference | .021 |
| High‐risk | 55 (96.5) | 2.29 (1.32‐3.97) | 1.54 (1.10‐2.16) | 1.34 (1.15‐1.56) | |||
| WBC count | <50 000 | 137 (82.5) | Reference | .077 | NA | NA | NA |
| ≥50 000 | 33 (86.8) | 1.41 (0.96‐2.07) | |||||
| AML type | 0 | 137 (82.0) | Reference | .266 | NA | NA | NA |
| 1 + 2 | 33 (89.2) | 1.24 (0.85‐1.82) | |||||
Events = number of deaths; % was calculated in relation to the number of cases corresponding to each category of the study.
Cytogenetic risk variable was transformed in two dummy variables in cox regression analysis
Abbreviations: 95% CI, 95% confidence interval; HR, hazard ratio; MIC, Minimizing approximated Information Criterion; NA, not selected to be included in the reduced model.
Crude HR.
Adjusted HR from unpenalized Cox PH regression model.
Adjusted HR from penalized Cox PH regression model estimated by MIC method.
Bold values denoted statistically significant results (P < 0.05).
The univariate effect of investigated parameters on OS indicated that an age above 65 years had a significant negative influence on survival (P < .001, HR = 2.15, 95% CI: 1.55‐2.99). Presence of the TNF‐α rs1800750 variant genotype (P = .005; HR = 4.22, 95% CI: 1.54‐11.52), or the FLT3‐ITD mutation (P = .009; HR = 1.70; 95% CI: 1.14‐2.54), and inclusion in the cytogenetic high risk group (P = .003; HR = 2.29; 95% CI: 1.32‐3.97) negatively influenced OS. In the case of AML patients with a cytogenetic risk established (n = 204) the treatment type (HD, LD, HD + HSCT) was significantly associated with OS (Log‐Rank test, χ 2(2) = 25.7, P < .001), the risk of death was higher in those treated with HD than in patients with LD (crude HR = 2.18, 95% CI: 1.57‐3.02). In addition, the effects of cytokines on OS were not modified after controlling for the treatment effect, of all polymorphisms only TNF‐α rs1800750 SNP having a significant effect on OS after controlling treatment effect (stratified Cox regression, P = .0003, stratification variable: treatment type, adjusted HR = 5.84, 95% CI: 2.09‐16.31).
A negative association was observed between ECOG status with a grade ≥2, LDH level ≥600 IU/L, a PLT count lower than 40 000/mm3 and OS (Table 4).
Multivariate Cox regression analysis based on the MIC estimation method retained only seven predictors from the 24 candidate predictors for OS in AML patients (namely age, TNF‐α rs1800629 and rs1800750, PLT count, bone marrow blast percentage, LDH level, and cytogenetic high risk). Based on our Cox proportional hazards regression findings, we considered that age above 65 years was an independent predictor for mortality in AML cases (P < .001, HR = 1.61, 95% CI: 1.37‐1.89). Following multivariate analysis, we observed a trend towards statistical significance for the association between TNF‐α rs1800629 variant genotype, PLT count, and mortality (P = .07, corrected HR = 1.17, 95% CI: 1.00‐1.38; P = .075, corrected HR = 1.17, 95% CI: 1.00‐1.38).
The TNF‐α rs1800750 variant genotype was a risk factor for death (P = .007, corrected HR = 1.2, 95% CI: 1.10‐1.47). Furthermore, we observed that blast percentage, LDH level, and cytogenetic high‐risk were independent significant predictors for death in AML (P = .04, corrected HR = 1.20, 95% CI: 1.03‐1.41; P = .022, corrected HR = 1.24, 95% CI: 1.05‐1.47; P = .021, corrected HR = 1.34, 95% CI: 1.15‐1.56, respectively).
4. DISCUSSION
Our study provides data regarding the impact of cytokine gene polymorphisms in AML patients and AML mortality predictors in a Romanian case‐control study.
We found that the TGF‐β1 rs1800470 and IFN‐γ rs2430561 variant genotypes were associated with AML susceptibility for the additive and dominant models. Furthermore, no evidence of association was observed between the investigated cytokine SNPs and AML in the allelic model, except for TNF‐α rs1800750 A allele (P = .002).
Regarding TGF‐β1 rs1800470, our findings were similar to those of a study performed in Brazil which demonstrated that the TGF‐β1 rs1800470 TT homozygous genotype was associated with an increased risk of developing hematological malignancies (OR = 4.07; 95% CI: 1.94‐8.52; P = .0002), with a 4‐fold increase in the risk of developing hematological cancers.18 Pehlivan et al found no significant differences between Turkish patients with Philadelphia positive CML and their controls.13 Contrary to our observations, no difference was observed in a Turkish population for the TGF‐β1 rs1800470 variant genotype.17
Regarding IFN‐γ +874T>A (rs2430561), no association was detected between the variant genotype or variant allele and AML risk in Turkey.17 In the meta‐analysis performed by Wu et al, no significant association was found between IFN‐γ +874T>A polymorphism and leukemia risk for all comparison models. In the case of subgroup analysis by leukemia type, a significantly increased risk for CML was observed in the dominant model (TT + TA vs AA, OR = 1.783, 95% CI: 1.236‐2.573, P = .002) and a decreased risk for CLL was found in the allelic, co‐dominant, and dominant models when separately using the fixed‐effect model (T vs A, OR = 0.660, 95% CI: 0.483‐0.902, P = .009; TT vs AA, OR = 0.472, 95% CI: 0.247‐0.902, P = .023 and TT + TA vs AA, OR = 0.457, 95% CI: 0.285‐0.734, P = .001).15 The difference in these findings may be due to the ethnicity of the studied populations, and the number of AML cases evaluated.
The TNF‐α rs1800629, IL‐10 rs1800872, and IL‐10 rs1800896 SNPs were not risk factors for AML development in our population, with similar findings being reported by Pehlivan et al.13 Similarly, a meta‐analysis of 19 publications comprising 1509 patients with leukemia and 4075 controls found no association between the TNF‐α rs1800629 polymorphism and leukemia risk.29 In contrast, a recent study showed statistically significant differences regarding the genotype and allele distribution of TNF‐α rs1800629 in CLL patients compared to controls (P = .00003 and P = .00007).30
In addition, our findings indicated that TNF‐α rs361525 did not confer susceptibility to AML in the additive and dominant models. No studies are currently available regarding the role of rs361525 in AML susceptibility, however, a closely‐related hematological malignancy case‐control study (123 Brazilian patients with MPN and 123 healthy subjects) showed that the rs361525 and rs1800629 variant genotypes were significantly higher in MPN cases (P = .04 and P = .02), providing a risk factor for developing MPN of 2.21 and 1.82, respectively.31 The rs1800750 heterozygous genotype was associated with a higher risk for Hodgkin's lymphoma in Mexican patients (OR = 4.41 95% CI: 1.21‐16.6, P = .02).32 Similarly, in our study, the heterozygous genotype was found only in AML cases but not in controls, and we considered that rs1800750 heterozygous genotype may be associated with an AML risk (P = .002). In the allelic model, we noticed that TNF‐α rs1800750 A allele was associated with AML susceptibility (P = .002).
Furthermore, we observed no differences regarding the frequency of IL‐6 rs1800795 genotypes or alleles among AML patients and controls, our data being similar to the results reported by Nursal et al.17 In contrast, the presence of the rs1800795 variant genotype could be associated with CML susceptibility in Turkish patients.13, 33
Research results regarding the role of IL‐10 SNPs in the predisposition to leukemia are contradictory. The IL‐10 −592C>A promoter SNP was investigated in 115 AML patients and 137 controls from China, where a significant difference regarding the −592AA genotype percentage (P = .014) and −592A allele frequency (P = .004) was observed. The −592AA genotype prevalence risk was 2.492 times higher than in CC genotype carriers (OR = 2.492; 95% CI: 1.013‐5.825).34 Moreover, a recent study reported that the presence of the IL‐10 rs1800872 variant allele was associated with a slightly increased risk of AML (adjusted OR = 1.30 95% CI: 1.01‐1.72).34 Nursal et al showed that variants of the IL‐10 (rs1800896 and rs1800872) gene may have a significant association with AML etiopathogenesis.17
Considering that no association could be detected between the IL‐10 rs1800896 allele or genotype frequency and CML risk, a recent study indicated that IL‐10 could be a useful survival biomarker in CML.13
In the present study, no relationship was found between IL‐10 rs1800872 and rs1800896 and AML predisposition. These findings are in line with certain studies reported previously 13; however, they are also in contradiction with several others.17, 34, 35
Regarding IL‐10 rs1800896, our findings were in line with those of Fei et al who found no significant differences between AML patients and controls when comparing rs1800896 allele and genotype frequencies.35 Hiroki et al observed no association between the IL‐10 rs1800896 variant genotype and IL‐10 plasma levels and concluded that rs1800896 was not associated with acute lymphoblastic leukemia (ALL) susceptibility nor with relapse risk.11
We also investigated the potential association between the IL‐10 and TNF‐α haplotypes and AML risk. The most frequent haplotypes of the IL‐10 rs1800896 and rs1800872 SNPs were CTGG (23.5% patients vs 25.1% controls) and CTGT (24.3% patients vs 20.7% controls). Logistic regression did not confirm any significant association between CTGT haplotype and AML risk (P = .337, OR = 1.22, 95% CI: 0.81‐1.83), with similar results being found for the CTGG haplotype (P = .876, OR = 0.97, 95% CI: 0.65‐1.83).
We observed that the most frequent TNF-α haplotypes (rs361525, rs1800629, and rs1800750) were h5 AGGGGG haplotype (30.1% patients vs 30.3% controls) and GGGGGG (60.6% patients vs 63.5% controls), but neither was correlated with AML risk (GGGGGG haplotype P = .145, OR = 0.63, 95% CI: 0.34‐1.17; AGGGGG haplotype P = .208, OR = 0.66, 95% CI: 0.34‐1.26). Moreover, we analyzed the impact of the IL‐10 and TNF‐α haplotypes on OS in our AML cohort. We did not find any differences in OS regarding the CTGG and CTGT haplotypes or any other IL‐10 haplotype (log‐Rank test, P = .809), nor between AGGGGG and GGGGGG haplotypes and the other TNF‐α haplotypes (log‐rank test, P = .482).
The current study showed a significantly lower survival rate in elderly AML patients compared to patients under 65 years of age. A shorter OS was observed in AML cases with cytogenetic high risk, and in those with decreased WBC counts. High LDH (>600 IU/L) levels and FLT3‐ITD mutation negatively influenced overall AML survival. The investigated SNPs had no effect on OS in AML, either when they were tested individually or in the case of combined variant genotypes (presence of >3 variant genotypes from all eight). The fact that no associations were found between AML patients' OS and the presence of TNF‐α (rs361525, rs1800750, and rs1800629), IL‐10 (rs1800896 and rs1800872), IL‐6 (rs1800795), TGF‐β1 (rs1800470), or IFN‐γ (rs2430561) variant genotypes suggests that these SNPs may not represent independent survival biomarkers in AML.
Moreover, in the univariate Cox regression analysis, we found that age above 65 years, TNF‐α rs1800750 variant genotype, FLT3‐ITD mutation, cytogenetic high risk, ECOG performance status ≥2, LDH level ≥600 IU/L, and PLT count lower than 40 000 cells/mm3 had an effect on OS in AML.
In multivariate Cox PH regression analysis, only age above 65 years, TNF‐α rs1800750 SNP, blast percentage, LDH level, and cytogenetic high‐risk were found to be independent significant predictors for OS in AML. We observed a trend towards statistical significance regarding the association between the TNF‐α rs1800629 variant genotype and OS. In contradiction, Kim et al reported a lower OS (estimated 20.1 months) in case of the GA variant genotype compared to GG homozygous genotype (estimated 54.6 months) for the IL‐10 rs1800896 SNP in AML patients.36 Similar to our findings, Kim et al, revealed that the IL‐10 rs1800871 and rs1800872 variant genotypes did not have an effect on OS.36 Similar results regarding this lack of effect on OS by IL‐10 rs1800871 have been previously reported.37
Pehlivan et al found no association between TNF‐α rs1800629, IL‐10 rs1800872, rs1800871, and rs1800896, IFN‐γ rs2430561, and TGF‐β1 rs1800470 (codons 10 and 25) polymorphisms and OS in their Turkish CML patients,13 being therefore in line with our observations.
The relationship between any two combined variant genotypes of the TGF‐β1, IFN‐γ, TNF‐α, IL‐6, and IL‐10 SNPs and AML risk was not evaluated because we only had a few cases with only one variant genotype and no cases with wild‐type homozygous genotype for all of the analyzed cytokine SNPs.
Our study contains a number of limitations, including the lack of data regarding cytokine expression and the lack of information regarding RUNX1 and CEBPA mutations, the lack of data about the plasma level of TNF‐α, IL‐10, IL‐6, TGF‐β1 and IFN‐γ in the patient samples. However, the robustness of this study is represented by the fact that it investigated a representative AML cohort in Romania, since, to our knowledge, there is currently no published data on the role of these polymorphisms in AML on Eastern European populations. This is the first study to investigate the association between cytokine SNPs and AML risk, and, as a result, our report presents novel observations not previously described in a complex heterogeneous disease characterized by genomic changes and the accumulation of blasts, most commonly in the bone marrow, resulting in bone marrow failure and leading to death.38, 39
Furthermore, our study investigated, for the first time, the relationship between somatic mutations in AML and cytokine SNPs in a single multivariate model, in which the estimation method allowed the selection of variables, model fitting, and stable regression coefficients.
In conclusion, based on our findings, we consider that TGF‐β1 rs1800470 and IFN‐γ rs2430561 variant genotypes were associated with AML susceptibility. Our study revealed that age above 65 years, PLT count (<40 000 cells/mm3), TNF‐α rs1800750 variant genotype, blast percentage (>70%), LDH level (≥600 IU/L), and cytogenetic high risk may be used as independent risk factors for assessing AML mortality.
CONFLICT OF INTERESTS
The authors report no conflict of interests.
ACKNOWLEDGMENTS
The authors of the manuscript would like to extend their thanks to the participants (patients and controls) in the present study. This work was supported by a grant of the Romanian National Authority for Scientific Research and Innovation, CNCS/CCCDI‐UEFISCDI, project no. PN‐III‐P2‐2.1‐PED‐2016‐1076 within PNCDI III, contract no. 147 PED/2017.
Bănescu C, Tripon F, Trifa AP, et al. Cytokine rs361525, rs1800750, rs1800629, rs1800896, rs1800872, rs1800795, rs1800470, and rs2430561 SNPs in relation with prognostic factors in acute myeloid leukemia. Cancer Med. 2019;8:5492–5506. 10.1002/cam4.2424
Claudia Bănescu, Florin Tripon and Adrian P. Trifa equally contributed to this paper.
REFERENCES
- 1. Bănescu C, Iancu M, Trifa AP, et al. Influence of XPC, XPD, XPF, and XPG gene polymorphisms on the risk and the outcome of acute myeloid leukemia in a Romanian population. Tumour Biol. 2016;37(7):9357‐9366. 10.1007/s13277-016-4815-6 [DOI] [PubMed] [Google Scholar]
- 2. Lee JS, Cheong HS, Koh Y, Ahn KS, Shin HD, Yoon SS. MCM7 polymorphisms associated with the AML relapse and overall survival. Ann Hematol. 2017;96(1):93‐98. 10.1007/s00277-016-2844-2. [DOI] [PubMed] [Google Scholar]
- 3. Multhoff G, Radons J. Radiation, inflammation, and immune responses in cancer. Front Oncol. 2012;2:58 10.3389/fonc.2012.00058. eCollection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Qu X, Tang Y, Hua S. Immunological approaches towards cancer and inflammation: a cross talk. Front Immunol. 2018;20(9):563 10.3389/fimmu.2018.00563. eCollection 2018. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Georgescu AM, Bănescu C, Badea I, et al. IL‐6 gene polymorphisms and sepsis in ICU adult Romanian patients: a prospective study. Rev Romana Med Lab. 2017;25(1):75‐89. 10.1515/rrlm-2016-0044 [DOI] [Google Scholar]
- 6. Sanjabi S, Zenewicz LA, Kamanaka M, Flavell RA. Anti‐ and Pro‐inflammatory roles of TGF‐β, IL‐10, and IL‐22 in immunity and autoimmunity. Curr Opin Pharmacol. 2009;9(4):447‐453. 10.1016/j.coph.2009.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dong M, Blobe GC. Role of transforming growth factor‐beta in hematologic malignancies. Blood. 2006;107(12):4589‐4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sanchez‐Correa B, Bergua JM, Campos C, et al. Cytokine profiles in acute myeloid leukemia patients at diagnosis: survival is inversely correlated with IL‐6 and directly correlated with IL‐10 levels. Cytokine. 2013;61(3):885‐891. 10.1016/j.cyto.2012.12.023 [DOI] [PubMed] [Google Scholar]
- 9. Mondet J, Hussein K, Mossuz P. Circulating cytokine levels as markers of inflammation in Philadelphia negative myeloproliferative neoplasms: diagnostic and prognostic interest. Mediators Inflamm. 2015;2015:1–10. 10.1155/2015/670580.. Epub 2015 Oct 7. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carey A, Edwards DK, Eide CA, et al. Identification of interleukin‐1 by functional screening as a key mediator of cellular expansion and disease progression in acute myeloid leukemia. Cell Rep. 2017;18(13):3204‐3218. 10.1016/j.celrep.2017.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hiroki CH, Amarante MK, Petenuci DL, et al. IL‐10 gene polymorphism and influence of chemotherapy on cytokine plasma levels in childhood acute lymphoblastic leukemia patients: IL‐10 polymorphism and plasma levels in leukemia patients. Blood Cells Mol Dis. 2015;55(2):168‐172. 10.1016/j.bcmd.2015.06.004 [DOI] [PubMed] [Google Scholar]
- 12. Trifunović J, Miller L, Debeljak Ž, Horvat V. Pathologic patterns of interleukin 10 expression – a review. Biochem Med (Zagreb). 2015;25(1):36‐48. 10.11613/BM.2015.004. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pehlivan M, Sahin HH, Pehlivan S, et al. Prognostic importance of single‐nucleotide polymorphisms in IL‐6, IL‐10, TGF‐β1, IFN‐γ, and TNF‐α genes in chronic phase chronic myeloid leukemia. Genet Test Mol Biomarkers. 2014;18(6):403‐409. 10.1089/gtmb.2014.0011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Urbanowicz I, Mazur G, Stacherzak‐Pawlik J, et al. IFN gamma gene polymorphism may contribute to the susceptibility to CLL. Pathol Oncol Res. 2010;16(2):213‐216. 10.1007/s12253-009-9209-2 [DOI] [PubMed] [Google Scholar]
- 15. Wu Z, Sun Y, Zhu S, Tang S, Liu C, Qin W. Association of interferon gamma +874T/A polymorphism and leukemia risk: a meta‐analysis. Medicine (Baltimore). 2016;95(12):e3129 10.1097/MD.0000000000003129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Parlayan C, Ikeda S, Sato N, Sawabe M, Muramatsu M, Arai T. Comprehensive association analysis of 30 single nucleotide polymorphisms related with metabolic syndrome on cancer susceptibility in Japanese population: a case‐control study. Health Sci J. 2018;12(1):545 10.21767/1791-809X.1000545 [DOI] [Google Scholar]
- 17. Nursal AF, Pehlivan M, Sahin HH, Pehlivan S. The associations of IL‐6, IFN‐γ, TNF‐α, IL‐10, and TGF‐β1 functional variants with acute myeloid leukemia in Turkish patients. Genet Test Mol Biomarkers. 2016;20(9):544‐551. 10.1089/gtmb.2016.0036 [DOI] [PubMed] [Google Scholar]
- 18. Vitiello G, Guembarovski RL, Oliveira CECd, Amarante MK, Perim AL, Watanabe MAE. Involvement of transforming growth factor beta‐1 (TGFβ1) cytokine and FOXP3 transcription factor genetic polymorphisms in hematological malignancies. Braz Arch Biol Technol. 2015;58(4):553‐561. 10.1590/S1516-8913201500287 [DOI] [Google Scholar]
- 19. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391‐2405. [DOI] [PubMed] [Google Scholar]
- 20. Daneshmandi S, Pourfathollah AA, Pourpak Z, Heidarnazhad H, Kalvanagh PA. Cytokine gene polymorphism and asthma susceptibility, progress and control level. Mol Biol Rep. 2012;39(2):1845‐1853. 10.1007/s11033-011-0927-7 [DOI] [PubMed] [Google Scholar]
- 21. Moreno I, Martín G, Bolufer P, et al. Incidence and prognostic value of FLT3 internal tandem duplication and D835 mutations in acute myeloid leukemia. Haematologica. 2003;88(1):19‐24. [PubMed] [Google Scholar]
- 22. Brewin JN, Horne GA, Bisling KE, Stewart HJ, Chevassut TJ. Rapid detection of DNMT3A R882 codon mutations allows early identification of poor risk patients with acute myeloid leukemia. Leuk Lymphoma. 2013;54(6):1336‐1339. [DOI] [PubMed] [Google Scholar]
- 23. Azari‐Yam A, Bagheri SD, Tavakkoly‐Bazzaz J, et al. NPM1 mutation detection in acute myeloid leukemia: a method comparison study. Genet Test Mol Biomarkers. 2016;20(2):63‐66. [DOI] [PubMed] [Google Scholar]
- 24. Tripon F, Crauciuc GA, Moldovan VG, et al. Simultaneously FLT3, NPM1 and DNMT3A mutations in adult patients with acute myeloid leukemia – case study. Rev Romana Med Lab. 2019;27(2): 245–254. 10.2478/rrlm-2019-0022. [DOI] [Google Scholar]
- 25. Sarah EH, William ML, Laurent G et al. Ensembl variation resources. 2018. 10.1093/database/bay119. [DOI] [Google Scholar]
- 26. Pavlou M, Ambler G, Seaman SR, et al. How to develop a more accurate risk prediction model when there are few events. BMJ. 2015;11(351):h3868 10.1136/bmj.h3868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Su X, Wijayasinghe CS, Fan J, Zhang Y. Sparse estimation of Cox proportional hazards models via approximated information criteria. Biometrics. 2016;72(3):751‐759. 10.1111/biom.12484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eliades N‐G, Eliades DG. HAPLOTYPE ANALYSIS: Software for Analysis of Haplotype Data. Distributed by the authors. Goettingen, Germany: Forest Genetics and Forest Tree Breeding, Georg‐August University; 2009. http://www.uni-goettingen.de/en/134935.html [Google Scholar]
- 29. Gong LL, Han FF, Lv YL, et al. TNF‐α and LT‐α polymorphisms and the risk of leukemia: a meta‐analysis. Tumori. 2017;103(1):53–59. 10.5301/tj.5000549 [DOI] [PubMed] [Google Scholar]
- 30. Jevtovic‐Stoimenov T, Cvetkovic T, Despotovic M, et al. The influence of TNF alpha ‐308 G/A polymorphism on oxidative stress in patients with chronic lymphocytic leukemia. Leuk Res. 2017;54:66‐72. 10.1016/j.leukres.2017.01.018 [DOI] [PubMed] [Google Scholar]
- 31. Macedo LC, de Cesare QF, Pagliari‐E‐Silva S, et al. Association of TNF polymorphisms with JAK2 (V617F) myeloproliferative neoplasms in Brazilian patients. Blood Cells Mol Dis. 2016;57:54‐57. 10.1016/j.bcmd.2015.12.005 [DOI] [PubMed] [Google Scholar]
- 32. Torres‐Espíndola LM, Velázquez‐Cruz R, Falfán‐Valencia R, et al. Genetic polymorphism of tumor necrosis factor promoter region and susceptibility to develop Hodgkin lymphoma in a Mexican population. Leuk Lymphoma. 2014;55(6):1295‐1299. 10.3109/10428194.2013.842982 [DOI] [PubMed] [Google Scholar]
- 33. Mutlu P, Yalcin S, Elci P, Yildirim M, Cetin AT, Avcu F. Association of ‐174G/C interleukin/6 gene polymorphism with the risk of chronic lymphocytic, chronic myelogenous and acute myelogenous leukemias in Turkish patients. J BUON. 2014;19(3):787‐791. [PubMed] [Google Scholar]
- 34. Chenjiao Y, Zili F, Haibin C, et al. IL‐10 promoter polymorphisms affect IL‐10 production and associate with susceptibility to acute myeloid leukemia. Pharmazie. 2013;68(3):201‐206. [PubMed] [Google Scholar]
- 35. Fei C, Yao XM, Sun Y, Gu XZ, Yu LQ, Lai X. Interleukin‐10 polymorphisms associated with susceptibility to acute myeloid leukemia. Genet Mol Res. 2015;14(1):925‐930. 10.4238/2015 [DOI] [PubMed] [Google Scholar]
- 36. Kim M, Kim J, Kim JR, et al. FLT3 expression and IL10 promoter polymorphism in acute myeloid leukemia with RUNX1‐RUNX1T1. Mol Biol Rep. 2015;42:451‐456. 10.1007/s11033-014-3786-1 [DOI] [PubMed] [Google Scholar]
- 37. Rashed R, Shafik RE, Shafik NF, Shafik HE. Associations of interleukin‐10 gene polymorphisms with acute myeloid leukemia in human (Egypt). J Cancer Res Ther. 2018;14(5):1083‐1086. 10.4103/0973-1482.187367 [DOI] [PubMed] [Google Scholar]
- 38. Bănescu C, Iancu M, Trifa AP, et al. From six gene polymorphisms of the antioxidant system, only GPX Pro198Leu and GSTP1 Ile105Val modulate the risk of acute myeloid leukemia. Oxid Med Cell Longev. 2016;2016:1–10. 10.1155/2016/2536705. Epub 2015 Dec 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Estey EH. Acute myeloid leukemia: 2019 update on risk‐stratification and management. Am J Hematol. 2018;93(10):1267‐1291. 10.1002/ajh.25214 [DOI] [PubMed] [Google Scholar]


