Myeloproliferative neoplasms (MPN) are classified into chronic myeloid leukemia (CML) and Philadelphia chromosome-negative MPN (Ph-negative MPN). CML is characterized by the t(9;22)(q34;q11) translocation, resulting in the fusion protein BCR-ABL1. Ph-negative MPN are characterized by mutations in driver genes leading to activation of the JAK2-STAT5 pathway. First described in 2005, the JAK2V617F mutation is found in almost all cases of polycythemia vera and about half cases of essential thrombocythemia (ET) and primary myelofibrosis (PMF). Mutations of CALR were reported in 2013 in the majority of JAK2 no-mutated ET and PMF. The cooccurrence of both BCR-ABL1 and JAK2 or CALR is very rare. Here, we describe a case of CML associated with a mutation of CALR detectable after 3 months of treatment by imatinib.
A 76-year-old-man with a history of hypertension, diabetes, and dyslipidemia was referred for leukocytosis. The initial blood analysis revealed (i) an elevated white blood count (73.6 109/L, including 19% of myeloid immature cells and 2.3% of blast cells), (ii) a normal hemoglobin level (139 g/L) and (iii) a thrombocytosis (890 109/L). The bone marrow cytology and histology showed a major granulocytic hyperplasia, megakaryocytes were numerous, normal or with small hypolobated nuclei. Of note, only 2 megacaryocytes were large with hyperlobated nuclei on the biopsy.
A molecular screening for MPN was performed and revealed a BCR-ABL1 fusion transcript (cooccurrence of e13a2 and e14a2 transcripts). JAK2V617F, CALR, and MPLW515 were negative by conventional screening. Conventional cytogenetics was 46,XY,t(9;22)(q34;q11)[20]. A treatment by imatinib 400 mg daily was initiated leading to a normalization of leukocytes and platelets counts.
After 3 months, a reincrease of platelets count was observed (1022 109/L) despite an optimal molecular response to imatinib with a ratio BCRL-ABL1/ABL1 of 0.96% (Fig. 1). A new molecular screening for Ph-MPN driver genes was performed and revealed a CALR type 1 mutation (NM_004343:c.1099_1150del) with an allele burden of 27%. A treatment by hydroxycarbamide was added and platelets decreased gradually. Nine months later, a new bone marrow biopsy was performed that showed a myeloproliferative neoplasm of the megakaryocytic lineage and a grade 1 focal fibrosis. Finally, an essential thrombocythemia (ET) associated to CML was diagnosed. Seventeen months after the diagnosis of CML, the patient was in very good response for both CML (molecular response MR4.5) and ET (complete clinicohematologic response according to the ELN consensus).1 The CALR allele burden remained stable during the follow-up around 25% to 30% (Fig. 1).
Figure 1.
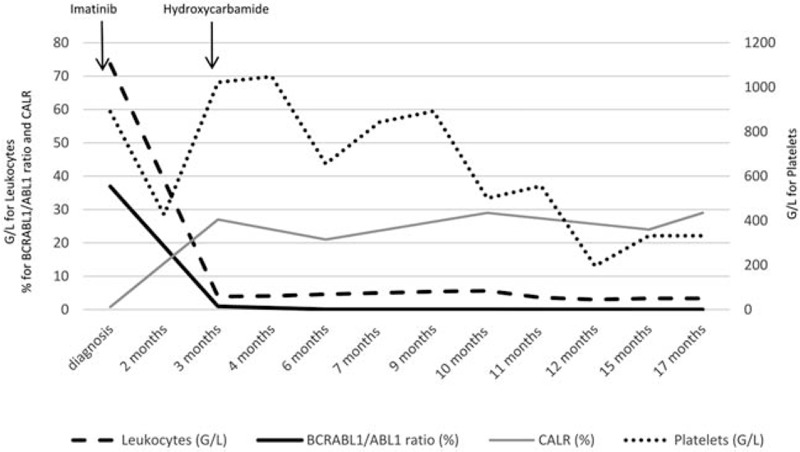
Biological data during the clinical course of the patient. BCR-ABL1 fusion transcript ratio was of 36.9% at diagnosis of CML while CALR allele burden was of 0.8% and was only detectable using digital PCR. At 3 months, BCR-ABL1 ratio decreased to 0.96% and CALR mutation allele burden increase to 27%. During the follow up, CML achieved a BCR-ABL1 molecular response MR4 at 1 year and MR4.5 at 15 months. The CALR mutation remained stable between 20% and 30% of allele burden.
We looked again for CALR mutation in the initial sample using digital PCR (QuantStudio, ThermoFisher, Waltham, USA), a more sensitive technology that allow detection up to 0.01% of allele burden.2 The type 1 CALR mutation was detected with 0.8% of allele burden (Fig. 2).
Figure 2.
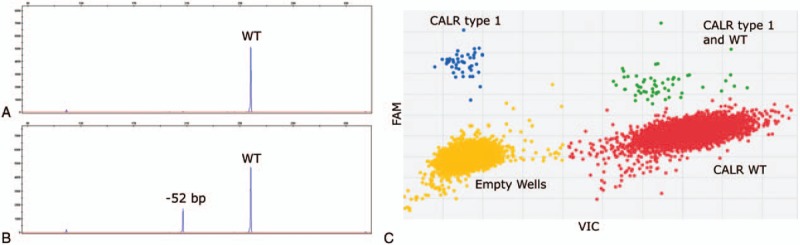
Molecular screening for CALR mutation. Screening for CALR mutation was performed by fragment analysis at diagnosis of CML (panel A), and 3 months later when the thrombocytosis appeared (panel B). Digital PCR at the time of diagnosis of CML was able to detect the CALR type 1 mutation with a VAF of 0.8% (panel C). VAF = variant allele frequency, WT = wild-type allele.
Cooccurrence of BCR-ABL1 and CALR is a very rare event with only few cases previously reported.3,4,5,6,7,8 In the first published case, Cabagnols et al showed that BCR-ABL1 fusion was secondary acquired in the CALR clone by analyzing the mutation hierarchy at clonal level. In 2 other cases, the authors concluded that BCR-ABL1 probably arises in the CALR-mutated clone, based on the evolution of BCR-ABL1 quantification and CALR allele burden.4,5 In another report, a CALR ET (or prefibrotic myelofibrosis) was diagnosed in a CML patient with complete BCR-ABL1 molecular response.7 Retrospectively, the CALR-mutation was detected at the diagnosis of CML 14 years earlier suggesting that the 2 molecular events occurred in separates clones. In the 2 last cases, one cannot assume about the order of acquisition of the molecular events.
Here, given the antiparallel evolution of the BCR-ABL1 transcript and CALR allele burden, it is reasonable to assume that the 2 events occur in 2 separate clones. At diagnosis, the CALR mutation was present at very low allele burden, then not detectable by fragment analysis (sensitivity around 5% of allele burden) because of the higher representation of BCR-ABL clone. The tyrosine kinase inhibitor treatment (TKI) leaded to a decrease of the BCR-ABL1 clone and allowed the expansion of CALR clone that can explain the short time between diagnosis of CML and ET. Not surprisingly, TKI was ineffective on CALR-mutated clone as in previous reports.3,5,6
Co-occurrence of BCR-ABL1 and JAK2V617F mutation was also described. It can arise either in a common clone as demonstrated by clonogenic assays9 or in separate clones as shown by observing the respective evolution of BCRABL1 transcript and JAK2V617F allele burden levels.10
The diagnosis of Ph-MPN in patients with CML is very rare. However, the persistence or appearance of an abnormal blood count during the course of the disease and despite a good molecular BCR-ABL1 response must prompt for searching JAK2V617F, CALR, or MPL mutations. Furthermore, this molecular screening for Ph-MPN should be renewed even it was done and was negative at diagnosis of CML. The present case report also suggests that CALR and BCR-ABL1 events can be found in separated clones.
Acknowledgments
The authors thank technicians of the laboratory of hematology of Angers hospital for technical achievement and Alain Zanetti for helpful discussion.
Footnotes
Citation: Blouet A, Rousselet MC, Le Bris Y, Ribourtout B, Bouvier A, Cottin L, Jouanneau-Courville R, Blanchet O, Ugo V, Luque Paz D. Imatinib Treatment of Chronic Myeloid Leukemia Reveals a Preexisting CALR-mutated Essential Thrombocythemia. HemaSphere, 2018;2:1. http://dx.doi.org/10.1097/HS9.0000000000000029
Authors’ contributions: AB and DLP collected data. M-CR analyzed bone-marrow biopsy. YLB, BR, AB, LC, and RJ-C performed molecular analysis. OB and VU reviewed the data. AB, VU, and DLP wrote the paper. All authors read and approved the final manuscript.
Funding/support: None.
The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Barosi G, Birgegard G, Finazzi G, et al. Response criteria for essential thrombocythemia and polycythemia vera: result of a European LeukemiaNet consensus conference. Blood 2009; 113:4829–4833. [DOI] [PubMed] [Google Scholar]
- 2.Mansier O, Migeon M, Etienne G, et al. JAK2V617F and CALR double mutations are more frequently encountered in patients with low JAK2V617F allelic burdens. Leuk Lymphoma 2016; 57:1949–1951. [DOI] [PubMed] [Google Scholar]
- 3.Cabagnols X, Cayuela J-M, Vainchenker W. A CALR mutation preceding BCR-ABL1 in an atypical myeloproliferative neoplasm. N Engl J Med 2015; 372:688–690. [DOI] [PubMed] [Google Scholar]
- 4.Bonzheim I, Mankel B, Klapthor P, et al. CALR-mutated essential thrombocythemia evolving to chronic myeloid leukemia with coexistent CALR mutation and BCR-ABL translocation. Blood 2015; 125:2309–2311. [DOI] [PubMed] [Google Scholar]
- 5.Loghavi S, Pemmaraju N, Kanagal-Shamanna R, et al. Insights from response to tyrosine kinase inhibitor therapy in a rare myeloproliferative neoplasm with CALR mutation and BCR-ABL1. Blood 2015; 125:3360–3363. [DOI] [PubMed] [Google Scholar]
- 6.Diamond JMS, de Almeida AM, Belo HJ, et al. CALR-mutated primary myelofibrosis evolving to chronic myeloid leukemia with both CALR mutation and BCR-ABL1 fusion gene. Ann Hematol 2016; 95:2101–2104. [DOI] [PubMed] [Google Scholar]
- 7.Dogliotti I, Fava C, Serra A, et al. CALR-positive myeloproliferative disorder in a patient with Ph-positive chronic myeloid leukemia in durable treatment-free remission: a case report. Stem Cell Investig 2017; 4:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seghatoleslami M, Ketabchi N, Ordo A, et al. Coexistence of p190 BCR/ABL transcript and CALR 52-bp deletion in chronic myeloid leukemia blast crisis: a case report. Mediterr J Hematol Infect Dis 2016; 8:e2016002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, Tripodi J, Kremyanskaya M, et al. BCR-ABL1 is a secondary event after JAK2V617F in patients with polycythemia vera who develop chronic myeloid leukemia. Blood 2013; 121:1238–1239. [DOI] [PubMed] [Google Scholar]
- 10.Hussein K, Bock O, Theophile K, et al. Chronic myeloproliferative diseases with concurrent BCR–ABL junction and JAK2V617F mutation. Leukemia 2007; 22:1059–1062. [DOI] [PubMed] [Google Scholar]


