Profound immune dysregulation is an increasingly recognized feature of myelodysplastic syndromes (MDS), contributing to ineffective hematopoiesis and driving disease progression. Immune dysregulation in MDS is highly complex and composed of many interdependent factors, including clonal hematopoietic cells with somatic mutations providing faulty signals to the immune system and altered cells of the bone marrow microenvironment contributing to inflammation and immunosuppression. Cellular components of disturbed immune regulation include T and natural killer cells, myeloid-derived suppressor cells as well as MSC. In this perspective, we highlight the role of the various players contributing to immune dysregulation in MDS and discuss novel therapeutic approaches currently being designed to improve treatment options.
MDS: A heterogeneous disease with distinct elements of immune dysregulation
MDS are clonal hematopoietic stem/progenitor cell (HSPC) disorders characterized by ineffective hematopoiesis, peripheral cytopenias, and a risk for transformation to acute myeloid leukemia (AML). Distinct acquired epigenetic and genetic mutations have been detected in MDS HSPC and are considered disease-initiating events.1 Recent work has also highlighted the role of the immune system as well as of the bone marrow mesenchymal stromal cells (MSC) compartment (ie, stem cell niche) in the development and progression of MDS.2,3 The interplay between clonal hematopoietic cells, cells of immune system, and the specific bone marrow microenvironment is central to how MDS manifests itself.
Dysregulation of the immune system has long been considered a defining feature of MDS. Autoimmune disorders can be commonly observed in MDS patients with varying frequency reported between 10% and 30%.4–6 Clinical presentation is quite diverse and encompasses disorders such as vasculitis (often presenting as Sweet syndrome), seronegative polyarthritis, neutrophilic dermatosis polychondritis, systemic lupus erythematodes (SLE), and Sjogren syndrome.6,7 Rarer manifestations include glomerulonephritis and polyneuropathy. Epidemiological studies have linked autoimmune disorders and MDS, and certain autoimmune diseases have a higher risk for development of MDS, most notably rheumatoid arthritis and SLE but also immune thrombocytopenia, autoimmune hemolytic anemia, myasthenia gravis, and giant cell arteritis.8,9 Furthermore, patients receiving azathioprine therapy for autoimmune disease (most commonly SLE) have a 7-fold higher risk of developing MDS.10
The immune system in MDS shows an inflammatory response (Fig. 1) with an increased release of inflammatory cytokines and immune mediators such as tumor-necrosis factor alpha (TNF-alpha), interferon-gamma (IFN-gamma), transforming-growth factor-ß, indoleamine-2,3-dioxygenase (IDO), nitric oxide (NO), and various interleukins such as interleukin-6 (IL-6) and interleukin-10 (IL-10).11 Cytokines are expressed by clonal hematopoietic cells as well as by MSC in the bone marrow microenvironment. In addition, activated T cells locally secrete IFN-gamma, which stimulates MSC and contributes to the inflammatory phenotype. Immune cell function is also impaired in MDS. Levels of regulatory T cells (T-regs) in the peripheral blood are decreased in low-risk MDS and counts of cytotoxic CD8+ T cells as well as natural killer (NK) cells are higher compared to healthy age-matched controls. By contrast, expansion of T-regs, especially memory T-regs, can be observed in higher-risk MDS, indicating an increasingly immunosuppressive state in advanced disease.12,13 MDS patients have increased numbers of myeloid-derived suppressor cells (MDSC), which are nonclonal immunosuppressive effector cells that also mediate a pro-inflammatory response.14,15 The main feature of MDSC is a potent suppression of T cell function.16 MDSC become activated by toll-like receptor (TLR) signaling as part of innate immune activation, specifically by calcium-binding proteins S100A9 and S100A8, which are TLR4 and CD33 ligands.14 TLRs have been found to be upregulated in the bone marrow of MDS patients and expression can be correlated with increased apoptosis in low-risk MDS.17 Recently, it has additionally been shown that S100A9 and reactive oxygen species produced by MDSC as well as clonal HSPC themselves activate the NLRP3 pattern recognition receptor, leading to inflammasome assembly and activation of inflammatory cell death.18 Induction of S100A9/S100A8 also leads to a p53-dependent differentiation defect in erythroblasts in MDS with (del5q).19
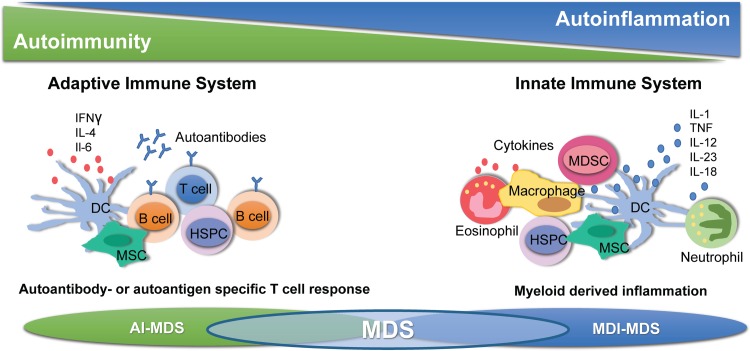
Figure 1.
Immune dysregulation in MDS. MDS is characterized by dysregulation of the adaptive as well as the innate immune system. Autoimmune mechanisms (AI-MDS, left panel) and myeloid-derived inflammation (MDI-MDS, right panel) contribute to specific phenotypes in low- and high-risk MDS, which can be targeted by immune modulatory therapies. DC = dendritic cell, HSPC = hematopoietic stem and progenitor cell, MDI = myelodysplastic syndrome, MDSC = myeloid-derived suppressor cell, MSC = mesenchymal stromal cell.
Finally, MSC as crucial components of the stem cell niche in the bone marrow are increasingly being recognized as important players in MDS pathogenesis and progression (Fig. 1). MSC are critical for regulating self-renewal, survival, and differentiation of HSPC.20 MSC communicate with HSPC directly by cell-cell contact and indirectly through secreted factors and production of extracellular matrix. In addition, MSC replenish osteoblasts as well as adipocytes in the bone marrow niche and have diverse immunoregulatory functions. In MDS, MSC and clonal HSPC exist in a close and codependent relationship. MDS MSC are essential for propagation of human MDS HSPC in vivo in xenograft models and cross-talk between MDS MSC and clonal HSPC has been shown to reinforce clonal dominance of MDS cells in the niche.21 MDS MSC are phenotypically and functionally altered and display a prominent inflammatory program, which contributes to negative regulation of normal hematopoiesis.2,22–24 MSC have profound immunosuppressive properties, which are mediated in part by production of IDO, which suppresses T cell proliferation and activation.25 While healthy MSC exhibit immunosuppressive effects and are thus used to treat graft-versus-host disease after allogeneic transplant, there is some evidence that MSC-mediated immunosuppression is decreased in low-risk MDS and may actually increase with disease progression to higher-risk MDS and secondary AML.26
In sum, the bulk of accumulated data show that the immune system is profoundly dysregulated in MDS. Activation of the innate immune system is considered a hallmark of MDS, and early stages of the disease are characterized by immune activation and inflammation. It is still unknown whether this is a cause or a consequence of (genetic) alterations within the HSPC pool. Progression to higher risk MDS and transformation to secondary AML is accompanied by dysregulation of the adaptive immune system and is defined by increased immunosuppression and progressive immune evasion mechanisms allowing unchecked proliferation of myeloid blasts.
Rationale for immune modulatory therapies in MDS
Immune modulatory therapies have long been employed for MDS, most commonly in the form of immunosuppressive therapy (IST) for (hypoplastic) low-risk MDS. More recently, the availability of checkpoint modulating agents and other T cell therapies and their clinical success in solid tumors have generated interest in these agents as a novel therapeutic approach for higher-risk MDS and secondary AML. The rationale for immune modulation as a treatment for MDS is based on 2 premises. First, in low-risk MDS the observed cytopenias are in part a result of immune activation and thus may be amenable to IST. Second, progression to higher-risk MDS or secondary AML is associated with immune evasion mechanisms enabling expansion of the leukemic clone, particularly myeloid blasts, which may be amenable to T cell therapies. Based on these 2 premises, immunosuppressive agents are primarily employed in low-risk MDS whereas more recently, immune modulatory drugs, including checkpoint modulators and other T cell activating therapies, have entered the scene as a novel approach to treat high-risk MDS and secondary AML.
Strategies for low-risk MDS
Immunosuppressive agents
IST with antithymocyte globulin (ATG, either horse or rabbit), with or without addition of cyclosporine (CSA), has been evaluated for treatment of low-risk MDS in a number of phase II clinical trials with small numbers of patients and response rates ranging from 16% to 67%.27 Various predictors of response have been described in these trials, most notably MDS-SLD (formerly refractory anemia, RA) with absence of ring sideroblasts, a hypoplastic bone marrow, DR15 HLA type, younger age (<60 years), female sex, trisomy 8, and short duration of transfusion dependence.28 Presence of a coexisting PNH clone had no influence on response to IST. However, a recent large retrospective analysis of 367 MDS patients treated with IST failed to confirm the predictive value of these previously described biomarkers of response.29 Interestingly, while the overall response rate to IST was 45%, the presence of SF3B1 mutations negatively affected response, which correlates with previous observations noting a decreased response in MDS with ring sideroblasts (MDS-RS, formerly RARS). To date, there is only 1 prospective randomized trial comparing ATG + CSA to best supportive care performed by the HOVON-SAKK cooperative group.30 In this trial of 74 low-risk transfusion-dependent MDS patients, hematologic response (CR, PR, and HI) at 6 months was 31% compared to 9% with a response duration of 16.4 months in the ATG + CSA arm. Response rates were highest for patients with a hypocellular bone marrow. However, transformation-free survival and overall survival did not differ between patients receiving ATG + CSA or best supportive care. Currently, ATG is still routinely used in the clinic in rare cases with hypoplastic MDS and a normal karyotype.
Very high response rates of 72% were also reported using the anti-CD52 monoclonal antibody alemtuzumab in a cohort of low-risk MDS patients with a high likelihood of response to IST based on HLA-DR15 expression, age, and duration of transfusion dependence.31 Interestingly, even cytogenetic responses including in patients with monosomy 7 have been observed. As CD52 is expressed on all lymphocyte subsets, with a higher density on T cells than on B cells, alemtuzumab induces widespread T cell depletion. However, due to licensing issues the drug is no longer available for the treatment of hematologic diseases.
Lenalidomide
Lenalidomide, an immune modulatory drug (ImiD), has a high rate of activity in low-risk MDS with del(5q), the most prevalent cytogenetic abnormality, with achievement of transfusion independence in 56% to 67% and cytogenetic complete remissions in 45%.32,33 Lenalidomide is thus considered the treatment of choice for transfusion-dependent low-risk MDS with del(5q), although in some countries first-line therapy with erythropoietin is also used in these patients.34 A recently completed randomized double-blind phase III European trial is investigating the efficacy of lenalidomide versus placebo in MDS del(5q) patients with a hemoglobin value <12 g/dL who have not yet become transfusion-dependent (SINTRA-Rev trial, EudraCT 2009-013619-36). The trial is designed to determine if lenalidomide can extend the period of transfusion independency of MDS del(5q) patients.
As an immunomodulatory agent with pleiotropic effects on the T cell repertoire as well as on the bone marrow microenvironment,35–37 lenalidomide has also been investigated in low-risk MDS without del(5q) with response rates around 25% and in higher-risk MDS and AML with del(5q). The results of these trials suggest that lenalidomide could be effective in a subset of patients with low-risk MDS without del(5q), but so far it has not been possible to identify prognostic markers that could be associated with response.38 Lenalidomide improves the function of MDS-derived MSC to support HSPC in in vitro culture models and thus may contribute to improved hematopoiesis by also affecting the stem cell niche.
Suppression of innate immune activation
Given the activation of the innate immune system with sustained inflammation in the bone marrow microenvironment mediated by MDSC and through TLR signaling, some clinical trials have been initiated to target these components. Because MDSC express high levels of CD33, there has been some interest in employing anti-CD33 directed therapies to deplete MDSC numbers in the bone marrow. Both a monoclonal antibody against CD33 (BI 836858) as well as a novel CD3/CD33 bispecific tetravalent antibody that recognizes both CD33 and CD3 (AMLV564) have been evaluated in preclinical models.39 The anti-CD33 antibody was shown to prevent CD33-mediated cytokine secretion in the bone marrow, correlating with a significant increase in hematopoietic colony formation in vitro as well as directly reducing MDSC through antibody-dependent cellular toxicity.40 In vitro treatment of bone marrow mononuclear cells from MDS patients with the bispecific antibody BI 836858 eliminated CD33+ MDSC and led to expansion of CD4+ and CD8+ T cells as well as improved hematopoietic colony formation in vitro.41 Both antibodies have now entered Phase I/II trials (NCT02240706 and NCT03516591). Interestingly, these preclinical studies also provided some first evidence that targeting CD33 may increase sensitivity to checkpoint inhibitors, thus augmenting immune response against the MDS clone.41
Targeting TLR signaling pathways has also been explored as means to modulate innate immune activation, for instance, by blocking TLR2 receptor. In vitro inhibition of TLR2 in cultured BM CD34 cells from patients with lower-risk MDS resulted in increased formation of erythroid colonies.42 However, for higher-risk MDS and secondary AML, more recent data suggest that targeting the innate immune system alone will be insufficient, as compensatory immune escape pathways become activated as the disease progresses, similar to what has been shown for solid tumors.43 This insight has prompted the concept of combining targets of innate immunity with checkpoint modulators.
Strategies for high-risk MDS
Countering immune evasion mechanisms
As outlined above, evasion of adaptive immune response is a feature of advanced MDS. Accordingly, recent efforts have focused on reactivating T cell responses through use of checkpoint modulators or novel T cell therapies.
Programmed cell death-1 (PD-1) is a negative regulatory receptor expressed on the surface of activated T cells as well as B cells and NK cells. It binds to PD-L1, which is a negative costimulatory ligand expressed on malignant cells, including MDS stem cells and leukemic blasts.43 IFN-gamma, produced by T cells as well as the bone marrow microenvironment, induces up-regulation of PD-L1 in leukemia cells. In a mouse model of AML, inhibition of the PD-1/PD-L1 pathway led to a significant reduction of AML burden in vivo and a prolonged murine survival, providing a preclinical rationale for use of checkpoint modulators in myeloid leukemia.39 Clinical trials of checkpoint modulation using PD-1 as well as PD-L1 inhibitors are currently underway in MDS and AML. The cytotoxic T-lymphocyte associated antigen 4 expressed on T cells is also part of an inhibitory checkpoint pathway and can be targeted by the monoclonal antibody ipilimumab to reverse T cell inhibition. So far, PD-1/PD-L1 inhibitors as well as CTLA4 inhibitors seem to have limited efficacy as single agents in advanced disease. In a recently reported Phase I trial of ipilimumab in MDS patients failing treatment with hypomethylating agents (HMA), responses consisted mainly of disease stabilization.44 By contrast, a second trial of single-agent ipilimumab in HMA failure patients reported an overall response rate of 30% and accrual is currently continuing.45 The Keynote-013 Phase Ib trial (NCT01953692) evaluated the PD-1 inhibitor pembrolizumab for patients with various advanced hematologic malignancies, including 28 MDS patients after treatment with HMA. The overall response rate was 4%.46 First-line pembrolizumab therapy, however, was shown to induce a clinical and molecular response in a patient with secondary AML receiving the drug for melanoma.47 Similar to pembrolizumab, no responses were seen in advanced MDS patients treated with the PD-1 inhibitor nivolumab after HMA failure.45 Interestingly, in MDS and secondary AML, PD-L1 expression levels are higher in hematopoietic cells than in those of healthy volunteers and resistance to the HMA azacitidine and decitabine is additionally associated with up-regulation of PD-1 and PD-L1 on leukemic cells, representing an immune evasion mechanism against HMA.43 However, this may be exploited therapeutically, as blockade of PD-L1 in combination with HMA therapy may actually increase the antitumor response. This combinatory approach is being tested in several ongoing clinical trials for higher-risk MDS and AML as a first-line therapy (pembrolizumab + azacitidine, NCT03094637). Nivolumab + azacitidine has shown impressive overall response rates of 80% in a small exploratory Phase II trial.45 Anti-PDL-1 antibodies, such as durvalumab and atezolizumab, are also being evaluated as first-line therapies in combination with HMA (NCT02775903 and NCT02508870), as is the combination of CTLA4 and PD-1 blockade with ipilimumab and nivolumab (NCT02530463).
Bispecific T cell engager antibody therapies
Finally, bispecific antibodies aimed at redirecting T cell killing by engagement of a specific target on the cancer cell are also being explored as novel therapeutic agents in MDS. Flotetuzumab is a CD3 × CD123 bispecific T cell engager antibody that has shown promising results in a recently presented Phase I trial of relapsed or refractory MDS and AML patients with a complete remission rate of 26% and an overall response rate of 42%.48 CD123 is expressed at high levels on leukemic stem cells and is differentially overexpressed in 93% of AML and 50% of MDS patients and previous work has shown a correlation between CD123+ cell frequency and prognosis.44,49 However, the anti-CD123 monoclonal antibody talacotuzumab recently failed to show any meaningful activity in advanced MDS and the trial was halted due to excess toxicity (NCT03011034). Thus, the early flotetuzumab results attest to the potency of harnessing a specific T cell response in myeloid leukemia. Interestingly and similar to observations with HMA, PD-L1 expression on leukemic blasts increased in patients no longer responding to flotetuzumab, suggesting the combination of flotetuzumab and checkpoint modulators such as PD-1/PD-L1 inhibitors may be beneficial. This may also be true for bispecific antibodies targeting CD3 and CD33, a molecule highly expressed on myeloid blasts in AML and MDS.50–52 (AMG330, NCT02520427; GEM33, EudraCT 2017-001707-77). Lysis of AML cells by T cells through engagement of AMG330 in vitro was shown to be augmented by inhibition of PD-L1.53 To this end, novel bifunctional checkpoint inhibitory T cell engaging antibodies combining T cell redirection to CD33 with locally restricted checkpoint blockade are also being developed.54 As these results are preclinical and bispecific antibodies are only yet in Phase I trials, whether combining bispecific antibodies with PD-1/PD-L1 inhibitors is feasible in terms of toxicity and efficacious in terms of clinical responses remains to be seen.
Summary
Immune modulatory approaches show high promise in the treatment of MDS. Given the heterogeneity of the disease, both in terms of risk stratification as well as highly variable genetic traits and the type of immune dysregulation present, it will be of utmost importance to correctly identify which patients will most likely benefit from which approach. For a small group of carefully selected low-risk MDS patients, IST with ATG shows high response rates with durable remissions and an acceptable toxicity profile. However, more recent efforts are focused on targeting the inflammatory phenotype (in particular the expanding MDSC compartment) in low-risk MDS induced through modulation of the innate immune system by targeting TLR signaling or MDSC directly through inhibition of CD33. By contrast, higher-risk MDS and AML is characterized by an immunosuppressive microenvironment and increased immune escape mechanisms allowing unchecked proliferation of immature progenitor cells. Thus, T cell directed therapies such as checkpoint modulation in combination with HMA or bispecific T cell engager antibody therapies to reverse immunosuppression and activate T cell responses are novel treatment options currently being investigated for this group of patients. The ideal target for bispecific T cell engager antibodies in myeloid disease has yet to be determined, but CD33 as well as CD123 seem to hold promise in patients with AML as well as MDS with high-risk features such as elevated blast counts.
As most of the clinical trials are still in their early stages, experience with these approaches for MDS is currently limited. Thus, critical issues such as optimal combination, dosing and scheduling of agents as well as identification of patient populations most likely to benefit from immune modulatory therapies remain to be answered by the currently ongoing clinical trials.
Acknowledgments
KSG received support from the Deutsche Forschungsgemeinschaft (SFB 1243) and the Deutsche Jose Carerras Leukämiestiftung (DJCLS 14 R/2018). UP received support from Deutsche Jose Carerras Leukämiestiftung and Boll-Stiftung. We would like to thank Silke Gloaguen and Anne Kubasch for editorial assistance.
Footnotes
Citation: Götze KS, Platzbecker U. Old Dogs, New Tricks: Revisiting Immune Modulatory Approaches for Myelodysplastic Syndromes. HemaSphere, 2018;2:6. http://dx.doi.org/10.1097/HS9.0000000000000162
Funding/support: Uwe Platzbecker received support from Deutsche Jose Carerras Leukämiestiftung and Boll-Stiftung.
Disclosure: Both authors have received honoraria and research support from Celgene, Amgen, Novartis, and Janssen.
References
- 1.Sperling AS, Gibson CJ, Ebert BL. The genetics of myelodysplastic syndrome: from clonal haematopoiesis to secondary leukaemia. Nat Rev Cancer 2017; 17:5–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bulycheva E, Rauner M, Medyouf H, et al. Myelodysplasia is in the niche: novel concepts and emerging therapies. Leukemia 2015; 29:259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Medyouf H. The microenvironment in human myeloid malignancies: emerging concepts and therapeutic implications. Blood 2017; 129:1617–1626. [DOI] [PubMed] [Google Scholar]
- 4.Okamoto T, Okada M, Mori A, et al. Correlation between immunological abnormalities and prognosis in myelodysplastic syndrome patients. Int J Hematol 1997; 66:345–351. [DOI] [PubMed] [Google Scholar]
- 5.Wilson AB, Neogi T, Prout M, et al. Relative risk of myelodysplastic syndromes in patients with autoimmune disorders in the General Practice Research Database. Cancer Epidemiol 2014; 38:544–549. [DOI] [PubMed] [Google Scholar]
- 6.Komrokji RS, Kulasekararaj A, Ali Al NH, et al. Autoimmune diseases and myelodysplastic syndromes. Am J Hematol 2016; 91:E280–E283. [DOI] [PubMed] [Google Scholar]
- 7.Mekinian A, Grignano E, Braun T, et al. Systemic inflammatory and autoimmune manifestations associated with myelodysplastic syndromes and chronic myelomonocytic leukaemia: a French multicentre retrospective study. Rheumatology (Oxford) 2016; 55:291–300. [DOI] [PubMed] [Google Scholar]
- 8.Anderson LA, Pfeiffer RM, Landgren O, et al. Risks of myeloid malignancies in patients with autoimmune conditions. Br J Cancer 2009; 100:822–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kristinsson SY, Björkholm M, Hultcrantz M, et al. Chronic immune stimulation might act as a trigger for the development of acute myeloid leukemia or myelodysplastic syndromes. J Clin Oncol 2011; 29:2897–2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ertz-Archambault N, Kosiorek H, Taylor GE, et al. Association of therapy for autoimmune disease with myelodysplastic syndromes and acute myeloid leukemia. JAMA Oncol 2017; 3:936–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gañán-Gómez I, Wei Y, Starczynowski DT, et al. Deregulation of innate immune and inflammatory signaling in myelodysplastic syndromes. Leukemia 2015; 29:1458–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mailloux AW, Sugimori C, Komrokji RS, et al. Expansion of effector memory regulatory T cells represents a novel prognostic factor in lower risk myelodysplastic syndrome. J Immunol 2012; 189:3198–3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kordasti SY, Ingram W, Hayden J, et al. CD4+CD25high Foxp3+ regulatory T cells in myelodysplastic syndrome (MDS). Blood 2007; 110:847–850. [DOI] [PubMed] [Google Scholar]
- 14.Chen X, Eksioglu EA, Zhou J, et al. Induction of myelodysplasia by myeloid-derived suppressor cells. J Clin Invest 2013; 123:4595–4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gabrilovich DI. Myeloid-derived suppressor cells. Cancer Immunol Res 2017; 5:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009; 9:162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maratheftis CI, Andreakos E, Moutsopoulos HM, et al. Toll-like receptor-4 is up-regulated in hematopoietic progenitor cells and contributes to increased apoptosis in myelodysplastic syndromes. Clin Cancer Res 2007; 13:1154–1160. [DOI] [PubMed] [Google Scholar]
- 18.Basiorka AA, McGraw KL, Eksioglu EA, et al. The NLRP3 inflammasome functions as a driver of the myelodysplastic syndrome phenotype. Blood 2016; 128:2960–2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneider RK, Schenone M, Ferreira MV, et al. Rps14 haploinsufficiency causes a block in erythroid differentiation mediated by S100A8 and S100A9. Nat Med 2016; 22:288–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bianco P, Cao X, Frenette PS, et al. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med 2013; 19:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medyouf H, Mossner M, Jann J-C, et al. Myelodysplastic cells in patients reprogram mesenchymal stromal cells to establish a transplantable stem cell niche disease unit. Cell Stem Cell 2014; 14:824–837. [DOI] [PubMed] [Google Scholar]
- 22.Geyh S, Öz S, Cadeddu RP, et al. Insufficient stromal support in MDS results from molecular and functional deficits of mesenchymal stromal cells. Leukemia 2013; 27:1841–1851. [DOI] [PubMed] [Google Scholar]
- 23.Ferrer RA, Wobus M, List C, et al. Mesenchymal stromal cells from patients with myelodysplastic syndrome display distinct functional alterations that are modulated by lenalidomide. Haematologica 2013; 98:1677–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen S, Zambetti NA, Bindels EMJ, et al. Massive parallel RNA sequencing of highly purified mesenchymal elements in low-risk MDS reveals tissue-context-dependent activation of inflammatory programs. Leukemia 2016; 30:1938–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Miguel MP, Fuentes-Julián S, Blázquez-Martínez A, et al. Immunosuppressive properties of mesenchymal stem cells: advances and applications. Curr Mol Med 2012; 12:574–591. [DOI] [PubMed] [Google Scholar]
- 26.Zhao W, Phinney DG, Bonnet D, et al. Mesenchymal stem cell biodistribution, migration, and homing in vivo. Stem Cells Int 2014; 2014:292109–292112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parikh AR, Olnes MJ, Barrett AJ. Immunomodulatory treatment of myelodysplastic syndromes: antithymocyte globulin, cyclosporine, and alemtuzumab. Semin Hematol 2012; 49:304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saunthararajah Y, Nakamura R, Wesley R, et al. A simple method to predict response to immunosuppressive therapy in patients with myelodysplastic syndrome. Blood 2003; 102:3025–3027. [DOI] [PubMed] [Google Scholar]
- 29.Stahl M, Deveaux M, de Witte TMM, et al. The use of immunosuppressive therapy (IST) in patients with the myelodysplastic syndromes (MDS): clinical outcomes and their predictors in a large international patient cohort. Blood 2017; 130 (suppl 1):422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Passweg JR, Giagounidis AAN, Simcock M, et al. Immunosuppressive therapy for patients with myelodysplastic syndrome: a prospective randomized multicenter phase III trial comparing antithymocyte globulin plus cyclosporine with best supportive care—SAKK 33/99. J Clin Oncol 2011; 29:303–309. [DOI] [PubMed] [Google Scholar]
- 31.Sloand EM, Olnes MJ, Shenoy A, et al. Alemtuzumab treatment of intermediate-1 myelodysplasia patients is associated with sustained improvement in blood counts and cytogenetic remissions. J Clin Oncol 2010; 28:5166–5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.List A, Dewald G, Bennett J, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med 2006; 355:1456–1465. [DOI] [PubMed] [Google Scholar]
- 33.Fenaux P, Giagounidis A, Selleslag D, et al. A randomized phase 3 study of lenalidomide versus placebo in RBC transfusion-dependent patients with low-/intermediate-1-risk myelodysplastic syndromes with del5q. Blood 2011; 118:3765–3776. [DOI] [PubMed] [Google Scholar]
- 34.Giagounidis A, Adès L. Lenalidomide for the treatment of MDS. Myelodysplast Syndromes 2018; 83:119–129. [Google Scholar]
- 35.Ximeri M, Galanopoulos A, Klaus M, et al. Effect of lenalidomide therapy on hematopoiesis of patients with myelodysplastic syndrome associated with chromosome 5q deletion. Haematologica 2010; 95:406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wobus M, Benath G, Ferrer RA, et al. Impact of lenalidomide on the functional properties of human mesenchymal stromal cells. Exp Hematol 2012; 40:867–876. [DOI] [PubMed] [Google Scholar]
- 37.Narla A, Dutt S, McAuley JR, et al. Dexamethasone and lenalidomide have distinct functional effects on erythropoiesis. Blood 2011; 118:2296–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santini V, Almeida A, Giagounidis A, et al. Randomized phase III study of lenalidomide versus placebo in RBC transfusion-dependent patients with lower-risk non-del(5q) myelodysplastic syndromes and ineligible for or refractory to erythropoiesis-stimulating agents. J Clin Oncol 2016; 34:2988–2996. [DOI] [PubMed] [Google Scholar]
- 39.Zhang L, Gajewski TF, Kline J. PD-1/PD-L1 interactions inhibit antitumor immune responses in a murine acute myeloid leukemia model. Blood 2009; 114:1545–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eksioglu EA, Chen X, Heider K-H, et al. Novel therapeutic approach to improve hematopoiesis in low risk MDS by targeting MDSCs with the Fc-engineered CD33 antibody BI 836858. Leukemia 2017; 31:2172–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng P, Eksioglu E, Chen X, et al. Immunodepletion of MDSC by AMV564, a novel tetravalent bispecific CD33/CD3 T cell engager restores immune homeostasis in MDS in vitro. Blood 2017; 130 (suppl 1):51–151. [Google Scholar]
- 42.Wei Y, Dimicoli S, Bueso-Ramos C, et al. Toll-like receptor alterations in myelodysplastic syndrome. Leukemia 2013; 27:1832–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang H, Bueso-Ramos C, DiNardo C, et al. Expression of PD-L1, PD-L2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia 2014; 28:1280–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jordan CT, Upchurch D, Szilvassy SJ, et al. The interleukin-3 receptor alpha chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia 2000; 14:1777–1784. [DOI] [PubMed] [Google Scholar]
- 45.Garcia-Manero G, Daver NG, Montalban-Bravo G, et al. A phase II study evaluating the combination of Nivolumab (Nivo) or Ipilimumab (Ipi) with azacitidine in pts with previously treated or untreated myelodysplastic syndromes (MDS). Blood 2016; 128:344. [Google Scholar]
- 46.Garcia-Manero G, Tallman MS, Martinelli G, et al. Pembrolizumab, a PD-1 inhibitor, in patients with myelodysplastic syndrome (MDS) after failure of hypomethylating agent treatment. Blood 2016; 128:345. [Google Scholar]
- 47.Kubasch AS, Wehner R, Bazzurri S, et al. Clinical, molecular, and immunological responses to pembrolizumab treatment of synchronous melanoma and acute myeloid leukemia. Blood Adv 2018; 2:1187–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uy GL, Godwin J, Rettig MP, et al. Preliminary results of a phase 1 study of flotetuzumab, a CD123 × CD3 bispecific Dart® protein, in patients with relapsed/refractory acute myeloid leukemia and myelodysplastic syndrome. Blood 2017; 130 (suppl 1):637. [Google Scholar]
- 49.Jin L, Lee EM, Ramshaw HS, et al. Monoclonal antibody-mediated targeting of CD123, IL-3 receptor α chain, eliminates human acute myeloid leukemic stem cells. Cell Stem Cell 2009; 5:31–42. [DOI] [PubMed] [Google Scholar]
- 50.Laszlo GS, Gudgeon CJ, Harrington KH, et al. Cellular determinants for preclinical activity of a novel CD33/CD3 bispecific T-cell engager (BiTE) antibody, AMG 330, against human AML. Blood 2014; 123:554–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Friedrich M, Henn A, Raum T, et al. Preclinical characterization of AMG 330, a CD3/CD33-bispecific T-cell-engaging antibody with potential for treatment of acute myelogenous leukemia. Mol Cancer Ther 2014; 13:1549–1557. [DOI] [PubMed] [Google Scholar]
- 52.Hoseini SS, Cheung NK. Acute myeloid leukemia targets for bispecific antibodies. Blood Cancer J 2017; 7:e522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krupka C, Kufer P, Kischel R, et al. Blockade of the PD-1/PD-L1 axis augments lysis of AML cells by the CD33/CD3 BiTE antibody construct AMG 330: reversing a T-cell-induced immune escape mechanism. Leukemia 2016; 30:484–491. [DOI] [PubMed] [Google Scholar]
- 54.Herrmann M, Krupka C, Deiser K, et al. Bifunctional PD-1 × αCD3 × αCD33 fusion protein reverses adaptive immune escape in acute myeloid leukemia. Blood 2018; doi: 10.1182/blood-2018-05-849802. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]