Abstract
Background
T1‐weighted dynamic contrast‐enhanced (DCE) perfusion magnetic resonance imaging (MRI) has been broadly utilized in the evaluation of brain tumors. We aimed at assessing the diagnostic accuracy of DCE‐MRI in discriminating between low‐grade gliomas (LGGs) and high‐grade gliomas (HGGs), between tumor recurrence and treatment‐related changes, and between primary central nervous system lymphomas (PCNSLs) and HGGs.
Methods
We performed this study based on the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis of Diagnostic Test Accuracy Studies criteria. We systematically surveyed studies evaluating the diagnostic accuracy of DCE‐MRI for the aforementioned entities. Meta‐analysis was conducted with the use of a random effects model.
Results
Twenty‐seven studies were included after screening of 2945 possible entries. We categorized the eligible studies into three groups: those utilizing DCE‐MRI to differentiate between HGGs and LGGs (14 studies, 546 patients), between recurrence and treatment‐related changes (9 studies, 298 patients) and between PCNSLs and HGGs (5 studies, 224 patients). The pooled sensitivity, specificity, and area under the curve for differentiating HGGs from LGGs were 0.93, 0.90, and 0.96, for differentiating tumor relapse from treatment‐related changes were 0.88, 0.86, and 0.89, and for differentiating PCNSLs from HGGs were 0.78, 0.81, and 0.86, respectively.
Conclusions
Dynamic contrast‐enhanced‐Magnetic resonance imaging is a promising noninvasive imaging method that has moderate or high accuracy in stratifying gliomas. DCE‐MRI shows high diagnostic accuracy in discriminating between HGGs and their low‐grade counterparts, and moderate diagnostic accuracy in discriminating recurrent lesions and treatment‐related changes as well as PCNSLs and HGGs.
Keywords: dynamic contrast‐enhanced MRI, gliomas, lymphoma, meta‐analysis, perfusion
Dynamic contrast‐enhanced‐Magnetic resonance imaging has high diagnostic accuracy in grading gliomas and moderate accuracy in surveillance of high‐grade gliomas and differentiation from primary central nervous system lymphomas. DCE‐MRI is a promising noninvasive imaging technique in diagnosing different types of brain tumors.
![]()
1. INTRODUCTION
Gliomas account for approximately 28% of all central nervous system tumors and 80% of all malignant brain tumors.1 The 2016 World Health Organization classification divides gliomas into grade I to IV, with grades I and II considered to be low‐grade gliomas (LGGs) and grades III and IV considered high‐grade gliomas (HGGs), on the basis of their histology and molecular features.2 Primary central nervous system lymphoma (PCNSL) most commonly occurs in the elderly3 and comprises 2.1% of primary intracranial tumors.1
The treatment options and prognosis are heavily dependent on the histological types and the recurrence status. The present standard therapy of HGGs is surgical resection and concomitant chemoradiation.4 Chemoradiation may knowingly result in radiation necrosis and pseudoprogression, which may notoriously resemble recurrence and tumor progression.5 Therefore, it is crucially important to utilize a noninvasive imaging technique that can differentiate them for the patient management.
Although magnetic resonance imaging (MRI) is routinely applied to classify brain tumors, conventional MRI has shortcomings.6, 7, 8, 9 To overcome such limitations, previous studies have reported combining conventional MRI with multimodal techniques, which increase the diagnostic accuracy.9, 10, 11
Perfusion‐weighted imaging is commonly used for the assessment and classification of intracranial tumors, and may be performed as dynamic susceptibility contrast‐enhanced (DSC) MRI, T1‐based dynamic contrast‐enhanced (DCE) MRI, and arterial spin labeling (ASL).12, 13, 14, 15 The most common MR perfusion technique in clinical practice is DSC‐MRI.8, 12 However, DCE‐MRI has added benefits of higher spatial resolution, more reliable quantification measurement of microvasculature and permeability indices, and reduced susceptibility artifacts with respect to DSC‐MRI.16, 17
A number of single‐center studies in mainly small cohorts have shown the potential of DCE‐MRI.18, 19, 20 Our work extends previous studies with a systematic large‐scale meta‐analysis and aims at evaluating the diagnostic value of DCE‐MRI. To achieve these aims, we have specifically explored whether using DCE measurements can successfully differentiate LGGs from HGGs, tumor recurrence from treatment‐related changes, and PCNSLs from HGGs.
2. MATERIALS AND METHODS
This study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis of Diagnostic Test Accuracy Studies criteria.21 This systematic review was registered in the PROSPERO online database of systematic reviews (CRD42018108948).
2.1. Search strategy
The search was systematically conducted on June 8, 2017 using PubMed, Ovid Embase, and the Cochrane Library. The detail of the search strategy is presented in the Supplementary Material 1.
2.2. Selection criteria
The abstracts of all articles retrieved in the initial search were screened by board‐certified neuroradiologists and in‐training neuroradiologists with research experience in perfusion imaging in neuro‐oncology. Selected full text manuscripts were reviewed to determine their relevance in detail. Both processes were executed by independent reviewers according to the following criteria. The inclusion criteria were: (a) DCE performed on brain tumor patients prior or during treatment; (b) study assessed diagnostic or prognostic value of DCE parameters. The exclusion criteria were: (a) no DCE (T1‐weighted perfusion) was performed; (b) no brain tumor patients were examined; (c) the study was conducted in pediatric population (<18 years old); (d) animal/laboratory study; (e) review articles, case reports, letters, commentaries, or conference proceedings; (f) brain tumor histology was not confirmed; (g) non‐English articles. In cases of discrepancies between two reviewers, a third one resolved the case.
For the meta‐analysis, selected full manuscripts were reviewed by two independent reviewers and in cases of discrepancies between two reviewers, all discrepancies were resolved by consensus. The inclusion criteria were: (a) the studies assessed the diagnostic accuracy of DCE‐MRI for discriminating between HGGs and LGGs, between recurrence and treatment‐related changes, and between PCNSLs and HGGs. The exclusion criteria were: (a) patient population clearly overlapped with other studies cohorts; (b) the information for extracting or calculating true‐negative (TN), false‐negative (FN), true‐positive (TP), and false‐positive (FP) values was not listed. Studies assessing the prognostic value of DCE‐MRI were excluded due to their small number. If overlapping studies showed no distinct information, the study with more patients was chosen.
2.3. Data extraction
Data were extracted from the included studies. Data included sensitivity and specificity to calculate subsequently the TN, FN, TP, and FP for each of the diagnostic task under investigation, number of patients, age of patients, study design, tumor histology, MRI field strength, whether DCE‐MRI was followed with DSC‐MRI or not, methods of a region of interest (ROI) analysis, deconvolution with arterial input function, and DCE model. The same two reviewers, who performed full‐text screening, independently conducted data extraction, and all inconsistencies were resolved by consensus.
2.4. Study quality assessment
We assessed the study quality based on the Quality Assessment of Diagnostic Accuracy Studies (QUADAS‐2) instrument (see Supplementary Material 2).22 Each study was evaluated for potential bias and quality by two independent reviewers experienced in neuro‐oncological imaging and advanced MRI techniques. Disagreements were resolved by consensus.
2.5. Statistical analysis
True‐negative, FN, TP, and FP values were calculated from the number of patients, and their sensitivity and specificity for statistical analysis. Two studies showed complete patients data but did not present calculations of sensitivity and specificity.23, 24 Therefore, we calculated these from the published patient data in each article using commercially available (MedCalc version 18.5 for Windows) software (Ostend, Belgium). Our statistical analysis explored the diagnostic accuracy of DCE in the following comparisons: HGGs vs LGGs, recurrence vs treatment‐related changes, and PCNSLs vs HGGs. Specifically, DTA meta‐analysis, subgroup analysis, heterogeneity, and publication bias were executed with the use of the MIDAS in STATA 15.0 (College Station, TX).
In DTA meta‐analysis, the pooled sensitivity, specificity, positive likelihood ratios (PLRs), negative likelihood ratios (NLRs), diagnostic odds ratios (DORs), and their 95% CIs were calculated for each comparison. The values of DCE parameters with the highest diagnostic accuracy were used. Random effects models were applied to address the expected heterogeneity. The accuracy was determined using a summary receiver operating characteristic curve (SROC) plot. To quantify error and accuracy, the area under the curve (AUC) was calculated. AUC values of more than 0.9 represented high accuracy and 0.7 ≤ AUC ≤ 0.9 reflected moderate accuracy.25
The pooled sensitivity and specificity were calculated in subgroups (studies number ≥4) created based on DCE perfusion imaging derived parameters (K trans, ve, and vp), applied pharmacokinetic model (model‐independent and two‐compartment model approaches), and methods of ROI analysis (whole lesion volume, lesion “hot‐spot,” and operator‐selected tumor part).
Heterogeneity was tested with the use of the quantity I 2. An I 2 >50% indicated substantial heterogeneity. The publication bias was evaluated for the analyses including >10 studies26 with the use of funnel plot asymmetry test.27, 28 P < 0.10 indicated significant asymmetry and low publication bias.27, 28
3. RESULTS
A total of 2945 articles were confirmed using our electronic database search. After removing duplicate articles and screening the studies titles and abstracts, 245 articles meeting the inclusion criteria underwent full‐text assessment resulting in 27 relevant studies.12, 13, 15, 18, 19, 20, 23, 24, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47 A flowchart of the selection procedure is summarized in Figure 1.
Figure 1.
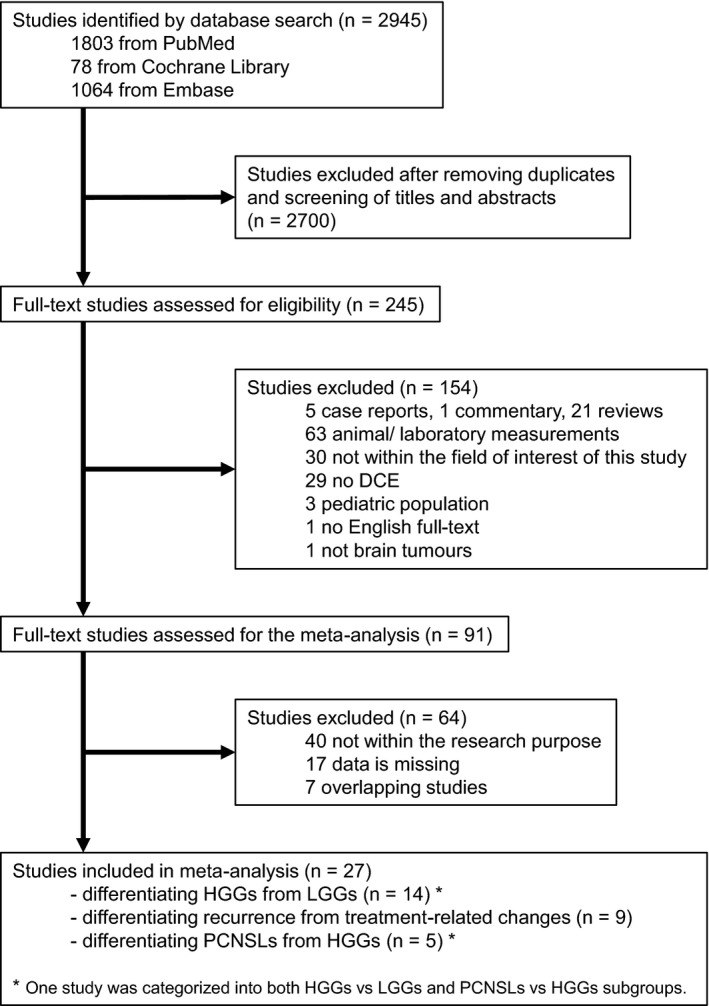
Flowchart describing the study selection process. One study was categorized in two categories (HGGs vs LGGs, PCNSLs vs HGGs). DCE, dynamic contrast‐enhanced; HGG, high‐grade glioma; LGG, low‐grade glioma; PCNSL, primary CNS lymphoma
3.1. Eligible studies characteristics
We categorized the 27 eligible studies into three groups assessing the role of DCE‐MRI in differentiation: HGGs from LGGs (14 studies12, 18, 23, 24, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38: 190 LGG and 356 HGG patients), recurrence from treatment‐related changes (9 studies13, 19, 39, 40, 41, 42, 43, 44, 45: 179 patients with relapse and 119 subjects with histologically/clinico‐radiologically verified treatment‐related changes) and PCNSLs from HGGs (5 studies15, 20, 34, 46, 47: 68 PCNSLs and 156 HGGs patients). One study was categorized into both HGGs vs LGGs and PCNSLs vs HGGs subgroups.34 All features of the included studies are demonstrated in Table 1 and Supplementary Material 3. The sensitivity and specificity of each DCE‐derived parameter are listed in Supplementary Material 4.
Table 1.
The characteristics of the studies included in the meta‐analysis
| High‐grade gliomas vs Low‐grade gliomas | Recurrence vs Treatment‐related changes | Primary central nervous system lymphomas vs High‐grade gliomas | |
|---|---|---|---|
| Patients, N | 546 (14 studies) | 298 (9 studies) | 224 (5 studies) |
| LGG: 190 | Recurrence: 179 | PCNSL: 68 | |
| HGG: 356 | Treatment‐related change: 119 | HGG: 156 | |
| DCE model | |||
| Two‐compartment model | 437 (12 studies) |
183 (6 studies) |
182 (4 studies) |
| Model independent | 109 (2 studies) | 139 (4 studies) | 42 (1 study) |
| DCE parameters | |||
| K trans | 352 (8 studies) | 183 (6 studies) | 125 (2 studies) |
| ve | 193 (5 studies) | 57 (2 studies) | 146 (3 studies) |
| vp | 235 (6 studies) | 61 (2 studies) | 36 (1 study) |
| Region of interest | |||
| Whole volume | 131 (4 studies) | 243 (7 studies) | 203 (4 studies) |
| Hot‐spot | 415 (10 studies) | 55 (2 studies) | 21 (1 study) |
| Country | |||
| China | 4 studies | 2 studies | |
| USA | 3 studies | 3 studies | 1 study |
| India | 2 studies | ||
| Canada | 2 studies | ||
| Korea | 1 study | 3 studies | 1 study |
| Italy | 1 study | ||
| Germany | 1 study | 2 studies | 1 study |
| Denmark | 1 study | ||
Abbreviations: DCE, dynamic contrast‐enhanced; HGG, high‐grade glioma; LGG, low‐grade glioma; PCNSL, primary CNS lymphoma.
3.2. Qualitative assessment
The results of the qualitative assessment are shown in Figure 2. Many studies had high bias in the patient selection and in the conduct or interpretation of the index test because of retrospective study design and a single rater. In more than 10 studies, it was unclear whether radiologists were blinded to histology or whether the interval between MRI and surgery was appropriate.
Figure 2.
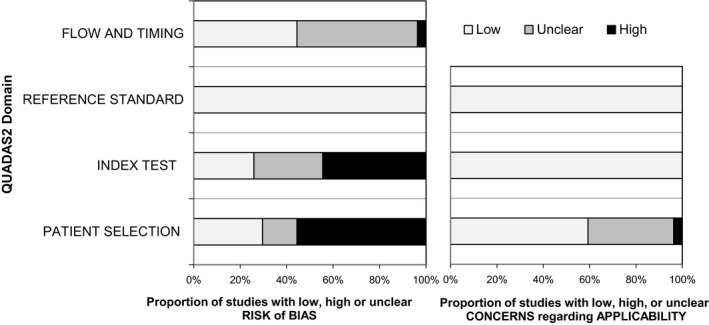
Results of the QUADAS2 quality assessment of the included studies. The risk of bias in four different domains and concerns regarding applicability in three domains are shown
3.3. Diagnostic test accuracy analysis: HGGs vs LGGs
3.3.1. Overall diagnostic accuracy
The pooled sensitivity was 0.93 and the pooled specificity was 0.90. Table2 shows PLR, NLR, and DOR. Figure 3A demonstrates the SROC plot with AUC of 0.96, implying high diagnostic accuracy. The sensitivity showed mild heterogeneities (I 2 = 57.25%), specificity was also heterogeneous (I 2 = 41.57%). The funnel plot revealed publication bias (P = 0.010).
Table 2.
Results of pooled estimates of studies
| Study number | Sensitivity | Specificity | PLR | NLR | DOR | AUC | I 2 sensitivity | I 2 specificity | |
|---|---|---|---|---|---|---|---|---|---|
| Diagnostic test accuracy analysis: high‐grade gliomas vs low‐grade gliomas | |||||||||
| All | 14 | 0.93 [0.87‐0.96] | 0.90 [0.82‐0.94] | 9.2 [5.1‐16.6] | 0.08 [0.05‐0.15] | 113 [42‐305] | 0.96 [0.94‐0.98] | 57.25 [31.82‐82.69] | 41.57 [4.74‐78.41] |
| Two‐compartment model | 12 | 0.91 [0.85‐0.95] | 0.89 [0.81‐0.94] | 8.4 [4.5‐15.6] | 0.10 [0.06‐0.17] | 82 [31‐216] | 0.96 [0.93‐0.97] | 47.97 [13.28‐82.67] | 40.25 [0.00‐80.85] |
| model‐independent | 2 | Study number is too small | |||||||
| Ktrans | 8 | 0.93 [0.84‐0.97] | 0.91 [0.82‐0.96] | 10.2 [4.9‐21.1] | 0.08 [0.04‐0.18] | 128 [44‐368] | 0.97 [0.95‐0.98] | 67.59 [43.47‐91.71] | 44.58 [0.00‐89.71] |
| ve | 5 | 0.87 [0.77‐0.93] | 0.95 [0.82‐0.99] | 18.9 [4.4‐80.1] | 0.13 [0.07‐0.25] | 141 [22‐883] | 0.96 [0.93‐0.97] | 35.46 [0.00‐98.77] | 43.83 [0.00‐100.00] |
| vp | 6 | 0.83 [0.67‐0.92] | 0.91 [0.77‐0.97] | 9.0 [3.2‐25.9] | 0.19 [0.09‐0.41] | 49 [9‐256] | 0.94 [0.92‐0.96] | 75.46 [55.49‐95.43] | 45.74 [0.00‐96.03] |
| hot‐spot ROI | 10 | 0.95 [0.89‐0.98] | 0.90 [0.82‐0.95] | 9.3 [5.0‐17.2] | 0.05 [0.02‐0.13] | 175 [52‐594] | 0.96 [0.94‐0.98] | 66.15 [43.47‐88.83] | 39.94 [0.00‐84.35] |
| whole volume ROI | 4 | 0.85 [0.73‐0.92] | 0.92 [0.68‐0.98] | 10.9 [2.2‐53.7] | 0.16 [0.08‐0.32] | 67 [9‐514] | 0.92 [0.90‐0.94] | 0.00 [0.00‐100.00] | 58.80 [13.48‐100.00] |
| Diagnostic test accuracy analysis: recurrence vs treatment‐related changes | |||||||||
| All | 9 | 0.88 [0.74‐0.95] | 0.86 [0.78‐0.91] | 6.4 [3.8‐10.5] | 0.13 [0.06‐0.32] | 47 [14‐156] | 0.89 [0.86‐0.91] | 72.77 [54.46‐91.08] | 0.00 [0.00‐100.00] |
| Two‐compartment model | 6 | 0.77 [0.65‐0.86] | 0.85 [0.75‐0.92] | 5.2 [2.9‐9.3] | 0.27 [0.17‐0.44] | 19 [8‐47] | 0.87 [0.84‐0.90] | 45.62 [0.00‐96.02] | 0.00 [0.00‐100.00] |
| model‐independent | 4 | 0.94 [0.86‐0.98] | 0.85 [0.74‐0.93] | 6.5 [3.4‐12.3] | 0.07 [0.03‐0.16] | 93 [29‐300] | 0.96 [0.94‐0.97] | 0.00 [0.00‐100.00] | 0.00 [0.00‐100.00] |
| Ktrans | 6 | 0.75 [0.63‐0.84] | 0.79 [0.68‐0.87] | 3.6 [2.3‐5.8] | 0.32 [0.21‐0.49] | 11 [5‐25] | 0.82 [0.78‐0.85] | 40.32 [0.00‐95.54] | 0.00 [0.00‐100.00] |
| ve | 2 | Study number is too small | |||||||
| vp | 2 | Study number is too small | |||||||
| hot‐spot ROI | 2 | Study number is too small | |||||||
| whole volume ROI | 7 | 0.91 [0.73‐0.97] | 0.88 [0.78‐0.93] | 7.3 [3.8‐13.8] | 0.11 [0.03‐0.34] | 68 [14‐328] | 0.91 [0.88‐0.93] | 76.12 [58.35‐93.89] | 0.00 [0.00‐100.00] |
| Diagnostic test accuracy analysis: primary central nervous system lymphomas vs high‐grade gliomas | |||||||||
| All | 5 | 0.78 [0.63‐0.89] | 0.81 [0.67‐0.90] | 4.1 [2.1‐7.7] | 0.27 [0.14‐0.51] | 15 [5‐50] | 0.86 [0.83‐0.89] | 51.10 [2.09‐100.00] | 69.63 [41.11‐98.16] |
| Two‐compartment model | 4 | 0.75 [0.53‐0.89] | 0.83 [0.69‐0.92] | 4.5 [2.0‐10.3] | 0.30 [0.14‐0.67] | 15 [3‐70] | 0.86 [0.83‐0.89] | 45.87 [0.00‐100.00] | 67.52 [32.79‐100.00] |
| model‐independent | 1 | Study number is too small | |||||||
| Ktrans | 2 | Study number is too small | |||||||
| ve | 3 | Study number is too small | |||||||
| vp | 1 | Study number is too small | |||||||
| hot‐spot ROI | 1 | Study number is too small | |||||||
| whole tumor ROI | 4 | 0.82 [0.67‐0.91] | 0.81 [0.64‐0.91] | 4.3 [2.0‐9.2] | 0.22 [0.11‐0.47] | 19 [5‐77] | 0.88 [0.85‐0.90] | 50.13 [0.00‐100.00] | 77.41 [54.83‐99.99] |
The numbers in the parentheses are 95% confidence intervals.
Abbreviations: AUC, area under the curve; DOR, diagnostic odds ratio; NLR, negative likelihood ratio; PLR, positive likelihood ratio; ROI, region of interest.
Figure 3.
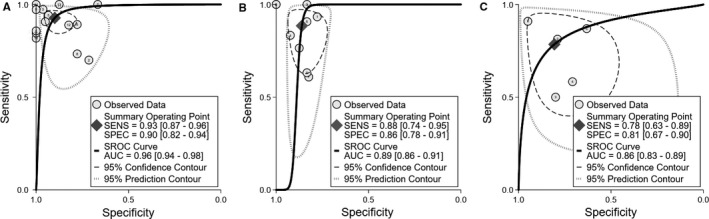
SROC plot of differentiating (A) HGGs from LGGs, (B) recurrence from treatment‐related changes, and (C) PCNSLs from HGGs. AUC, area under the curve; HGG, high‐grade glioma; LGG, low‐grade glioma; PCNSL, primary CNS lymphoma; SENS, sensitivity; SPEC, specificity; SROC, Summary receiver operating characteristic curve
3.3.2. Subgroup analyses
The results of the subgroup analyses are shown in Table 2. Sensitivity (0.95) was higher for studies with the hot‐spot method of ROI. AUC (0.97) was the highest for the studies that used K trans. Heterogeneity was lower for the studies that used ve. The model‐independent parameters were not entitled for subgroup meta‐analysis due to the small number of studies.
3.4. Diagnostic test accuracy analysis: recurrence vs treatment‐related changes
3.4.1. Overall diagnostic accuracy
The pooled sensitivity was 0.88 and the pooled specificity was 0.86. Table 2 shows PLR, NLR, and DOR. Figure 3B exhibits the SROC plot with AUC of 0.89, suggesting moderate diagnostic accuracy. The sensitivity analysis showed substantial heterogeneity (I 2 = 72.77%) and the specificity analysis presented low heterogeneity (I 2 = 0.00%).
3.4.2. Subgroup analyses
Table 2 summarizes the results of the subgroup analyses. Sensitivity (0.94) and AUC (0.96) were the highest for studies using model‐independent approaches. The subgroup analysis for the two‐compartment model approach, the model‐independent approach, and K trans estimation had no obvious heterogeneity. Articles with ve, vp calculation, and “hot‐spot” ROI placement were not eligible for further subgroup meta‐analysis.
3.5. Diagnostic test accuracy analysis: PCNSLs vs HGGs
3.5.1. Overall diagnostic accuracy
The pooled sensitivity and specificity were 0.78 and 0.81, respectively. Table 2 shows PLR, NLR, DOR, and AUC. Figure 3C presents the SROC plot with AUC of 0.86, demonstrating moderate diagnostic accuracy. The sensitivity and specificity were characterized by mild heterogeneity (I 2 = 51.10% and 69.63%, respectively).
3.5.2. Subgroup analyses
The results of the subgroup analyses are detailed in Table 2. We could perform subgroup analysis only for studies with two‐compartment model approaches (N = 4) and whole volume analysis (N = 4).
4. DISCUSSION
Our results suggest that DCE‐MRI can stage gliomas into HGGs and LGGs with high diagnostic performance, whereas the accuracy in discriminating between tumor recurrence and unspecific treatment‐induced changes, and between PCNSLs and mimicking HGGs is slightly lower. The overall diagnostic performance results indicate that DCE‐MRI can be successfully utilized in the current neuro‐oncological clinical practice.
Our work adds to the existing literature and a previous systematic review and meta‐analysis, which had compared the diagnostic value of selected advanced MRI techniques, including DCE‐MRI, in brain tumors.8, 48, 49, 50 We believe that this is the first meta‐analysis to perform subgroup analyses addressing the type of ROI analysis, the applied pharmacokinetic model, and DCE‐MRI derived parameters. DCE‐MRI as perfusion surrogate measures is relatively understudied because data noise and model fitting instabilities have a remarkable effect on the modeling process.51 Parameter values and diagnostic accuracy differ also depending on the methods of ROI selection with the optimal strategy to be still an open debate.
Among the applied ROI methods for stratifying gliomas, “hot‐spot” measurement had higher accuracy than whole volume ROI, in line with the report by Santarosa et al38 Although “hot‐spot” is presumed to reflect accurate staging, whole lesion measurement is reproducible, comprehensive but can be time consuming.
To differentiate between recurrence and treatment‐related changes, the model‐independent showed clearly higher sensitivity and AUC than for the 2‐compartment model‐derived perfusion biomarkers, as reported by Hamilton et al19 Model‐independent parameters are generally preferred because temporal resolution requirements are relaxed and the potential for fit failure owing to signal noise is irrelevant.52
There are some limitations in our study. First, the analysis of studies aiming at grading gliomas revealed publication bias and the composition of the two groups was imbalanced. Most analyses indicated substantial heterogeneity in terms of MR field strength, different types of MR coils, pulse sequence parameters, volume of contrast agent, injection time, which all could affect the outcomes. Some studies performed DCE using only half of contrast agent for DCE‐MRI, followed with DSC‐MRI.12, 38 ROI methodology, DCE parameters, and DCE models (most studies were on the basis of the two‐compartment Tofts‐Kermode model) also differed substantially prompting us to perform subgroup analyses, which in turn indicated substantial heterogeneity. Model‐independent analysis papers also reported different parameters on each study. The study designs of the included studies revealed only retrospective analyses, lack of consensus and blinding in placing ROIs exposing the studies to substantial bias. Another limitation is the small number studies included in subgroup analyses, and we acknowledge that further studies are needed for adding credibility. Last but not least, in the era of integrated histomolecular glioma classification, there was insufficient number of studies which evaluated the diagnostic accuracy of molecular subtype using DCE‐MRI.53
In conclusion, our results suggest that DCE‐MRI is a promising noninvasive imaging method that has good accuracy in diagnosing different types of brain tumors. Specifically, DCE‐MRI has high diagnostic performance in stratifying gliomas in high‐ or low‐grade, and moderate diagnostic accuracy in differentiating recurrence from treatment‐related changes, and PCNSLs from HGGs. Significant efforts for the standardization of the acquisition parameters and the postprocessing should be, however, intensely made.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
SO, AU, JU, ST, and SB were involved in conception and design. SO, AU, IL, JU, ML, SMH, ES, ST, and SB were involved in acquisition of data (screening and data extraction). SO, ARG, JPG, XG, and SB were involved in statistical analysis and interpretation of data. All authors were involved in drafting, editing and critical revision for important intellectual content. All authors gave final approval of the version to be published.
Supporting information
Okuchi S, Rojas‐Garcia A, Ulyte A, et al. Diagnostic accuracy of dynamic contrast‐enhanced perfusion MRI in stratifying gliomas: A systematic review and meta‐analysis. Cancer Med. 2019;8:5564–5573. 10.1002/cam4.2369
Sachi Okuchi, Antonio Rojas‐Garcia and Agne Ulyte contributed equally to this work
Funding information
This research is supported by the National Institute for Health Research (NIHR) University College London Hospitals Biomedical Research Centre (UCLH BRC) and the NIHR Collaboration for Leadership in Applied Health Research and Care North Thames at Bart's Health NHS Trust (NIHR CLAHRC North Thames). The views expressed in this article are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care.
REFERENCES
- 1. Ostrom QT, Gittleman H, Farah P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 2013;15(suppl 2):ii1–ii56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803‐820. [DOI] [PubMed] [Google Scholar]
- 3. Shiels MS, Pfeiffer RM, Besson C, et al. Trends in primary central nervous system lymphoma incidence and survival in the U.S. Br J Haematol. 2016;174:417‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987‐996. [DOI] [PubMed] [Google Scholar]
- 5. Yang I, Aghi MK. New advances that enable identification of glioblastoma recurrence. Nat Rev Clin Oncol. 2009;6:648‐657. [DOI] [PubMed] [Google Scholar]
- 6. Ginsberg LE, Fuller GN, Hashmi M, Leeds NE, Schomer DF. The significance of lack of MR contrast enhancement of supratentorial brain tumors in adults: histopathological evaluation of a series. Surg Neurol. 1998;49:436‐440. [DOI] [PubMed] [Google Scholar]
- 7. Al‐Okaili RN, Krejza J, Woo JH, et al. Intraaxial brain masses: MR imaging‐based diagnostic strategy–initial experience. Radiology. 2007;243:539‐550. [DOI] [PubMed] [Google Scholar]
- 8. van Dijken B, van Laar PJ, Holtman GA, van der Hoorn A. Diagnostic accuracy of magnetic resonance imaging techniques for treatment response evaluation in patients with high‐grade glioma, a systematic review and meta‐analysis. Eur Radiol. 2017;27:4129‐4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zonari P, Baraldi P, Crisi G. Multimodal MRI in the characterization of glial neoplasms: the combined role of single‐voxel MR spectroscopy, diffusion imaging and echo‐planar perfusion imaging. Neuroradiology. 2007;49:795‐803. [DOI] [PubMed] [Google Scholar]
- 10. Hu X, Wong KK, Young GS, Guo L, Wong ST. Support vector machine multiparametric MRI identification of pseudoprogression from tumor recurrence in patients with resected glioblastoma. J Magn Reson Imaging. 2011;33:296‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kickingereder P, Wiestler B, Sahm F, et al. Primary central nervous system lymphoma and atypical glioblastoma: multiparametric differentiation by using diffusion‐, perfusion‐, and susceptibility‐weighted MR imaging. Radiology. 2014;272:843‐850. [DOI] [PubMed] [Google Scholar]
- 12. Nguyen TB, Cron GO, Perdrizet K, et al. Comparison of the diagnostic accuracy of DSC‐ and dynamic contrast‐enhanced MRI in the preoperative grading of astrocytomas. AJNR Am J Neuroradiol. 2015;36:2017‐2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shin KE, Ahn KJ, Choi HS, et al. DCE and DSC MR perfusion imaging in the differentiation of recurrent tumour from treatment‐related changes in patients with glioma. Clin Radiol. 2014;69:e264‐e272. [DOI] [PubMed] [Google Scholar]
- 14. Roy B, Awasthi R, Bindal A, et al. Comparative evaluation of 3‐dimensional pseudocontinuous arterial spin labeling with dynamic contrast‐enhanced perfusion magnetic resonance imaging in grading of human glioma. J Comput Assist Tomogr. 2013;37:321‐326. [DOI] [PubMed] [Google Scholar]
- 15. Kickingereder P, Sahm F, Wiestler B, et al. Evaluation of microvascular permeability with dynamic contrast‐enhanced MRI for the differentiation of primary CNS lymphoma and glioblastoma: radiologic‐pathologic correlation. AJNR Am J Neuroradiol. 2014;35:1503‐1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cha S. Update on brain tumor imaging: from anatomy to physiology. AJNR Am J Neuroradiol. 2006;27:475‐487. [PMC free article] [PubMed] [Google Scholar]
- 17. Essig M, Shiroishi MS, Nguyen TB, et al. Perfusion MRI: the five most frequently asked technical questions. AJR Am J Roentgenol. 2013;200:24‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arevalo‐Perez J, Peck KK, Young RJ, Holodny AI, Karimi S, Lyo JK. Dynamic contrast‐enhanced perfusion MRI and diffusion‐weighted imaging in grading of gliomas. J Neuroimaging. 2015;25:792‐798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hamilton JD, Lin J, Ison C, et al. Dynamic contrast‐enhanced perfusion processing for neuroradiologists: model‐dependent analysis may not be necessary for determining recurrent high‐grade glioma versus treatment effect. AJNR Am J Neuroradiol. 2015;36:686‐693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lin X, Lee M, Buck O, et al. Diagnostic accuracy of T1‐weighted dynamic contrast‐enhanced‐MRI and DWI‐ADC for differentiation of glioblastoma and primary CNS lymphoma. AJNR Am J Neuroradiol. 2017;38:485‐491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McInnes M, Moher D, Thombs BD, et al. Preferred reporting items for a systematic review and meta‐analysis of diagnostic test accuracy studies: the PRISMA‐DTA statement. JAMA. 2018;319:388‐396. [DOI] [PubMed] [Google Scholar]
- 22. Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529‐536. [DOI] [PubMed] [Google Scholar]
- 23. Ludemann L, Hamm B, Zimmer C. Pharmacokinetic analysis of glioma compartments with dynamic Gd‐DTPA‐enhanced magnetic resonance imaging. Magn Reson Imaging. 2000;18:1201‐1214. [DOI] [PubMed] [Google Scholar]
- 24. Roberts HC, Roberts TP, Brasch RC, Dillon WP. Quantitative measurement of microvascular permeability in human brain tumors achieved using dynamic contrast‐enhanced MR imaging: correlation with histologic grade. AJNR Am J Neuroradiol. 2000;21:891‐899. [PMC free article] [PubMed] [Google Scholar]
- 25. Swets JA. Measuring the accuracy of diagnostic systems. Science. 1988;240:1285‐1293. [DOI] [PubMed] [Google Scholar]
- 26. Ioannidis JP, Trikalinos TA. The appropriateness of asymmetry tests for publication bias in meta‐analyses: a large survey. CMAJ. 2007;176:1091‐1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang L, Min Z, Tang M, Chen S, Lei X, Zhang X. The utility of diffusion MRI with quantitative ADC measurements for differentiating high‐grade from low‐grade cerebral gliomas: evidence from a meta‐analysis. J Neurol Sci. 2017;373:9‐15. [DOI] [PubMed] [Google Scholar]
- 28. Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005;58:882‐893. [DOI] [PubMed] [Google Scholar]
- 29. Arevalo‐Perez J, Kebede AA, Peck KK, et al. Dynamic contrast‐enhanced MRI in low‐grade versus anaplastic oligodendrogliomas. J Neuroimaging. 2016;26:366‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jia Z, Geng D, Liu Y, Chen X, Zhang J. Low‐grade and anaplastic oligodendrogliomas: differences in tumour microvascular permeability evaluated with dynamic contrast‐enhanced magnetic resonance imaging. J Clin Neurosci. 2013;20:1110‐1113. [DOI] [PubMed] [Google Scholar]
- 31. Jung SC, Yeom JA, Kim J‐H, et al. Glioma: application of histogram analysis of pharmacokinetic parameters from T1‐weighted dynamic contrast‐enhanced MR imaging to tumor grading. AJNR Am J Neuroradiol. 2014;35:1103‐1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li X, Zhu Y, Kang H, et al. Glioma grading by microvascular permeability parameters derived from dynamic contrast‐enhanced MRI and intratumoral susceptibility signal on susceptibility weighted imaging. Cancer Imaging. 2015;15:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nguyen TB, Cron GO, Mercier JF, et al. Diagnostic accuracy of dynamic contrast‐enhanced MR imaging using a phase‐derived vascular input function in the preoperative grading of gliomas. AJNR Am J Neuroradiol. 2012;33:1539‐1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhao J, Yang ZY, Luo BN, Yang JY, Chu JP. Quantitative evaluation of diffusion and dynamic contrast‐enhanced MR in tumor parenchyma and peritumoral area for distinction of brain tumors. PLoS ONE. 2015;10:e0138573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jain KK, Sahoo P, Tyagi R, et al. Prospective glioma grading using single‐dose dynamic contrast‐enhanced perfusion MRI. Clin Radiol. 2015;70:1128‐1135. [DOI] [PubMed] [Google Scholar]
- 36. Jia Z, Geng D, Xie T, Zhang J, Liu Y. Quantitative analysis of neovascular permeability in glioma by dynamic contrast‐enhanced MR imaging. J Clin Neurosci. 2012;19:820‐823. [DOI] [PubMed] [Google Scholar]
- 37. Roy B, Gupta RK, Maudsley AA, et al. Utility of multiparametric 3‐T MRI for glioma characterization. Neuroradiology. 2013;55:603‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Santarosa C, Castellano A, Conte GM, et al. Dynamic contrast‐enhanced and dynamic susceptibility contrast perfusion MR imaging for glioma grading: preliminary comparison of vessel compartment and permeability parameters using hotspot and histogram analysis. Eur J Radiol. 2016;85:1147‐1156. [DOI] [PubMed] [Google Scholar]
- 39. Yun TJ, Park C‐K, Kim TM, et al. Glioblastoma treated with concurrent radiation therapy and temozolomide chemotherapy: differentiation of true progression from pseudoprogression with quantitative dynamic contrast‐enhanced MR imaging. Radiology. 2015;274:830‐840. [DOI] [PubMed] [Google Scholar]
- 40. Bisdas S, Naegele T, Ritz R, et al. Distinguishing recurrent high‐grade gliomas from radiation injury: a pilot study using dynamic contrast‐enhanced MR imaging. Acad Radiol. 2011;18:575‐583. [DOI] [PubMed] [Google Scholar]
- 41. Thomas AA, Arevalo‐Perez J, Kaley T, et al. Dynamic contrast enhanced T1 MRI perfusion differentiates pseudoprogression from recurrent glioblastoma. J Neurooncol. 2015;125:183‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Suh CH, Kim HS, Choi YJ, Kim N, Kim SJ. Prediction of pseudoprogression in patients with glioblastomas using the initial and final area under the curves ratio derived from dynamic contrast‐enhanced T1‐weighted perfusion MR imaging. AJNR Am J Neuroradiol. 2013;34:2278‐2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Narang J, Jain R, Arbab AS, et al. Differentiating treatment‐induced necrosis from recurrent/progressive brain tumor using nonmodel‐based semiquantitative indices derived from dynamic contrast‐enhanced T1‐weighted MR perfusion. Neuro Oncol. 2011;13:1037‐1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Seeger A, Braun C, Skardelly M, et al. Comparison of three different MR perfusion techniques and MR spectroscopy for multiparametric assessment in distinguishing recurrent high‐grade gliomas from stable disease. Acad Radiol. 2013;20:1557‐1565. [DOI] [PubMed] [Google Scholar]
- 45. Larsen VA, Simonsen HJ, Law I, Larsson HB, Hansen AE. Evaluation of dynamic contrast‐enhanced T1‐weighted perfusion MRI in the differentiation of tumor recurrence from radiation necrosis. Neuroradiology. 2013;55:361‐369. [DOI] [PubMed] [Google Scholar]
- 46. Lu S, Gao Q, Yu J, et al. Utility of dynamic contrast‐enhanced magnetic resonance imaging for differentiating glioblastoma, primary central nervous system lymphoma and brain metastatic tumor. Eur J Radiol. 2016;85:1722‐1727. [DOI] [PubMed] [Google Scholar]
- 47. Choi YS, Lee H‐J, Ahn SS, et al. Primary central nervous system lymphoma and atypical glioblastoma: differentiation using the initial area under the curve derived from dynamic contrast‐enhanced MR and the apparent diffusion coefficient. Eur Radiol. 2017;27:1344‐1351. [DOI] [PubMed] [Google Scholar]
- 48. Patel P, Baradaran H, Delgado D, et al. MR perfusion‐weighted imaging in the evaluation of high‐grade gliomas after treatment: a systematic review and meta‐analysis. Neuro Oncol. 2017;19:118‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Xu W, Wang Q, Shao A, Xu B, Zhang J. The performance of MR perfusion‐weighted imaging for the differentiation of high‐grade glioma from primary central nervous system lymphoma: a systematic review and meta‐analysis. PLoS ONE. 2017;12:e0173430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liang J, Liu D, Gao P, et al. Diagnostic values of DCE‐MRI and DSC‐MRI for differentiation between high‐grade and low‐grade gliomas: a comprehensive meta‐analysis. Acad Radiol. 2018;25:338‐348. [DOI] [PubMed] [Google Scholar]
- 51. Roberts C, Issa B, Stone A, Jackson A, Waterton JC, Parker GJ. Comparative study into the robustness of compartmental modeling and model‐free analysis in DCE‐MRI studies. J Magn Reson Imaging. 2006;23:554‐563. [DOI] [PubMed] [Google Scholar]
- 52. Chung WJ, Kim HS, Kim N, Choi CG, Kim SJ. Recurrent glioblastoma: optimum area under the curve method derived from dynamic contrast‐enhanced T1‐weighted perfusion MR imaging. Radiology. 2013;269:561‐568. [DOI] [PubMed] [Google Scholar]
- 53. Seow P, Wong J, Ahmad‐Annuar A, Mahajan A, Abdullah NA, Ramli N. Quantitative magnetic resonance imaging and radiogenomic biomarkers for glioma characterisation: a systematic review. Br J Radiol. 2018;91:20170930. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


