Abstract
The MabThera and Involved field Radiotherapy study investigated efficacy and safety of involved field (IF) radiotherapy in combination with the anti-CD20 antibody Rituximab for early-stage follicular lymphoma (FL) in a prospective, single-arm multicenter phase 2 design. Eighty-five stage I–II FL patients received 8 cycles of Rituximab (375 mg/m2) and IF irradiation (30/40 Gy). The primary endpoint was progression-free survival (PFS) 2 years from treatment start. Secondary endpoints were overall survival (OS), complete response rates, toxicity, quality of life, and minimal residual disease (MRD) response with protocol defined visits up to month 30. For the primary endpoint, PFS at 2 years was 85% for the intention-to-treat set. Long-term data were captured in selected sites and evaluated as post hoc analysis in the per protocol (PP) set: PFS and OS were 78% and 96% at 5 years with a median follow-up of 66 or 78 months, respectively. There were 17/76 recurrences in the PP set, of which 14 were outside the radiation volume only. MRD analyses revealed a clonal marker in 36% of patients at diagnosis. All but 1 marker positive patients experienced a molecular treatment response. There were 13 serious adverse events (4 related to the therapy) during the first 30 months. IF radiotherapy combined with Rituximab is well tolerated and highly efficient with low rates of recurrence in the first years in early-stage FL. The efficacy is comparable with more aggressive therapy approaches without compromising the quality of life and maintains for an extended follow-up of more than 5 years.
Introduction
Treatment of early-stage nodal follicular lymphoma (FL) has been debated for many years.1 Radiation therapy is an effective treatment in this setting. Although large radiation volumes seem to reduce the relapse rate,2,3 the National Comprehensive Cancer Network guidelines recommend radiation treatment only of the pathological involved regions (involved site radiation therapy) without prophylactic treatment of additional lymph node areas predominantly due to the higher toxicity profile of large field radiation. Since the anti-CD20 antibody Rituximab proved to eliminate minimal residual disease (MRD) in advanced stages4–6 and enhances radiation sensitivity in vitro,7 a combination of Rituximab and radiation limited to the involved region might solve this dilemma.
The German Low-Grade Lymphoma Study Group (GLSG) in cooperation with the German working group of radiation oncology (ARO) conducted a prospective, single-arm, multicenter phase II study investigating the efficacy and toxicity of an involved-field radiotherapy in combination with Rituximab treatment (MIR = MabThera and Involved field Radiotherapy) in early-stage nodal FL.
Results
Patients
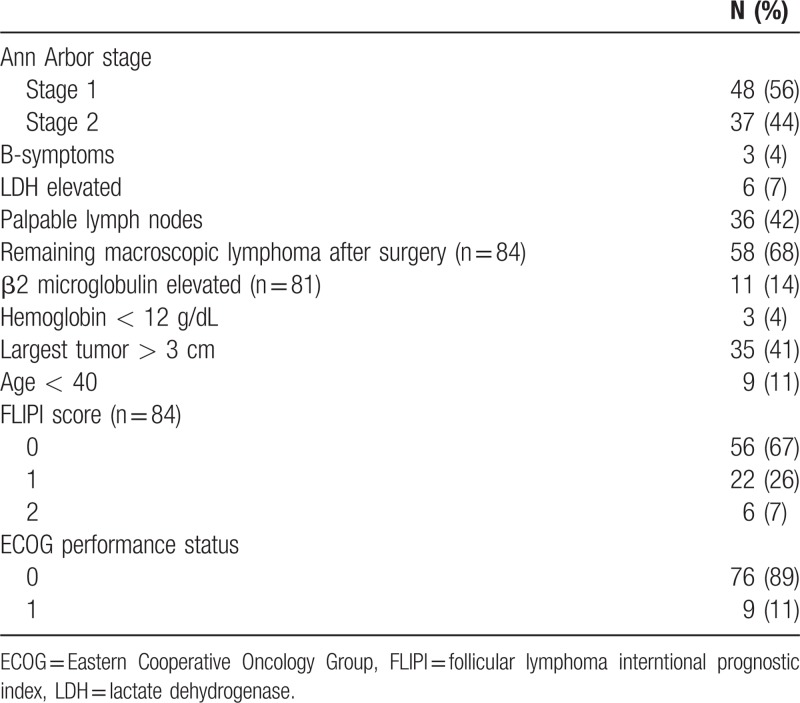
Eighty-five patients (47 males/38 females) in 16 centers had been registered between February 2008 and October 2010. Median age was 55 years (min/max: 21/75 years). Further details of the patients’ characteristics are shown in Table 1.
Table 1.
Patient Characteristics of the 85 Patients Included in the Study
The localization of the lymphoma manifestations was supradiaphragmal in 48% (30% cervical involvement) and infradiaphragmal in 52% (29% inguinal involvement).
All 85 patients started the therapy and were included in the intention to treat (ITT) population, which was the primary analysis set. The per protocol (PP) set was used for sensitivity analysis. Nine patients were excluded for the PP set due to violation of the inclusion criteria (stage [n = 3], histology [n = 1]) or application of <50% Rituximab (allergic reaction to Rituximab [n = 1], noncompliance [n = 1], withdrawal of consent [n = 2], missing Rituximab documentation [n = 1]).
Out of 16 centers, 13 participated for the second period of extended follow-up data collection beyond month 30. Data of 60 patients were available for the post hoc analysis of the long-term efficacy.
Efficacy
Progression-free survival
Fifteen patients (18%) of the ITT population and 14 of the PP set showed a recurrence during the protocol defined follow-up period with a median follow-up of 29.6 months. Progression-free survival (PFS) at 24 months as the primary endpoint was 85% in the ITT set (90% confidence interval [CI] [77; 91%], 1-sided P = 0.026), and 86% (90% CI [77; 91%], 1-sided P = 0.025) for the PP set.
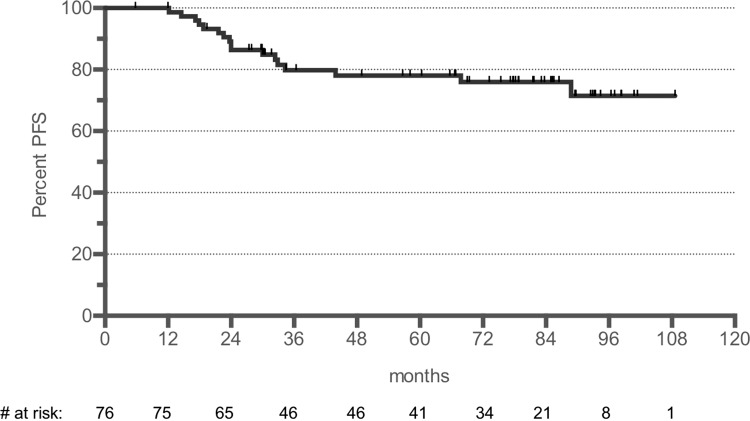
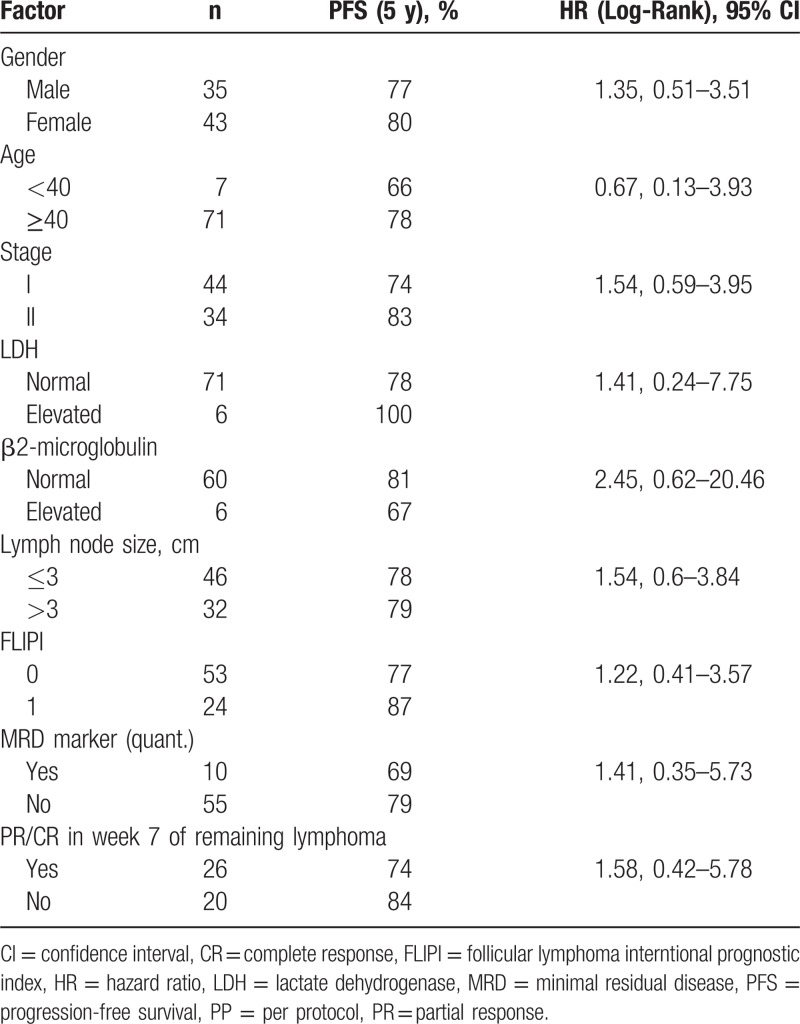
The post hoc defined PFS at 5 years of the extended follow-up period has been estimated to 78% with a median time follow-up period of 66 months for the PP set (Fig. 1). No prognostic markers for PFS could be identified (Table 2).
Figure 1.
PFS of the PP set (median follow-up 66 months). PFS = progression-free survival, PP = per protocol.
Table 2.
Univariate Analysis of Prognostic Factors for PFS (PP Set)
Overall survival
There were 2 deaths during the protocol defined 30 months follow-up. Both deaths were due to secondary cancers. Overall survival (OS) at 2 years was 97% (95% CI = [90; 99]) for the ITT and 97% (89; 99) for the PP set.
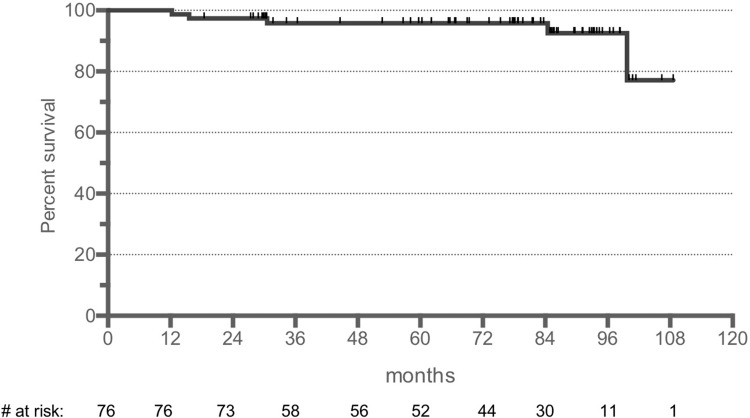
At 5 years, the OS was 96% with a median follow-up of 78 months in the post hoc analysis of the extended follow-up period (Fig. 2). There were no lymphoma-related deaths.
Figure 2.
Overall survival of the PP set (median follow-up 78 months). PP = per protocol.
CR rate in the first 6 months
The complete response (CR) rates for the ITT set were 51%, 73%, and 85% in week 7 (after 4 cycles Rituximab), week 18, and month 6, respectively. CR rates in patients with initially macroscopic residual lymphoma were 29% (95% confidence intervall = [18; 42], 15 of 52 patients) in week 7, 63% ([49; 74], 35 of 56 patients) in week 18, and 79% ([65; 88], 37 of 47 patients) at month 6.
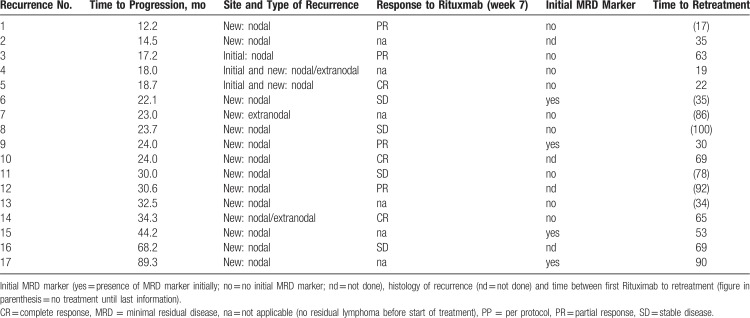
Recurrences
A total of 17 recurrences were observed in the PP set. Three recurrences were within the radiation volume. Two recurrences occurred as aggressive lymphoma (65 and 90 months) outside the radiation volume. Details of the recurrences are listed in Table 3.
Table 3.
Characterization of the Recurrences of the PP Set
Circulating lymphoma cells at diagnosis and MRD
Screening polymerase chain reaction (PCR) for the presence of a t(14;18) translocation or a clonal immunoglobulin heavy chain (IGH) rearrangement was performed in 64/83 stage I/II study patients with available diagnostic samples (77 samples, 64 peripheral blood [PB], 13 bone marrow [BM]); 24/64 patients (38%) had a clonal marker prior to treatment with the majority showing a t(14;18) translocation (19/24), 3 patients showed a t(14;18) and IGH rearrangement, and 2 patients a clonal IGH rearrangement only. Distribution of t(14;18) breakpoints was major breakpoint region (MBR) in 19 patients, 3’ MBR in 1 and minor cluster region in 2 patients.
BM was more frequently positive (6/13, 46%) compared with PB (22/62, 35%) revealing occult BM infiltration at diagnosis.
Patients with an MRD marker did not differ from the whole study cohort with respect to age, performance status, lactate dehydrogenase (LDH), follicular lymphoma interntional prognostic index, and PFS. However, a clonal marker was detected more frequently in patients with Ann Arbor stage II (15/26, 58%) compared with stage I (8/38, 21%; P = 0.0038).
Of 24 patients with an MRD marker, 22 were investigated by a t(14;18) real-time quantitative PCR (RQ-PCR) in diagnostic and follow-up samples. At baseline, levels of circulating lymphoma cells (CLC) in the PB were low with 12 patients (57%) showing CLC level below the quantitative range (QR). Nine patients showed CLC above the QR (median CLC level 2.3 × 10−4, range: 1.0 × 10−4 to 4.3 × 10−3). In BM samples, 3/5 showed a median infiltration of 3.2 × 10−4, range: 2.1 × 10−4 to 5.8 × 10−4 while 2 were below QR.
Protocol-defined MRD negativity at week 18 by t(14;18) RQ-PCR was observed in 20/21 (95%) patients with an available sample. Of 21 patients, 14 (67%) achieved a CR, 1 patient a CRu, and 6 patients a partial response (PR) (30%). The only patient with persistent MRD positivity had a PR at restaging in week 18, remained marker positive, and showed no recurrence up to an extended follow-up of 67 months.
Of 19 patients without progression after treatment, 15 (79%) were consistently MRD negative in PB at all follow-up time visits. In 4 patients in remission (21%), MRD positivity reoccurred but below the QR of 10−4. All 4 patients showed fluctuating MRD levels with alternating MRD positivity/negativity during follow-up.
Three initially marker positive patients showed clinical progression at months 22, 24, and 33 after start of treatment. In 2 patients, follow-up samples were MRD positive at or prior to time of relapse. In the third patient, no sample at relapse was available.
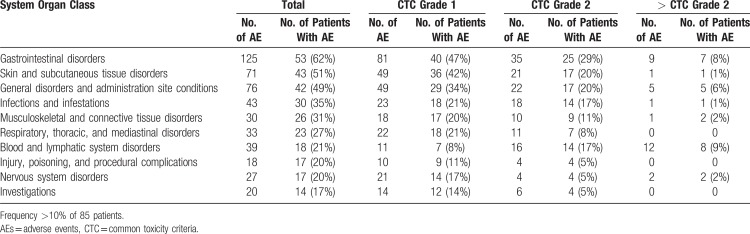
Toxicity
A total of 529 adverse events were reported in 95% (81/85) of the patients during treatment (335 [63%]) and protocol-defined follow-up (192 [36%]). Most of the adverse advents were minor (common toxicity criteria (CTC) grade 1 [321 (61%)] or 2 [164 (31%)]). Twenty-three adverse events (4%) were rated grade 3. There was only 1 grade 4 toxicity (lymphopenia <0.2 × 109/L). Treatment had to be terminated due to side effects in 1 patient (allergic reaction to Rituximab).
The most common adverse events organized by organ class and CTC grade are summarized in Table 4.
Table 4.
Most Common AEs According to Organ Class and CTC Grade
Thirteen patients experienced serious adverse events within the 30 months follow-up. Four of these events were classified as possibly related to therapy: allergic reaction to Rituximab, bacteremia, pulmonary embolism, and stomatitis. All patients completely recovered from those side effects.
Secondary neoplasia
Two patients died of secondary cancer during the protocol-defined period: A peritoneal mesothelioma was diagnosed 6 months after the first Rituximab application and 4 months after radiation of the right cervical and left axillary region. The patient died 12.3 months after start of treatment. A metastatic nonsmall cell lung cancer was diagnosed in a second patient 12 months after radiation of the left cervical and right hilar region. The patient had a history of heavy smoking (>80 pack years) and died 22.6 months after start of therapy. Both secondary cancers were judged not related to therapy. A third patient developed a small cell lung cancer at month 80.
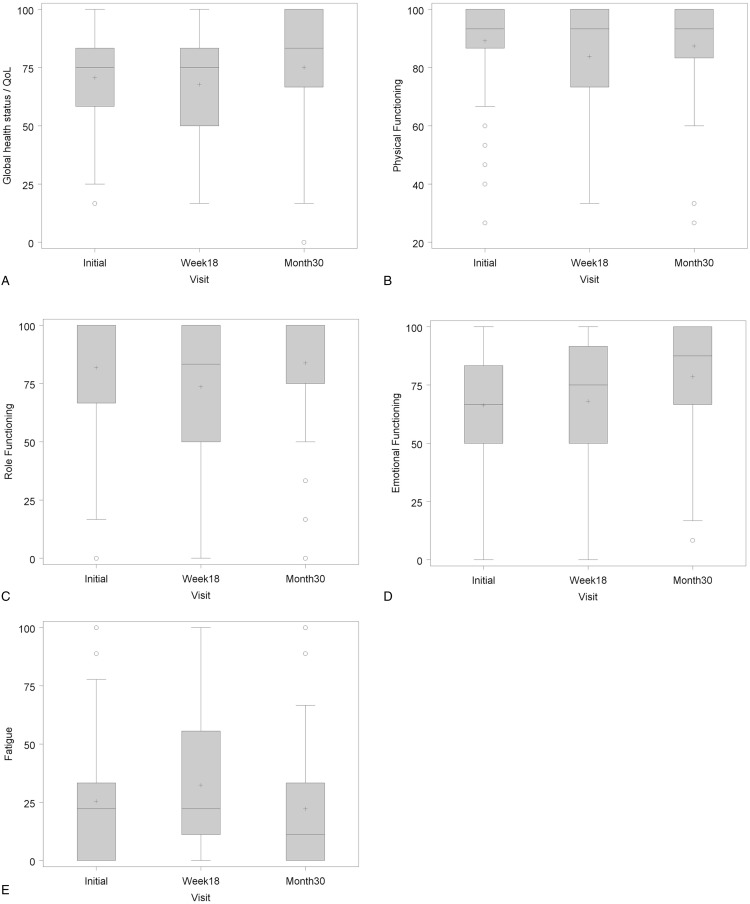
Quality of life
The treatment had no substantial influence on patients’ quality of life. There was a minor impairment of the global health status, the physical and social performance and occurrence of fatigue at the end of the therapy. However, there was also a trend to overall improvement of quality of life at the end of follow-up (Fig. 3).
Figure 3.
Quality of life according to the QLQ-C30 questionnaire. (A) Global health status, (B) physical functioning, (C) role functioning, (D) emotional functioning, and (E) fatigue.
Discussion
Treatment of early-stage FL has been a controversial issue for the last decades. Especially in elderly patients, a watch and wait strategy might be considered since only 25% receive therapy within the first 5 years after diagnosis and the 10 years survival is 85% in a small cohort of patients.8 On the other hand, radiation therapy has been shown to be very effective and early radiation therapy seems to improve disease-specific survival and OS based on an SEER database US National Cancer Database analysis.9,10 The MIR concept might be an alternative curative approach ensuring quality of life and with only a minimal risk of severe side effects.
However, larger treatment fields proved to be more effective for longtime control than treatment of only smaller volumes.2,3 Since larger radiation volumes are associated with more toxicity, the NCCN guidelines still recommend only localized radiation therapy for those patients. The ARO 98-01 trial randomized total lymphatic irradiation against extended field irradiation in early-stage FL. Preliminary results showed recurrences mainly in the nonirradiated areas with a significant better PFS of patients treated with total lymphatic irradiation.11 The Stanford data2 and the ARO98-01 data11 implicate that a part of the patients with early-stage FL actually do not have a limited stage but present with occult systemic disease with a need of a systemic treatment. This is also supported by the results of our PCR-based analysis of CLC in the diagnostic PB in our study. In 22 stage I/II patients (35%), CLC were detected in PB at diagnosis. Also, submicroscopic BM infiltration detected by PCR was frequent with 6/13 cases (46%) despite a negative BM histology.
Friedberg et al12 reported that systemic chemotherapy was advantageous compared with limited field irradiation alone in stage 1 FL. A combination of chemotherapy and involved field (IF) irradiation improved PFS compared with IF radiation only, but was hampered by toxicity.13–15 Recently, the results of randomized trial were published investigating Cyclophosphamide, Vincristine, Prednisone (CVP) chemotherapy as additional systemic treatment in combination with IF radiotherapy versus radiotherapy alone16 Rituximab was added later to the systemic therapy in 31 patients. Radiation therapy alone was significantly inferior in PFS compared with the combined modality approach. The best results were achieved using Rituximab, CVP, and radiation with a PFS of approximately 86% at 5 years.
The current MIR study combined an IF radiation therapy only with Rituximab. The PFS at 2 years was at least as good as in prospective large radiation field series but with significantly lower toxicity.3,11 The presented PFS at 5 years of 78% was close to the data of MacManus of 86% without the accompanying toxicity of chemotherapy.16 However, our data show limitation since it is based on a post hoc analysis of prospectively collected long-term data. This might result in a bias due to less extensive follow-up examinations or drop-outs. This could also be an explanation for the reduction of recurrences during the extended follow-up period. However, other published data also show a marked decrease of the PFS in initial years and a trend to a plateau thereafter.2,3,12,17 Our data confirm the results of a larger retrospective Italian study, which suggested an improved patient outcome by the addition of Rituximab to limited field radiation therapy17: The 5 years PFS of the current study is even superior to their data (5 years PFS 68%). The Italian study also included FL grade 3a and/or applied only 4 cycles Rituximab instead of 8 cycles in the MIR study.17
Response duration was associated with a continuous MRD response as 15/19 patients (79%) without progression were consistently MRD negative in PB at all follow-up time points. Only few relapsing patients had follow-up samples for MRD evaluation. However, it seems that MRD reappearance is associated with clinical relapse. A possible treatment concept evolving from our results could be Rituximab retreatment already at MRD relapse to prevent clinical recurrences. Alternatively, Obinutuzumab may result in a significant higher efficacy compared with Rituximab based on the studies in CLL and advanced FL.18–20
Given the higher rate of outfield recurrences in the current study, radiation did have an additional effect to Rituximab with an increase of the CR rate from 29% to 79%. It is not clear, if a lower radiation dose would also show the same efficacy. Lowry et al21 published a randomized trial for radiation alone with 24 Gy versus 40 Gy (2 Gy fraction size) showing no benefit for the higher dose group in indolent lymphomas. Therefore, 12 × 2 Gy might have given similar results. However, a WHO diagnosis could be made in only 80% of the indolent lymphomas in this British trial and of those, only 60% were proven FL according to central pathologic review.21 In the current study, all specimen underwent a central pathologic review, which confirmed an FL grade 1 or 2 corresponding to the inclusion criteria. Only 1 patient had to be excluded from the PP population due to the diagnosis of Hodgkin lymphoma in the central review.
Radiation therapy in combination with rituximab induced MRD response at week 18 in all patients except one. This demonstrates the impact of Rituximab lymphoma cell clearance as data from IF radiation therapy alone detected MRD responses in only 60%. In contrast to IF radiation alone where only patients with a low-level of CLC (<1:100,000) achieved PCR negativity, lymphoma cell clearance in the MIR study was independent from the pretherapeutic CLC load.22
There are only few publications analyzing the presence of CLC at diagnosis in small series with early-stage FL. Lambrechts et al23 detected t(14;18) positive cells in 75% of a series of 12 early-stage I/II FL. Pulsoni22 investigated a series of 24 early-stage FL and detected CLC in 66% of all cases. This higher detection frequency may in part result from the relatively contamination prone-nested PCR approach applied in both studies and the fact that nested PCR approaches detects t(14;18) positive cells in about 40% of healthy individuals.24 By contrast, the MRD detection of 34% in our series was confirmed by a quality controlled RQ-PCR assay25 and standardized data analysis according to EuroMRD guideline,26 thereby reflecting in the most precise frequency determination of CLC in FL. The results show that in 1/3 of patients with early-stage disease lymphoma cells have spread from the affected lymph node to PB or BM.
Presence of a clonal marker is obviously dependent on stage as a clonal marker was detected more frequently in patients with Ann Arbor stage II (58%) compared with stage I (21%) in our series. It seems safe to assume that detection of a molecular marker at diagnosis is a sensitive indicator for tumor burden, since in advanced stage III/IV FL the frequency of CLC detection increases up to 80% (t(14;18) and IGVH multiplex PCR) and is associated with a high tumor load.27 In contrast to Ruella et al,17 our data did not reveal initial MRD positivity as a negative prognostic marker. This might be explained by the fact that Rituximab was applied only 4 times in the Italian study instead of 8 times in the MIR study. However, Ruella et al also investigated only BM, while most of our analyses are based on blood samples. Also, 57% of MRD positive samples in the MIR study showed only a minor infiltration below the QR assuming a low systemic lymphoma burden.
Compared with advanced FL,28 level of CLC measured by RQ-PCR is low in stage I/II FL in the MIR study with 57% showing CLC level below the QR of 10−4 reflecting a lower overall tumor burden.
Rituximab and IF radiotherapy were well tolerated. There were only 4 serious adverse events possibly related to the treatment, which is clearly a lower rate than for the more aggressive approaches combining chemotherapy and small field radiation. MacManus et al16 reported 45 grade 3 or 4 toxicities were counted in 69 patients receiving CVP or R-CVP in addition to IF radiotherapy. There were 3 cases of secondary cancers (ie, non-small cell lung cancer, mesothelioma, and small cell lung cancer) in our cohort, 2 of these occurred within 12 months after initiation of the lymphoma therapy and were outside of the radiation fields. A correlation to the study treatment seems to be unlikely, since a large meta-analysis shows that the addition of Rituximab to standard treatment is not associated with an increased risk of secondary malignancies.29
The low toxicity profile of the MIR schedule is also reflected by the quality of life data. The MIR treatment had no significant negative influence on quality of life similar to patients with advanced disease receiving only Rituximab.30 Quality of life scores 1 year after radiotherapy only did not differ from the normal population in the PHAROS-registry using the same EORTC-C30 questionnaire as in the current study. By contrast, after immunochemotherapy patients reported significant higher fatigue scores 1 year after therapy compared with patients who received radiotherapy only or to the normal population.31 In the current study, fatigue was not significantly influenced by immuno-radiotherapy with only slightly elevated fatigue scores at week 18.
In conclusion, IF radiotherapy in combination with Rituximab is well tolerated and shows low rates of recurrence in early-stage FL. Results are comparable with more aggressive therapeutic approaches but without compromising the quality of life and maintain also for an extended follow-up of more than 5 years.
Materials and methods
Study design and patient's entry criteria
Details of the study design are published elsewhere.32 Shortly, the MIR study recruited patients with CD20 positive nodal FL grade 1/2 according to the WHO classification 2001, in localized stage I/II (Ann Arbor), younger than 76 years and with Eastern Cooperative Oncology Group performance status 0 to 2. Patients with bulky disease (>7 cm), prior radiotherapy, prior chemotherapy or immunotherapy, or a prior diagnosis of a malignant neoplasia were excluded. The primary endpoint was PFS at 24 months as defined by Cheson.33 Secondary endpoints were CR rate after Rituximab monotherapy (week 7) and completion of treatment (week 18), relapse pattern, OS, toxicity, and quality of life. The study was approved by local ethical committees of the Medical Faculty of the University of Heidelberg (AFmu-085/2007), the federal Paul-Ehrlich Institute and the German agency for radiation protection and registered (ClinicalTrials.govID: NCT00509184). All patients gave their written informed consent.
Since long-term follow-up is of interest for the community, the study centers were encouraged to further follow the patients after the last protocol defined visit at month 30 (extended evaluation period). However, frequency and extent (clinical and imaging) of follow-up examinations were at discretion of the local investigator and the data were not centrally monitored. These data were retrospectively collected for a post hoc analysis of PFS and OS at 5 years.
Treatment
Treatment consisted of 4 once per week administrations of Rituximab (MabThera, Roche Pharma, 375 mg/m2). In week 7, patients received a restaging and radiation planning CT of the involved region. Four further weekly administrations of Rituximab were given in weeks 9 to 12. Radiation treatment of the involved lymph node regions (adapted from Yahalom and Mauch34) was initiated in week 9 and applied in 2 Gy fractions (5 times/wk) up to a total dose of 30 Gy. In case of remaining lymphoma after initial Rituximab therapy in week 7, the residual region was boosted with 5 × 2 Gy in week 12.
Follow-up
Protocol-defined follow-up visits were performed in week 18 and months 6, 12, 18, 24, and 30. These visits included the assessment of medical status, a physical examination, and analysis of blood cell counts and LDH. Three-dimensional imaging (CT/MRI) of neck, thorax, abdomen, and pelvis was mandatory for all time points except of week 18 (involved region only).
Further follow-up examination during the extended evaluation period was in the centers discretion as mentioned above.
Quality assurance
Histologic specimens were centrally reviewed by the GLSG pathology reference panel. Staging imaging series were centrally reviewed and extent of radiation was recommended accordingly. For data of the first 30 months, core data monitoring was performed in all patients and additionally 100% source data were verified in 20% of the patients.
Statistics
Primary endpoint
PFS estimation for the primary endpoint was based on the prior trial ARO 98-01 (extended field radiotherapy vs total lymphatic radiotherapy in early stages of nodal FL). An interim analysis of this trial showed a PFS of 75% at 2 years.35 The MIR study aimed to improve this outcome. Kaplan-Meier estimates and 2-sided 90% CIs were calculated at 6, 12, 18, 24, and 30 months after start of treatment. The null hypothesis (PFS at 24 months ≤ 75%) is rejected if the lower boundary of the 2-sided 90% CI at month 24 is above 75% corresponding to a 1-sided test with α = 0.05.
Secondary endpoints
Remission was evaluated according to Cheson 1999.33 Complete remission rate (CR/CRu) at week 7 and week 18 was evaluated only for patients with remaining lymphoma after the diagnostic biopsy procedure. In addition to the protocol-predefined endpoints, the rate of CR at month 6 has been calculated.
OS was calculated from the beginning of therapy until death or last follow-up (Kaplan-Meier method).
QLQ-C30 (EORTC, version 3.0) questionnaires were used for the assessment of the quality of life before treatment as well as in week 18 and month 30. Missing values were imputed using the LOCF (last observation carried forward) or BOCF (baseline observation carried forward) method.
Toxicity was evaluated according to CTCAE version 3.0 scoring system.
Kaplan-Meier estimates were used for the post hoc evaluation of PFS and OS at 5 years during the extended evaluation period.
MRD analysis
MRD marker screening was performed prior to start of Rituximab in PB and BM. MRD was assessed at week 18 and months 12, 24, and 30.
DNA was extracted with the Qiagen Blood Mini Kit (Qiagen, Hilden, Germany). In PB or BM lymphoma cells were assessed by t(14;18) or IGH rearrangement multiplex PCR as published.36 RQ-PCR assays with generic primers for the t(14;18) breakpoints and/or allele-specific primers for the Ig rearrangements were performed on an ABI PRISM 7700 thermal cycler (Applied Biosystems, CA). MRD quantification was performed as previously described25 and evaluated according to EuroMRD criteria.26
Assays were designed to reach a sensitivity of 1 × 10−5 with Albumin as control gene to correct for DNA amount or PCR inhibitors.37 MRD positivity of a sample was defined if any of the triplicates were positive by RQ-PCR analysis. Molecular complete remission was defined as absence of PCR-detectable neoplastic cells in PB and/or BM at any time point with a sensitivity of at least 10−4. MRD analyses in PB and/or BM were pooled, and higher MRD values were encountered for calculation.
Acknowledgments
The authors would like to thank Dr Thomas Welzel for his help in the central radiological review process and Oliver Göring of the coordination center for clinical trials (KKS Heidelberg) for his help in the data management. A special thank belongs to Anja Freiberger (KKS Heidelberg) for her excellent project management.
Author Contributions
Klaus Herfarth: idea, planning, central QA, statistics, discussion, writing.
Peter Borchmann, Karin Hohloch, Volker Budach, Rita Engenhart-Cabillic, Ulrich Keller, Gabriele Reinartz, Hans-Theodor Eich, Jan Dürig, Thomas Wiegel: discussion, writing.
Sven Schnaidt: statistics, discussion, writing.
Marianne Engelhard, Andreas Viardot, Mathias Witzens-Harig: idea, discussion, writing.
Clemens F. Hess: discussion.
Bernd Dörken: discussion.
Wolfgang Hiddemann: planning, discussion, writing.
Eva Hoster: planning, statistics, discussion, writing.
Christiane Pott: planning, MRD analysis, discussion, writing.
Martin Dreyling: idea, planning, discussion, writing.
Supplementary Material
Footnotes
Citation: Herfarth K, Borchmann P, Schnaidt S, Hohloch K, Budach V, Engelhard M, Viardot A, Engenhart-Cabillic R, Keller U, Reinartz G, Eich H-T, Witzens-Harig M, Hess CF, Dörken B, Dürig J, Wiegel T, Hiddemann W, Hoster E, Pott C, Dreyling M. Rituximab With Involved Field Irradiation for Early-stage Nodal Follicular Lymphoma: Results of the MIR Study. HemaSphere, 2018;2:6. http://dx.doi.org/10.1097/HS9.0000000000000160.
Christiane Pott and Martin Dreyling shared senior authorship: CP (MRD data) and MD (clinical data).
Funding/support: The study was partly supported by research funding From Roche Pharma Grenzach, Germany to KH.
Participating centers in Germany were Charité Berlin (Campus Benjamin Franklin and Campus Charité Mitte), Helios Hospital Berlin-Buch, University Hospital Carl Gustav Carus Dresden, University Hospital Essen, University Medical Center Göttingen, Hannover Medical School, Heidelberg University Hospital, University Hospital Cologne, University Medical Center Mannheim, University Hospital Marburg, Hospital of the Ludwigs-Maximilians-University (LMU) Munich, University Hospital rechts der Isar Munich, Münster University Hospital, Ulm University Medical Center.
Disclosure: Klaus Herfarth: reports grants, personal fees, and nonfinancial support from Roche Pharma, during the conduct of the study.
Peter Borchmann, Sven Schnaidt: No conflicts of interest.
Karin Hohloch: Roche Pharma: Travel support and advisory board.
Volker Budach, Marianne Engelhard, Rita Engenhart-Cabillic, Gabriele Reinartz, Hans-Theodor Eich, Mathias Witzens-Harig, Clemens F. Hess, Bernd Dörken, Thomas Wiegel, Christiane Pott: Has nothing to disclose.
Andreas Viardot: personal fees from Roche, Amgen, Pfizer, Gilead outside the submitted work.
Ulrich Keller: Roche Pharma: Consultancy and travel support.
Jan Dürig: Roche Pharma: Honoraria, consultancy, and advisory committee.
Wolfgang Hiddemann: reports grants and personal fees from Roche AG, during the conduct of the study; grants and personal fees from Roche AG, grants and personal fees from Gilead, grants and personal fees from Janssen, outside the submitted work.
Eva Hoster: Roche: institutional research funding and travel support.
Martin Dreyling: Roche: Speakers honoraria, scientific advisory board, institutional support of academic trials.
References
- 1.Dreyling M, Ghielmini M, Rule S, et al. Newly diagnosed and relapsed follicular lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017; 28:3109. [DOI] [PubMed] [Google Scholar]
- 2.Mac Manus MP, Hoppe RT. Is radiotherapy curative for stage I and II low-grade follicular lymphoma? Results of a long-term follow-up study of patients treated at Stanford University. J Clin Oncol 1996; 14:1282–1290. [DOI] [PubMed] [Google Scholar]
- 3.Stuschke M, Hoederath A, Sack H, et al. Extended field and total central lymphatic radiotherapy in the treatment of early stage lymph node centroblastic-centrocytic lymphomas: results of a prospective multicenter study. Study Group NHL-fruhe Stadien. Cancer 1997; 80:2273–2284. [DOI] [PubMed] [Google Scholar]
- 4.Rambaldi A, Lazzari M, Manzoni C, et al. Monitoring of minimal residual disease after CHOP and rituximab in previously untreated patients with follicular lymphoma. Blood 2002; 99:856–862. [DOI] [PubMed] [Google Scholar]
- 5.Galimberti S, Guerrini F, Morabito F, et al. Quantitative molecular evaluation in autotransplant programs for follicular lymphoma: efficacy of in vivo purging by Rituximab. Bone Marrow Transplant 2003; 32:57–63. [DOI] [PubMed] [Google Scholar]
- 6.Brugger W, Hirsch J, Grunebach F, et al. Rituximab consolidation after high-dose chemotherapy and autologous blood stem cell transplantation in follicular and mantle cell lymphoma: a prospective, multicenter phase II study. Ann Oncol 2004; 15:1691–1698. [DOI] [PubMed] [Google Scholar]
- 7.Skvortsova I, Popper BA, Skvortsov S, et al. Pretreatment with rituximab enhances radiosensitivity of non-Hodgkin's lymphoma cells. J Radiat Res (Tokyo) 2005; 46:241–248. [DOI] [PubMed] [Google Scholar]
- 8.Advani R, Rosenberg SA, Horning SJ. Stage I and II follicular non-Hodgkin's lymphoma: long-term follow-up of no initial therapy. J Clin Oncol 2004; 22:1454–1459. [DOI] [PubMed] [Google Scholar]
- 9.Pugh TJ, Ballonoff A, Newman F, et al. Improved survival in patients with early stage low-grade follicular lymphoma treated with radiation: a Surveillance, Epidemiology, and End Results database analysis. Cancer 2010; 116:3843–3851. [DOI] [PubMed] [Google Scholar]
- 10.Vargo JA, Gill BS, Balasubramani GK, et al. What is the optimal management of early-stage low-grade follicular lymphoma in the modern era? Cancer 2015; 121:3325–3334. [DOI] [PubMed] [Google Scholar]
- 11.Engelhard M, Unterhalt M, Hansmann M, et al. Follicular lymphoma: curability by radiotherapy in limited stage nodal disease? Updated results of a randomized trial. Ann Oncol 2011; 22 (suppl 4):iv90–iv91. [Google Scholar]
- 12.Friedberg JW, Byrtek M, Link BK, et al. Effectiveness of first-line management strategies for stage I follicular lymphoma: analysis of the National LymphoCare Study. J Clin Oncol 2012; 30:3368–3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelsey SM, Newland AC, Hudson GV, et al. A British National Lymphoma Investigation randomised trial of single agent chlorambucil plus radiotherapy versus radiotherapy alone in low grade, localised non-Hodgkins lymphoma. Med Oncol 1994; 11:19–25. [DOI] [PubMed] [Google Scholar]
- 14.Seymour JF, McLaughlin P, Fuller LM, et al. High rate of prolonged remissions following combined modality therapy for patients with localized low-grade lymphoma. Ann Oncol 1996; 7:157–163. [DOI] [PubMed] [Google Scholar]
- 15.Seymour JF, Pro B, Fuller LM, et al. Long-term follow-up of a prospective study of combined modality therapy for stage I–II indolent non-Hodgkin's lymphoma. J Clin Oncol 2003; 21:2115–2122. [DOI] [PubMed] [Google Scholar]
- 16.MacManus M, Fisher R, Roos D, et al. Randomized trial of systemic therapy after involved-field radiotherapy in patients with early-stage follicular lymphoma: TROG 99.03. J Clin Oncol 2018; 36:2918–2925. [DOI] [PubMed] [Google Scholar]
- 17.Ruella M, Filippi AR, Bruna R, et al. Addition of rituximab to involved-field radiation therapy prolongs progression-free survival in stage I–II follicular lymphoma: results of a multicenter study. Int J Radiat Oncol Biol Phys 2016; 94:783–791. [DOI] [PubMed] [Google Scholar]
- 18.Goede V, Fischer K, Engelke A, et al. Obinutuzumab as frontline treatment of chronic lymphocytic leukemia: updated results of the CLL11 study. Leukemia 2015; 29:1602–1604. [DOI] [PubMed] [Google Scholar]
- 19.Sehn LH, Goy A, Offner FC, et al. Randomized phase II trial comparing obinutuzumab (GA101) with rituximab in patients with relapsed CD20+ indolent B-cell non-Hodgkin lymphoma: final analysis of the GAUSS study. J Clin Oncol 2015; 33:3467–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marcus R, Davies A, Ando K, et al. Obinutuzumab for the first-line treatment of follicular lymphoma. N Engl J Med 2017; 377:1331–1344. [DOI] [PubMed] [Google Scholar]
- 21.Lowry L, Smith P, Qian W, et al. Reduced dose radiotherapy for local control in non-Hodgkin lymphoma: a randomised phase III trial. Radiat Oncol 2011; 100:86–92. [DOI] [PubMed] [Google Scholar]
- 22.Pulsoni A, Starza ID, Frattarelli N, et al. Stage I/II follicular lymphoma: spread of bcl-2/IgH+ cells in blood and bone marrow from primary site of disease and possibility of clearance after involved field radiotherapy. Br J Haematol 2007; 137:216–220. [DOI] [PubMed] [Google Scholar]
- 23.Lambrechts AC, Hupkes PE, Dorssers LC, et al. Translocation (14;18)-positive cells are present in the circulation of the majority of patients with localized (stage I and II) follicular non-Hodgkin's lymphoma. Blood 1993; 82:2510–2516. [PubMed] [Google Scholar]
- 24.Limpens J, Stad R, Vos C, et al. Lymphoma-associated translocation t(14;18) in blood B cells of normal individuals. Blood 1995; 85:2528–2536. [PubMed] [Google Scholar]
- 25.Pott C, Bruggemann M, Ritgen M, et al. MRD detection in B-cell non-Hodgkin lymphomas using Ig gene rearrangements and chromosomal translocations as targets for real-time quantitative PCR. Methods Mol Biol 2013; 971:175–200. [DOI] [PubMed] [Google Scholar]
- 26.van der Velden VH, Cazzaniga G, Schrauder A, et al. Analysis of minimal residual disease by Ig/TCR gene rearrangements: guidelines for interpretation of real-time quantitative PCR data. Leukemia 2007; 21:604–611. [DOI] [PubMed] [Google Scholar]
- 27.Pott C, Hoster E, Kehden B, et al. Minimal residual disease in patients with follicular lymphoma treated with obinutuzumab or rituximab as first-line induction immunochemotherapy and maintenance in the phase 3 GALLIUM study. Blood 2016; 128:613.27492312 [Google Scholar]
- 28.Hirt C, Schuler F, Kiefer T, et al. Rapid and sustained clearance of circulating lymphoma cells after chemotherapy plus rituximab: clinical significance of quantitative t(14;18) PCR monitoring in advanced stage follicular lymphoma patients. Br J Haematol 2008; 141:631–640. [DOI] [PubMed] [Google Scholar]
- 29.Fleury I, Chevret S, Pfreundschuh M, et al. Rituximab and risk of second primary malignancies in patients with non-Hodgkin lymphoma: a systematic review and meta-analysis. Ann Oncol 2016; 27:390–397. [DOI] [PubMed] [Google Scholar]
- 30.Ardeshna KM, Qian W, Smith P, et al. Rituximab versus a watch-and-wait approach in patients with advanced-stage, asymptomatic, non-bulky follicular lymphoma: an open-label randomised phase 3 trial. Lancet Oncol 2014; 15:424–435. [DOI] [PubMed] [Google Scholar]
- 31.Oerlemans S, Issa DE, van den Broek EC, et al. Impact of therapy and disease-related symptoms on health-related quality of life in patients with follicular lymphoma: results of the population-based PHAROS-registry. Eur J Haematol 2014; 93:229–238. [DOI] [PubMed] [Google Scholar]
- 32.Witzens-Harig M, Hensel M, Unterhalt M, et al. Treatment of limited stage follicular lymphoma with Rituximab immunotherapy and involved field radiotherapy in a prospective multicenter phase II trial-MIR trial. BMC Cancer 2011; 11:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol 1999; 17:1244–1253. [DOI] [PubMed] [Google Scholar]
- 34.Yahalom J, Mauch P. The involved field is back: issues in delineating the radiation field in Hodgkin's disease. Ann Oncol 2002; 13 (suppl 1):79–83. [DOI] [PubMed] [Google Scholar]
- 35.Engelhard M, Stuschke M, Hansmann M, et al. Follicular lymphoma, immunocytoma, and mantle cell lymphoma: randomised evaluation of curative radiotherapy in limited stage nodal disease. Ann Oncol 2005; 16 (suppl 5):108. [Google Scholar]
- 36.van Dongen JJ, Langerak AW, Bruggemann M, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia 2003; 17:2257–2317. [DOI] [PubMed] [Google Scholar]
- 37.Pongers-Willemse MJ, Verhagen OJ, Tibbe GJ, et al. Real-time quantitative PCR for the detection of minimal residual disease in acute lymphoblastic leukemia using junctional region specific TaqMan probes. Leukemia 1998; 12:2006–2014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.