Supplemental Digital Content is available in the text
Abstract
Registry data are important for monitoring the impact of new therapies on treatment algorithms and outcomes, and for guiding clinical decision making in multiple myeloma (MM). This observational study analyzed real-world data from patients in the Population-based HAematological Registry for Observational Studies who were treated for symptomatic MM from 2008 to 2013 in the Netherlands. The primary endpoint was overall survival (OS) from initiation of first-line treatment. Secondary endpoints included OS and progression-free survival per treatment line, treatment patterns, and treatment response. Between 2008 and 2013, 917, 583, 283, and 139 patients had initiated first, second, third, and fourth treatment lines, respectively. Thalidomide-based regimens were the most frequently used first-line treatment (66%); bortezomib- and lenalidomide-based regimens were most often used in the second line (41% and 27%, respectively). The median OS (95% confidence interval) ranged from 37.5 months (34.8–41.8 months) in the first line to 9.2 months (6.2–12.3 months) in the fourth line. Univariate analyses showed that survival benefits were most apparent in younger patients (≤65 vs >65 years). These analyses provide important real-world information on treatment patterns and outcomes in patients with MM.
Introduction
Multiple myeloma (MM) accounts for 1% of all cancers, and in 2012 the number of new cases in Europe was estimated at 38,900.1,2 In the Netherlands, the incidences of MM in 2012 were 6.6 and 4.0 cases per 10,000 people for men and women, respectively.2 There have been improvements in diagnosis, early intervention, treatment, and supportive care for patients with MM over the past 15 years, and as a result, patient outcomes in Europe have improved significantly.3,4 Data from the Dutch Cancer Registry show that 5-year overall survival (OS) improved substantially in patients with MM diagnosed in 2004 to 2009 compared with those diagnosed in 1989 to 1993.5 Although survival rates have improved over time, relapse rates and mortality remain high, indicating a need for more effective treatment options. Until 2014, the immunomodulatory drugs (IMiDs) thalidomide6 and lenalidomide,7 and the proteasome inhibitor bortezomib8 were the mainstays of treatment in Europe. It is important to understand how these treatment patterns and agents have impacted patient outcomes in routine clinical practice.
While clinical trials are considered the gold standard for assessing novel treatment options, they often have strict inclusion criteria that exclude, for example, patients with comorbidities and/or a poor health status. Registries differ from clinical trials in that they collect real-world data on all patients and are therefore likely to reflect the characteristics of the general patient population. Disease-specific registries such as the Population-based HAematological Registry for Observational Studies (PHAROS) in the Netherlands are valuable sources of real-world evidence. PHAROS was established in 2010 and has collected treatment data from adult patients (aged ≥18 years) diagnosed with hematological malignancies (including MM) in the Netherlands from January 2004 onward.9 These data provide information on treatment patterns and patient outcomes in daily clinical practice for a broad patient population often not captured in clinical trials.
Previously, data from PHAROS have been used to describe and evaluate patterns of treatment sequences and the impact of sequence ordering on progression-free survival (PFS) and OS for nontransplant-eligible patients diagnosed between 2004 and 2011,5,10,11 as well as to assess cost-effectiveness.9,12 In this study, data from patients who received initial treatment for symptomatic MM between January 1, 2008 and December 31, 2013 were analyzed to assess real-world treatment patterns and long-term patient outcomes in the Netherlands. The routine use of thalidomide-based regimens as well as the approval of bortezomib and lenalidomide during this period may have impacted treatment patterns and patient outcomes over time; therefore, this time frame was selected for analysis.
Results
Patient and disease characteristics
The total study population in PHAROS included 1887 patients with MM diagnosed from 2004 onward, of whom 917 had initiated first-line treatment in or after 2008 and who comprised the first treatment line analysis set; 583, 283, and 139 patients had initiated second, third, and fourth treatment lines in or after 2008, respectively.
Considering trial participation (data not shown), 15% of all patients in the first-line treatment cohort participated in a clinical trial at first line (138 out of 917 patients). Out of the 538 patients who had initiated a second treatment line, 47 participated in a clinical trial at that line (8%). In the later-line treatment cohorts, trial participation was 3% for third-line treatment (9 patients out of 283) and 8% for fourth-line treatment (11 patients out of 139).
Baseline patient characteristics at the initiation of a treatment line were generally similar across treatment lines (Table 1), with the exception of the year of diagnosis, which was related to the inclusion criteria. The median age at treatment initiation was 70 years in the first treatment line, 71 years in the second and third treatment lines, and 72 years in the fourth treatment line. Over one-third of patients had more than 1 comorbidity at diagnosis (35–42% across treatment lines). In the first-line treatment cohort, 18% of patients had undergone high-dose therapy with stem cell transplantation (SCT) as their initial treatment. In the second treatment line cohort, 23% of the patients had received this high-dose treatment with SCT in either the current or previous treatment line. For patients included in the third and fourth treatment line cohort, this was 25% and 21%, respectively.
Table 1.
Patient and Disease Characteristics for Patients Initiating a Line of Treatment in 2008 to 2013 at the Start of Each Treatment Line
Treatment patterns
In the first treatment line, thalidomide-based regimens were most frequently used (66%), followed by bortezomib-based regimens (15%) (Table 2). The proportion of patients receiving a regimen containing thalidomide in the second, third, and fourth treatment lines was 19%, 14%, and 5%, respectively.
Table 2.
Treatment Regimens Used at Each Treatment Line for Patients Initiating a Line of Treatment During 2008 to 2013
In the second treatment line, bortezomib- and lenalidomide-based regimens were used most often (41% and 27%, respectively). In subsequent treatment lines, lenalidomide-based regimens were most common (44% and 34% in the third and fourth treatment lines, respectively), followed by bortezomib-based regimens (28% and 29%, respectively).
Patient outcomes
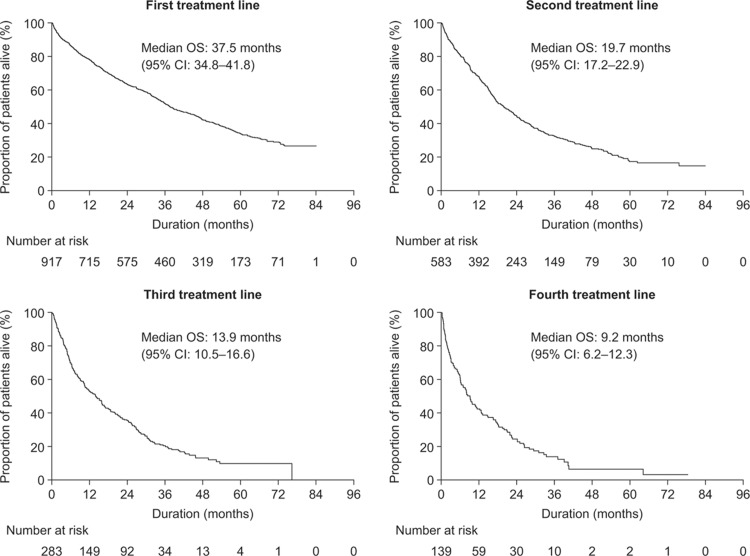
The median follow-up for OS from the first treatment line was 62.4 months (95% confidence interval [CI]: 60.6–64.3 months). The median OS was 37.5 months (95% CI: 34.8–41.8 months), 19.7 months (95% CI: 17.2–22.9 months), 13.9 months (95% CI: 10.5–16.6 months), and 9.2 months (95% CI: 6.2–12.3 months) for patients receiving first, second, third, and fourth treatment lines in or after 2008, respectively (Fig. 1).
Figure 1.
Kaplan–Meier curves of overall survival from first, second, third, and fourth treatment lines for patients initiating treatment during 2008 to 2013. CI = confidence interval, OS = overall survival.
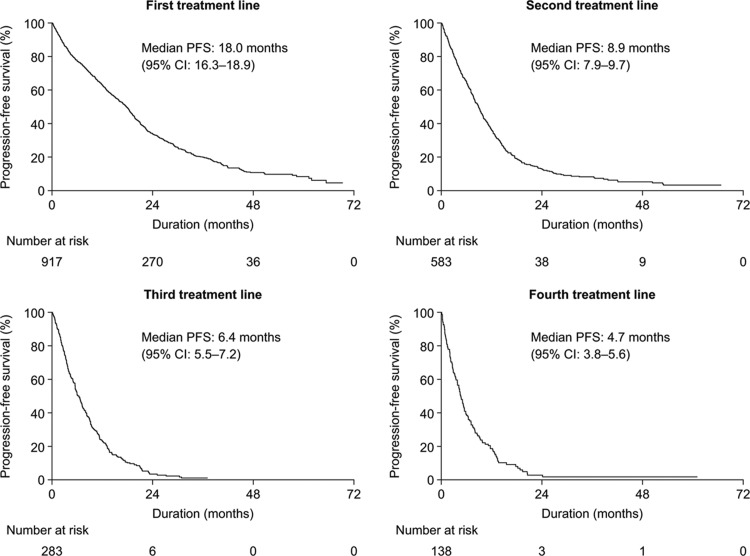
A similar trend was also observed for PFS. The median follow-up for PFS from the first treatment line was 38.8 months (95% CI: 36.4–44.0 months). The median PFS in the first treatment line was 18.0 months (95% CI: 16.3–18.9 months). In subsequent treatment lines, the median PFS decreased to 8.9 months (95% CI: 7.9–9.7 months), 6.4 months (96% CI: 5.5–7.2 months), and 4.7 months (95% CI: 3.8–5.6 months) following the initiation of the second, third, and fourth treatment lines, respectively (Fig. 2).
Figure 2.
Kaplan–Meier curves of progression-free survival from first, second, third, and fourth treatment lines for patients initiating treatment during 2008 to 2013. CI = confidence interval, PFS = progression-free survival.
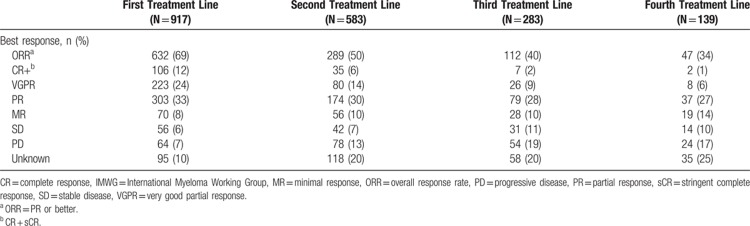
The depth of response also decreased at later lines of treatment (Table 3). In the first treatment line, 69% of patients achieved a partial response (PR) or better, and 12% of patients had a complete response (CR) or stringent CR (sCR). In the fourth treatment line, only 34% of patients achieved a PR or better, and only 1% had achieved a CR or sCR. It should be noted, however, that the percentage of patients for whom the level of response was unknown increased in later lines. Almost one-quarter of patients (24%) achieved a very good PR in the first treatment line, while in the second, third, and fourth treatment lines, the rate had decreased to 14%, 9%, and 6%, respectively. Concurrently, the proportion of patients with progressive disease increased from 7% in the first treatment line to 13%, 19%, and 17% in the second, third, and fourth treatment lines, respectively.
Table 3.
Best Response by Treatment Line (per IMWG Criteria) for Patients Initiating a Line of Treatment in 2008 to 2013
Age at treatment initiation and survival outcomes
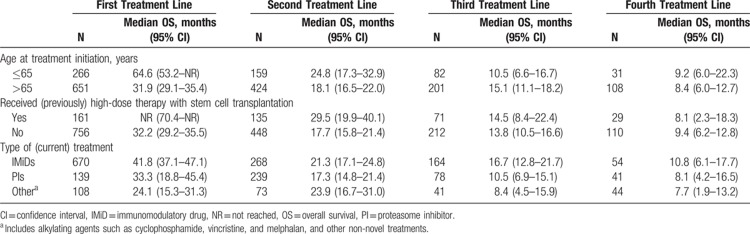
In the first treatment line, the median OS in patients aged 65 years or younger at the initiation of treatment was double that of those who were older at the initiation of treatment (64.6 months [95% CI: 53.2 months to not reached [NR] vs 31.9 months [95% CI: 29.1–35.4 months]) (Table 4). The median OS was also longer for younger patients (aged ≤65 years) than for older patients (aged >65 years) in the second and fourth treatment lines, although the CIs were overlapping, and patient numbers were low in later treatment lines.
Table 4.
Overall Survival in Patients Initiating a Line of Treatment During 2008 to 2013, by Subgroup
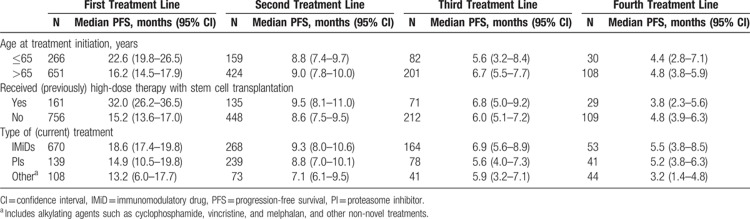
A longer median PFS was observed in younger patients (aged ≤65 years) than in those older than 65 years at the initiation of first-line treatment: 22.6 months (95% CI: 19.8–26.5 months) versus 16.2 months (95% CI: 14.5–17.9 months). This difference was not observed in later treatment lines (Table 5).
Table 5.
Progression-Free Survival in Patients Initiating a Line of Treatment in 2008 to 2013, by Subgroup
High-dose therapy with SCT and survival outcomes
For patients who received high-dose therapy with SCT (in the first treatment line), the median OS from initiation of the first treatment line was NR (95% CI: 70.4 months to NR) compared with 32.2 months (95% CI: 29.2–35.5 months) for those who had not received SCT in the first line (Table 4). For patients who were in the second treatment line and had ever received SCT (ie, in the first or second line), the median OS was 29.5 months (95% CI: 19.9–40.1 months) compared with 17.7 months (95% CI: 15.8–21.4 months) for those who had not received SCT in the first or second treatment line. A trend for longer OS in patients who had received SCT (in the previous or current line) was also observed in the third line, but CIs were overlapping (14.5 months [95% CI: 8.4–22.4 months] compared with 13.8 months [95% CI: 10.5–16.6 months]). A longer OS was not observed in fourth-line analyses (8.1 months [95% CI: 2.3–18.3 months] compared with 9.4 months [95% CI: 6.2–12.8 months]).
In the first line, PFS in patients who received SCT was double that observed in those who did not receive SCT (32.0 months [95% CI: 26.2–36.5 months] vs 15.2 months [95% CI: 13.6–17.0 months]) (Table 5). The results of these analyses must be interpreted with caution; older patients are less likely to be eligible for SCT than younger patients, and this analysis was not corrected for age.
Treatment type and survival outcomes
The median OS in the first line was longer in patients receiving IMiDs (41.8 months [95% CI: 37.1–47.1 months]) or proteasome inhibitors (33.3 months [95% CI: 18.8–45.4 months]) than in those receiving “other agents” (“other agents” included alkylating agents such as cyclophosphamide and melphalan, or other non-novel treatments) (24.1 months [95% CI: 15.3–31.3 months]). This trend was less pronounced in subsequent treatment lines.
The median PFS was 18.6 months (95% CI: 17.4–19.8 months), 14.9 months (95% CI: 10.5–19.8 months), and 13.2 months (95% CI: 6.0–17.7 months) in patients receiving IMiDs, proteasome inhibitors, and treatment with “other agents” in the first treatment line, respectively (Table 5). PFS was longer in individuals treated with IMiDs or proteasome inhibitors than in those who received “other agents” in the first, second, and third treatment lines.
International Staging System stage at diagnosis and survival outcomes
Across all lines of treatment, patients with International Staging System (ISS) stage I disease at diagnosis had a longer median OS than those diagnosed with ISS stage II or III disease, ranging from 62.2 months (95% CI: 51.7 months to NR), 37.5 months (95% CI: 33.3–46.1 months), and 30.3 months (95% CI: 22.8–36.8 months), respectively, in the first treatment line, to 17.3 months (95% CI: 7.7–22.3 months), 7.0 months (95% CI: 2.3–19.5 months), and 5.2 months (95% CI: 2.3–9.5 months), respectively, in the fourth treatment line (Supplementary Table 1, Supplemental Digital Content).
A similar pattern was observed for median PFS, ranging from 22.0 months (ISS I) (95% CI: 20.1–26.2 months), 19.0 months (ISS II) (95% CI: 17.6–20.8 months), and 13.0 months (ISS III) (95% CI: 10.5–17.2 months) in the first treatment line, to 5.6 months (ISS I) (95% CI: 3.8–7.8 months), 3.8 months (ISS II) (95% CI: 2.3–8.0 months), and 3.5 months (ISS III) (95% CI: 2.0–5.0 months) in the fourth treatment line. In the third and fourth treatment lines, the difference in PFS was smaller than in the first and second treatment lines (Supplementary Table 2, Supplemental Digital Content). For both survival analyses, ISS stage was assessed at diagnosis only; therefore, as patients progressed into later treatment lines, the proportions of patients with low ISS stage decreased.
Discussion
This large, retrospective analysis of PHAROS revealed important insights into real-world treatment patterns and outcomes in patients with symptomatic MM in the Netherlands. Most patients who initiated treatment from 2008 up to and including 2013 received thalidomide-based regimens in the first treatment line and bortezomib- or lenalidomide-based regimens in subsequent lines. This reflects the approval and reimbursement status of these agents in Europe6–8 and the treatment recommendations in the Netherlands13–16 at the time patients initiated treatment lines.
Other studies have also assessed real-world treatment patterns in patients with MM. Analysis of the Austrian Myeloma Registry found that 48% of patients receiving first-line treatment received bortezomib, and 33% received an IMiD.4 In the second treatment line, 48% of patients received bortezomib-based treatment, and 54% received an IMiD-based regimen.4 Similar results were obtained from an analysis of data from the Czech Registry of Monoclonal Gammopathies (RMG)17 and a large observational patient chart review across 7 European countries.18 In PHAROS, a larger proportion of patients received an IMiD in the first treatment line than in the Austrian and Czech registries. This might partly be explained by the age of patients at diagnosis in the different registries, the treatment guidelines in the Netherlands, and the fact that thalidomide was the only agent that had been approved for upfront treatment at the start of the study period; bortezomib was approved later in the period. The use of IMiDs and bortezomib in the second treatment line was similar across the 3 registries.
There have been significant increases in the use of bortezomib over time, following its initial approval in Europe in 2004 and subsequent label updates.8 It is likely that treatment patterns will change in the coming years with the approval of next-generation novel agents and treatment combinations. Since 2013 several new agents have received regulatory approval in Europe, including bendamustine,19 pomalidomide,20 panobinostat,21 carfilzomib,22 ixazomib,23 daratumumab,24 and elotuzumab.25 It is important to note that when interpreting the treatment patterns seen in this analysis, reimbursement status and Dutch MM guidelines play important roles in influencing treatment decisions in the Netherlands. Only a small proportion of patients in the PHAROS cohort may have received 1 or more of these next-generation novel agents as part of a clinical trial or an early access program. Furthermore, uptake of new agents may vary by region and by clinic owing to local institutional practice9; therefore, an updated analysis of PHAROS in the coming years is warranted.
In our study, the medians for OS and PFS from initiation of the first treatment line were approximately 3 and 1.5 years, respectively. In subsequent treatment lines, the median OS and PFS decreased substantially. In our study, patients were included if they initiated a treatment line in 2008 or later, and so those in later lines (ie, fourth line) may have received older agents in previous treatment lines; this may have impacted on survival. Nevertheless, decreased OS and PFS were also observed in the European patient chart review of 7 countries18 and, although the patient population was slightly different from that of PHAROS, in an analysis of the Czech RMG.17
Our analyses of PHAROS revealed that age at treatment initiation was associated with longer survival. This is supported by findings from the Europe-wide EUROCARE-5 study in which 1-year age-relative OS decreased from 81.6% in patients aged between 55 and 64 years to 59.7% in those older than 75 years.26 This may be explained by the fact that elderly patients are more likely to have comorbidities, including renal impairment, cardiovascular disease, diabetes, and deep vein thrombosis, which can impact upon treatment decisions and patient outcomes.27 We also found that patients who received an SCT in a current or previous line had a longer median OS and PFS than those who had not received such treatment. In the Netherlands, patients aged over 65 years are generally not eligible for high-dose induction therapy and SCT (at least at the time of data collection)13; therefore, their outcomes are likely to be worse than those who are eligible for these treatments. Other population studies have also demonstrated the benefit of SCT on survival.28,29
In our study, patients with ISS stage I disease at diagnosis had a longer OS and PFS than those diagnosed with ISS stage II or III disease. This is supported by an analysis of the Greek Myeloma Study group30 and in another study in which 5-year survival rates were also found to be significantly higher in patients with ISS stage I disease than in those with stage II or III disease (52%, 42%, and 28%, respectively).3
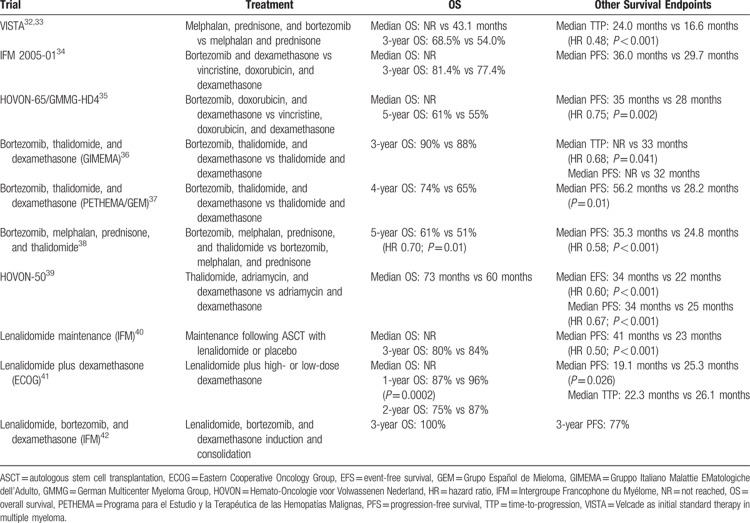
In clinical trials, OS and PFS have been shown to be considerably longer than those reported here (Table 6). In addition, the proportion of patients in PHAROS achieving a CR or sCR during the first treatment line was only 12%, despite the availability of a number of treatment options in clinical practice. Although the inclusion and exclusion criteria of clinical trials vary, in general, elderly patients and those who are in poor health are often not included; hence, patients in clinical trials may not be representative of the general population. This analysis of PHAROS suggests that clinical trial data may not fully reflect the real-world outcomes experienced by patients with symptomatic MM. Registry studies are therefore valuable because they provide data on a heterogeneous patient population that more accurately reflects patients encountered in clinical practice.9 In addition, in real-world practice, dose reductions and modifications may be more likely than in clinical trials, which may impact on survival.
Table 6.
Survival Outcomes in Randomized Phase 2 and 3 Clinical Trials in the Frontline Setting (2008–2013)
This study has some limitations. First, if progression data were not available, PFS was estimated using the start date of a new treatment line as a proxy for the date of progression. Consequently, PFS may have been overestimated in this analysis. Second, in some instances, prognostic data, such as ISS stage at diagnosis, were missing. Thus, proportions and outcomes should be interpreted with caution. In general, registries could be improved if data could be retrieved more easily and consistently from hospital records. Third, owing to the advanced age of patients in PHAROS (median age 71 years), the proportion of patients who had (previously) undergone SCT was lower than might be expected in a typical MM patient population, for example. The majority were elderly patients, and during the study period these were not eligible for SCT in the Netherlands if their age was ≥66 years. Fourth, response in real-world practice is not always measured and/or registered regularly. As a consequence, a comparison with response as measured in clinical trials may be biased.
This analysis of PHAROS provides important information on treatment patterns and outcomes in patients with symptomatic MM in real-world clinical practice in the Netherlands. Most patients received IMiDs and proteasome inhibitors in the first and subsequent treatment lines. With each successive treatment line, OS, PFS, and depth of response decreased. Despite the availability of a number of treatment options, there remains a significant unmet need in MM. The data presented here will provide a useful baseline with which the impact on survival of treatment patterns, including new classes and combinations of agents, can be compared. This information is important for patients, physicians, and payers, and may help to inform treatment decisions in the future.
Materials and methods
Study design
This was a noninterventional observational study using retrospective data from patient charts entered into PHAROS. The study was approved by the ethical committee of the Erasmus University Medical Center Rotterdam in the Netherlands (MEC-2011-200). Patients were followed up from diagnosis until death, loss to follow-up, or end of the observation period.
Data collection and study population
All patients diagnosed with MM (aged ≥18 years) in the Netherlands between January 1, 2004 and December 31, 2011 are included in PHAROS. Data in PHAROS were obtained from hospital records using standardized case report forms. Data were collected between January 2010 and December 2013. OS data were updated in December 2014 for this analysis. Information was collected on diagnosis, comorbidities before and after diagnosis, hospitalization, treatment, supportive care, treatment response (assessed by the International Myeloma Working Group criteria31), infections, treatment-related adverse events, and post-treatment outcomes (including response status and survival).
Patients were included in the analysis for each treatment line that was initiated from 2008 up to and including 2013, and could therefore be evaluated in more than 1 treatment line. We performed the analyses per line. In the first-line analysis, all patient are included who had their first line in 2008 or later. In the second-line analysis, we included patients who had a second line in or after 2008. These could be the same patients but also patients who had a first line in 2007 or earlier; the same applies for the third line and fourth line.
Patients who were enrolled in clinical studies were included in the analysis; patients with smouldering MM (SMM) at diagnosis were excluded. Patients with SMM who progressed to symptomatic MM were included from the time that they initiated MM treatment.
Study endpoints
The primary endpoint of the study was OS from initiation of the first treatment line. The secondary endpoints included OS from the second, third, and fourth line, PFS, best treatment response, and treatment received, all by treatment line. We also performed analyses of OS and PFS according to age, ISS stage at diagnosis, (previous) SCT status, and treatment type.
Statistical analyses
No formal hypotheses were tested. Patients were grouped according to treatment line. Patients who initiated a treatment line between 2008 and 2013 were included for each treatment line; therefore, individuals could have been included in multiple treatment lines if these were initiated within the time frame of these analyses. Patient characteristics and most commonly used treatment regimens were summarized by line of therapy. A line of therapy was considered as a period during which a patient was treated with a specific antitumor regimen and the period following treatment. A treatment line ended when a new treatment was initiated, the end of the follow-up period was reached, or the patient died. A new treatment line was defined as the initiation of treatment with a new antitumor drug regimen when the patient appeared to be refractory, or after disease progression. Changes in dosing were not considered a new line of therapy. Retreatment with the same antitumor regimen was considered a new line of therapy only if it followed disease progression. Patients may have been diagnosed with MM (or with SMM), but did not immediately start treatment; hence, it was possible that there was a delay between diagnosis and first-line treatment initiation.
All analyses were descriptive, and no formal comparisons were made. OS and PFS were estimated using Kaplan–Meier curves. Treatment responses were described by treatment line and depth of response, and patient characteristics were summarized using descriptive statistics.
Supplementary Material
Acknowledgments
The authors are grateful to the various registration teams of the Netherlands Cancer Registry for the data collection. PHAROS is a cooperation of HOVON (Dutch–Belgian Cooperative Trial Group for Hematology Oncology), the Institute for Medical Technology Assessment at Erasmus University Rotterdam, and the Comprehensive Cancer Organization, the Netherlands (IKNL).
Footnotes
Citation: Verelst SGR, Blommestein HM, De Groot S, Gonzalez-McQuire S, DeCosta L, de Raad JB, Uyl-de Groot CA, Sonneveld P. Long-term Outcomes in Patients With Multiple Myeloma: A Retrospective Analysis of the Dutch Population-based HAematological Registry for Observational Studies (PHAROS). HemaSphere, 2018;2:4. http://dx.doi.org/10.1097/HS9.0000000000000045
Funding/support: PHAROS is financially supported by ZonMW 2009-2012 (No. 80-82500-98-01007) and through unrestricted grants from: Bayer, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Mundipharma, Novartis, Roche, and Sanofi.
Funding for this research was provided by Amgen (Europe) GmbH. Data analysis and statistical support were provided by the Institute for Medical Technology Assessment at Erasmus University Rotterdam, the Netherlands and were funded by Amgen (Europe) GmbH. Medical writing support was provided by Liz Hartfield of Oxford PharmaGenesis, Oxford, UK and was funded by Amgen (Europe) GmbH.
Disclosure: Author contributions and potential conflicts of interest: SGRV, HMB, LD, SG-M, and JBD conceived and designed the study; SG-M, JBD, PS, CAU, SGRV, HMB, and SD were involved in data acquisition; all authors were involved in the interpretation of the analyses and writing of the paper. All authors approved the final version of the paper and take responsibility for its content.
SGRV: no conflicts of interest; HMB: research support from Bristol-Myers Squibb; SD: research support from Amgen; SG-M, LD, and JBD: employees of Amgen and hold Amgen stock; CAU: research support from Astellas, Bayer, Bristol-Myers Squibb, Celgene, Gilead, GlaxoSmithKline, Janssen-Cilag, MSD, Mundipharma, Novartis, Roche, Therakos, and Sanofi; PS: research support and honoraria from Amgen, Celgene, Janssen, Karyopharm, SkylineTx, and Takeda.
References
- 1.Moreau P, San Miguel J, Ludwig H, et al. Multiple myeloma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24 (suppl 6):vi133–vi137. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 2013; 49:1374–1403. [DOI] [PubMed] [Google Scholar]
- 3.Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood 2008; 111:2516–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willenbacher E, Weger R, Rochau U, et al. Real-world use of 3rd line therapy for multiple myeloma in Austria: an Austrian Myeloma Registry (AMR) analysis of the therapeutic landscape and clinical outcomes prior to the use of next generation myeloma therapeutics. PLoS ONE 2016; 11:e0147381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verelst SGR, Van Norden Y, Coebergh JWW, et al. Multiple myeloma: treatment and survival in the era of novel agents. Clin Lymphoma Myeloma Leuk 2013; 13 (suppl 1):S228. [Google Scholar]
- 6.Celgene Europe Ltd. Thalidomide Celgene summary of product characteristics. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000823/WC500037050.pdf (Accessed July 21, 2016). [Google Scholar]
- 7.Celgene Europe Ltd. Revlimid summary of product characteristics. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000717/WC500056018.pdf (Accessed July 21, 2016). [Google Scholar]
- 8.Janssen-Cilag Ltd. Velcade summary of product characteristics. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000539/WC500048471.pdf (Accessed July 21, 2016). [Google Scholar]
- 9.Blommestein HM, Franken MG, Uyl-de Groot CA. A practical guide for using registry data to inform decisions about the cost effectiveness of new cancer drugs: lessons learned from the PHAROS registry. Pharmacoeconomics 2015; 33:551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verelst S, van Norden Y, Blommestein H, et al. Treatment sequencing and efficacy in newly diagnosed non-transplant eligible myeloma patients in the Netherlands: a population based study. Blood 2012; 120:4284. [Google Scholar]
- 11.Verelst S, van Norden Y, Sonneveld P. Age versus frailty: what should determine treatment choice in the elderly myeloma patients these days? Clin Lymphoma Myeloma Leuk 2015; 15 (suppl 3):e69–e70. [Google Scholar]
- 12.Blommestein HM, Verelst SG, de Groot S, et al. A cost-effectiveness analysis of real-world treatment for elderly patients with multiple myeloma using a full disease model. Eur J Haematol 2016; 96:198–208. [DOI] [PubMed] [Google Scholar]
- 13.Lokhorst H, Huijgens PC, Raymakers R, et al. Modern treatment methods for multiple myeloma: guidelines from the Dutch Haemato-Oncology Association (HOVON). Ned Tijdschr Geneeskd 2005; 149:808–813. [PubMed] [Google Scholar]
- 14.Lokhorst HM, Zweegman S, Kersten MJ, et al. Richtlijnen behandeling multipel myeloom anno 2008. Ned Tijdschr Hematol 2008; 5:150–156. [Google Scholar]
- 15.Sonneveld P, Zweegman S, Vellenga E, et al. Richtlijnen behandeling plasmacelaandoeningen anno 2010. Ned Tijdschr Hematol 2010; 7:84–95. [Google Scholar]
- 16.Zweegman S, Lokhorst HM, Levin M-D, et al. Richtlijnen behandeling multipel myeloom 2012. Ned Tijdschr Hematol 2012; 9:300–320. [Google Scholar]
- 17.Hájek R, Jarkovsky J, Campioni M, et al. Long-term outcomes and treatment patterns in patients with symptomatic multiple myeloma in the real-world setting: a retrospective analysis of the Czech RMG registry. Value Health 2016; 19:A158. [Google Scholar]
- 18.Yong K, Delforge M, Driessen C, et al. Multiple myeloma: patient outcomes in real-world practice. Br J Haem 2016; 175:252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Astellas Pharma GmbH. Levact summary of product characteristics. Available from: http://www.mhra.gov.uk/home/groups/spcpil/documents/spcpil/con1427431950045.pdf. (Accessed March 22, 2017). [Google Scholar]
- 20.Celgene Europe Ltd. Imnovid summary of product characteristics. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002682/WC500147717.pdf (Accessed March 6, 2017). [Google Scholar]
- 21.Novartis Europharm Ltd. Farydak summary of product characteristics. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003725/WC500193298.pdf (Accessed July 21, 2016). [Google Scholar]
- 22.Amgen. Kyprolis summary of product characteristics. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003790/WC500197692.pdf (Accessed September 5, 2016). [Google Scholar]
- 23.Takeda Pharma. NINLARO summary of product characteristics. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003844/WC500217620.pdf (Accessed January 5, 2017). [Google Scholar]
- 24.Janssen-Cilag International NV. DARZALEX summary of product characteristics. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004077/WC500207296.pdf (Accessed July 21, 2016). [Google Scholar]
- 25.Bristol-Myers Squibb Pharma EEIG. Empliciti summary of product characteristics. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003967/WC500206673.pdf (Accessed August 16, 2016). [Google Scholar]
- 26.De Angelis R, Minicozzi P, Sant M, et al. Survival variations by country and age for lymphoid and myeloid malignancies in Europe 2000–2007: results of EUROCARE-5 population-based study. Eur J Cancer 2015; 51:2254–2268. [DOI] [PubMed] [Google Scholar]
- 27.Mehta J, Cavo M, Singhal S. How I treat elderly patients with myeloma. Blood 2010; 116:2215–2223. [DOI] [PubMed] [Google Scholar]
- 28.Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med 1996; 335:91–97. [DOI] [PubMed] [Google Scholar]
- 29.Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med 2003; 348:1875–1883. [DOI] [PubMed] [Google Scholar]
- 30.Kastritis E, Zervas K, Symeonidis A, et al. Improved survival of patients with multiple myeloma after the introduction of novel agents and the applicability of the International Staging System (ISS): an analysis of the Greek Myeloma Study Group (GMSG). Leukemia 2009; 23:1152–1157. [DOI] [PubMed] [Google Scholar]
- 31.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia 2006; 20:1467–1473. [DOI] [PubMed] [Google Scholar]
- 32.Mateos MV, Richardson PG, Schlag R, et al. Bortezomib plus melphalan and prednisone compared with melphalan and prednisone in previously untreated multiple myeloma: updated follow-up and impact of subsequent therapy in the phase III VISTA trial. J Clin Oncol 2010; 28:2259–2266. [DOI] [PubMed] [Google Scholar]
- 33.San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med 2008; 359:906–917. [DOI] [PubMed] [Google Scholar]
- 34.Harousseau JL, Attal M, Avet-Loiseau H, et al. Bortezomib plus dexamethasone is superior to vincristine plus doxorubicin plus dexamethasone as induction treatment prior to autologous stem-cell transplantation in newly diagnosed multiple myeloma: results of the IFM 2005-01 phase III trial. J Clin Oncol 2010; 28:4621–4629. [DOI] [PubMed] [Google Scholar]
- 35.Sonneveld P, Schmidt-Wolf IG, van der Holt B, et al. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized phase III HOVON-65/GMMG-HD4 trial. J Clin Oncol 2012; 30:2946–2955. [DOI] [PubMed] [Google Scholar]
- 36.Cavo M, Pantani L, Petrucci MT, et al. Bortezomib-thalidomide-dexamethasone is superior to thalidomide-dexamethasone as consolidation therapy after autologous hematopoietic stem cell transplantation in patients with newly diagnosed multiple myeloma. Blood 2012; 120:9–19. [DOI] [PubMed] [Google Scholar]
- 37.Rosinol L, Oriol A, Teruel AI, et al. Superiority of bortezomib, thalidomide, and dexamethasone (VTD) as induction pretransplantation therapy in multiple myeloma: a randomized phase 3 PETHEMA/GEM study. Blood 2012; 120:1589–1596. [DOI] [PubMed] [Google Scholar]
- 38.Palumbo A, Bringhen S, Larocca A, et al. Bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide compared with bortezomib-melphalan-prednisone for initial treatment of multiple myeloma: updated follow-up and improved survival. J Clin Oncol 2014; 32:634–640. [DOI] [PubMed] [Google Scholar]
- 39.Lokhorst HM, van der Holt B, Zweegman S, et al. A randomized phase 3 study on the effect of thalidomide combined with adriamycin, dexamethasone, and high-dose melphalan, followed by thalidomide maintenance in patients with multiple myeloma. Blood 2010; 115:1113–1120. [DOI] [PubMed] [Google Scholar]
- 40.Attal M, Lauwers-Cances V, Marit G, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med 2012; 366:1782–1791. [DOI] [PubMed] [Google Scholar]
- 41.Rajkumar SV, Jacobus S, Callander NS, et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol 2010; 11:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roussel M, Lauwers-Cances V, Robillard N, et al. Front-line transplantation program with lenalidomide, bortezomib, and dexamethasone combination as induction and consolidation followed by lenalidomide maintenance in patients with multiple myeloma: a phase II study by the Intergroupe Francophone du Myelome. J Clin Oncol 2014; 32:2712–2717. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.