Supplemental Digital Content is available in the text
Abstract
Despite significant progress in the treatment of pediatric acute myeloblastic leukemia (AML), relapse remains the commonest cause of death. Randomized ELAM02 trial questioned if maintenance therapy with interleukin-2 (IL2), for 1 year, improves disease-free survival (DFS). Patients aged 0 to 18 years, with newly diagnosed AML (excluding patients with acute promyelocytic leukemia or down syndrome AML) were enrolled. They received 1 course of induction treatment (cytarabine and mitoxantrone) and 3 courses of consolidation treatment (high-dose cytarabine in courses 1 and 3). According to the cytogenetics risk, patients not undergoing hematopoietic stem cell transplantation, still in complete remission (CR) after the third course of consolidation treatment, were eligible for randomization to 1 year of maintenance therapy with monthly courses of IL2 or no maintenance treatment. There were 438 evaluable patients, 154 of whom were randomized to the IL2/no maintenance groups. Relapse occurred in 28 patients from the IL2+ group and 29 patients in the IL2− group. Survival was similar in the 2 groups, with a 4-year DFS of 62% without IL2 and 66% with IL2 (P = 0.75). In the CBF population, 4-year DFS was 55% without IL2 and 78% with IL2 (P = 0.07). No deaths from toxicity or excess of serious adverse events related to IL2 treatment were recorded. Prolonged IL2 for maintenance therapy after intensive chemotherapy is feasible and safe in pediatric AML patients in their first CR. Such treatment did not improve DFS in this study, but a positive trend was observed in favor of IL2 maintenance therapy among core binding factor acute myeloblastic leukemia.
Introduction
Pediatric acute myeloblastic leukemia (AML) is a rare disease, affecting 75 to 80 patients under the age of 15 years annually in France.1 Intensive treatment and effective supportive care result in an overall survival (OS) of 60% to 75%, relapse rate (RR) of 35% to 45%, and an event-free survival (EFS) of 45% to 60%.2–4 Salvage therapy has improved, but relapse remains the commonest cause of death, and innovative therapeutic approaches are required to reduce RR. The standard treatment for pediatric AML is 4 or 5 courses of chemotherapy.5–7 Induction therapy generally consists of an anthracycline or an anthracenedione and 7 to 10 days of cytarabine, and is followed by a high-dose cytarabine postremission treatment.6–9 The MRC AML12 study showed mitoxantrone to be superior to daunorubicin for induction therapy.2 Four (rather than 5) courses of intensive chemotherapy were demonstrated to be optimal.2,6 Allogeneic hematopoietic stem cell transplantation (HSCT) is currently the only postconsolidation treatment capable of reducing the risk of relapse, thereby increasing disease-free survival (DFS) in patients at high risk.10 Standard maintenance chemotherapy is not effective, as demonstrated in the CCG 123 and LAME91 randomized studies.5,11,12
Interleukin-2 (IL2) is a cytokine with potent immunomodulatory activity that stimulates tumor-specific cytotoxic T lymphocytes and natural killer (NK) cells,13,14 mimicking the immune graft-versus-leukemia effect of HSCT.15 Adult patients with acute leukemia with high levels of cytokine production and NK activity have a lower risk of relapse.16 Preliminary nonrandomized clinical trials on patients with recurrent AML showed a potential effect of IL2, with remission and long-term survival observed in some cases.17,18 The potential antileukemic effects of IL213,14,19 and its limited toxicity at high doses17,18,20 suggested that the prolonged administration of lower doses might be a useful consolidation treatment to prevent relapse in patients not undergoing HSCT.20,21
ELAM02 was a prospective, national, multicenter, randomized controlled trial (RCT) assessing the benefits of 1 year of IL2 maintenance therapy in patients with primary pediatric AML. After 4 cycles of chemotherapy, patients not undergoing HSCT were randomized to 2 groups, with and without IL2 maintenance therapy. Low doses of IL2 were administered in 5-day courses, as in the EORTC-GIMENA trial.22,23 We report here the final results for IL2 maintenance treatment in this study.
Patients and methods
Patients below 18 years of age with French-American-British de novo AML subtypes24–26 (excluding AML-M3), postmyelodysplasia AML or isolated myeloid sarcoma were eligible provided they had no syndrome associated with predisposition to leukemia, such as down syndrome or inherited bone marrow failure syndromes. Patients previously exposed to chemotherapy or radiotherapy were not eligible. The study was sponsored by the Assistance Publique-Hôpitaux de Paris and was conducted in accordance with the Helsinki Declaration. The patients’ parents gave their signed informed consent for participation in the study. The eligibility of each patient was checked. Cytogenetic abnormalities were defined according to International System for Human Cytogenetic Nomenclature criteria.27 Marrow morphology and cytogenetics were reviewed centrally at diagnosis. The trial was registered at www.clinicaltrial.gov as NCT00149162. The criteria for HSCT were modified by an amendment to the trial in June 2010. The database was censored on June 24, 2014. Median follow-up (FU) was 49 months [28–70].
Biological analyses
Cytogenetic (karyotype and fluorescent in situ hybridization) and molecular analyses were performed on bone marrow samples at diagnosis. In brief, more than 50 recurrent gene rearrangements and KMT2A-partial tandem duplication were screened by reverse transcription polymerase chain reaction amplification assay and 36 genes recurrently mutated in myeloid malignancies were studied by high-throughput sequencing, as published and described previously.28
Treatments
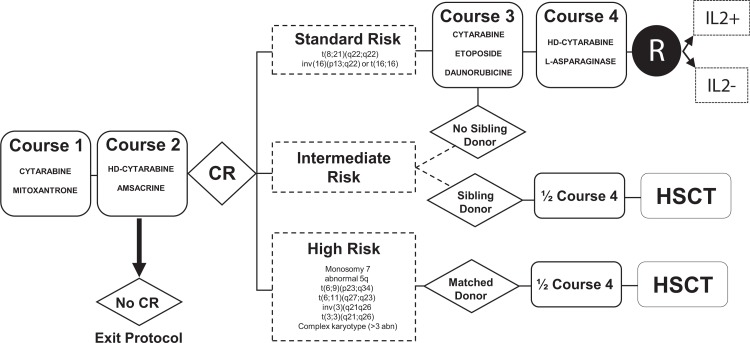
Figure 1 shows the study scheme. Induction therapy (course 1), consisted of 200 mg/m2 per day cytarabine administered by continuous intravenous infusion (IV) for 7 days (days 1–7) and mitoxantrone (12 mg/m2 per day) administered IV over a period of 1 hour, for 5 consecutive days (days 1–5). Bone marrow response was assessed on day 15, and was repeated on day 20 if there were 5% to 25% (M2) or more than 25% (M3) blasts at the first assessment. For patients M3 on day 15 or 20, course 2 was administered on day 21, regardless of blood formula. Course 2 consisted of high-dose (3 g/m2) cytarabine IV for 3 hours, twice daily, for 3 days (days 1 to 3) and amsacrine (100 mg/m2) IV for 1 hour, for 3 days (days 1–3). Patients not eligible for HSCT received 2 additional courses. Course 3 consisted of cytarabine (200 mg/m2 per day) by continuous IV for 4 days (days 1–4), etoposide (100 mg/m2 per day) by IV for 1 hour for 4 days (days 1–4) and daunorubicin (40 mg/m2 per day) administered IV for 2 hours on 4 days (days 1–4). Course 4 combined high-dose (3 g/m2) cytarabine IV for 3 hours twice daily for two 2-day sequences (days 1 and 2 and then days 8 and 9) with l-asparaginase (6000 U/m2 per day) administered by 1-hour IV on days 2 and 9, 6 hours after the end of cytarabine infusion. For patient under the age of 1 year or weighing <10 kg on day 1 of a course of chemotherapy, a dose reduction was applied: 33% for children under 6 months of age and 25% for children aged 6 to 12 months or weighing <10 kg. All patients received intrathecal therapy (IT) on day 1 of course 1. Prophylactic neuromeningeal treatment, including 4 rounds of IT (days 1 and 7 of course 1; days 1 and 4 of course 2), was administered to patients with AML-M4, M4Eo, M5 or white blood count ≥50 × 109/L at diagnosis. In cases of central nervous system involvement, defined as cranial palsy, epiduritis, or cerebrospinal fluid with blasts (regardless of the number of elements) or at least 5 elements per mm3, neuromeningeal treatment included 6 administrations of IT (days 1, 4, and 7 of course 1; days 1, 4, and 7 of course 2; with leucovorin rescue (15 mg/m2 at H24 and H36 of the 1st and 2nd administration of IT). IT doses (methotrexate plus methylprednisolone and cytarabine) are provided in Supplemental Table S1 (Supplemental Digital Content).
Figure 1.
Treatment scheme for the ELAM02 trial. abn = abnormality, CR = complete remission, d = day, HSCT = hematopoietic stem cell transplantation, R = randomization.
Remission
Marrow assessment to determine CR status took place no later than 35 days after course 1 for patients with <5% blasts (M1) on day 15, or on day 28 of course 2 for patients M3 on day 15 or day 20 of course 1. CR was expected to occur by the end of course 2 at the latest. CR was defined as all of the following: <5% blasts in bone marrow, no extramedullary disease, absolute neutrophil count (ANC) ≥1.0 × 109/L, and platelet count ≥ 80 × 109/L. Patients with refractory disease after course 2 left the trial.
Allogeneic hematopoietic stem cell transplantation
HSCT was recommended for all patients in CR after course 2, except those with AML with t(8;21)(q22;q22), provided that an HLA-identical sibling donor was available and there was no active severe infection or organ failure. HSCT with a matched unrelated donor or unrelated cord blood was recommended for patients at high risk according to one of the following criteria: monosomy 7, 5q deletion, t(9;22)(q34;q11) or t(6;9)(p23;q34) and refractory disease after course 2. Risk classification was modified in 2010, as indicated in Supplemental Table S2 (Supplemental Digital Content). These patients are not analyzed here.
IL2 randomization
Patients not undergoing HSCT and still in CR after course 4 were eligible for randomization to receive recombinant IL2 (PROLEUKIN®, aldesleukine, Chiron, Novartis) provided they had no current infection or organ failure. The control group received no maintenance treatment. Centralized blocked-balanced randomization was performed in a 1:1 ratio. Details are provided in the “Results” section.
Statistical analysis
The outcomes for the ELAM02 trial were remission status after courses 1 and 2 of chemotherapy, OS, EFS, and DFS. OS was defined as the time from study entry to death, or last contact for patients without event; EFS was the time from study enrollment to treatment failure, relapse, death, or last contact for patients without event; and DFS was the time from remission to relapse, death, or last contact for patients without event.
For patients randomized for IL2 maintenance therapy, the primary outcome measure was DFS, defined as time from randomization until death or relapse of any type. The secondary outcome measures were regimen total toxicity and biological prognostic factors.
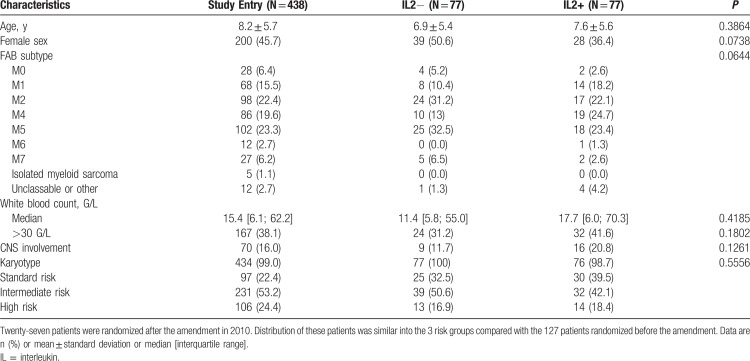
The baseline characteristics of the patients are expressed as frequencies and percentages for qualitative variables and as means and standard deviations or medians and interquartile ranges for continuous variables.
Survival was estimated by Kaplan-Meier methods (Greenwood variance) and compared in log-rank tests. All tests were 2-sided, with P values <0.05 considered significant. Analyses were performed with SAS V.9.3 software (SAS Institute, Cary, NC).
Data were entered through the Webtrial by Quanticsoft portal IGLAM.
Results
Patients
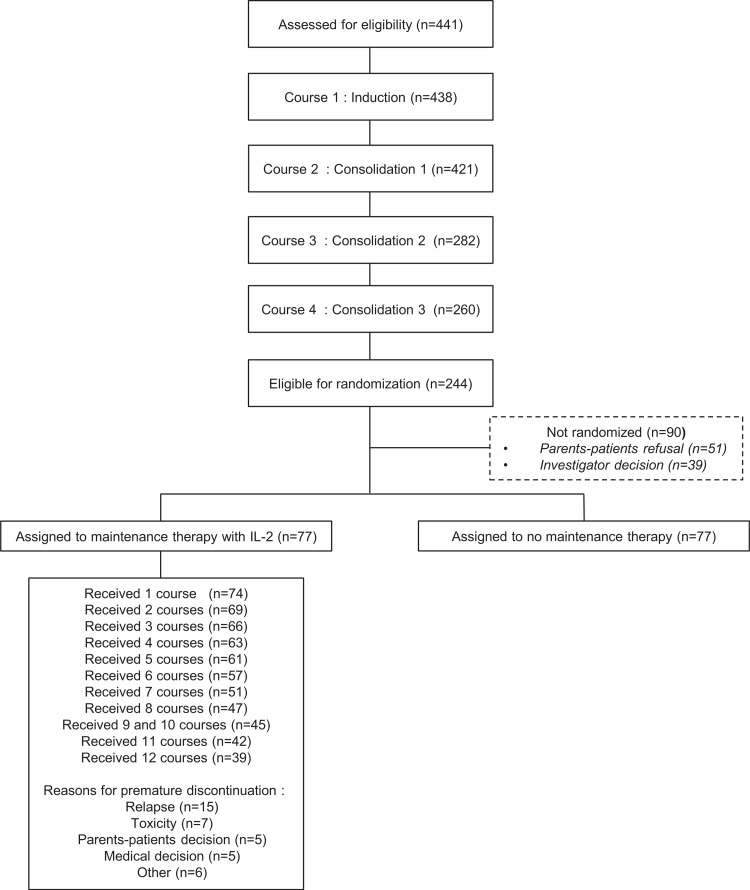
Between March 2005 and December 2011, 441 patients were enrolled in the ELAM02 trial. Three were excluded for not meeting the inclusion criteria. There were thus 438 eligible patients (Fig. 2), and their main characteristics are described in Table 1. At the end of the courses 1 and 2, 391 patients (89%) achieved CR. HSCT was required for 119 patients; 244 (56%) patients not undergoing HSCT and completing all 4 courses of treatment were eligible for IL2 randomization. Ninety (37%) of these patients were not randomized, mostly due to parental refusal (n = 51), or for medical reasons (n = 39). Baseline characteristics of randomized versus nonrandomized patients were similar (Supplemental Table S3, Supplemental Digital Content). In total, 154 patients (63%) were randomized into 2 equal groups of 77 patients (with and without IL2 maintenance therapy). The 4-year EFS of the 438 patients enrolled in the trial was 57% (95% confidence interval [CI]: 52–62), and 4-year OS was 73% (95% CI: 68–77).
Figure 2.
Flowchart.
Table 1.
Baseline Characteristics of the Patients
Recommendations for IL2 administration
Patients randomized to the IL2+ arm were treated by subcutaneous IL2 injection for 5 days (2.5 million U/m2 on day 1; followed by 5 million U/m2 from days 2–5) each month, for 12 cycles. Paracetamol was systematically administered immediately before and 6 hours after IL2 injection. Treatment was administered at specialized centers, during hospitalization for 5 days or day hospitalization if treatment was well tolerated. The following criteria had to be met to start an IL2 treatment cycle: no fever or current infection, platelets >1011/L, ANC >1 × 109/L, normal bilirubin, and creatinine levels. In cases of adverse effects, such as severe (grade ≥ 3) clinical (fever >40°C, hypotension requiring IV fluids) and/or biological (thrombocytopenia [grade ≥ 3]), renal dysfunction (grade ≥ 2), liver dysfunction (grade ≥ 3)) toxicities, the IL2 dose was halved. Treatment was stopped if adverse effects persisted.
Administration and safety of IL2
The mean and median numbers of cycles administered to the 77 patients receiving IL2 were 12 (range: 5–12) and 8.6 ± 4.2, respectively. Treatment was stopped before cycle 6 in 20 patients (26%), and after cycle 6 in 18 patients (23%), due to relapse (15 patients), persistent toxicity (7 patients) or parental or medical decisions (16 patients) (Supplemental Fig. S1, Supplemental Digital Content). Thirty-nine patients (51%) received all 12 planned cycles of IL2 and 57 (74%) received at least 6 cycles. The main toxic effects observed were fever in 72% of patients, chills in 20% and liver test abnormalities in 36% (20% grade 1, 10% grade 2, and 6% grade 3). Neither death from toxicity nor excess serious adverse events related to IL2 treatment were recorded.
Effect of IL2 on outcome
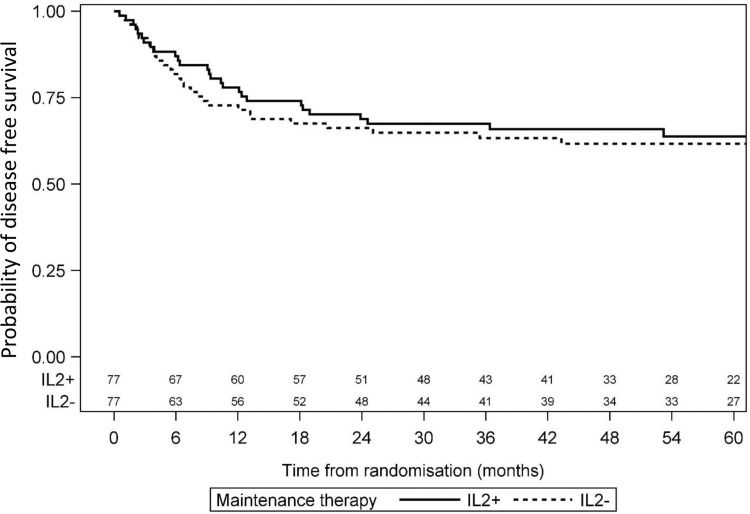
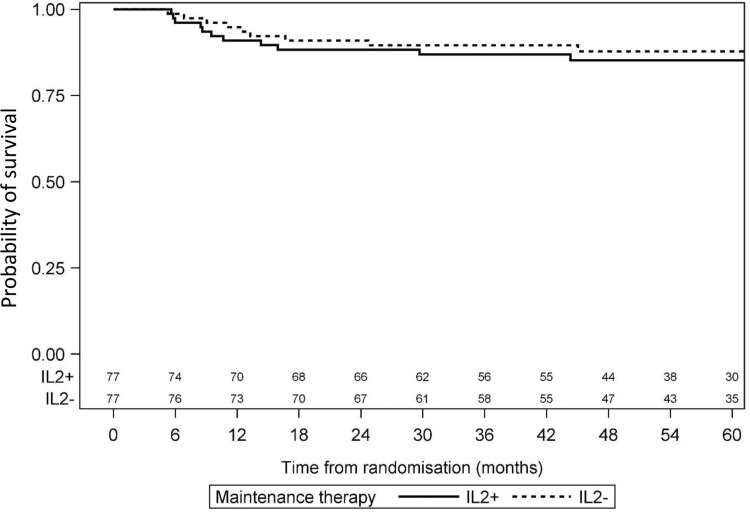
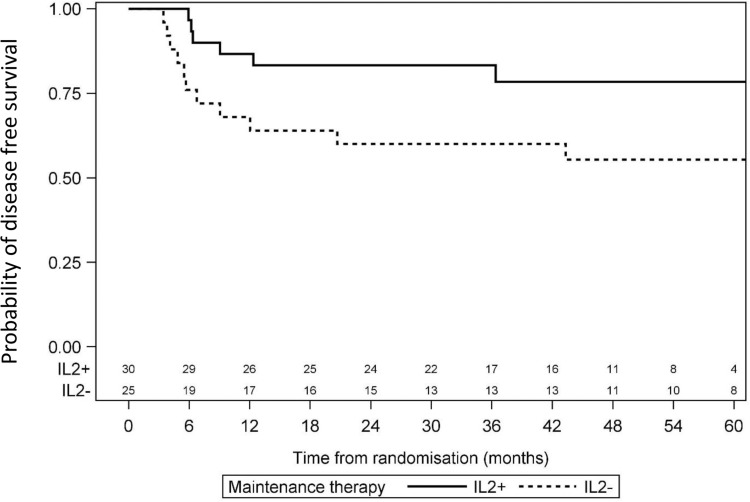
Median FU was 55 months (34–71) for the randomized patients, with 24 months of FU for the last patient randomized. Relapse occurred in 28 patients from the IL2+ group and 29 patients in the IL2− group. Risk of relapse was similar in the 2 groups, with a HR of 0.92 (95% CI: 0.55–1.55). All deaths were relapse-related. Four-year DFS was 62% (95% CI: 51–73) for the IL2− group and 66% (95% CI: 55–77) for the IL2+ group (P = 0.74) (Fig. 3). Four-year OS was 88% (95% CI: 80–95) for the IL2− group and 85% (95% CI: 77–93) for the IL2+ group (P = 0.49; Fig. 4). In the CBF population, 4-year DFS was 55% (95% CI: 36–75) for the IL2− group and 78% (95% CI: 63–94) for the IL2+ group (P = 0.07) (Fig. 5).
Figure 3.
Comparison of disease-free survival between IL2− and IL2+ patients.
Figure 4.
Comparison of overall survival between IL2− and IL2+ patients.
Figure 5.
Comparison of disease-free survival between IL2− and IL2+ patients among CBF AML. CBF AML = core binding factor acute myeloblastic leukemia.
Discussion
The French ELAM02 randomized trial was the second prospective study to investigate whether postremission immunotherapy with IL2 could reduce the risk of relapse in children with AML. The first trial (CCG-2961) was reported by the COG and included 289 patients equally randomized to the 2 groups (with and without IL2) from 1996 to 2002 (Supplemental Table S4, Supplemental Digital Content). IL2 was given as a single short consolidation therapy after CR, in patients not undergoing HSCT, after high-dose cytarabine/asparaginase intensification. IL2 was administered by continuous infusion for 4 days, at a dose of 9 million U/m2 per day. After 4 rest days, IL2 treatment was resumed as a single 10-day cycle of 1.6 million U/m2 per day administered by continuous infusion.29 No differences in DFS or OS were shown.10 In the ELAM02 trial, IL2 was administered as subcutaneous injections on 5 days each month, for 1 year. This IL2 regimen was tolerable and safe, but of no clear benefit in children without high-risk AML who were not candidates for HSCT after intensive chemotherapy. IL2 maintenance therapy did not worsen the prognosis of patients with relapses and, therefore, did not alter leukemic cell sensitivity at relapse.
Several other RCTs of IL2 as a consolidation monotherapy have been conducted in adults.30–32 Five such trials and the CCG-2961 trial were pooled in a meta-analysis published in 2011 (Supplemental Table S4, Supplemental Digital Content), which concluded that IL2 alone was less effective than remission maintenance therapy for AML patients in CR1.33 A recent Cochrane analysis pooled 3 additional RCTs, including the ELAM02 trial, and analyzed 1665 patients in total. This meta-analysis included 6 trials studying DFS in 1426 participants and 5 trials studying OS in 1355 participants. It found no difference in DFS or OS between the IL2 and no-treatment groups. No mortality due to adverse events was reported.34 However, given the heterogeneity of patient populations, IL2 doses and schedules, and trial efficacies, with very different DFS values for the control arms, it remains possible that IL2 maintenance therapy provides some benefit in selected patients.34,35 For instance, in patients with CBF leukemia, there was a trend toward beneficial effects of IL2 treatment, as the 4-year DFS was 55% (95% CI: 36–75) for the IL2− group and 78% (95% CI: 63–94) for the IL2+ group (P = 0.07).
A combination of IL2 and histamine dihydrochloride (HDC/IL2) was shown to improve leukemia-free survival (LFS) as a postconsolidation maintenance treatment in a randomized phase 3 trial in adults with AML (median age, 57 years) from 1998 to 2000. In this trial, 320 patients were equally randomized to the control arm and a treatment arm receiving 10 consecutive 3-week cycles of HDC/IL2, followed by rest for 3 to 6 weeks, for a total of 18 months. The patients in the control arm received no treatment. Three-year LFS was significantly better in the HDC/IL2 group (40%) than in the control group (26%).36 IL2 has generally been investigated as a single agent in most trials, so a randomized study comparing IL2 and HDC/IL2 would be of interest. This issue was addressed in a Bayesian meta-analysis evaluating the extra benefit of adding HDC to IL2 to treat AML. Based on 2 models comparing HDC/IL2 with standard of care (SC) or IL2 alone, HDC/IL2 was found to be more beneficial than either SC or IL2 alone.37 It has recently been shown that the early recovery of lymphocyte levels in blood after allogeneic HSCT is associated with a lower risk of relapse for patients with AML.38 Based on these results, a post hoc analysis was conducted in the HDC/IL2 trial.36 Patients treated with HDC/IL2 and displaying lymphocyte induction between cycles 1 and 3 and between cycles 1 and 4 had an LFS of 57%, whereas patients with no lymphocyte induction had an LFS of 27%.39 Together, these results suggest that IL2 may be beneficial in some patients, particularly if combined with HDC.
One of the main limitations of the ELAM02 trial was the withdrawal of 41% of the eligible patients, mostly due to patient/parental refusal, or medical reasons. This is a typical finding for trials in which randomization occurs late in the course of the disease, particularly if the experimental treatment is compared with an absence of further treatment. Nevertheless, more patients withdrew than expected, and this may have reduced the power of the statistical analysis. By comparison, the rate of withdrawal in the CCG-2961 trial was 25%.10
OS in the previous French LAME 89/91 study was 60%; in the ELAM02 study it was 73%. EFS was similar in the 2 studies, but the inclusion criteria were not entirely comparable, as patients with AML-M7 were excluded from LAME 89/91. This better OS was related to better salvage therapy for patients with relapses or refractory disease. Overall, these results are similar to those obtained by other international pediatric groups.7
Future efforts to improve outcome in children with AML should focus on reducing the risk of relapse. Three parallel approaches would be likely to yield progress. First, treatment optimization should make it possible to limit the disease as much as possible rapidly after induction therapy, through the use of agents targeting a common feature shared by leukemic cells. Surface antigens remain the most common features, so immunotherapy is a promising approach to AML treatment.40 Gemtuzumab ozogamicin was shown to be effective in some trials in adults with AML41–44 and in the randomized pediatric trial COG AAML0531.4 New alternative immunotherapy strategies are also promising. Recent results for acute B-cell leukemia treatment with bispecific T-cell engagers, such as blinatumomab, or T-cell chimeric antigen receptors, should be developed to target AML cells.45–47 If IL2 alone has not been shown to be effective in reducing RR, a trend in favor of IL2 was observed in CBF leukemia. Therefore, IL2 should be tested prospectively in this specific subgroup and/or more interestingly, HDC/IL2 combination. Second, multiple treatment strategies should be developed for AMLs, as single approaches to the treatment of all AMLs are likely to become obsolete. Molecular screening should highlight new subgroups of AMLs and identify potential treatment targets.48 The key issue will be identifying the most relevant treatment target. Third, choosing the right drug is important, but the correct choices of timing and dose are essential to treatment optimization. Minimal residual disease (MRD) monitoring is a promising approach, as the correlation between MRD and outcome has been clearly demonstrated.49–51 Quantifying MRD, after induction therapy, makes it possible to detect poor responders, who are candidates for more intensive or alternative therapies. MRD monitoring should become standard practice for AML, as is already the case for ALL.52
All these developments will require cooperation between international groups working on pediatric AML.
Acknowledgments
The authors thank the SFCE and the principal investigators and their staff at the 28 SFCE centers for all their efforts in conducting this trial. The authors would also like to thank the members of the Data Safety Monitoring Board: François Doz, Marie-Cécile Le Deley, Jean-Pierre Marie, and Emmanuel Raffoux. The authors thank all the CRAs and Dominique Damas the data manager from URC-Est—APHP (Prof. Simon Tabassome). The authors thank Audrey Guilmatre for her comments and manuscript correction. The authors thank the patients and their parents for participating in this study. The authors thank the ARHME and Enfants et Santé associations for financial support.
Supplementary Material
Footnotes
Citation: Petit A, Ducassou S, Leblanc T, Pasquet M, Rousseau A, Ragu C, Cachanado M, Nelken B, Bertrand Y, Michel G, Gandemer V, Cuccuini W, Fenneteau O, Lapillonne H, Auvrignon A, Baruchel A, Leverger G, on behalf of SFCE. Maintenance Therapy With Interleukin-2 for Childhood AML: Results of ELAM02 Phase III Randomized Trial. HemaSphere, 2018;2:6. http://dx.doi.org/10.1097/HS9.0000000000000159
Funding/support: This study was supported by a grant from the French Ministry of Health (PHRC-K 2003 no. 03142). Novartis provided the PROLEUKIN. The sponsor was Assistance Publique-Hôpitaux de Paris (Département de la Recherche Clinique et du Développement, Clinical Research and Development Department). CONECT-AML (COllaborative Network for Children and Teenagers with Acute Myeloblastic Leukemia) is supported by a grant from InCA, Fondation ARC, Ligue nationale contre le cancer, and Association Laurette Fugain (InCa-ARC-LIGUE_11905). Supported by PHRC National 2003, ELAM02 protocol N/REF: AOM03142, P030441.
Disclosure: The authors have indicated they have no potential conflicts of interest to disclose.
Clinical trial information: NCT00149162.
Author contributions: AA, AB, VG, TL, GL, GM, BN, and YB conceived the study; MC and AR performed statistical analysis; AA, MC, GL, AP, AR, and CR analyzed and interpreted data; AP, GL, and CR wrote the manuscript; all authors approved the manuscript. This article was written on behalf of the 28 participating centers of the SFCE and the investigators: Nathalie Aladjidi, Corinne Armari, Claire Berger, Laurence Blanc, Pascale Blouin, Benoit Brethon, Hervé Chambost, Gérard Couillault, Jean-Hugues Dalle, Anne Deville, Catherine Devoldere, Catherine Dollfus, Jean Donadieu, Cécile Dumesnil De Maricourt, Natacha Entz-Werle, Sylvie Fasola, Fanny Fouyssac, Claire Galambrun, Stéphanie Gorde-Grosjean, Stéphanie Haouy, Mathilde Jehanne, Charlotte Jubert, Justina Kanold, Anne Lambilliotte, Anne Lutun, Judith Landman-Parker, Odile Lejars, Patrice Lutz, Aude Marie-Cardine, Françoise Mazingue, Odile Minckes, Frédéric Millot, Claire Oudin, Caroline Oudot, Catherine Paillard, Isabelle Pellier, Yves Perel, Dominique Plantaz, Geneviève Plat, Maryline Poiree, Fanny Rialland, Yves Reguerre, Pierre Rohrlich, Claudine Schmitt, Pascale Schneider, Nicolas Sirvent, Jean-Louis Stephan, Marie-Dominique Tabone, Caroline Thomas, Jean-Pierre Vannier, Karima Yacouben.
References
- 1.Clavel J, Goubin A, Auclerc MF, et al. Incidence of childhood leukaemia and non-Hodgkin's lymphoma in France: National Registry of Childhood Leukaemia and Lymphoma, 1990–1999. Eur J Cancer Prev. 2004;13:97–103. [DOI] [PubMed] [Google Scholar]
- 2.Gibson BE, Webb DK, Howman AJ, et al. Results of a randomized trial in children with acute myeloid leukaemia: medical research council AML12 trial. Br J Haematol. 2011;155:366–376. [DOI] [PubMed] [Google Scholar]
- 3.Creutzig U, Zimmermann M, Bourquin JP, et al. Randomized trial comparing liposomal daunorubicin with Idarubicin as induction for pediatric acute myeloid leukemia: results from study AML-BFM 2004. Blood. 2013;122:37–43. [DOI] [PubMed] [Google Scholar]
- 4.Gamis AS, Alonzo TA, Meshinchi S, et al. Gemtuzumab ozogamicin in children and adolescents with de novo acute myeloid leukemia improves event-free survival by reducing relapse risk: results from the randomized phase III Children's Oncology Group trial AAML0531. J Clin Oncol. 2014;32:3021–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perel Y, Auvrignon A, Leblanc T, et al. Treatment of childhood acute myeloblastic leukemia: dose intensification improves outcome and maintenance therapy is of no benefit—multicenter studies of the French LAME (Leucemie Aigue Myeloblastique Enfant) Cooperative Group. Leukemia. 2005;19:2082–2089. [DOI] [PubMed] [Google Scholar]
- 6.Gibson BE, Wheatley K, Hann IM, et al. Treatment strategy and long-term results in paediatric patients treated in consecutive UK AML trials. Leukemia. 2005;19:2130–2138. [DOI] [PubMed] [Google Scholar]
- 7.Creutzig U, van den Heuvel-Eibrink MM, Gibson B, et al. Diagnosis and management of acute myeloid leukemia in children and adolescents: recommendations from an international expert panel. Blood. 2012;120:3187–3205. [DOI] [PubMed] [Google Scholar]
- 8.Bloomfield CD, Lawrence D, Byrd JC, et al. Frequency of prolonged remission duration after high-dose cytarabine intensification in acute myeloid leukemia varies by cytogenetic subtype. Cancer Res. 1998;58:4173–4179. [PubMed] [Google Scholar]
- 9.Lie SO, Abrahamsson J, Clausen N, et al. Long-term results in children with AML: NOPHO-AML Study Group—report of three consecutive trials. Leukemia. 2005;19:2090–2100. [DOI] [PubMed] [Google Scholar]
- 10.Lange BJ, Smith FO, Feusner J, et al. Outcomes in CCG-2961, a children's oncology group phase 3 trial for untreated pediatric acute myeloid leukemia: a report from the children's oncology group. Blood. 2008;111:1044–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wells RJ, Woods WG, Buckley JD, et al. Treatment of newly diagnosed children and adolescents with acute myeloid leukemia: a Children's Cancer Group study. J Clin Oncol. 1994;12:2367–2377. [DOI] [PubMed] [Google Scholar]
- 12.Perel Y, Auvrignon A, Leblanc T, et al. Impact of addition of maintenance therapy to intensive induction and consolidation chemotherapy for childhood acute myeloblastic leukemia: results of a prospective randomized trial, LAME 89/91. Leucamie Aique Myeloide Enfant. J Clin Oncol. 2002;20:2774–2782. [DOI] [PubMed] [Google Scholar]
- 13.Adler A, Albo V, Blatt J, et al. Interleukin-2 induction of lymphokine-activated killer (LAK) activity in the peripheral blood and bone marrow of acute leukemia patients: II. Feasibility of LAK generation in children with active disease and in remission. Blood. 1989;74:1690–1697. [PubMed] [Google Scholar]
- 14.Lauria F, Raspadori D, Rondelli D, et al. In vitro susceptibility of acute leukemia cells to the cytotoxic activity of allogeneic and autologous lymphokine activated killer (LAK) effectors: correlation with the rate and duration of complete remission and with survival. Leukemia. 1994;8:724–728. [PubMed] [Google Scholar]
- 15.Lowdell MW, Koh MB. Immunotherapy of AML: future directions. J Clin Pathol. 2000;53:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tajima F, Kawatani T, Endo A, et al. Natural killer cell activity and cytokine production as prognostic factors in adult acute leukemia. Leukemia. 1996;10:478–482. [PubMed] [Google Scholar]
- 17.Maraninchi D, Blaise D, Viens P, et al. High-dose recombinant interleukin-2 and acute myeloid leukemias in relapse. Blood. 1991;78:2182–2187. [PubMed] [Google Scholar]
- 18.Meloni G, Foa R, Vignetti M, et al. Interleukin-2 may induce prolonged remissions in advanced acute myelogenous leukemia. Blood. 1994;84:2158–2163. [PubMed] [Google Scholar]
- 19.Becknell B, Caligiuri MA. Interleukin-2, interleukin-15, and their roles in human natural killer cells. Adv Immunol. 2005;86:209–239. [DOI] [PubMed] [Google Scholar]
- 20.Maraninchi D, Vey N, Viens P, et al. A phase II study of interleukin-2 in 49 patients with relapsed or refractory acute leukemia. Leuk Lymphoma. 1998;31:343–349. [DOI] [PubMed] [Google Scholar]
- 21.Cortes JE, Kantarjian HM, O’Brien S, et al. A pilot study of interleukin-2 for adult patients with acute myelogenous leukemia in first complete remission. Cancer. 1999;85:1506–1513. [PubMed] [Google Scholar]
- 22.Willemze R, Suciu S, Mandelli F, et al. Value of low dose IL-2 as maintenance following consolidation treatment or autologous transplantation in acute myelogenous leukemia (AML) patients aged 15–60 years who reached CR after high dose (HD-AraC) vs standard dose (SD-AraC) cytosine arabinoside during induction: results of the AML-12 trial of EORTC and GIMEMA Leukemia Groups [abstract]. Blood. 2009;114:791.19182202 [Google Scholar]
- 23.Willemze R, Suciu S, Meloni G, et al. High-dose cytarabine in induction treatment improves the outcome of adult patients younger than age 46 years with acute myeloid leukemia: results of the EORTC-GIMEMA AML-12 trial. J Clin Oncol. 2014;32:219–228. [DOI] [PubMed] [Google Scholar]
- 24.Bennett JM, Catovsky D, Daniel MT, et al. Proposed revised criteria for the classification of acute myeloid leukemia. A report of the French-American-British Cooperative Group. Ann Intern Med. 1985;103:620–625. [DOI] [PubMed] [Google Scholar]
- 25.Bennett JM, Catovsky D, Daniel MT, et al. The chronic myeloid leukaemias: guidelines for distinguishing chronic granulocytic, atypical chronic myeloid, and chronic myelomonocytic leukaemia. Proposals by the French-American-British Cooperative Leukaemia Group. Br J Haematol. 1994;87:746–754. [DOI] [PubMed] [Google Scholar]
- 26.Bennett JM, Catovsky D, Daniel MT, et al. Proposal for the recognition of minimally differentiated acute myeloid leukaemia (AML-MO). Br J Haematol. 1991;78:325–329. [DOI] [PubMed] [Google Scholar]
- 27.ISCN (International System for Human Cytogenetic Nomenclature). In: Mitelman F, ed. Guidelines for Cancer Cytogenetics. Supplement to an International System for Human Cytogenetic Nomenclature. Basel, Switzerland: Karge; 1991. [Google Scholar]
- 28.Marceau-Renaut A, Duployez N, Ducourneau B, et al. Molecular profiling defines distinct prognostic subgroups in childhood AML: a report from the French ELAM02 Study Group. HemaSphere. 2018;2:e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sievers EL, Lange BJ, Sondel PM, et al. Children's cancer group trials of interleukin-2 therapy to prevent relapse of acute myelogenous leukemia. Cancer J Sci Am. 2000;6 (suppl 1):S39–S44. [PubMed] [Google Scholar]
- 30.Pautas C, Merabet F, Thomas X, et al. Randomized study of intensified anthracycline doses for induction and recombinant interleukin-2 for maintenance in patients with acute myeloid leukemia age 50 to 70 years: results of the ALFA-9801 study. J Clin Oncol. 2010;28:808–814. [DOI] [PubMed] [Google Scholar]
- 31.Blaise D, Attal M, Reiffers J, et al. Randomized study of recombinant interleukin-2 after autologous bone marrow transplantation for acute leukemia in first complete remission. Eur Cytokine Netw. 2000;11:91–98. [PubMed] [Google Scholar]
- 32.Baer MR, George SL, Caligiuri MA, et al. Low-dose interleukin-2 immunotherapy does not improve outcome of patients age 60 years and older with acute myeloid leukemia in first complete remission: Cancer and Leukemia Group B Study 9720. J Clin Oncol. 2008;26:4934–4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buyse M, Squifflet P, Lange BJ, et al. Individual patient data meta-analysis of randomized trials evaluating IL-2 monotherapy as remission maintenance therapy in acute myeloid leukemia. Blood. 2011;117:7007–7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mao C, Fu XH, Yuan JQ, et al. Interleukin-2 as maintenance therapy for children and adults with acute myeloid leukaemia in first complete remission. Cochrane Database Syst Rev. 2015;11:CD010248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grosso D, Weiss MA. Posttreatment interleukin-2 in patients with acute myeloid leukemia: the end of a long road for patients and clinical trials? Cancer. 2014;120:940–941. [DOI] [PubMed] [Google Scholar]
- 36.Brune M, Castaigne S, Catalano J, et al. Improved leukemia-free survival after postconsolidation immunotherapy with histamine dihydrochloride and interleukin-2 in acute myeloid leukemia: results of a randomized phase 3 trial. Blood. 2006;108:88–96. [DOI] [PubMed] [Google Scholar]
- 37.Berry SM, Broglio KR, Berry DA. Addressing the incremental benefit of histamine dihydrochloride when added to interleukin-2 in treating acute myeloid leukemia: a Bayesian meta-analysis. Cancer Invest. 2011;29:293–299. [DOI] [PubMed] [Google Scholar]
- 38.Michelis FV, Messner HA, Loach D, et al. Early lymphocyte recovery at 28 d post-transplant is predictive of reduced risk of relapse in patients with acute myeloid leukemia transplanted with peripheral blood stem cell grafts. Eur J Haematol. 2014;93:273–280. [DOI] [PubMed] [Google Scholar]
- 39.Hallner A, Aurelius J, Thoren FB, et al. Immunotherapy with histamine dihydrochloride and low-dose interleukin-2 favors sustained lymphocyte recovery in acute myeloid leukemia. Eur J Haematol. 2015;94:279–280. [DOI] [PubMed] [Google Scholar]
- 40.Grosso DA, Hess RC, Weiss MA. Immunotherapy in acute myeloid leukemia. Cancer. 2015;121:2689–2704. [DOI] [PubMed] [Google Scholar]
- 41.Buckley SA, Walter RB. Antigen-specific immunotherapies for acute myeloid leukemia. Hematol Am Soc Hematol Educ Program. 2015;2015:584–595. [DOI] [PubMed] [Google Scholar]
- 42.Burnett AK, Hills RK, Milligan D, et al. Identification of patients with acute myeloblastic leukemia who benefit from the addition of gemtuzumab ozogamicin: results of the MRC AML15 trial. J Clin Oncol. 2011;29:369–377. [DOI] [PubMed] [Google Scholar]
- 43.Burnett AK, Russell NH, Hills RK, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy improves survival in older patients with acute myeloid leukemia. J Clin Oncol. 2012;30:3924–3931. [DOI] [PubMed] [Google Scholar]
- 44.Castaigne S, Pautas C, Terre C, et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet. 2012;379:1508–1516. [DOI] [PubMed] [Google Scholar]
- 45.Schlegel P, Lang P, Zugmaier G, et al. Pediatric posttransplant relapsed/refractory B-precursor acute lymphoblastic leukemia shows durable remission by therapy with the T-cell engaging bispecific antibody blinatumomab. Haematologica. 2014;99:1212–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Annesley CE, Brown P. Novel agents for the treatment of childhood acute leukemia. Ther Adv Hematol. 2015;6:61–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kolb EA, Meshinchi S. Acute myeloid leukemia in children and adolescents: identification of new molecular targets brings promise of new therapies. Hematol Am Soc Hematol Educ Program. 2015;2015:507–513. [DOI] [PubMed] [Google Scholar]
- 49.Loken MR, Alonzo TA, Pardo L, et al. Residual disease detected by multidimensional flow cytometry signifies high relapse risk in patients with de novo acute myeloid leukemia: a report from Children's Oncology Group. Blood. 2012;120:1581–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rubnitz JE, Inaba H, Dahl G, et al. Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: results of the AML02 multicentre trial. Lancet Oncol. 2010;11:543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ivey A, Hills RK, Simpson MA, et al. Assessment of minimal residual disease in standard-risk AML. N Engl J Med. 2016;374:422–433. [DOI] [PubMed] [Google Scholar]
- 52.Grimwade D, Freeman SD. Defining minimal residual disease in acute myeloid leukemia: which platforms are ready for “prime time”? Hematol Am Soc Hematol Educ Program. 2014;2014:222–233. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.