Abstract
Phosphatidylinositol 3-kinase-delta (PI3Kδ) signaling is critical for proliferation, survival, homing, and tissue retention of malignant B cells. Idelalisib, a selective oral inhibitor of PI3Kδ, has shown considerable single-agent activity in patients with heavily pretreated chronic lymphocytic leukemia (CLL). This study evaluated the safety and clinical activity of idelalisib in combination with bendamustine (IB) or rituximab (IR) or both (IBR) in patients with relapsed or refractory (R/R) CLL. Idelalisib was given continuously at 100 or 150 mg twice daily in combination with rituximab (375 mg/m2 weekly × 8 doses), bendamustine (70 or 90 mg/m2, days 1 and 2 every 4 weeks × 6 cycles) or BR (rituximab, 375 mg/m2 every 4 weeks and bendamustine, 70 mg/m2, days 1 and 2 every 4 weeks × 6 cycles). The primary endpoint was safety; secondary endpoints included overall response rate (ORR), duration of response (DOR), and progression-free survival (PFS). Fifty-two patients (median age 64 years) with a median of 3 prior therapies were enrolled. ORR was 84.6% (89.5% IR group, 77.8% IB group, and 86.7% IBR group). The overall median PFS was 25.6 months, and median DOR was 26.6 months. The most common grade ≥3 adverse events (≥10% of patients) were pneumonia (19.2%), diarrhea (13.5%), and febrile neutropenia (17.3%). Idelalisib-based combination therapy with bendamustine and/or rituximab was highly active, resulting in durable tumor control in patients with heavily pretreated R/R CLL. However, its tolerability profile suggests that these regimens should be used cautiously in this patient population. ClinicalTrials.gov ID: NCT01088048.
Introduction
Treatment options for relapsed or refractory (R/R) chronic lymphocytic leukemia (CLL) include the same chemoimmunotherapy regimens used in the frontline setting, including fludarabine (F) and cyclophosphamide (C) or bendamustine (B), combined with rituximab (R), referred to as FCR and BR, respectively.1,2
The B-cell receptor signaling pathway is activated in malignant B cells.3 Activation of the B-cell receptor, as well as integrin, cytokine and chemokine receptors, in turn activates phosphatidylinositol 3-kinase-delta (PI3Kδ), providing strong proliferative and survival signals and tissue migration and retention advantages to the malignant B cell.4 Idelalisib is a potent, highly selective, oral, small-molecule inhibitor of PI3Kδ.5 In lymphoid cell lines and primary patient samples, idelalisib blocks PI3Kδ and serine/threonine kinase signaling and promotes apoptosis.5–7
In a phase 1 trial of 52 patients with R/R CLL, idelalisib demonstrated an overall response rate (ORR) of 72% with a median progression-free survival (PFS) of 15.8 months.8 This heavily pretreated patient population included 24% of patients with del(17p)/TP53 mutations and 91% with unmutated IGHV. More than two-thirds of the patients experienced nodal responses and resolution of cytopenias. No dose-limiting toxicities were observed. The low rates of myelosuppression and immunosuppression with idelalisib suggested that it could be safely combined with other active drugs.
A subsequent randomized, double-blind, placebo-controlled, phase 3 trial was conducted comparing rituximab with or without idelalisib in patients with heavily pretreated R/R CLL who were not considered fit for standard chemoimmunotherapy.9 The addition of idelalisib to rituximab significantly improved ORR, PFS, and overall survival (OS). The PFS benefit was also seen in patients with poor prognostic features. Although grade 3 or 4 adverse events (AEs) were common in both groups, there was no significant overall increase in the rate of AEs in the idelalisib plus rituximab group, albeit with short follow up.
Given the role of PI3Kδ in B-cell malignancies, the pharmacologic profile of idelalisib, and the demonstrated safety and efficacy of idelalisib monotherapy in patients with R/R CLL, we performed a phase 1, open-label study in patients with indolent non-Hodgkin lymphoma (iNHL), CLL, and mantle-cell lymphoma. Here, we report the cohort of patients with previously treated CLL and characterize the tolerability and clinical activity of idelalisib in combination with rituximab, bendamustine, or both.
Methods
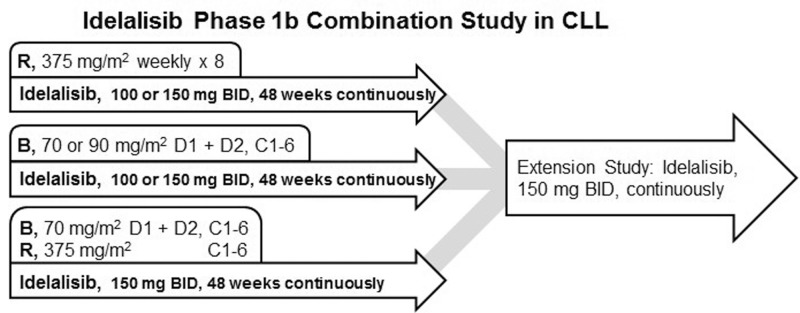
The present report summarizes data derived from a larger, phase 1, open-label study that evaluated idelalisib in various combinations for R/R iNHL, mantle cell lymphoma, or CLL (ClinicalTrials.gov identifier: NCR01088048) that was initiated in 2010 (Fig. 1). A total of 115 patients with CLL were included. Results through September 1, 2015 for 52 patients treated with idelalisib in combination with rituximab (IR, n = 19), with bendamustine (IB, n = 18), or with bendamustine plus rituximab (IBR, n = 15) are reported here; these results informed the subsequent development of idelalisib for the treatment of CLL. The remaining 63 patients were allocated to exploratory combinations of idelalisib with ofatumumab, fludarabine, chlorambucil, rituximab/chlorambucil, or rituximab/lenalidomide, and these results will be reported elsewhere. The primary study evaluated patients through 48 weeks of continuous idelalisib treatment and was followed by an extension study offering continued idelalisib monotherapy for patients who were deriving clinical benefit.
Figure 1.
The design of the primary and extension studies examining the efficacy and safety of idelalisib in combination with rituximab and/or bendamustine in patients with R/R CLL. B = bendamustine, BID = twice daily, C = cycles, CLL = chronic lymphocytic leukemia, D = day, R = rituximab.
The studies were conducted at 11 centers in the United States according to the principles of Good Clinical Practice after review and approval by the US Food and Drug Administration. Institutional review boards at each study site approved the study protocols, and all patients provided written informed consent. All authors had full access to study data and were involved in data interpretation, manuscript preparation, revision, and final approval.
Eligibility criteria
Patients with a documented diagnosis of CLL as established by the International Workshop on CLL (IWCLL)10 were eligible for the study. Key eligibility for the study included patients with R/R CLL (refractory defined as not responding to a standard regimen or progressing within 6 months of the last course of a standard regimen); symptomatic disease requiring treatment based upon the IWCLL criteria; absolute neutrophil count >1000/mm3, and platelet count >75,000/μL, unless cytopenias were related to underlying CLL; adequate liver and kidney function; and no prior allogeneic transplant.
Study treatments
The study drug was provided as capsules or tablets containing 100 or 150 mg of active idelalisib; doses of 50 and 75 mg were available for dose reductions. Idelalisib was taken twice daily (BID) with water at approximately 12-hour intervals. The starting dosage of idelalisib was determined based on safety, efficacy, and pharmacokinetic data from phase 1 healthy volunteer11 and monotherapy8 studies. In the absence of disease progression or unacceptable toxicity, patients continued idelalisib through 48 weeks on the primary study (twelve 28-day cycles) and indefinitely on the extension study.
Subjects with CLL were enrolled by investigator choice into multiple cohorts that incorporated interim safety and efficacy data from the study and other new findings. The following treatment regimens were evaluated: idelalisib 100 mg BID with rituximab 375 mg/m2 weekly × 8 doses; idelalisib 100 mg BID with bendamustine 90 mg/m2 × 6 cycles; idelalisib 150 mg BID with rituximab 375 mg/m2 weekly × 8 doses; idelalisib 150 mg BID with bendamustine 70 or 90 mg/m2 × 6 cycles; and idelalisib 150 mg BID with bendamustine 70 mg/m2 plus rituximab 375 mg/m2 × 6 cycles. All patients remained on single-agent idelalisib at the same dose until unacceptable toxicity or disease progression. Dose-limiting toxicities were assessed in the IR and IB cohorts after cycle 1 and defined as grade ≥3 nonhematologic toxicity or grade 4 hematologic toxicity persisting for at least 7 days, considered to be related to 1 or more drugs in the regimen.
Combination partners were administered at doses and schedules that were standard at the time the study was planned and initiated. For all treatment days, idelalisib was administered before bendamustine or rituximab, and bendamustine was administered before rituximab (on day 1 of the cycle). Bendamustine was administered as a 30-minute intravenous infusion at a dose of 70 or 90 mg/m2 on days 1 and 2 of each of the 6, 28-day cycles.12 Rituximab was administered as an intravenous infusion at a dose of 375 mg/m2 weekly for 8 cycles, or on day 1 of each of the 6, 28-day cycles when combined with bendamustine. Premedication with an analgesic/antipyretic and an antihistamine was administered before each infusion. Concomitant treatment was allowed for AEs, concurrent illness, or symptom management, as deemed medically necessary by the investigator.
Recent safety signals in the first-line setting in CLL and relapsed iNHL/small lymphocytic leukemia suggest that idelalisib may be associated with neutropenia and an increased risk for serious infections, particularly Pneumocystis pneumonia and cytomegalovirus infection/reactivation.13 In the absence of such safety signals at the time, this trial was designed and conducted, a proactive recommendation to use granulocyte colony-stimulating factor or prophylactic antibiotics was not made, and data on the ad hoc use of these or other supportive care agents were not collected.
Study assessments
Screening assessments included documentation of disease diagnosis, staging (Rai and Binet systems), prognostic factors (interphase fluorescence in situ hybridization), cytogenetic abnormalities, IGHV mutational status, recording of B systemic symptoms, World Health Organization (WHO) performance status score, physical examination, clinical laboratory tests, immunophenotyping of peripheral blood, a bone marrow biopsy (if not done within 6 weeks prior to study initiation), and computed tomography scans. At subsequent visits, AEs and concomitant medications were recorded, and standard laboratory tests (hematology, serum chemistry) were conducted.
By physical examination and complete blood count, response was evaluated every 8 weeks for the first 6 months, then every 12 weeks. The best overall response was the best response recorded from the start of treatment until disease progression or discontinuation. Complete response (CR), partial response (PR), stable disease, and progressive disease (PD) were assessed using standard IWCLL criteria.10 Computed tomography scans (or magnetic resonance imaging) were used to assess lymph-node size. Nodal responses were calculated from the sum of the product of the greatest perpendicular diameters (SPD) and percent change in SPD from baseline to each subsequent assessment time point. Patients who had PD at any point stopped study drug treatment and discontinued the study. Patients who derived clinical benefit were given the option to enroll in a long-term safety extension protocol that allowed continued treatment with idelalisib.
Statistical analysis
Each regimen was analyzed separately; no cross-regimen comparisons were made. Unless otherwise noted, data from the primary and extension study were considered together. All efficacy and safety analyses were based on the intent-to-treat (ITT) analysis set, which included all subjects who received at least 1 dose of study drug (idelalisib or combination therapy). The ORR was calculated as the proportion of subjects whose best overall response was a CR or PR and is presented with a 2-sided 95% exact confidence interval (CI). Duration of response (DOR), PFS, and OS were summarized using the Kaplan-Meier method. Survival curves were plotted based on the Kaplan-Meier method. DOR was evaluated in responding patients.
Results
Patient characteristics
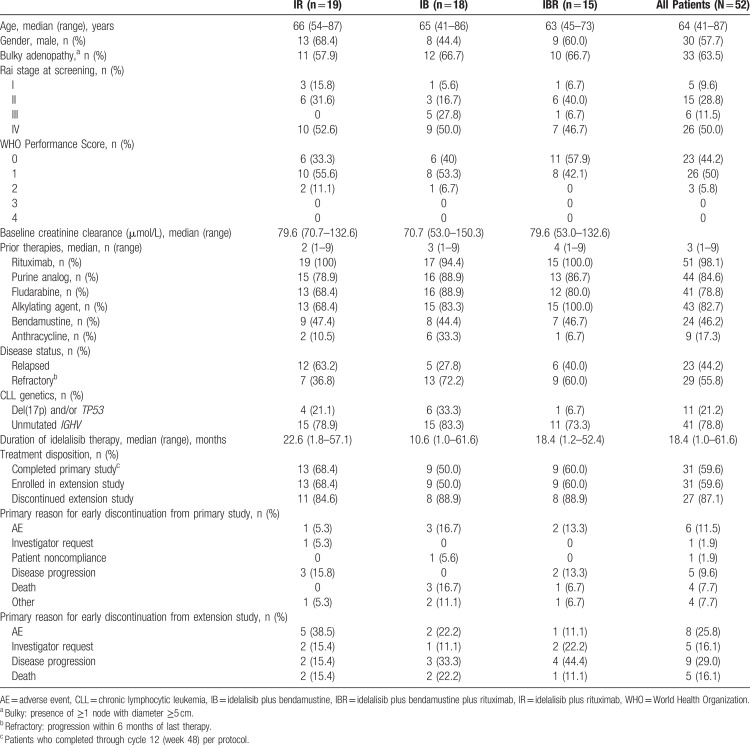
Patient characteristics by treatment arm, as well as for all patients enrolled in the study, are listed in Table 1. The majority of patients (57.7%) were male, and the median age was 64 years (range: 41–87). Almost two-thirds of patients (63.5%) had bulky adenopathy (defined as the presence of ≥1 node with diameter ≥5 cm), and most had advanced-stage disease (61.5% Rai stage III or IV). Approximately 94% of patients had a WHO performance score of 0 or 1; the remaining 6% had a WHO performance score of 2. Adverse prognostic factors were common: Unmutated IGHV14 was detected in 78.8% of patients, and 21.2% of cases had del(17p) or a TP53 mutation.15 No Richter transformations were reported, although the study did not systematically collect that information. Participants were heavily pretreated with a median of 3 prior therapies (range: 1–9). None of the patients enrolled in the study received prior therapy with a Bruton tyrosine kinase inhibitor or venetoclax. Approximately 56% of patients had disease that was refractory to their last prestudy therapy, and 65% of patients had disease that was refractory to rituximab (defined as not responding or progressing within 6 months of the last course).
Table 1.
Patient Baseline Characteristics and Disposition
Patient disposition
All patients in the ITT analysis set (N = 52) received idelalisib (Table 1). The median exposure for patients in the primary and extension studies was 18.4 months (range: 1.0–61.6). Most patients (84.6%) received 150 mg BID, while the remainder (4 patients [7.7%] in the IR arm and 4 patients [7.7%] in the IB arm) received 100 mg BID. At 6 months, 38 patients (73.1%) were still receiving idelalisib. At 1 year, 31 patients (59.6%) were still receiving idelalisib. All of these patients continued idelalisib therapy in the extension study.
Across the primary and extension studies, 6 patients (11.5%) had a treatment-emergent AE (TEAE), which led to a reduced dose of idelalisib. One of these patients was on idelalisib 100 mg BID, and the other 5 patients were on idelalisib 150 mg BID. Specific TEAEs leading to dose reduction included increased transaminase or alkaline phosphatase levels, ischemic colitis, febrile neutropenia, neutropenia, pyrexia, and erythematous rash. Twenty patients experienced a TEAE leading to idelalisib discontinuation; in 14 of these patients, TEAE was the primary reason for discontinuation. The most common TEAE causing idelalisib discontinuation was diarrhea/colitis (6 patients). The only other TEAE that led to discontinuation in more than 1 patient was rash (2 patients). Fourteen patients discontinued due to PD, 6 due to investigator request, 1 due to patient noncompliance, and 4 for other reasons.
Safety profile
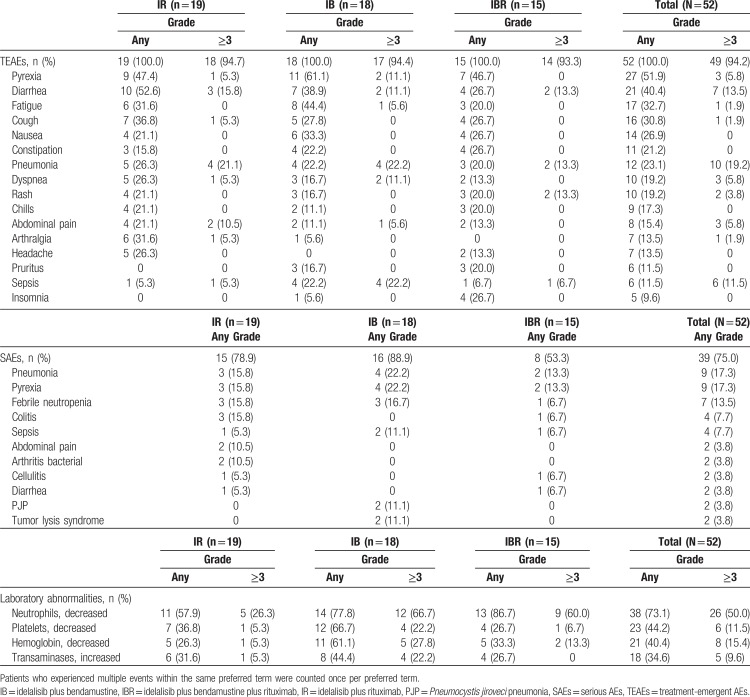
The incidences of TEAEs and laboratory abnormalities occurring in ≥15% of patients are shown in Table 2. Most of the AEs were consistent with those expected for heavily pretreated patients with R/R CLL.
Table 2.
Incidence of TEAEs (≥20% of Patients in Any Cohort; Primary and Extension Studies), SAEs (≥2 Patients in Any Cohort; Primary and Extension Studies), and Laboratory Abnormalities (Primary Study Only)
All 52 patients in the study experienced at least 1 TEAE. Forty-nine patients (94.2%) experienced TEAEs that were grade ≥3 in severity. The most frequently (≥20%) reported investigator-assessed TEAEs of any grade or attribution were neutropenia, pyrexia, diarrhea, fatigue, cough, nausea, anemia, pneumonia, and constipation. The most frequently reported TEAEs of grade ≥3 across cohorts were neutropenia and pneumonia. Laboratory abnormalities occurring in ≥15% of patients (in the primary study only) included lymphocytopenia, leukopenia, hypertriglyceridemia, neutropenia, elevated transaminases, anemia, thrombocytopenia, increased alkaline phosphatase, hyponatremia, high cholesterol, hypokalemia, increased serum bilirubin, hypoalbuminemia, and hypoglycemia.
A total of 39 patients (75.0%) across both studies experienced at least 1 serious AE (SAE). SAEs occurring in more than 5% of patients overall included pneumonia, pyrexia, febrile neutropenia, colitis, and sepsis. Eight patients (15.4%) experienced a TEAE leading to death during the primary or extension study; death was attributed to sepsis in 2 patients (septic shock, intracranial hemorrhage, dyspnea, multiorgan failure, fungal pneumonia, Pneumocystis jiroveci pneumonia [PJP], and subdural hematoma in 1 patient each).
Efficacy
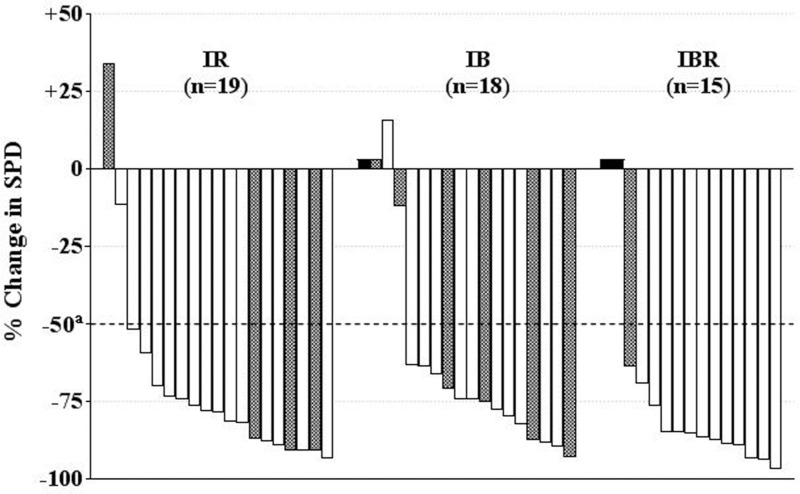
Idelalisib reduced lymphadenopathy in most patients in the primary study (Fig. 2). The overall mean lymph-node response compared with baseline was −73.2% (95% CI: −81.0, −65.4) (P < 0.001). All cohorts showed a similar mean reduction: −70.1% for IR (95% CI: −85.3, −54.9), −67.6% for IB (95% CI: −83.0, −52.1), and −84.6% for IBR (95% CI: −90.4, −78.8).
Figure 2.
Best on-treatment change in lymph-node area by patient for combined primary and extension studies. The overall mean lymph-node response compared with baseline was −73.2%. aCriterion for lymphadenopathy response according to Hallek et al.10 Shaded bars represent the presence of del(17p) or TP53 mutation; black bars represent patients without a follow-up tumor assessment (unevaluable). IB = idelalisib plus bendamustine, IBR = idelalisib plus bendamustine plus rituximab, IR = idelalisib plus rituximab, SPD = sum of the product of the greatest perpendicular diameters of measured lymph nodes.
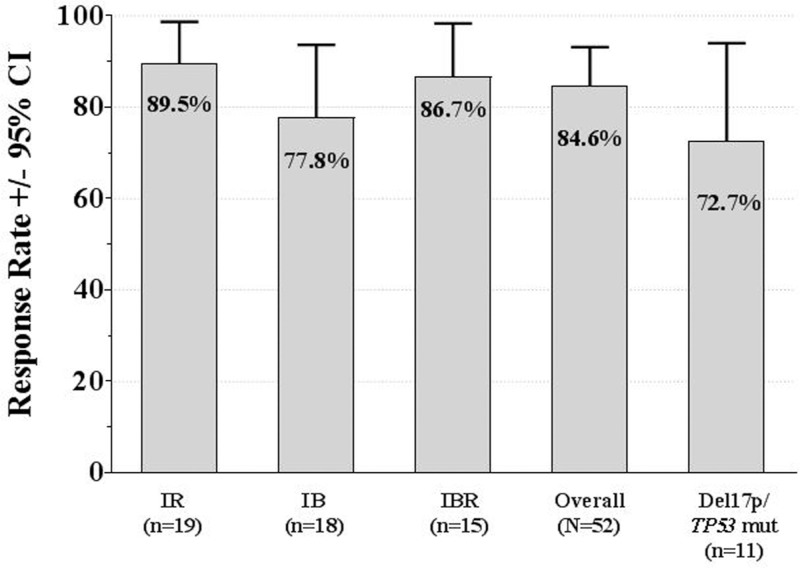
Using standard IWCLL response criteria,10 44 (84.6%) patients achieved an objective response (95% CI: 71.9, 93.1) across cohorts (Fig. 3). In the patients treated with IR, the ORR was 89.5% with no CRs and 17 (89.5%) PRs. In the patients treated with IB, the ORR was 77.8%, with 1 (5.6%) CR and 13 (72.2%) PRs. In the patients treated with IBR, the ORR was 86.7%, with 2 (13.3%) CRs and 11 (73.3%) PRs. As shown in Fig. 3, the response rates remained high (72.7%, 95% CI: 39.0, 94.0) in patients with del(17p) or mutated TP53.
Figure 3.
Overall response rates (intent-to-treat analysis) for combined primary and extension studies. Response according to International Workshop on Chronic Lymphocytic Leukemia criteria.10 CI = confidence interval, IB = idelalisib plus bendamustine, IBR = idelalisib plus bendamustine plus rituximab, IR = idelalisib plus rituximab, mut = mutation.
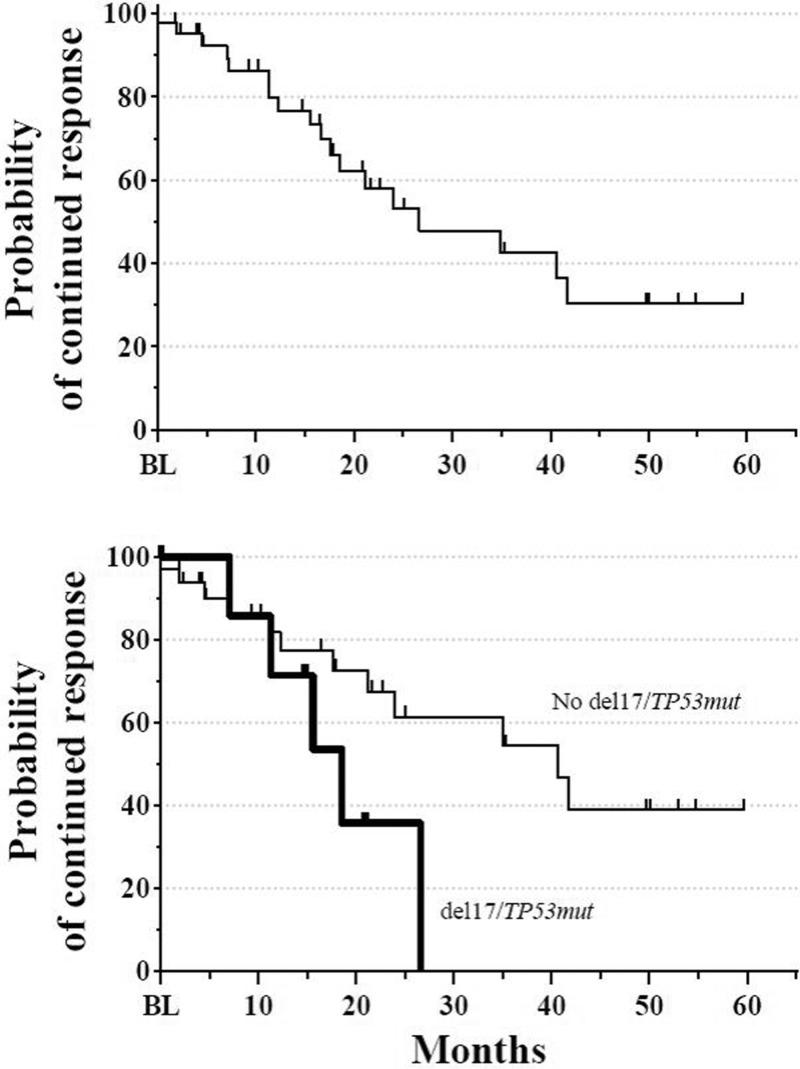
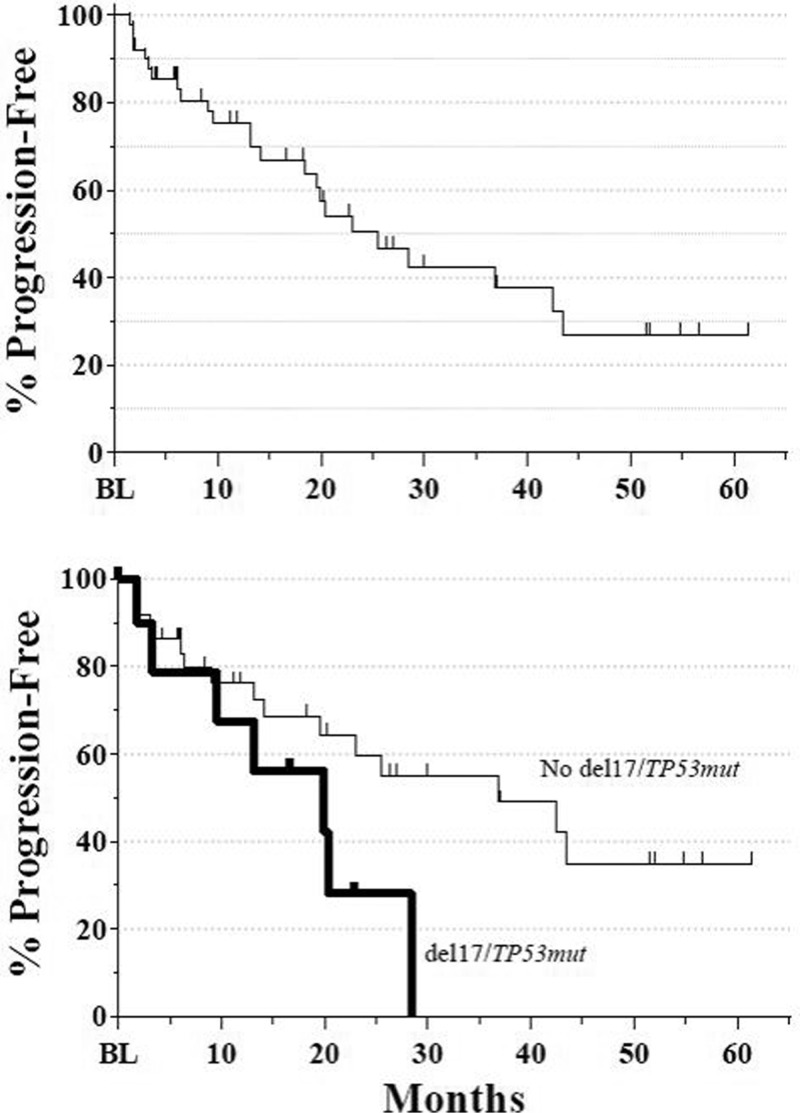
The median time to response was 1.9 months, but responses up to 8.3 months after initiation of therapy were observed. As depicted in Fig. 4, the overall median DOR was 26.6 months: 34.9 months for the IR cohort, 16.7 months for the IB cohort, and 21.2 months for the IBR cohort. In patients with adverse del(17p) or TP53 mutations, the median DOR was 18.5 months. The overall median PFS was 25.6 months (Fig. 5): 36.8 months for the IR cohort, 18.5 months for the IB cohort, and 23.0 months for the IBR cohort. Again, patients with adverse cytogenetics also benefited. The overall median PFS for patients with either del(17p) or TP53 mutations was 19.9 months versus 36.8 months for those without. Median OS has not yet been reached. At 12 months, the OS rate was 88.1% (95% CI: 78.1, 98.0), and at 24 months, it was 82.0% (95% CI: 69.7, 94.3).
Figure 4.
(A) Overall DOR for combined primary and extension studies. Median DOR was 26.6 months (N = 44). Extension study assessments based on standard of care. (B) DOR in patients with (thick line) or without (thin line) del(17p)/TP53 mutations. Median DOR was 18.5 months for patients with del(17p) and/or TP53 mutations (n = 8) and 40.6 months for patients without del(17p)/TP53 mutations (n = 33). DOR = duration of response.
Figure 5.
(A) Overall PFS for combined primary and extension studies. Median PFS was 25.6 months (N = 52). Extension study assessments based on standard of care. (B) PFS in patients with (thick line) or without (thin line) del(17p)/TP53 mutations. Median PFS was 19.9 months for patients with either del(17) or TP53 mutations (n = 11) and 36.8 months for patients without del(17p)/TP53 mutations (n = 38). PFS = progression-free survival.
Discussion
In this phase 1 study, the use of idelalisib in combination with rituximab, bendamustine, or both resulted in durable, high response rates in heavily pretreated patients with CLL, including many who were refractory to chemoimmunotherapy and who had other unfavorable prognostic characteristics, including adverse cytogenetics, bulky adenopathy, and advanced-stage disease. Approximately 38.5% of patients discontinued due to AEs.
The AEs observed were consistent with known side effects of the agents used or the underlying disease; there were no novel safety events. Pneumonia was the most common SAE (17%) and was observed most frequently in the IB group (22%). Rash was observed in 19% of patients but was equally distributed between the 3 groups, suggesting a relationship to idelalisib. However, this incidence was lower than reported in prior idelalisib monotherapy trials.8 Pneumonitis was rare and observed in only 1 patient. Asymptomatic hepatic enzyme elevation is a consistent AE reported in all trials with idelalisib. Of the 5 patients (9.6%) who experienced grade ≥3 aspartate aminotransferase (AST) or alanine aminotransferase (ALT) elevation, 4 resumed treatment (3 in the IB group and 1 in the IR group). This is similar to the incidence observed in patients previously treated with idelalisib monotherapy,8 as well as with idelalisib plus rituximab.9 As in the randomized trial of rituximab with or without idelalisib, despite the high rate of AEs of any grade, the combination was overall tolerable. The addition of bendamustine, rituximab, or both did not lead to notably greater toxicity or higher rates of treatment discontinuation, although the study was not powered to detect differences between cohorts.
Subsequent phase 3 trials with idelalisib involving R/R CLL patients demonstrated similar or higher rates of the AEs associated with idelalisib use. In a randomized trial of idelalisib with ofatumumab versus ofatumumab alone, the combination resulted in grade ≥3 AE rates of 20%, 23%, 5%, and 8% for pneumonia, diarrhea, or colitis, pneumonitis and AST or ALT elevation, respectively.16 A second randomized trial compared idelalisib, bendamustine, and rituximab to bendamustine and rituximab. The idelalisib combination arm resulted in grade ≥3 AE rates of 11%, 9%, 1.4%, and 21% for the same toxicities.17 By contrast, much higher rates of hepatotoxicity were observed in a phase 2 study of idelalisib monotherapy in previously untreated CLL patients; 54% experienced grade ≥3 transaminitis, with evidence for an immune-mediated mechanism. The severity was greater in the younger, treatment-naive patients.18 In a trial of previously untreated patients limited to those ≥65 years of age treated with idelalisib and rituximab, 42% experienced grade ≥3 diarrhea and/or colitis and 23% AST or ALT elevation.19
Importantly, 3 phase 3 trials were terminated prematurely when an increased rate of SAEs and increased mortality were noted in the treatment regimens that included idelalisib.13 Most events were infections, including sepsis and opportunistic infections such as PJP and cytomegalovirus infections. These trials involved previously untreated CLL patients and iNHL patients with disease characteristics that differed from the approved indication for idelalisib.
The primary objective of this phase 1 study was to characterize the safety of idelalisib in combination with rituximab, bendamustine, or both in previously treated CLL patients. Secondary objectives included the clinical efficacy of these combinations; however, the study was not designed to compare the different treatment arms. In general, the responses in all treatment arms were similar. The ORR of 85% was significantly higher than reported rates with the 3 respective treatment regimens when used without idelalisib.20–22 The difference was even greater in the subset of patients with del(17p) or TP53 mutations or unmutated IGHV, highlighting the activity of idelalisib in these difficult-to-treat populations. In addition, the observed ORR (89%) in the IR group was comparable to that reported in a larger group of similar patients (81%)9 and slightly higher than with idelalisib monotherapy in a prior trial (72%).8
Nodal responses were rapid, clinically significant and similar in all treatment groups and were independent of patient characteristics. Importantly, the DOR and PFS were longer than those reported in prior studies with each of the treatment regimens without idelalisib and in the idelalisib monotherapy trial.18,20–22
The present data demonstrate that idelalisib, a first-in-class, selective oral inhibitor of PI3Kδ, in combination with rituximab and/or bendamustine, offers significant and rapid reductions in lymphadenopathy and durable tumor control in heavily pretreated patients with CLL. Based on these results, phase 3 trials evaluating the efficacy of idelalisib in combination with rituximab or bendamustine plus rituximab were initiated and subsequently completed.9,17 Idelalisib in combination with bendamustine plus rituximab improved PFS compared with bendamustine plus rituximab alone.17 Although these trials demonstrated a benefit from the addition of idelalisib to bendamustine and/or rituximab, significant SAEs and infections associated with combination therapy should lead to judicious and cautious use of these regimens in patients with previously treated CLL.
Acknowledgments
The authors thank the patients for their dedication to this clinical trial and the clinical personnel at each of the study sites for their diligence in caring for patients and collecting study data. The authors also acknowledge study team members at Novella Clinical, Inc (Columbus, OH), Cancer Genetics, Inc (Rutherford, NJ), and INC Research (Raleigh, NC). This clinical study was sponsored and funded by Gilead Sciences, Inc (Foster City, CA). Editorial support was provided by Impact Communication Partners, Inc (New York, NY).
Footnotes
Citation: Coutre SE, Flinn IW, de Vos S, Barrientos JC, Schreeder MT, Wagner-Johnson ND, Sharman JP, Boyd TE, Fowler N, Dreiling L, Kim Y, Mitra S, Rai K, Leonard JP, Furman RR. Idelalisib in Combination With Rituximab or Bendamustine or Both in Patients With Relapsed/Refractory Chronic Lymphocytic Leukemia. HemaSphere, 2018;2:3. http://dx.doi.org/10.1097/HS9.0000000000000039
Funding/support: This clinical study was sponsored and funded by the Gilead Sciences, Inc (Foster City, CA, USA).
Author contributions: SEC performed the research and wrote and edited the manuscript; IWF, SdV, JCB, MTS, NDW-J, JPS, TEB, NF, KR, JPL, and RRF performed the research; LD and SM analyzed and interpreted the data and wrote and/or edited the manuscript; YK analyzed the data and wrote and/or edited the manuscript. Timothy DiChiara, PhD, Medical Writer at Gilead Sciences, assisted in the preparation of the manuscript.
Disclosure: SEC received research grants and advisory board fees from Gilead Sciences, Inc. IWF received institutional research funding from Acerta, BeiGene, Celgene, Constellation, Curis, Forma, Forty Seven, Genentech, Gilead Sciences, Inc, Incyte, Verastem, Janssen, Kite Pharma, Pharmacyclics, Portola Pharmaceuticals, Seattle Genetics, TG Therapeutics, Inc, and Trillium Therapeutics. SD and JPL received research grants from Gilead Sciences, Inc. JCB received research grants and consultancy fees from Gilead Sciences, Inc. RRF has received consultancy fees from Gilead Sciences, Inc, Genentech, Pharmacyclics, Janssen Pharmaceutica, AbbVie Inc, Sunesis Pharmaceuticals, Verastem, and TG Therapeutics, Inc. NDW-J received research grants from Gilead Sciences, Inc, Merck & Co., Regeneron, and Astex; she has received advisory board fees from Pharmacyclics, Juno Therapeutics, ADC Therapeutics, Janssen Pharmaceutica, and Gilead Sciences, Inc. JPS received institutional research grants and consultancy fees from and served on a speaker's bureau for Gilead Sciences, Inc; he has also received grants from Acerta, Pharmacyclics, AbbVie Inc, Genentech, and TG Therapeutics, Inc. NF received institutional research grants from and served on scientific advisory boards for Gilead Sciences, Inc, Roche, Celgene, and Infinity. LD, SM, and YK are employed by Gilead Sciences, Inc. MTS, TEB, and KR declare no conflicts of interest.
References
- 1.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Non-Hodgkin's Lymphomas, v3.2016. Available from: http://www.nccn.org/professionals/physician_gls/pdf/nhl.pdf. Accessed April 19, 2017. [Google Scholar]
- 2.Byrd JC, Rai K, Peterson BL, et al. Addition of rituximab to fludarabine may prolong progression-free survival and overall survival in patients with previously untreated chronic lymphocytic leukemia: an updated retrospective comparative analysis of CALGB 9712 and CALGB 9011. Blood 2005; 105:49–53. [DOI] [PubMed] [Google Scholar]
- 3.Burger JA, Chiorazzi N. B cell receptor signaling in chronic lymphocytic leukemia. Trends Immunol 2013; 34:592–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herman SE, Johnson AJ. Molecular pathways: targeting phosphoinositide 3-kinase p110-delta in chronic lymphocytic leukemia. Clin Cancer Res 2012; 18:4013–4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lannutti BJ, Meadows SA, Herman SE, et al. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood 2011; 117:591–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herman SE, Gordon AL, Wagner AJ, et al. Phosphatidylinositol 3-kinase-δ inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood 2010; 116:2078–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davids MS, Deng J, Wiestner A, et al. Decreased mitochondrial apoptotic priming underlies stroma-mediated treatment resistance in chronic lymphocytic leukemia. Blood 2012; 120:3501–3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown JR, Byrd JC, Coutre SE, et al. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110δ, for relapsed/refractory chronic lymphocytic leukemia. Blood 2014; 123:3390–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med 2014; 370:997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood 2008; 111:5446–5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Webb HK, Chen H, Yu AS, et al. Clinical pharmacokinetics of CAL 101, a p110δ isoform-selective PI3K inhibitor, following single- and multiple-dose administration in healthy volunteers and patients with hematological malignancies. Blood 2010; 116:1774. [Google Scholar]
- 12.Cheson BD, Byrd JC, Rai KR, et al. Novel targeted agents and the need to refine clinical end points in chronic lymphocytic leukemia. J Clin Oncol 2012; 30:2820–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.United States Food and Drug Administration. FDA alerts healthcare professionals about clinical trials with Zydelig (idelalisib) in combination with other cancer medicines. FDA website. Available at: https://www.fda.gov/Drugs/DrugSafety/ucm490618.htm. Accessed March 8, 2017.
- 14.Hamblin TJ, Davis Z, Gardiner A, et al. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood 1999; 94:1848–1854. [PubMed] [Google Scholar]
- 15.Rossi D, Cerri M, Deambrogi C, et al. The prognostic value of TP53 mutations in chronic lymphocytic leukemia is independent of Del17p13: implications for overall survival and chemorefractoriness. Clin Cancer Res 2009; 15:995–1004. [DOI] [PubMed] [Google Scholar]
- 16.Jones JA, Robak T, Brown JA, et al. Efficacy and safety of idelalisib in combination with ofatumumab for previously treated chronic lymphocytic leukaemia: an open-label, randomised phase 3 trial. Lancet Haematol 2017; 4:e114–e126. [DOI] [PubMed] [Google Scholar]
- 17.Zelenetz AD, Barrientos JC, Brown JR, et al. Idelalisib or placebo in combination with bendamustine and rituximab in patients with relapsed or refractory chronic lymphocytic leukaemia: interim results from a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol 2017; 18:297–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lampson BL, Kasar SN, Matos TR, et al. Idelalisib given front-line for treatment of chronic lymphocytic leukemia causes frequent immune-mediated hepatotoxicity. Blood 2016; 128:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Brien SM, Lamanna N, Kipps TJ, et al. A phase 2 study of idelalisib plus rituximab in treatment-naïve older patients with chronic lymphocytic leukemia. Blood 2015; 126:2686–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huhn D, von Schilling C, Wilhelm M, et al. Rituximab therapy of patients with B-cell chronic lymphocytic leukemia. Blood 2001; 98:1326–1331. [DOI] [PubMed] [Google Scholar]
- 21.Bergmann MA, Goebeler ME, Herold M, et al. Efficacy of bendamustine in patients with relapsed or refractory chronic lymphocytic leukemia: results of a phase I/II study of the German CLL Study Group. Haematologica 2005; 90:1357–1364. [PubMed] [Google Scholar]
- 22.Fischer K, Cramer P, Busch R, et al. Bendamustine combined with rituximab in patients with relapsed and/or refractory chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol 2001; 29:3559–3566. [DOI] [PubMed] [Google Scholar]