Abstract
Deregulated Janus Kinase 2 (JAK2) activation is central to the pathogenesis of most myeloproliferative neoplasms (MPNs), of which essential thrombocythemia (ET) is the most common entity. Patients with ET are risk-stratified according to their risk of thrombo-hemorrhagic complications. High-risk patients are offered treatments to reduce their platelet count using cytoreductive therapy. The disease course is often long and therapy intolerance is not infrequent. Ruxolitinib, a Janus Kinase (JAK) 1/JAK2 inhibitor, has demonstrated efficacy in patients with both myelofibrosis (MF) and polycythemia vera and is well tolerated. Side effects include predictable cytopenias and an augmented risk of infections. Ruxolitinib has been investigated in a small group of ET patients who were refractory/intolerant to hydroxycarbamide (HC) and demonstrated improvements in both symptoms and splenomegaly. Of note, a proportion of treated patients (13.2%) also had a significant reduction in platelet counts. However, these results require further validation in comparison with conventional therapy. Recently, a randomized-controlled phase 2 study (MAJIC-ET) assessed the role of Ruxolitinib in patients refractory or intolerant to HC. This study revealed that Ruxolitinib demonstrated some clinical efficacy but was only superior in terms of symptom control. In clinical practice, some individuals with ET do exhaust all potential treatment options and there may well be a role for Ruxolitinib in such patients or those with a significant symptom burden. However, in the wider context the goal of therapy with the use of JAK inhibitor therapy in ET needs to be defined carefully and we explore this within this timely review article.
Introduction
Essential thrombocythemia (ET), an acquired clonal hemopoietic stem cell disorder, is classified as a BCR-ABL1-negative myeloproliferative neoplasm (MPN).1 Other disorders may present with a thrombocytosis and must be carefully excluded at the time of diagnosis.2
Deregulated Janus Kinase 2 (JAK2) activation is central to the pathogenesis of most MPNs, including ET. A number of genetic mutations are contributory to JAK-STAT pathway activation. An acquired single point mutation in JAK2 (valine to phenylalanine at position 617) (JAK2 V617F), present in 50% to 60% of ET patients, leads to a constitutively active tyrosine kinase.3 Activating mutations in exon 10 of the thrombopoietin receptor (MPL) have been described in between 8% and 10% of ET patients.4 Lastly, Calreticulin (CALR) mutations affecting exon 9 were described by 2 groups in both ET and myelofibrosis (MF) patients lacking JAK2/MPL mutations and are present in approximately 25% of cases.5,6 This translates that up to 20% of patients with ET have an as yet unidentified genetic aberration, the so-called “triple negative ET” cohort, which consists of a heterogeneous group of patients with varying clinical outcomes.7–9 These patients may have other mutations within either JAK2 or MPL genes and it is widely assumed that deregulated JAK-STAT activation is also important in these individuals.8,9
ET is generally regarded as a benign disease but carries an inherent risk of progression to post-ET MF, myelodysplasia, or indeed acute myeloid leukemia (AML). Moreover, patients with ET have a shorter than normal life expectancy with an estimated median survival between 20 and 33 years,10 though this is perhaps likely to be inaccurate due to a lack of long-term robust data. Most complications are related to arterial or venous thrombosis.11 Bleeding due to platelet dysfunction or acquired von Willebrand syndrome occurs especially with high platelet counts.12,13 Constitutional symptoms are common and often inadequately recognized and managed.14,15 A unique clinical assessment tool called the MPN Symptom Assessment Form Total Symptom Score had been devised and validated in MPN patients.16
Some conditions can resemble ET in the clinical setting; the recent World Health Organization (WHO) revision has particularly highlighted the entity prefibrotic-MF (pre-MF).17 Pre-MF is defined by the presence of megakaryocytic proliferation and atypia with reticulin fibrosis grade 0 to 1 on bone marrow; a driver molecular mutation (JAK2V617F, CALR, or MPL), and one of anemia, leukocytosis, splenomegaly and an elevated LDH. Several studies have reviewed patients previously classified as ET and re-stratified a considerable proportion of patients as pre-MF (180/1071: 16.8%)18 and also with patients reported as diagnosed with MF patients.19 ET, pre-MF, and indeed as also highlighted by the WHO, polycythemia vera (PV), share similar characteristics, for instance, all affect young patients (<60 years) but the pre-MF population presents more frequently with splenomegaly, extreme leukocytosis and thrombocytosis than ET.18,20 Regarding nondrivers mutations, it seems the mutational landscape and distribution of ASXL1, SRSF2, U2AF1, SF3B1, EZH2 or IDH1/221 are comparable to that seen in overt-MF. Treatment for pre-MF has not been well defined. Several studies have suggested that the incidence of transformation to MF and AML is higher compared with ET. While in comparison with overt-MF population, the incidence of leukemia is lower and overall survival higher for pre-MF. Therefore, it is very important that in studies evaluating pathogenesis or therapeutics of ET that these other entities are excluded.
ET patients are traditionally risk-stratified based upon their age and/or history of vascular complications.18 A more recent risk scoring system entitled the International Prognostic Score of thrombosis (IPSET-thrombosis) utilized age, thrombosis history, cardiovascular risk factors, and JAK2 mutation status, resulting in 3 distinct risk groups. This system defined the risk of thrombosis better than the traditional 2-tiered model.22 However, limitations of the IPSET-thrombosis system are a lack of prospective validation and the large intermediate-risk group for which the optimal management strategy is uncertain.
Ruxolitinib (Novartis, Basel, Switzerland), a selective JAK 1/2 inhibitor, is currently approved for treatment of MF,23–25 and moreover recently received approval for patients with PV who are refractory/intolerant to hydroxycarbamide (HC).26 Here we discuss the rationale of the potential use of Ruxolitinib in ET.
Current treatment of ET
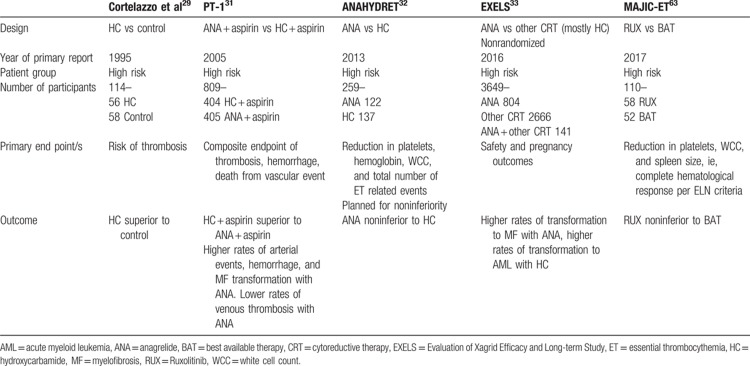
Treatment for patients with ET varies according to individual risk stratification, and ranges from aspirin alone to the use of cytoreductive therapy as shown in Table 1.10 Treatments in ET are not intended to be curative but rather directed at reducing the thrombo-hemorrhagic risk. Unless contra-indicated, most patients should be started on aspirin. A retrospective analysis showed that aspirin for low-risk ET reduced venous thromboembolism in those with the JAK2 mutation and arterial thrombosis in patients with cardiac risk factors.27 Although recently the benefit of aspirin in patients with CALR mutations was questioned our own practice is to use this agent unless bleeding occurs or there is another contra-indication.28 Patients in the high-risk group require cytoreductive therapy to reduce the augmented risk of thrombosis.10
Table 1.
Previous Clinical Trials in High-Risk ET
A landmark trial that investigated the efficacy of HC in high-risk ET was published in 1995.29 Patients were randomized into receiving HC to keep the platelet count <600 × 109/L or received no myelosuppression. Antiplatelet agents were permitted but not mandated. This trial demonstrated that HC treatment resulted in significantly less thrombosis compared with no cytoreduction (3.6% vs 24%).29
Anagrelide (Agrylin/Xagrid, Shire Pharmaceuticals, UK), an inhibitor of cyclic AMP phosphodiesterase III, was initially designed as an antiplatelet agent, and was subsequently found to inhibit both megakaryocyte differentiation and proliferation.30 It is approved for second-line therapy for ET in the EU, and for therapy of MPN by the Food and Drug Administration (FDA). Two randomized studies, primary thrombocythemia-1 (PT-1)31 and ANAHYDRET,32 compared the efficacy and safety of HC versus anagrelide in combination with aspirin for PT-1 and no aspirin in ANAHYDRET.31,32 Both confirmed the efficacy and tolerability of anagrelide as a second-line agent, PT-1 demonstrating that anagrelide was inferior to HC and ANAHYDRET was a noninferiority study that met its primary endpoint. The efficacy and safety of anagrelide in ET was further clarified with the results of the Evaluation of Xagrid Efficacy and Long-term Study (EXELS) study (NCT00567502). This large phase 4 study demonstrated that anagrelide was most frequently prescribed for young patients and confirmed the results from the PT-1 study.33
Interferon-α may lead to up to 80% hematological responses as defined by reduction of hematocrit, white blood cell (WBC), and platelet count.34 However, it can cause significant side effects leading to discontinuation in up to 25% of cases. Hence, it is usually reserved for younger patients or those who are pregnant.34 The efficacy of HC and interferon-α therapy has not yet been compared, however, the results of the MPD-RC 112 trial investigating the efficacy of pegylated interferon-α against HC as a first-line treatment in high-risk ET and PV is awaited.
Other potential therapies for ET include alkylating agents such as Pipobroman and Busulfan. Pipobroman is effective in achieving hematological response but is clearly leukemogenic.35,36 On the other hand, the leukemia-risk of Busulfan was modest when it was used as a short single course.37 The use of these alkylating agents has been reserved for patients with no other option or limited life expectancy. Particular caution should be observed on sequential use of HC and Busulfan that can certainly result in higher leukemia risk.38
Recent therapeutic advances in MPN utilize the knowledge of the deregulated JAK/STAT pathway, which is targetable with JAK inhibitors and other agents.24,25 This review focuses on Ruxolitinib in ET but other JAK inhibitors exist with good efficacy in MF, such as Pacritinib,39 Momelotinib,39–41 and Fedratinib.42,43
Other novel agents are currently being investigated in MPNs including ET. These agents usually target pathways downstream of JAK/STAT activation, such as the phosphatydylinositol-3’-kinase pathway. Targeting the telomere has additionally emerged as an area of interest in myeloid disorders. Imetelstat is a telomerase inhibitor that exhibited good efficacy in both MF and ET and was also observed to cause molecular responses in some patients who had clonality markers, such as JAK2 V617F mutation.44,45 Moreover, Givinostat and Vorinostat, both histone-deacetylase inhibitors, have demonstrated improvements in splenomegaly, symptomatology, and allele burden in phase II studies of patients with ET.46,47
Despite the multiple agents available or undergoing exploration in ET, there is still a significant unmet medical need. In particular, there are only limited treatment options for patients who develop resistance or intolerance to first-line therapy. This group of patients requires a novel agent or combination therapy to control their symptoms and reduce complication risks. In a recent report, the Landmark survey identified that a further treatment aspiration for patients was to reduce risk of transformation although no current therapies have been shown to achieve this.48
Pharmacology of Ruxolitinib
Ruxolitinib (INCB18424) has a molecular weight of 306.4 g/mol. Its chemical name is (R)-3-(4-(7H-pyrrolo[2,3d-]pyrimidin-4-yl)-1H-pyrazol-1-yl)-3-cyclopentylpropanenitrile phosphate, with a molecular formula of C17H18N6. In vitro, Ruxolitinib reduced proliferation of the JAK2 V617F Ba/F3 cell line and interleukin-6 signaling. These findings were replicated in primary PV samples.49,50 In a JAK2 V617F murine model, Ruxolitinib improved survival, splenomegaly, and normalized the cytokine profile. The half maximal inhibitory concentration (IC50) values of Ruxolitinib for JAK1 and JAK2 are 3.3 and 2.8 nM, respectively.49Table 2 summarizes the characteristics of Ruxolitinib.
Table 2.
Characteristics of Ruxolitinib
Oral Ruxolitinib is rapidly absorbed. Maximal plasma concentration was achieved within 1 to 2 hours in fasted subjects, coinciding with maximal inhibition of STAT3 phosphorylation.51 Metabolism is predominantly by CYP3A4 and to a minor degree by CYP2C9 resulting in a less potent metabolite, M18. Inducers or inhibitors of CYP3A4 significantly affect Ruxolitinib metabolism.52 Ruxolitinib is mostly excreted in the urine and to a small extent in the feces.53 The half-life of Ruxolitinib and metabolites is about 6 hours. A phase 1/2 trial established the tolerated starting dose of Ruxolitinib in MF to be 15 mg twice a day followed by individualized titration.54
Clinical efficacy of Ruxolitinib
Phase 3 studies, COMFORT-1 and COMFORT-2, led to approval of Ruxolitinib for the management of symptoms and splenomegaly in MF. COMFORT-1 (NCT00952289) included 309 patients randomized 1:1 to either Ruxolitinib or placebo; 41.9% in the Ruxolitinib group had a 35% spleen volume reduction compared with 0.7% in the placebo group. Symptom improvement of more than 50% was observed in 45.9% of Ruxolitinib group and 5.3% of the placebo group. These benefits were seen regardless of JAK2 mutation status.24 A survival advantage was subsequently demonstrated in the Ruxolitinib arm but may have even been underestimated by the crossover design of the trial.55 COMFORT-2 (NCT00934544) included 219 patients randomized in a 2:1 fashion to Ruxolitinib or best available therapy (BAT). The primary end point, a 35% reduction of spleen volume at 48 weeks, was attained by 28% of the Ruxolitinib group. Patients in the Ruxolitinib-treated group also reported significant symptom improvement.25 At 144 weeks, splenomegaly response was sustained and improved survival compared with BAT was additionally demonstrated.23
Two studies investigating the role of Ruxolitinib in PV have been recently reported. The first was a phase 2 trial in high-risk PV/ET patients who were refractory or intolerant to HC (NCT00726232). Thirty-four PV patients received Ruxolitinib for a median of 35.0 months, 97% achieving a hematocrit <45% without phlebotomy by week 24. Moreover, spleen size reduction was observed in 63% of patients by week 144. Maintained symptom improvement was observed as early as week 4.56 This study established a starting dose of 10 mg twice daily. The second, RESPONSE (NCT01243944), was a phase 3 study in PV patients demonstrating resistance/intolerance to HC. Here patients were randomized 1:1 to receive either Ruxolitinib or BAT. Crossover occurred at week 32 if primary endpoints were not met or later. A total of 222 patients were randomized. The composite primary endpoints, hematocrit control and 35% spleen volume reduction, were reached in 20.9% of Ruxolitinib and only 0.9% of BAT groups, respectively. A 50% reduction in the MPN-SAF total symptom score occurred in 49% of the Ruxolitinib group but only 0.9% in the BAT group.57 Recent 80-week follow-up data from the RESPONSE study were presented during 2015, these data were reassuring for prolonged maintenance of responses.58
Although these studies demonstrate a possible role of Ruxolitinib in PV and led to its approval for this indication by the FDA and European Medicines Agency, the relevance of the primary end points in this disorder, particularly spleen size reduction might be questioned. Certainly the limited therapy options for such patients are also demonstrated by the fact that over 50% of BAT patients went back to receive HC. Currently, there is no evidence that Ruxolitinib reduces the risk of PV transforming to MF and AML. Nonetheless, Ruxolitinib appeared useful in maintaining adequate hematocrit control in patients with high-risk PV who are refractory to HC. This and control of leukocytes and inflammation may have translated to a lower than expected risk of thromboembolic events in patients who received Ruxolitinib in the RESPONSE trial (1.2 events per 100 patient years).57 This may well be due, at least in part, to the effect of Ruxolitinib on reducing both the WBC count and other cells involve in moderating inflammation and immune response like natural killers and T regulatory cells; and inflammatory markers (eg, IL6, TNF-α, or C-reactive protein) which may be responsible patient's constitutional symptoms.59 However, this was not prespecified as an endpoint in the study and due to the crossover aspect of the trial cannot be evaluated further. Two other studies have evaluated the efficacy of Ruxolitinib in PV: RELIEF,60 and RESPONSE 261 demonstrating similar results. In the real-world setting, data from the MAJIC-PV trial are awaited.
Safety and tolerability of Ruxolitinib in MF and PV
The safety profile of Ruxolitinib in MPN was primarily established in the COMFORT trials. Discontinuation rates were low in these 2 studies, ranging from 8% to 11% within study periods. Grade 3 to 4 nonhematological malignancies were uncommon. Common adverse events included fatigue, diarrhea, weight gain, and dyspnea.24,25
Hematological toxicity was noted to be more prevalent in the Ruxolitinib arm as compared to BAT in COMFORT-2. Anemia was mostly managed with dose modifications (5%), transfusions, or both. More patients in the Ruxolitinib arm required at least 1 unit of packed red cells (51% vs 38% in BAT arm) though the mean number of transfusions per month was not significantly different.25 Thrombocytopenia usually led to dose modification or interruption in both studies. In COMFORT-1, grade 3 to 4 bleeding episodes took place with similar rate in both Ruxolitinib and placebo arms. Bruising occurred more often in patients on Ruxolitinib but was grade 3 in only 1 patient.24 Progression to AML was similar in both arms of these studies.24,25
In the phase 1/2 PV/ET study, grade 3/4 leukopenia was observed in 3 patients (7.7%) while grade 3 anemia was observed in 1 patient only (2.6%). Two patients had more than grade 3 infections, both involving the respiratory tract. Nonhematological adverse events included weight gain, diarrhea, cough, and headache.62 Similar data are available from the RESPONSE trial, both the initial report and the 80-week follow-up data.58 In MAJIC-ET, hematological toxicity was again notably more common in the Ruxolitinib group with grade 3/4 anemia occurring in 21% compared with 0% in the BAT group, and 2 patients discontinued Ruxolitinib treatment because of anemia.63 Grade 3 infections were also more frequent with Ruxolitinib occurring in 15.5%, compared with 3.5% of those treated with BAT, although no grade 4 infections were reported.
There is increasing evidence that Ruxolitinib is potently immunosuppressive as higher incidences of herpes infections, urinary and respiratory infections have occurred in Ruxolitinib-treated patients across all the phase 3 trials reported to date. Increased risk of basal-cell and squamous-cell carcinomas was demonstrated in the RESPONSE trial but did not result in interruption of therapy. However, it was noted that more patients with a prior history of these conditions were assigned to the Ruxolitinib and these patients had higher exposure previously to HC.57 Case reports of Cryptococcus neoformans pneumonia,64Pneumocystis jiroveci pneumonitis,65 bilateral toxoplasmosis retinitis,66 and hepatitis B reactivation67 among others have been described as complications in patients receiving Ruxolitinib as discussed in a recent review.68 Significant morbidity involving Ruxolitinib and infection have also been reported, for example, an Epstein-Barr virus-driven lymphoproliferative disorder69 and a case of progressive multifocal leukoencephalopathy (PML) associated with JC-Virus 4070 resulted in permanent disability. Well-described effects on T cell subsets,71 natural killer cells,72 and dendritic cell function and migration73 have also been described and may contribute to the higher rate of atypical infectious complications occurring in a number of patients.68
Ruxolitinib in ET
Table 3 summarizes the current studies investigating the use of Ruxolitinib in ET. An open label, phase 2 study investigating the efficacy and safety of Ruxolitinib in patients with high-risk PV and ET who are refractory or intolerant to HC has been reported. The PV aspect of the study was discussed above. There were 39 high-risk ET patients resistant to HC who were treated with Ruxolitinib within this trial for a median exposure of 205.6 weeks. All patients were followed up for a period of 48 months. At the time of data cut off, 61.5% of patients were still receiving Ruxolitinib.62 Efficacy was as follows: 13.2% of those with platelet >400 × 109/L had reduction to <400 × 109/L; 72.7% with WBC count >10 × 109/L had a WBC <10 × 109/L at 48 months. These data suggest more than modest efficacy and the median exposure period is actually long for a phase II study though it is appropriate for a disease with a protracted course such as ET. All of the ET patients with a palpable spleen had >50% reduction; improvement in symptoms were also reported.
Table 3.
Current Clinical Trials Assessing Ruxolitinib in ET
In a recent publication of a cohort of patients from this study, Pieri et al's report that 3 patients (2 ET and 1 PV) achieved and maintained a complete molecular response (CMR) with Ruxolitinib at 5-year review.56 At the time of CMR, the patient with PV also had complete hematological remission while the patients with ET had partial hematological remission, although their bone marrows displayed persistent morphological features of MPN. Furthermore, the patient with PV had a persistent TET2 Y867H mutation at 5 years. These findings, if replicated, could be of significant importance and it will also be essential to look at molecular responses for other “nondriver mutations” in addition, such as ASXL1. The only other therapies reported to induce molecular responses in ET are interferon alpha and imetelstat as discussed above. Data for effects on clones such as TET2 as discussed above suggest interferon may not be comprehensively effective74 and there was also an admixed clonal response for imetelstat where clonal emergence with therapy was identified.75
MAJIC-ET, a phase 2 trial in which 110 patients with high-risk ET either intolerant or resistant to HC, were randomized on a 1:1 basis between Ruxolitinib and BAT, has recently reported initial results.63 BAT consisted predominantly of HC (71.1%), anagrelide (48.1%), and interferon (40.4%), with many patients in this group receiving more than 1 line of therapy at different time points. The primary outcome was achievement of a CR as defined by a platelet count of <400 × 109/L, white cell count <10 × 109/L, and a normal spleen size. Ruxolitinib was shown to be noninferior to BAT with 46.5% achieving CR compared with 44.2% in the BAT group. Similarly, PR was largely equivalent in the 2 groups, occurring in 46.5% treated with Ruxolitinib compared with 51.9% of those receiving BAT. There was no evidence of a difference in the duration of overall response between Ruxolitinib and BAT, and OS and PFS at 1 year were also similar. Safety and tolerability were similar to that reported in PV and MF.
Molecular analysis was also performed in MAJIC-ET, with the overall mean allele burden for JAK2, MPL, and CALR mutations not reduced after 1 year of treatment with Ruxolitinib. Interestingly, a single CMR was seen in a JAK2 V617F positive patient and 2 occurred in CALR-mutated patients treated with Ruxolitinib. With regard to disease transformation, there were no statistically significant differences between the 2 groups. Importantly the validity of CMR was called into question as a consequence of one of these patients attaining a CMR and then progressing to PET-MF. Thrombosis was seen in the Ruxolitinib group on 11 occasions in 10 patients, compared with 5 events in 3 patients in the BAT group; however, thrombosis-free probability between the 2 groups, although borderline, was not statistically significant (P = 0.09).
Overall 85 patients completed a symptom burden questionnaire at baseline and at least once during treatment. The maximum total symptom score reduction taken from any point during the first 12 months of treatment was significantly greater in the Ruxolitinib group with a median reduction of 32% compared with 0% in the BAT group. Ruxolitinib was especially noted to result in improvements in pruritus, depression, concentration and resulted in a greater ability to perform normal activities compared with BAT.
While the results from MAJIC-ET do not suggest that Ruxolitinib should be preferred to currently available therapies in the management of most patients with ET, it has shown similar efficacy and therefore could be considered as an alternative option if available. Furthermore, for a subset of patients with particularly troublesome symptoms, it has shown to be efficacious and should be considered at an earlier time point where possible. The relatively high costs of Ruxolitinib treatment and potential for toxicities, particularly infective complications, relating to the long-term exposure required in patients with ET, are probably the main barriers to routine implementation. Further studies are also warranted to evaluate, in more depth, the role of Ruxolitinib in preventing both thrombosis and transformation.
Conclusion
Though the use of Ruxolitinib as a first-line therapy has been well established in MF, usage in ET, and perhaps PV, needs further evaluation. This is in part due to the fact that these patients with ET have near-normal life expectancy, unlike in MF, and hence potential exposure to this agent could be long. Survival benefits may therefore be harder to detect in ET. Similarly, the long-term use of Ruxolitinib in patients with ET has to be balanced with the potential risks, albeit small, of infection and secondary malignancy, particularly cutaneous squamous cell cancers, and by the lack of long-term follow-up data. Nonetheless, Ruxolitinib may play a crucial role in patients with high-risk ET patients refractory/intolerant of HC since there are limited options of therapy currently available. For example, more than half of the BAT in RESPONSE study was HC. ET is a chronic long-term condition and while other second-line therapies exist many patients become intolerant of them and/or are too young to be considered for agents such as busulfan. Future studies will also need to directly address the question of Ruxolitinib's impact on symptom improvement and reduction of thrombotic events. In fact, a recent study demonstrated possible biological reasons behind reduction of thrombosis by Ruxolitinib.76
As the first therapy that specifically targets the deregulated JAK-STAT signaling in MPN, Ruxolitinib has the potential to transform therapeutic options for these disorders. In MF, Ruxolitinib has been shown to have a profound effect on symptoms, spleen size, and improved survival. In HC resistant/intolerant PV, control of the blood count, symptoms, and splenomegaly were demonstrated for patients where treatment options may be limited. Importantly there was a suggestion of reduced thrombotic events, but no evidence of effects upon transformation of disease to MF or AML in PV patients.
To date there is much less data concerning efficacy of Ruxolitinib in ET yet there is a well-defined need for new therapeutic approaches. This condition, the commonest of the classical MPNs, often requires treatment over the course of many decades, patients often cycle through many of the conventional therapies which do usually control the blood count and reduce the risk of thrombosis but do not affect the natural history of the disease or symptom burden which can be profound. These data to date suggest that Ruxolitinib can control myeloproliferation in ET though its efficacy in controlling thrombocytosis per se is relatively low, control of the leukocyte count, which may be of augmented importance, is more impressive. The relevance of improvement in quality of life also cannot be overemphasized where some patients have a high symptom burden from a disease that will be present for several decades. Recently available preliminary data suggesting some ET patients may enter a molecular remission with Ruxolitinib are tantalizing. However, the economic justification for using this expensive therapy will also be more challenging in ET. Furthermore, while considered relatively safe, Ruxolitinib is an immunosuppressant: increasing propensity for infection and developing skin cancers, especially in those predisposed. Hence, a thorough risk-benefit assessment is mandated before considering Ruxolitinib as an agent with therapeutic benefit in ET.
Footnotes
Citation: Gunawan A, Harrington P, Garcia-Curto N, McLornan D, Radia D, Harrison C. Ruxolitinib for the Treatment of Essential Thrombocythemia. HemaSphere, 2018;2:4 http://dx.doi.org/10.1097/HS9.0000000000000056.
Funding/support: None.
Disclosure: DM: Honoraria and Speaker Bureau/Advisory Board: Jazz Pharma; Novartis and Celgene. CH receives research support, honoraria, and speaker fees from Novartis. AG, PH, and NG-C have indicated they have no potential conflicts of interest to disclose.
AG, PH, and NG-C: Participated in writing the paper. DM and CH: Participated in writing and reviewing the paper. DR: Participated in reviewing the paper.
References
- 1.Swerdlow SH, Jaffe ES. International Agency for Research on Cancer, World Health Organization. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: International Agency for Research on Cancer; 2008. [Google Scholar]
- 2.Alimam S, Wilkins BS, Harrison CN. How we diagnose and treat essential thrombocythaemia. Br J Haematol 2015; 171:306–321. [DOI] [PubMed] [Google Scholar]
- 3.Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 2005; 7:387–397. [DOI] [PubMed] [Google Scholar]
- 4.Pikman Y, Lee BH, Mercher T, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med 2006; 3:e270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nangalia J, Massie CE, Baxter EJ, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med 2013; 369:2391–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klampfl T, Gisslinger H, Harutyunyan AS, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med 2013; 369:2379–2390. [DOI] [PubMed] [Google Scholar]
- 7.Tefferi A, Thiele J, Vannucchi AM, et al. An overview on CALR and CSF3R mutations and a proposal for revision of WHO diagnostic criteria for myeloproliferative neoplasms. Leukemia 2014; 28:1407–1413. [DOI] [PubMed] [Google Scholar]
- 8.Cabagnols X, Favale F, Pasquier F, et al. Presence of atypical thrombopoietin receptor (MPL) mutations in triple negative essential thrombocythemia patients. Blood 2015; 127:333–342. [DOI] [PubMed] [Google Scholar]
- 9.Milosevic Feenstra JD, Nivarthi H, Gisslinger H, et al. Whole exome sequencing identifies novel MPL and JAK2 mutations in triple negative myeloproliferative neoplasms. Blood 2015; 127:325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tefferi A, Barbui T. Polycythemia vera and essential thrombocythemia: 2015 update on diagnosis, risk-stratification and management. Am J Hematol 2015; 90:162–173. [DOI] [PubMed] [Google Scholar]
- 11.Passamonti F, Thiele J, Girodon F, et al. A prognostic model to predict survival in 867 World Health Organization-defined essential thrombocythemia at diagnosis: a study by the International Working Group on Myelofibrosis Research and Treatment. Blood 2012; 120:1197–1201. [DOI] [PubMed] [Google Scholar]
- 12.Budde U, Schaefer G, Mueller N, et al. Acquired von Willebrand's disease in the myeloproliferative syndrome. Blood 1984; 64:981–985. [PubMed] [Google Scholar]
- 13.Lancellotti S, Dragani A, Ranalli P, et al. Qualitative and quantitative modifications of von Willebrand factor in patients with essential thrombocythemia and controlled platelet count. J Thromb Haemost 2015; 13:1226–1237. [DOI] [PubMed] [Google Scholar]
- 14.Mesa RA, Scherber RM, Geyer HL. Reducing symptom burden in patients with myeloproliferative neoplasms in the era of Janus kinase inhibitors. Leuk Lymphoma 2015; 56:1989–1999. [DOI] [PubMed] [Google Scholar]
- 15.Mesa RA, Niblack J, Wadleigh M, et al. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): an international Internet-based survey of 1179 MPD patients. Cancer 2007; 109:68–76. [DOI] [PubMed] [Google Scholar]
- 16.Emanuel RM, Dueck AC, Geyer HL, et al. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol 2012; 30:4098–4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016; 127:2391–2405. [DOI] [PubMed] [Google Scholar]
- 18.Barbui T, Barosi G, Birgegard G, et al. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. J Clin Oncol 2011; 29:761–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guglielmelli P, Pacilli A, Rotunno G, et al. Presentation and outcome of patients with 2016 WHO diagnosis of prefibrotic and overt primary myelofibrosis. Blood 2017; 129:3227–3236. [DOI] [PubMed] [Google Scholar]
- 20.Rumi E, Boveri E, Bellini M, et al. Clinical course and outcome of essential thrombocythemia and prefibrotic myelofibrosis according to the revised WHO 2016 diagnostic criteria. Oncotarget 2017; 8:101735–101744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mudireddy M, Shah S, Lasho T, et al. Prefibrotic versus overtly fibrotic primary myelofibrosis: clinical, cytogenetic, molecular and prognostic comparisons. Br J Haematol 2017; Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 22.Barbui T, Finazzi G, Carobbio A, et al. Development and validation of an International Prognostic Score of thrombosis in World Health Organization-essential thrombocythemia (IPSET-thrombosis). Blood 2012; 120:5128–5133. [DOI] [PubMed] [Google Scholar]
- 23.Cervantes F, Vannucchi AM, Kiladjian JJ, et al. Three-year efficacy, safety, and survival findings from COMFORT-II, a phase 3 study comparing Ruxolitinib with best available therapy for myelofibrosis. Blood 2013; 122:4047–4053. [DOI] [PubMed] [Google Scholar]
- 24.Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of Ruxolitinib for myelofibrosis. N Engl J Med 2012; 366:799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with Ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med 2012; 366:787–798. [DOI] [PubMed] [Google Scholar]
- 26.Tefferi A, Pardanani A. Myeloproliferative neoplasms: a contemporary review. JAMA Oncol 2015; 1:97–105. [DOI] [PubMed] [Google Scholar]
- 27.Alvarez-Larran A, Cervantes F, Pereira A, et al. Observation versus antiplatelet therapy as primary prophylaxis for thrombosis in low-risk essential thrombocythemia. Blood 2010; 116:1205–1210. [DOI] [PubMed] [Google Scholar]
- 28.Alvarez-Larran A, Pereira A, Guglielmelli P, et al. Antiplatelet therapy versus observation in low-risk essential thrombocythemia with a CALR mutation. Haematologica 2016; 101:926–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cortelazzo S, Finazzi G, Ruggeri M, et al. Hydroxyurea for patients with essential thrombocythemia and a high risk of thrombosis. N Engl J Med 1995; 332:1132–1136. [DOI] [PubMed] [Google Scholar]
- 30.Tomer A. Effects of anagrelide on in vivo megakaryocyte proliferation and maturation in essential thrombocythemia. Blood 2002; 99:1602–1609. [DOI] [PubMed] [Google Scholar]
- 31.Harrison CN, Campbell PJ, Buck G, et al. Hydroxyurea compared with anagrelide in high-risk essential thrombocythemia. N Engl J Med 2005; 353:33–45. [DOI] [PubMed] [Google Scholar]
- 32.Gisslinger H, Gotic M, Holowiecki J, et al. Anagrelide compared with hydroxyurea in WHO-classified essential thrombocythemia: the ANAHYDRET Study, a randomized controlled trial. Blood 2013; 121:1720–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Birgegard G, Besses C, Griesshammer M, et al. Treatment of essential thrombocythemia in Europe: a prospective long-term observational study of 3649 high-risk patients in the Evaluation of Anagrelide Efficacy and Long-term Safety study. Haematologica 2018; 103:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kiladjian JJ, Chomienne C, Fenaux P. Interferon-alpha therapy in bcr-abl-negative myeloproliferative neoplasms. Leukemia 2008; 22:1990–1998. [DOI] [PubMed] [Google Scholar]
- 35.De Sanctis V, Mazzucconi MG, Spadea A, et al. Long-term evaluation of 164 patients with essential thrombocythaemia treated with pipobroman: occurrence of leukaemic evolution. Br J Haematol 2003; 123:517–521. [DOI] [PubMed] [Google Scholar]
- 36.Najean Y, Rain JD. Treatment of polycythemia vera: the use of hydroxyurea and pipobroman in 292 patients under the age of 65 years. Blood 1997; 90:3370–3377. [PubMed] [Google Scholar]
- 37.Shvidel L, Sigler E, Haran M, et al. Busulphan is safe and efficient treatment in elderly patients with essential thrombocythemia. Leukemia 2007; 21:2071–2072. [DOI] [PubMed] [Google Scholar]
- 38.Finazzi G, Ruggeri M, Rodeghiero F, et al. Second malignancies in patients with essential thrombocythaemia treated with busulphan and hydroxyurea: long-term follow-up of a randomized clinical trial. Br J Haematol 2000; 110:577–583. [DOI] [PubMed] [Google Scholar]
- 39.Komrokji RS, Seymour JF, Roberts AW, et al. Results of a phase 2 study of pacritinib (SB1518), a JAK2/JAK2(V617F) inhibitor, in patients with myelofibrosis. Blood 2015; 125:2649–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pardanani A, Laborde RR, Lasho TL, et al. Safety and efficacy of CYT387, a JAK1 and JAK2 inhibitor, in myelofibrosis. Leukemia 2013; 27:1322–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beauverd Y, McLornan DP, Harrison CN. Pacritinib: a new agent for the management of myelofibrosis? Expert Opin Pharmacother 2015; 16:2381–2390. [DOI] [PubMed] [Google Scholar]
- 42.Pardanani A, Harrison C, Cortes JE, et al. Safety and efficacy of fedratinib in patients with primary or secondary myelofibrosis: a randomized clinical trial. JAMA Oncol 2015; 1:643–651. [DOI] [PubMed] [Google Scholar]
- 43.Pardanani A, Tefferi A, Jamieson C, et al. A phase 2 randomized dose-ranging study of the JAK2-selective inhibitor fedratinib (SAR302503) in patients with myelofibrosis. Blood Cancer J 2015; 5:e335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baerlocher GM, Oppliger Leibundgut E, Ottmann OG, et al. Telomerase inhibitor imetelstat in patients with essential thrombocythemia. N Engl J Med 2015; 373:920–928. [DOI] [PubMed] [Google Scholar]
- 45.Tefferi A, Lasho TL, Begna KH, et al. A pilot study of the telomerase inhibitor imetelstat for myelofibrosis. N Engl J Med 2015; 373:908–919. [DOI] [PubMed] [Google Scholar]
- 46.Rambaldi A, Dellacasa CM, Finazzi G, et al. A pilot study of the Histone-Deacetylase inhibitor Givinostat in patients with JAK2V617F positive chronic myeloproliferative neoplasms. Br J Haematol 2010; 150:446–455. [DOI] [PubMed] [Google Scholar]
- 47.Andersen CL, McMullin MF, Ejerblad E, et al. A phase II study of vorinostat (MK-0683) in patients with polycythaemia vera and essential thrombocythaemia. Br J Haematol 2013; 162:498–508. [DOI] [PubMed] [Google Scholar]
- 48.Harrison CN, Koschmieder S, Foltz L, et al. The impact of myeloproliferative neoplasms (MPNs) on patient quality of life and productivity: results from the international MPN Landmark survey. Ann Hematol 2017; 96:1653–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quintas-Cardama A, Vaddi K, Liu P, et al. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood 2010; 115:3109–3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quintas-Cardama A, Kantarjian H, Cortes J, et al. Janus kinase inhibitors for the treatment of myeloproliferative neoplasias and beyond. Nat Rev Drug Discov 2011; 10:127–140. [DOI] [PubMed] [Google Scholar]
- 51.Shi JG, Chen X, McGee RF, et al. The pharmacokinetics, pharmacodynamics, and safety of orally dosed INCB018424 phosphate in healthy volunteers. J Clin Pharmacol 2011; 51:1644–1654. [DOI] [PubMed] [Google Scholar]
- 52.Shi JG, Chen X, Emm T, et al. The effect of CYP3A4 inhibition or induction on the pharmacokinetics and pharmacodynamics of orally administered Ruxolitinib (INCB018424 phosphate) in healthy volunteers. J Clin Pharmacol 2012; 52:809–818. [DOI] [PubMed] [Google Scholar]
- 53.Shilling AD, Nedza FM, Emm T, et al. Metabolism, excretion, and pharmacokinetics of [14C]INCB018424, a selective Janus tyrosine kinase 1/2 inhibitor, in humans. Drug Metab Dispos 2010; 38:2023–2031. [DOI] [PubMed] [Google Scholar]
- 54.Verstovsek S, Kantarjian H, Mesa RA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med 2010; 363:1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Verstovsek S, Mesa RA, Gotlib J, et al. Efficacy, safety, and survival with Ruxolitinib in patients with myelofibrosis: results of a median 3-year follow-up of COMFORT-I. Haematologica 2015; 100:479–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pieri L, Pancrazzi A, Pacilli A, et al. JAK2V617F complete molecular remission in polycythemia vera/essential thrombocythemia patients treated with Ruxolitinib. Blood 2015; 125:3352–3353. [DOI] [PubMed] [Google Scholar]
- 57.Vannucchi AM, Kiladjian JJ, Griesshammer M, et al. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N Engl J Med 2015; 372:426–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kiladjian JJ, Vanucchi AM, Griesshammer M, et al. Ruxolitinib versus best available therapy in patients with polycythaemia vera: 80-week follow-up from the RESPONSE trial. Paper presented at: 20th Congress of the European Hematology Association, Vienna, Austria; June 22, 2015. [Google Scholar]
- 59.McLornan DP, Khan AA, Harrison CN. Immunological consequences of JAK inhibition: friend or foe? Curr Hematol Malig Rep 2015; 10:370–379. [DOI] [PubMed] [Google Scholar]
- 60.Mesa R, Vannucchi AM, Yacoub A, et al. The efficacy and safety of continued hydroxycarbamide therapy versus switching to Ruxolitinib in patients with polycythaemia vera: a randomized, double-blind, double-dummy, symptom study (RELIEF). Br J Haematol 2017; 176:76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Passamonti F, Griesshammer M, Palandri F, et al. Ruxolitinib for the treatment of inadequately controlled polycythaemia vera without splenomegaly (RESPONSE-2): a randomised, open-label, phase 3b study. Lancet Oncol 2017; 18:88–99. [DOI] [PubMed] [Google Scholar]
- 62.Passamonti F, Rumi E, Della Porta MG, et al. INCB018424, a selective inhibitor of JAK1 and JAK2, downregulates the expression of Leukocyte Alkaline Phosphatase (LAP) on circulating granulocytes in patients with polycythemia vera and essential thrombocythemia (Abstract). Paper presented at: 51st ASH Annual Meeting and Exposition 2009, New Orleans, LA. [Google Scholar]
- 63.Harrison CN, Mead AJ, Panchal A, et al. Ruxolitinib vs best available therapy for ET intolerant or resistant to hydroxycarbamide. Blood 2017; 130:1889–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wysham NG, Sullivan DR, Allada G. An opportunistic infection associated with Ruxolitinib, a novel janus kinase 1,2 inhibitor. Chest 2013; 143:1478–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee SC, Feenstra J, Georghiou PR. Pneumocystis jiroveci pneumonitis complicating Ruxolitinib therapy. BMJ Case Rep 2014; 2014:pii: bcr2014204950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goldberg RA, Reichel E, Oshry LJ. Bilateral toxoplasmosis retinitis associated with Ruxolitinib. N Engl J Med 2013; 369:681–683. [DOI] [PubMed] [Google Scholar]
- 67.Caocci G, Murgia F, Podda L, et al. Reactivation of hepatitis B virus infection following Ruxolitinib treatment in a patient with myelofibrosis. Leukemia 2014; 28:225–227. [DOI] [PubMed] [Google Scholar]
- 68.Galli S, McLornan D, Harrison C. Safety evaluation of Ruxolitinib for treating myelofibrosis. Expert Opin Drug Saf 2014; 13:967–976. [DOI] [PubMed] [Google Scholar]
- 69.Palmason R, Linden O, Richter J. Case-report: EBV driven lymphoproliferative disorder associated with Ruxolitinib. BMC Hematol 2015; 15:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wathes R, Moule S, Milojkovic D. Progressive multifocal leukoencephalopathy associated with Ruxolitinib. N Engl J Med 2013; 369:197–198. [DOI] [PubMed] [Google Scholar]
- 71.Parampalli Yajnanarayana S, Stubig T, Cornez I, et al. JAK1/2 inhibition impairs T cell function in vitro and in patients with myeloproliferative neoplasms. Br J Haematol 2015; 169:824–833. [DOI] [PubMed] [Google Scholar]
- 72.Schonberg K, Rudolph J, Vonnahme M, et al. JAK inhibition impairs NK cell function in myeloproliferative neoplasms. Cancer Res 2015; 75:2187–2199. [DOI] [PubMed] [Google Scholar]
- 73.Heine A, Held SA, Daecke SN, et al. The JAK-inhibitor Ruxolitinib impairs dendritic cell function in vitro and in vivo. Blood 2013; 122:1192–1202. [DOI] [PubMed] [Google Scholar]
- 74.Kiladjian JJ, Masse A, Cassinat B, et al. Clonal analysis of erythroid progenitors suggests that pegylated interferon alpha-2a treatment targets JAK2V617F clones without affecting TET2 mutant cells. Leukemia 2010; 24:1519–1523. [DOI] [PubMed] [Google Scholar]
- 75.Leibundgut EO, Haubitz M, Burrington B, et al. Dynamics of mutations in patients with ET treated with Imetelstat. 57th ASH Annual meeting and Exposition, Orlando, FL; 2015. [Google Scholar]
- 76.Keohane C, McLornan DP, Sanchez K, et al. The effects of JAK inhibitor therapy upon novel markers of thrombosis in myeloproliferative neoplasms. Haematologica 2015; 100:e348–e350. [DOI] [PMC free article] [PubMed] [Google Scholar]