Abstract
Intricate systems of checkpoints such as the programmed cell death protein 1 (PD-1)/programmed death ligand 1 (PD-L1) axis regulate adaptive immune responses to protect against tissue damage. However, diverse cancers can exploit these pathways to evade or suppress antitumor immunity, leading to tumor progression. Correspondingly, immune checkpoint inhibitors that block PD-1/PD-L1 signaling have shown marked therapeutic efficacy in certain cancers, such as Hodgkin lymphoma. Reed-Sternberg cells, the hallmark cells of Hodgkin lymphoma, commonly overexpress PD-1 ligands, and recent clinical trials have demonstrated impressive response rates with the PD-1 inhibitors nivolumab and pembrolizumab in relapsed or refractory Hodgkin lymphoma, leading to their FDA approval in this setting. Current efforts are underway to improve clinical responses by incorporating PD-1 inhibitors into earlier treatment regimens and identifying therapeutic agents that synergize with PD-1 inhibitors. This review summarizes our understanding of the PD-1/PD-L1 axis in Hodgkin lymphoma, recent clinical studies of anti-PD-1 monotherapy and promising combination immunotherapy in the pipeline.
Introduction
With current front-line treatment regimens of chemotherapy alone or in combination with radiation, over 80% of patients with Hodgkin lymphoma achieve long-term cure.1 For those patients with relapsed or refractory disease, approximately 50% are cured with high-dose salvage chemotherapy and autologous hematopoietic stem cell transplantation. However, patients who relapse after stem cell transplant (SCT) have traditionally had a poor prognosis. The antibody–drug conjugate brentuximab vedotin, an anti-CD30 monoclonal antibody coupled to a microtubule polymerization inhibitor monomethyl auristatin E, was the first drug approved by the US Food and Drug Administration (FDA) for relapsed classical Hodgkin lymphoma after autologous SCT. Overall response rates from a phase II trial are 75% with a complete response rate of 34%,2 but long-term remissions are rare.
Research over the past 3 decades has unveiled multiple regulatory pathways that act to dampen immune activation and prevent autoimmunity. For example, in lymphoid organs, T cells express the surface molecule cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), which competes with CD28 for binding to the costimulatory molecules B7-1 and B7-2 on antigen-presenting cells and results in inhibitory signaling.3 In peripheral tissues, target cells express PD-1 ligands that can engage PD-1 on T cells to induce T cell exhaustion and impaired effector responses.4 Taking advantage of these inhibitory pathways, many cancers also express PD-1 ligands and thereby suppress antitumor immunity.4 Recent efforts in developing immune checkpoint inhibitors—antibodies against CTLA-4 or PD-1/PD-L1—have led to their approval in several malignancies including relapsed or refractory Hodgkin lymphoma, in which response rates of PD-1 inhibitors nivolumab or pembrolizumab exceed 60%.5 Despite this success, complete remissions and durable responses are still uncommon, and thus current research efforts seek to expand the therapeutic potential of PD-1 blockade through novel immunotherapy combinations. In this review, we will highlight our understanding of the biology of the PD-1 pathway in Hodgkin lymphoma, recent clinical trials establishing the therapeutic efficacy of PD-1 blockade in relapsed and refractory disease, and promising combination strategies incorporating PD-1 inhibitors under current investigation.
Biology of PD-1 and checkpoint inhibition
Encoded by the PDCD1 gene, PD-1 is a type 1 transmembrane protein belonging to immunoglobulin superfamily of receptors that contains an extracellular IgV domain, a transmembrane domain, and an intracellular domain bearing an immune receptor tyrosine-based inhibitory motif (ITIM).6 PD-1 was established as a negative regulator of T cell responses through experiments showing development of a lupus-like syndrome in mice deficient for PD-1 (Table 1).7 This phenotype is less severe than CTLA-4-null mice, which develop a fatal lymphoproliferative disorder by 3 to 4 weeks of age.8,9 PD-1 is not expressed on naïve T cells but is induced during antigen-mediated T cell activation. Clearance of the activating antigen leads to downregulation of PD-1 expression, but persistent antigen such as in chronic viral infection or cancer leads to sustained and high expression of PD-1.10 Several transcription factors regulate PD-1 expression in activated T cells, including NFATC1, FOXO1, T-bet, and BLIMP1, and epigenetic regulation such as through alterations in DNA methylation patterns or histone modifications may also play a role.10
Table 1.
Phenotypes of Mice Deficient in Checkpoint Inhibitors

PD-1 recognizes 2 ligands: PD-L1 (also known as B7-H1; CD274) is expressed by a wide variety of cell types including both hematopoietic and nonhematopoietic cells, while PD-L2 (B7-DC; CD273) expression tends to be more restricted to dendritic cells, macrophages, and B cells.10,11,12,13,14 Unlike PD-1-deficient mice, mice lacking PD-L1 and PD-L2 do not develop a spontaneous lupus-like syndrome but have increased susceptibility to autoimmunity in various disease models.15,16 Ligand binding of PD-1 leads to recruitment of the protein tyrosine phosphatase Src homology domain-containing phosphatase 2 to the T cell receptor complex, resulting in the dephosphorylation of signaling molecules such as ZAP70 and dampening of T cell signaling.10 Consequently, this attenuated signaling causes T cell exhaustion, or the progressive loss of effector functions and potential. Early studies showed that various cancer cells express PD-L1 on the surface and that tumor infiltrating lymphocytes often express PD-1, suggesting that the PD-1/PD-L1 pathway can be exploited by tumors to suppress the antitumor immune response and allow for tumor growth.4,17
Applying these observations and subsequent proof-of-concept studies to patients, PD-1 inhibitors have been introduced into the clinic in several cancer types with varying efficacy. PD-1 blockade was first successfully applied to metastatic melanoma with objective response rates approximately 40% and with durable responses in many patients.18,19 These advances soon led to the expansion of anti-PD-1 agents to nonsmall-cell lung cancer,20 renal cell carcinoma,21 bladder cancer,22 head and neck squamous cell cancers,23 and tumors exhibiting mismatch repair deficiency,24 with approvals for other solid tumors likely in the near future. Moreover, PD-1 blockade has had marked success in some hematologic malignancies, particularly in Hodgkin lymphoma.
Role of PD-1 in Hodgkin lymphoma
Classical Hodgkin lymphoma is characterized by rare clonal, multinucleated Reed-Sternberg cells that are situated within a milieu of infiltrating inflammatory cells. In most patients, Reed-Sternberg cells robustly express PD-L1 or PD-L2, which are encoded by genes within the chromosomal region 9p24.1.25 For example, one study showed that 105 of 108 patients (97%) with newly diagnosed Hodgkin lymphoma had alterations of the PDL1 and PDL2 loci, including 56% with copy gain and 36% with amplification.26 These genetic alterations may have prognostic significance, as 9p24.1 amplifications were associated with advanced stage disease and shortened progression-free survival.26
Multiple signaling pathways have been shown to regulate PD-L1 and PD-L2 expression in Reed-Sternberg cells (Fig. 1A), although our understanding of these mechanisms is limited. Treatment of classical Hodgkin lymphoma cell lines with MEK/ERK inhibitors in vitro downregulated PD-L1 expression, suggesting that ERK/MAPK signaling promotes PD-L1 expression.27 Chromosome 9p24.1 also contains the JAK2 locus, and inhibition of JAK2 decreased PD-L1 transcription.25 Furthermore, in Epstein-Barr virus (EBV)-associated Hodgkin lymphoma cells with normal PDL1 copy number, EBV-latent membrane protein (LMP1) induced PD-L1 expression through the AP-1 and JAK-STAT pathways.28
Figure 1.
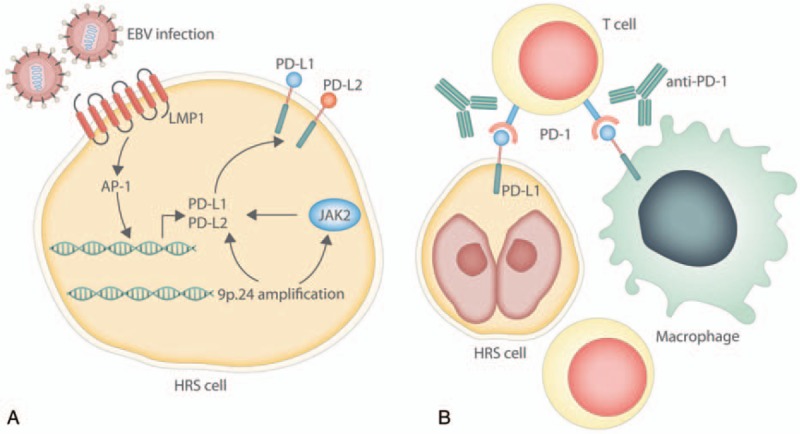
PD-1 blockade in Hodgkin lymphoma. (A) PD-L1 and PD-L2 are upregulated in Hodgkin Reed-Sternberg (HRS) cells through several mechanisms, including amplification of chromosome 9p.24 which encodes the PDL1 and PDL2 loci. JAK2 is also encoded on chromosome 9p.24, and JAK-STAT signaling promotes PD-L1 and PD-L2 expression. In EBV-associated Hodgkin lymphoma, latent membrane protein 1 (LMP1) can promote PD-L1 and PD-L2 expression through AP-1. (B) T cells within the tumor microenvironment express PD-1, which recognizes PD-L1 and PD-L2 expressed on HRS cells, as well as other leukocytes such as macrophages. Antibodies such as nivolumab and pembrolizumab block PD-1 to disrupt PD-1/PD-L1 signaling.
In addition to phenotypically characterizing Reed-Sternberg cells, PD-L1 and PD-L2 have functional consequences on immune suppression of antitumor responses. Reed-Sternberg cells typically account for less than 5% of cells within the tumor, which predominantly consist of T cells as well as macrophages, eosinophils, B cells, plasma cells, neutrophils, and fibroblasts.29 Interestingly, T cells from Hodgkin lymphoma tissue samples as well as peripheral blood from patients expressed PD-1,30 and increased PD-1 expression on intratumoral T cells has been associated with poor prognosis.31 Another recent study suggests that high expression of PD-1/PD-L1 on leukocytes within the tumor microenvironment, but not PD-L1/PD-L2 on Reed-Sternberg cells, is associated with inferior overall survival.32 Importantly, patient Hodgkin lymphoma cells treated with antibodies inhibiting PD-1/PD-L1 signaling showed increased IFN-γ production, suggesting that antitumor responses of Hodgkin lymphoma infiltrating T cells are suppressed by the PD-1 axis.30 Preclinical data also support a role for PD-1 blockade in other hematological malignancies,5 providing a rationale for assessing the clinical activity of PD-1 inhibition in patients.
The exact mechanism of action for PD-1 inhibition in Hodgkin lymphoma is poorly characterized (Fig. 1B). In some solid tumors such as melanoma and nonsmall-cell lung cancer, high levels of nonsynonymous mutational burden have been associated with improved objective responses and more durable clinical benefit with immune checkpoint inhibitors.33,34 These observations suggest that anti-PD-1 activity relies on the recognition of neoantigens created by these nonsynonymous mutations. However, Reed-Sternberg cells are rare within the tumor environment and are less likely to harbor a high mutational load. In addition, one proposed mechanism of action for checkpoint inhibition is CD8+ T cell recognition of neoantigens presented on major histocompatibility complex (MHC) class I on tumor cells.35 Correspondingly, loss of MHC class I such as from mutations in beta-2-microglobulin (B2M) have been associated with acquired resistance to PD-1 blockade in melanoma.36 Yet, Reed-Sternberg cells frequently harbor inactivating mutations in B2M, resulting in loss of expression of the MHC class I complex which is critical for engagement with CD8+ T cells.37 Moreover, decreased MHC class I expression was associated with poorer clinical outcomes independent of PD-L1 and PD-L2 amplification.38 This suggests that CD8+ T cell effector responses may not be solely responsible for the clinical efficacy of checkpoint inhibitors. Others have proposed that in Hodgkin lymphoma, PD-L1 may be involved in transmitting trophic signals that promote tumor growth independent of regulating the immune response to the tumor.39
While most studies have focused on PD-L1 and PD-L2 expression by Reed-Sternberg cells, these cells comprise only a small population within the tumor, and so it is possible that other inflammatory cells within the tumor microenvironment may also play a role in immune suppression. Interestingly, tumor-associated macrophages have been shown to express PD-L1, and increased levels of tumor-associated macrophages are associated with poor clinical outcomes in patients with advanced disease.40,41 In fact, recent data suggest that the majority of PD-L1 in Hodgkin lymphoma tumors is expressed by tumor-associated macrophages, which are in close contact with PD-L1+ Reed-Sternberg cells as well as PD-1+ CD4+ T cells.42 In other models, tumor-associated macrophages have been shown to express PD-1, and blockade of PD-1 enhances macrophage phagocytosis to restrict tumor growth. Thus, PD-1 inhibition in Hodgkin lymphoma may also target tumor-associated macrophages within the immune environment.
Clinical experience of anti-PD-1 monotherapy in Hodgkin lymphoma
As preliminary data suggested that Reed-Sternberg cells express PD-1 ligands and that PD-1 blockade may have activity in Hodgkin lymphoma, several clinical trials have assessed the safety and efficacy of PD-1-blocking antibodies in patients (Table 2). Nivolumab is a fully human IgG4 antibody targeting PD-1 that has been approved for the treatment of advanced stage melanoma, nonsmall-cell lung cancer, renal cell carcinoma, and other malignancies. In a phase I trial of 23 heavily pretreated patients with relapsed or refractory Hodgkin lymphoma treated with nivolumab 3 mg/kg every 2 weeks, the overall response rate was 87%, with a complete response rate of 17%.43 The rate of progression-free survival at 24 weeks was 86%. Analysis of pretreatment specimens demonstrated increased expression of PD-L1 and PD-L2 in Reed-Sternberg cells as well as phospho-STAT3 expression suggestive of active JAK-STAT signaling.
Table 2.
Clinical Trial Results of PD-1 Inhibitor Monotherapy
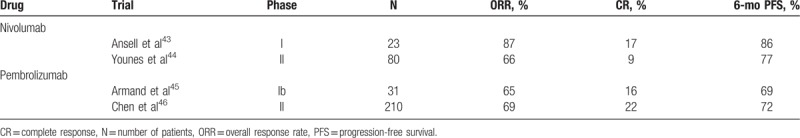
Moreover, a second multicenter phase II study (CheckMate 205) of 80 patients with classical Hodgkin lymphoma who had failed to respond to autologous stem cell transplantation or had relapsed after brentuximab vedotin further demonstrated the therapeutic benefit of nivolumab.44 With a median follow-up of 8.9 months, there was an objective response rate of 66.3% with a complete response seen in 9% of patients. The toxicity profile of nivolumab was similar to those established from studies of nivolumab in other malignancies, with the most common adverse events being fatigue, infusion reaction, and rash. These studies demonstrate that PD-1 blockade with nivolumab is safe and effective in Hodgkin lymphoma, leading to FDA approval (May 2016) and European Medicines Agency (EMA) approval (November 2016) of nivolumab for patients who had failed to respond or progressed after autologous stem cell transplantation and brentuximab vedotin.
In addition to nivolumab, a second humanized IgG4 antibody pembrolizumab was also recently approved by the FDA (March 2017) and EMA (May 2017) for patients with Hodgkin lymphoma who had failed multiple lines of therapy. A phase I study of 31 heavily pretreated patients with relapsed or refractory Hodgkin lymphoma (KEYNOTE-013) demonstrated an overall response rate of 65% with a complete response rate of 16%.45 These impressive response rates were supported by a phase II study (KEYNOTE-087) involving 210 patients with relapsed or refractory Hodgkin lymphoma, with an overall response rate of 69%, compete response rate of 22.4%, and a 6-month progression-free survival of 63.4%.46 Thus, treatment outcomes with single agent nivolumab and pembrolizumab are comparable, and response rates are similar between patients who have and have not received prior brentuximab vedotin (Table 3). Importantly, while previously there were no approved options for patients who had failed brentuximab vedotin after transplant, PD-1 inhibitors have expanded the treatment armamentarium for these patients. A phase III trial is currently underway comparing pembrolizumab versus brentuximab vedotin in patients with relapsed or refractory classical Hodgkin lymphoma (NCT02684292).
Table 3.
Outcomes of PD-1 Inhibitors in Patients With and Without Prior Brentuximab Vedotin Treatment

Several antibodies targeting PD-L1 have also been developed for clinical use. Avelumab is a fully human IgG1 monoclonal antibody that selectively binds to PD-L1.47 Early data from a phase I study of avelumab in patients with heavily pretreated Hodgkin lymphoma suggest that avelumab has an acceptable toxicity profile and has clinical activity, with a 54.8% overall response rate among 31 patients.48 Thus, anti-PD-L1 therapy is also a promising strategy in Hodgkin lymphoma.
While checkpoint inhibitors have shown promising results in advanced Hodgkin lymphoma, one concern is the effect of anti-PD-1 therapy on toxicities related to allogeneic hematopoietic stem cell transplantation. Graft-versus-host disease (GVHD) is a major cause of morbidity and mortality after allogeneic hematopoietic stem cell transplantation and is mediated by alloreactive donor T cells that recognize recipient antigens as foreign.49 Preclinical models suggested that while PD-1 blockade can augment graft-versus-tumor effects, it may also result in higher rates of acute GVHD and accelerate GVHD lethality.50,51,52 Retrospective analyses have assessed outcomes for patients with advanced lymphoma treated with anti-PD-1 therapy before or after allogeneic hematopoietic SCT. In a study of 39 patients (of which 31 patients had Hodgkin lymphoma) who underwent allogeneic hematopoietic stem cell transplantation after receiving a PD-1 inhibitor, the 1-year cumulative incidence of acute grade 2 to 4 and grade 3 to 4 GVHD were 44% and 23%, respectively, with a 1-year cumulative incidence of chronic GVHD of 41%.53 There were 3 treatment-related deaths attributed to early acute GVHD, and the incidence of grade 4 GVHD was slightly higher than prior studies (13%), although there did not appear to be any increased risk of relapse. Another retrospective study of 20 Hodgkin lymphoma patients treated with nivolumab after relapse following allogeneic hematopoietic stem cell transplantation showed an acceptable rate of GVHD of 30% (6 patients, all of whom had previously had GVHD).54 In contrast, a separate study of 31 patients with relapsed lymphoma after allogeneic hematopoietic stem cell transplantation and treated with pembrolizumab or nivolumab showed a higher GVHD rate of 55%, with eight deaths attributed to new-onset GVHD.55 These data should be interpreted with caution as they were retrospective in nature, patients were treated with heterogeneous conditioning regimens, and they were compared to historical controls, among other limitations. Nevertheless, anti-PD-1 therapy appears to be feasible before or after hematopoietic stem cell transplantation but may carry a risk of increased early immune toxicities, and therefore further prospective studies are warranted.
Prospective combination immunotherapy with PD-1 inhibition in Hodgkin lymphoma
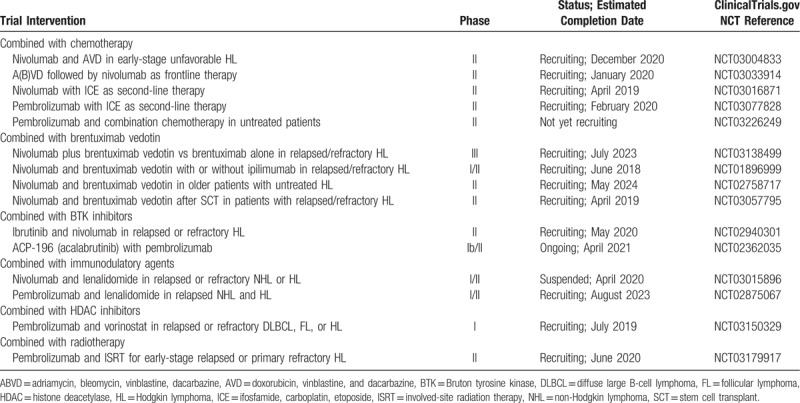
Despite the clinical success of PD-1 agents in Hodgkin lymphoma, many patients never achieve complete remissions or eventually relapse, with no standard treatment options currently approved after failing SCT, brentuximab vedotin and anti-PD-1 therapy. Consequently, many clinical studies are underway to either incorporate anti-PD-1 agents into earlier phases of treatment in combination with standard chemotherapy regimens. Other studies are rationally combining PD-1 inhibitors with active agents in Hodgkin lymphoma for potentially synergistic effects (Table 4).
Table 4.
Ongoing Clinical Trials of PD-1 Inhibitor Combination Therapy
Combined with chemotherapy
For the past several decades, combination chemotherapy with doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) has been the standard regimen for most patients with advanced Hodgkin lymphoma.56 In the second-line for relapsed or refractory disease, several salvage chemotherapy regimens have been used, such as ifosfamide, carboplatin, and etoposide (ICE).1 While these regimens are established for initial or second-line treatment, it is not known whether immunotherapy with PD-1 inhibition has a role in earlier treatment strategies. Notably, there is a strong rationale for combining immunotherapy with chemotherapy, as chemotherapeutic agents can trigger immunogenic tumor cell death that leads to the release of danger signals, Toll-like receptor (TLR) ligands, ATP, and other immunomodulatory factors.57 These signals are recognized by innate immune effectors such as dendritic cells that can stimulate T cells or inhibit immunosuppressive pathways, resulting in control of tumor growth. Thus, it is possible that PD-1 blockade may synergize with chemotherapy, especially in tumors that are chemotherapy-sensitive. Current studies are underway to add nivolumab to salvage regimens such as ICE (NCT03016871) or incorporate nivolumab into frontline therapy, either currently or after A(B)VD (NCT03004833, NCT03033914). The combination of nivolumab with frontline chemotherapy appears to be well-tolerated as recently reported from a cohort of CheckMate 205, in which untreated patients with newly diagnosed advanced stage Hodgkin lymphoma were given four doses of nivolumab followed by nivolumab plus chemotherapy (doxorubicin, vinblastine, dacarbazine).58 Ten patients (20%) had serious adverse events, most commonly febrile neutropenia, and common immune-mediated adverse events included hypothyroidism (16%) and elevated transaminases (8%), consistent with prior experience with nivolumab and doxorubicin, vinblastine, and dacarbazine chemotherapy separately.
Combined with brentuximab vedotin
In the relapsed or refractory setting after autologous stem cell transplantation, brentuximab vedotin has an overall response rate of 75% and complete response rate of 34%.2 Brentuximab vedotin has also been used as consolidation therapy after transplant for patients with high risk of relapse, with improvements in progression-free survival in a phase III trial.59 As above with chemotherapy, brentuximab vedotin may target malignant cells to augment cytotoxicity and tumor antigen release, thereby stimulating an immune response which can be further boosted by checkpoint inhibition. Multiple clinical trials combining brentuximab vedotin and nivolumab are underway, and preliminary results are promising with objective response rates of 85% and complete response rates exceeding 60%.60
Combined with ibrutinib
Ibrutinib is an oral inhibitor of Bruton tyrosine kinase (BTK), which is a key enzyme downstream of the B-cell receptor.61 Ibrutinib is currently approved in the treatment of multiple hematologic malignancies including chronic lymphocytic leukemia, mantle cell lymphoma and Waldenström macroglobulinemia.62 While ibrutinib has been shown to have modest clinical activity in other hematologic malignancies such as diffuse large B-cell lymphoma, its activity in Hodgkin lymphoma has not been reported in prospective clinical trials. Nevertheless, there is some evidence that ibrutinib may also be effective in Hodgkin lymphoma. For instance, 2 patients with progressive Hodgkin lymphoma after multiple therapeutic regimens including autologous or allogeneic hematopoietic stem cell transplantation demonstrated complete or near-complete responses with ibrutinib treatment.63 Interestingly, in mice, ibrutinib enhanced the therapeutic effect of anti-PD-L1 treatment in several cancer models, including those that are intrinsically insensitive to ibrutinib treatment.64 In addition to inhibiting BTK, ibrutinib has also been shown to target other tyrosine kinases including interleukin-2-inducible T cell kinase (ITK), which is critical for the survival of Th2 cells.65 Therefore, ibrutinib may skew T cells to a Th1 phenotype which have increased antitumor properties, perhaps explaining the combinatorial effect of ibrutinib and checkpoint blockade. Evaluating these potentially synergistic effects in patients, clinical trials combining immune checkpoint blockade with ibrutinib or second-generation BTK inhibitors are currently underway (NCT02362035, NCT02940301).
Combined with immunomodulatory drugs
Previous studies have suggested a potential role for CTLA-4 inhibition in Hodgkin lymphoma, with early reports demonstrating CTLA-4 expression in tumor infiltrating T cells.66,67 Data for CTLA-4 inhibition in patients is limited, although a phase I study of the CTLA-4 inhibitor ipilimumab in patients with hematologic malignancies progressing after allogeneic hematopoietic stem cell transplantation reported complete responses for 2 of 14 Hodgkin lymphoma patients.68 As combined checkpoint blockade has shown efficacy in solid malignancies,18 several studies are assessing combinations of ipilimumab and nivolumab in hematologic malignancies. Preliminary data from CheckMate 039 (NCT01592370) suggest an overall response rate of 74% and complete response rate of 19% with combined ipilimumab and nivolumab in primarily transplant-naïve Hodgkin lymphoma patients.69 These responses are comparable to anti-PD-1 monotherapy in the relapsed/refractory setting, and so the role of combined checkpoint blockade remains unclear.
Other immunomodulatory drugs such as lenalidomide act through several potential mechanisms, with possible direct effects on malignant cells and indirect activity through modulation of the tumor microenvironment.70 Lenalidomide is currently approved for the treatment of multiple myeloma and myelodysplastic syndrome with deletion of chromosome 5q, and it is also active in several B cell malignancies.71,72 A phase II clinical trial of lenalidomide in 38 relapsed or refractory classical Hodgkin lymphoma patients demonstrated an objective overall response rate of 19%,73 providing a rationale for studying lenalidomide in combination with other agents. In preclinical models of multiple myeloma, lenalidomide treatment modulated PD-1 and PD-L1 expression on effector and tumor cells, and also enhanced checkpoint inhibitor-induced cytotoxicity of multiple myeloma cells.49 Thus, these data suggest that immunomodulatory agents like lenalidomide may have synergistic effects with PD-1 blockade, a hypothesis which is being tested in clinical trials combining pembrolizumab or nivolumab with lenalidomide in patients with Hodgkin or non-Hodgkin lymphoma (NCT02875067, NCT03015896). Of note, recent trials in multiple myeloma combining pembrolizumab and immunomodulatory agents (lenalidomide, pomalidomide) have been placed on clinical hold by the FDA due to excessive toxicity, and so the safety of this combination will need to be further established.
Combined with HDAC inhibitors
Histone deacetylases (HDACs) are a family of enzymes that deacetylate lysine residues on histone and nonhistone proteins, and they regulate several pathways implicated in oncogenesis such as cell cycle progression, apoptosis, angiogenesis, and immunity.74 Epigenetic changes have been implicated in the malignant phenotype of Reed-Sternberg cells, and previous studies revealed that Hodgkin lymphoma cell lines and primary tumor tissue highly express class I and II HDACs.75,76 Several HDAC inhibitors have been developed for clinical use, and preclinical studies demonstrated cytotoxic effects of HDAC inhibitors on Hodgkin lymphoma cells.77,78 In a phase II study of 129 heavily pretreated patients with relapsed or refractory Hodgkin lymphoma, treatment with panobinostat, a pan-HDAC inhibitor, resulted in an overall response rate of 27% and complete response rate of 4%.79 Interestingly, HDAC inhibitors can induce a wide array of immunologic changes in malignant cells and the tumor microenvironment, including expression of PD-1 or PD-1 ligands. For example, correlative studies from the above phase II trial found decreased expression of PD-1 on peripheral blood T cells with panobinostat treatment.80 In in vitro studies of melanoma cell lines and patient tumors, HDAC inhibition upregulated PD-L1 and PD-L2 expression, and combined treatment with panobinostat and anti-PD-1 in vivo in mouse studies promoted tumor regression and survival to a greater extent than monotherapy.81 HDAC inhibition also synergized with anti-PD-1 treatment in a lung cancer model.82 Overall, these data suggest that combining HDAC inhibition with PD-1 blockade may improve clinical responses through both direct cytotoxic effects as well as regulation of antitumor immunity, which is being tested in clinical trials (ie, NCT03150329 combining vorinostat, a HDAC inhibitor currently approved in cutaneous T cell lymphoma, and pembrolizumab).
Combined with radiotherapy
Radiation therapy is often used with curative intent in the treatment of localized malignancies, such as in early-stage Hodgkin lymphoma or bulky tumors. Not only does radiotherapy exert a direct cytotoxic effect on malignant cells, it also modulates the tumor microenvironment and antitumor immune responses.83 For example, radiation treatment causes the release of tumor antigens and danger signals, as well as proinflammatory cytokines and chemokines. This promotes the migration and maturation of antigen-presenting cells, which stimulate cytotoxic T cell responses against the tumor. In rare cases, radiotherapy has been reported to induce an abscopal effect, or the regression of lesions at distant sites outside the field of radiation.
Given the success of checkpoint blockade in diverse malignancies and the immunomodulatory effects of radiation, several studies have evaluated the combination of these 2 modalities. For example, in a mouse model, ionizing radiation augmented PD-L1 expression in the tumor, and the combination of ionizing radiation and anti-PD-L1 demonstrated greater tumor regression than either treatment alone.84 Mechanistically, this activity depended on CD8+ T cell-mediated cytotoxicity of myeloid-derived suppressor cells. Synergistic effects of radiotherapy and PD-1 inhibition have also been observed in preclinical models of glioblastoma multiforme,85 melanoma,86 and others. Intriguingly, a recent case report of a patient with relapsed Hodgkin lymphoma treated with pembrolizumab and radiation to a mediastinal lymph node reported tumor regression outside the radiation field, suggesting that combined PD-1 blockade and radiotherapy may also be active in Hodgkin lymphoma and can promote an abscopal effect.87 Indeed, a phase II trial combining pembrolizumab with involved-site radiation therapy for relapsed or primary refractory Hodgkin lymphoma is currently being conducted (NCT03179917).
Concluding remarks
The advent of immune checkpoint inhibitors has vastly improved treatment options for many cancer types. While the pathologic cells of Hodgkin lymphoma, Reed-Sternberg cells, robustly overexpress PD-1 ligands, the interplay between Reed-Sternberg cells and the immune microenvironment as well as the mechanism of action for PD-1 inhibitors in this disease are still mysterious. Nevertheless, PD-1 inhibitors as monotherapy in the relapsed or refractory setting have shown remarkable clinical responses. Ongoing clinical investigation with novel combinations of PD-1 inhibitors will hopefully reveal promising strategies to improve cure rates in Hodgkin lymphoma.
Footnotes
Citation: Moy RH, Younes A. Immune Checkpoint Inhibition in Hodgkin Lymphoma. HemaSphere, 2018;2:1. http://dx.doi.org/10.1097/HS9.0000000000000020
Funding/support: Supported in part by the MSK Cancer Center Support Grant P30 CA 008748.
AY received research support for clinical trials and/or honorarium from BMS, Merck, Takeda, and Genentech.
References
- 1.von Tresckow B, Moskowitz CH. Treatment of relapsed and refractory Hodgkin lymphoma. Semin Hematol 2016; 53:180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma. J Clin Oncol 2012; 30:2183–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walunas TL, Bakker CY, Bluestone JA. CTLA-4 ligation blocks CD28-dependent T cell activation. J Exp Med 1996; 183:2541–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest 2015; 125:3384–3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Younes A, Ansell S, Fowler N, et al. The landscape of new drugs in lymphoma. Nat Rev Clin Oncol 2017; 14:335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riley JL. PD-1 signaling in primary T cells. Immunol Rev 2009; 229:114–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishimura H, Nose M, Hiai H, et al. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 1999; 11:141–151. [DOI] [PubMed] [Google Scholar]
- 8.Tivol EA, Borriello F, Schweitzer AN, et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 1995; 3:541–547. [DOI] [PubMed] [Google Scholar]
- 9.Waterhouse P, Penninger JM, Timms E, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science 1995; 270:985–988. [DOI] [PubMed] [Google Scholar]
- 10.Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 11.Tseng SY, Otsuji M, Gorski K, et al. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med 2001; 193:839–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Latchman Y, Wood CR, Chernova T, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2001; 2:261–268. [DOI] [PubMed] [Google Scholar]
- 13.Dong H, Zhu G, Tamada K, et al. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med 1999; 5:1365–1369. [DOI] [PubMed] [Google Scholar]
- 14.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000; 192:1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Latchman YE, Liang SC, Wu Y, et al. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc Natl Acad Sci U S A 2004; 101:10691–10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Chung Y, Bishop C, et al. Regulation of T cell activation and tolerance by PDL2. Proc Natl Acad Sci U S A 2006; 103:11695–11700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol 2015; 33:1974–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015; 373:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015; 372:320–330. [DOI] [PubMed] [Google Scholar]
- 20.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016; 375:1823–1833. [DOI] [PubMed] [Google Scholar]
- 21.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015; 373:1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016; 387:1909–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol 2016; 17:956–965. [DOI] [PubMed] [Google Scholar]
- 24.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015; 372:2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Green MR, Monti S, Rodig SJ, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood 2010; 116:3268–3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roemer MGM, Advani RH, Ligon AH, et al. PD-L1 and PD-L2 genetic alterations define classical Hodgkin lymphoma and predict outcome. J Clin Oncol 2016; 34:2690–2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamamoto R, Nishikori M, Tashima M, et al. B7-H1 expression is regulated by MEK/ERK signaling pathway in anaplastic large cell lymphoma and Hodgkin lymphoma. Cancer Sci 2009; 100:2093–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Green MR, Rodig S, Juszczynski P, et al. Constitutive AP-1 activity and EBV infection induce PD-L1 in Hodgkin lymphomas and posttransplant lymphoproliferative disorders: implications for targeted therapy. Clin Cancer Res 2012; 18:1611–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vardhana S, Younes A. The immune microenvironment in Hodgkin lymphoma: T cells, B cells, and immune checkpoints. Haematologica 2016; 101:794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamoto R, Nishikori M, Kitawaki T, et al. PD-1-PD-1 ligand interaction contributes to immunosuppressive microenvironment of Hodgkin lymphoma. Blood 2008; 111:3220–3224. [DOI] [PubMed] [Google Scholar]
- 31.Muenst S, Hoeller S, Dirnhofer S, et al. Increased programmed death-1+ tumor-infiltrating lymphocytes in classical Hodgkin lymphoma substantiate reduced overall survival. Hum Pathol 2009; 40:1715–1722. [DOI] [PubMed] [Google Scholar]
- 32.Hollander P, Kamper P, Smedby KE, et al. High proportions of PD-1+ and PD-L1+ leukocytes in classical Hodgkin lymphoma microenvironment are associated with inferior outcome. Blood Adv 2017; 1:1427–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 2014; 371:2189–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015; 348:124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riaz N, Morris L, Havel JJ, et al. The role of neoantigens in response to immune checkpoint blockade. Int Immunol 2016; 28:411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zaretsky JM, Garcia-Diaz A, Shin DS, et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med 2016; 375:819–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reichel J, Chadburn A, Rubinstein PG, et al. Flow sorting and exome sequencing reveal the oncogenome of primary Hodgkin and Reed-Sternberg cells. Blood 2015; 125:1061–1072. [DOI] [PubMed] [Google Scholar]
- 38.Roemer MGM, Advani RH, Redd RA, et al. Classical Hodgkin lymphoma with reduced β2M/MHC class I expression is associated with inferior outcome independent of 9p24.1 status. Cancer Immunol Res 2016; 4:910–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fridman WH, Zitvogel L, Sautès-Fridman C, et al. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 40.Steidl C, Lee T, Shah SP, et al. Tumor-associated macrophages and survival in classic Hodgkin's lymphoma. N Engl J Med 2010; 362:875–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan KL, Scott DW, Hong F, et al. Tumor-associated macrophages predict inferior outcomes in classic Hodgkin lymphoma: a correlative study from the E2496 Intergroup trial. Blood 2012; 120:3280–3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carey CD, Gusenleitner D, Lipschitz M, et al. Topological analysis reveals a PD-L1-associated microenvironmental niche for Reed-Sternberg cells in Hodgkin lymphoma. Blood 2017; 130:2420–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med 2015; 372:311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Younes A, Santoro A, Shipp M, et al. Nivolumab for classical Hodgkin's lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol 2016; 17:1283–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Armand P, Shipp MA, Ribrag V, et al. Programmed death-1 blockade with pembrolizumab in patients with classical Hodgkin lymphoma after brentuximab vedotin failure. J Clin Oncol 2016; 34:3733–3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen R, Zinzani PL, Fanale MA, et al. Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J Clin Oncol 2017; 35:2125–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boyerinas B, Jochems C, Fantini M, et al. Antibody-dependent cellular cytotoxicity activity of a novel anti-PD-L1 antibody avelumab (MSB0010718C) on human tumor cells. Cancer Immunol Res 2015; 3:1148–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen R, Gibbs AL, Collins GP, et al. Blockade of the PD-1 checkpoint with anti-PD-L1 antibody avelumab is sufficient for clinical activity in relapsed/refractory classical Hodgkin lymphoma (CHL). Hematol Oncol 2017; 35:67.28591424 [Google Scholar]
- 49.Ferrara JLM, Levine JE, Reddy P, et al. Lancet 2009; 373:1550–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blazar BR, Carreno BM, Panoskaltsis-Mortari A, et al. Blockade of programmed death-1 engagement accelerates graft-versus-host disease lethality by an IFN-gamma-dependent mechanism. J Immunol 2003; 171:1272–1277. [DOI] [PubMed] [Google Scholar]
- 51.Saha A, Aoyama K, Taylor PA, et al. Host programmed death ligand 1 is dominant over programmed death ligand 2 expression in regulating graft-versus-host disease lethality. Blood 2013; 122:3062–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Michonneau D, Sagoo P, Breart B, et al. The PD-1 axis enforces an anatomical segregation of CTL activity that creates tumor niches after allogeneic hematopoietic stem cell transplantation. Immunity 2016; 44:143–154. [DOI] [PubMed] [Google Scholar]
- 53.Merryman RW, Kim HT, Zinzani PL, et al. Safety and efficacy of allogeneic hematopoietic stem cell transplant after PD-1 blockade in relapsed/refractory lymphoma. Blood 2017; 129:1380–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herbaux C, Gauthier J, Brice P, et al. Efficacy and tolerability of nivolumab after allogeneic transplantation for relapsed Hodgkin lymphoma. Blood 2017; 129:2471–2478. [DOI] [PubMed] [Google Scholar]
- 55.Haverkos BM, Abbott D, Hamadani M, et al. PD-1 blockade for relapsed lymphoma post-allogeneic hematopoietic cell transplant: high response rate but frequent GVHD. Blood 2017; 130:221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gordon LI, Hong F, Fisher RI, et al. Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: an intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496). J Clin Oncol 2013; 31:684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol 2011; 8:151–160. [DOI] [PubMed] [Google Scholar]
- 58.Ramchandren R, Fanale MA, Rueda A, et al. Nivolumab for newly diagnosed advanced-stage classical Hodgkin lymphoma (cHL): results from the Phase 2 Checkmate 205 study. Blood 2017; 130 (suppl 1):651. [Google Scholar]
- 59.Moskowitz CH, Nademanee A, Masszi T, et al. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin's lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2015; 385:1853–1862. [DOI] [PubMed] [Google Scholar]
- 60.Herrera AF, Moskowitz AJ, Bartlett NL, et al. Interim results from a phase 1/2 study of brentuximab vedotin in combination with nivolumab in patients with relapsed or refractory Hodgkin lymphoma. Hematol Oncol 2017; 35 (S2):85–86. [Google Scholar]
- 61.Woyach JA, Johnson AJ, Byrd JC. The B-cell receptor signaling pathway as a therapeutic target in CLL. Blood 2012; 120:1175–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Castillo JJ, Treon SP, Davids MS. Inhibition of the Bruton tyrosine kinase pathway in B-cell lymphoproliferative disorders. Cancer J 2016; 22:34–39. [DOI] [PubMed] [Google Scholar]
- 63.Hamadani M, Balasubramanian S, Hari PN. Ibrutinib in refractory classic Hodgkin's lymphoma. N Engl J Med 2015; 373:1381–1382. [DOI] [PubMed] [Google Scholar]
- 64.Sagiv-Barfi I, Kohrt HEK, Czerwinski DK, et al. Therapeutic antitumor immunity by checkpoint blockade is enhanced by ibrutinib, an inhibitor of both BTK and ITK. Proc Natl Acad Sci U S A 2015; 112:E966–E972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dubovsky JA, Beckwith KA, Natarajan G, et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood 2013; 122:2539–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xerri L, Devilard E, Hassoun J, et al. In vivo expression of the CTLA4 inhibitory receptor in malignant and reactive cells from human lymphomas. J Pathol 1997; 183:182–187. [DOI] [PubMed] [Google Scholar]
- 67.Vandenborre K, Delabie J, Boogaerts MA, et al. Human CTLA-4 is expressed in situ on T lymphocytes in germinal centers, in cutaneous graft-versus-host disease, and in Hodgkin's disease. Am J Pathol 1998; 152:963–973. [PMC free article] [PubMed] [Google Scholar]
- 68.Bashey A, Medina B, Corringham S, et al. CTLA4 blockade with ipilimumab to treat relapse of malignancy after allogeneic hematopoietic cell transplantation. Blood 2009; 113:1581–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ansell S, Gutierrez ME, Shipp MA, et al. A phase 1 study of nivolumab in combination with ipilimumab for relapsed or refractory hematologic malignancies (CheckMate 039). Blood 2016; 128:183. [Google Scholar]
- 70.Kotla V, Goel S, Nischal S, et al. Mechanism of action of lenalidomide in hematological malignancies. J Hematol OncolJ Hematol Oncol 2009; 2:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McCarthy PL, Owzar K, Hofmeister CC, et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med 2012; 366:1770–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.List A, Kurtin S, Roe DJ, et al. Efficacy of lenalidomide in myelodysplastic syndromes. N Engl J Med 2005; 352:549–557. [DOI] [PubMed] [Google Scholar]
- 73.Fehniger TA, Larson S, Trinkaus K, et al. A phase 2 multicenter study of lenalidomide in relapsed or refractory classical Hodgkin lymphoma. Blood 2011; 118:5119–5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.West AC, Johnstone RW. New and emerging HDAC inhibitors for cancer treatment. J Clin Invest 2014; 124:30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gloghini A, Buglio D, Khaskhely NM, et al. Expression of histone deacetylases in lymphoma: implication for the development of selective inhibitors. Br J Haematol 2009; 147:515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Adams H, Fritzsche FR, Dirnhofer S, et al. Class I histone deacetylases 1, 2 and 3 are highly expressed in classical Hodgkin's lymphoma. Expert Opin Ther Targets 2010; 14:577–584. [DOI] [PubMed] [Google Scholar]
- 77.Buglio D, Mamidipudi V, Khaskhely NM, et al. The class-I HDAC inhibitor MGCD0103 induces apoptosis in Hodgkin lymphoma cell lines and synergizes with proteasome inhibitors by an HDAC6-independent mechanism. Br J Haematol 2010; 151:387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Buglio D, Georgakis GV, Hanabuchi S, et al. Vorinostat inhibits STAT6-mediated TH2 cytokine and TARC production and induces cell death in Hodgkin lymphoma cell lines. Blood 2008; 112:1424–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Younes A, Sureda A, Ben-Yehuda D, et al. Panobinostat in patients with relapsed/refractory Hodgkin's lymphoma after autologous stem-cell transplantation: results of a phase II study. J Clin Oncol 2012; 30:2197–2203. [DOI] [PubMed] [Google Scholar]
- 80.Oki Y, Buglio D, Zhang J, et al. Immune regulatory effects of panobinostat in patients with Hodgkin lymphoma through modulation of serum cytokine levels and T-cell PD1 expression. Blood Cancer J 2014; 4:e236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Woods DM, Sodré AL, Villagra A, et al. Upregulates PD-1 ligands in melanoma and augments immunotherapy with PD-1 blockade. Cancer Immunol Res 2015; 3:1375–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zheng H, Zhao W, Yan C, et al. HDAC inhibitors enhance T-cell chemokine expression and augment response to PD-1 immunotherapy in lung adenocarcinoma. Clin Cancer Res 2016; 22:4119–4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Weichselbaum RR, Liang H, Deng L, et al. Radiotherapy and immunotherapy: a beneficial liaison? Nat Rev Clin Oncol 2017; 14:365–379. [DOI] [PubMed] [Google Scholar]
- 84.Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 2014; 124:687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zeng J, See AP, Phallen J, et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys 2013; 86:343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015; 520:373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Michot J-M, Mazeron R, Dercle L, et al. Abscopal effect in a Hodgkin lymphoma patient treated by an anti-programmed death 1 antibody. Eur J Cancer 2016; 66:91–94. [DOI] [PubMed] [Google Scholar]


