Malaria is a life-threatening disease which typically causes symptoms that include fever, tiredness, vomiting, headaches, and anemia, and is transmitted through the bite of an infected Anopheles mosquito which carries the Plasmodium parasite. In those who have recently survived an infection, reinfection usually causes milder symptoms. Naturally acquired immunity in fact develops over time after repeated infections and the production of antimalarial antibodies is thought to play a crucial role.1,2 Humoral immunity to malarial parasites can protect against disease, although the precise mechanisms remain unclear.1,2 The important role of antibodies in the host defense against malaria was demonstrated when serum transferred from healthy malaria immune adults to hospitalized children with malaria resulted in a significant amelioration of symptoms and parasitemia.3
During infection, parasite-specific antibodies are generated, which help to reduce the amount of parasitized red blood cells (pRBCs) and decrease parasitemia.4–6 Infection-induced antibodies might also control parasite numbers during a secondary plasmodium infection. Since the number of pRBCs can be very elevated and correlate with disease severity in humans and experimental animals, strategies to reduce pRBC number and minimize parasite replication are therefore valuable to prevent or ameliorate malaria.1,2,4
Antibodies are highly effective at controlling pRBC numbers in the bloodstream but their function in vivo has remained almost completely unexplored. In vitro studies predict that antibodies may function in different ways, including phagocyte- and complement-mediated mechanisms.2,4 An increasing number of plasmodium antigens have been identified as relevant to acquired humoral immunity and are considered potential targets for vaccine.1,2 The targets of protective antibodies include proteins expressed on the sporozoite needed for hepatocyte invasion, or on the merozoite surface needed for erythrocyte invasion, and variant surface antigens (VSAs) delivered to the surface of the infected erythrocyte and important for sequestration and pathogenesis.2 Antimalarial IgG antibodies may function to block parasite invasion of hepatocytes and erythrocytes, opsonize parasites and pRBCs and activate complement-mediated processes.2 However, to date the mechanisms responsible for antibodies-induced parasite control have been poorly investigated in vivo.
A recent paper in PLoS Pathogens elucidated the molecular mechanisms underlying the protective effect of these antibodies in the control of blood-stage parasites.4 Akter et al took advantage of animal models of antibody-mediated immunity in the context of malaria infection, obtained by treating plasmodium-infected mice with immune serum from mice that resolved a primary plasmodium infection.4,5 This approach showed that immune sera successfully control parasitemia in infected mice and provided a model to study the mechanisms involved. A labeled pRBC adoptive transfer technique was applied to study parasite clearance and replication in vivo.4,5 Interestingly, the transfer of plasmodium-infected labeled RBCs in mice treated with immune sera failed to show any difference in parasite life-cycle stages of pRBCs (rings, trophozoites, schizonts) as well as in labeled pRBCs numbers postinfusion compared to nonimmune sera-treated mice, despite the observation of lower parasitemia. This result suggested that immune sera-mediated control of parasitemia is independent from pRBC clearance.
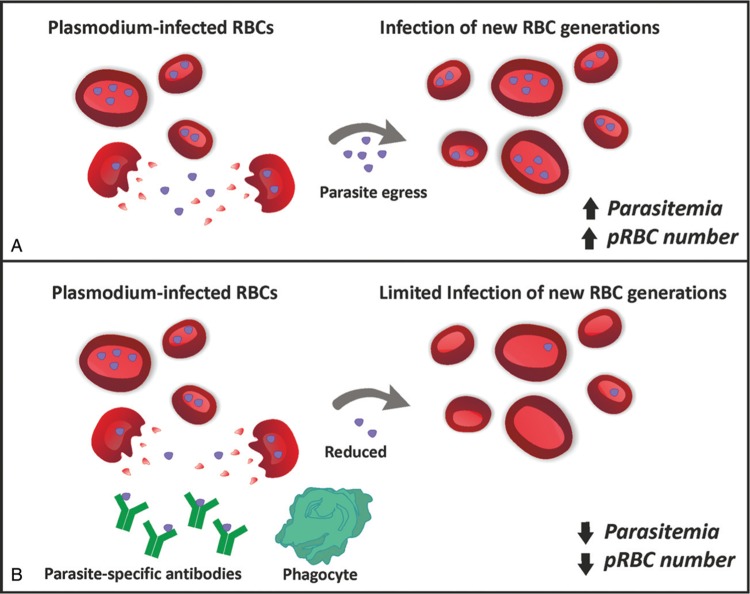
Further experiments revealed that antibodies in immune sera reduce parasite replication by blocking their ability to infect subsequent generation of uninfected RBCs (Fig. 1). Following the infusion of labeled pRBCs, which remain comparable over time in mice treated with immune sera vs control sera, the number of nonlabeled infected RBCs was instead drastically reduced by immune sera administration, demonstrating a crucial role for plasmodium-specific antibodies in blocking parasite transmission to new yet uninfected RBC generations.4–6 Experiments of phagocyte depletion in mice obtained through clodronate treatment showed that phagocyte functions are required, at least in part, to mediate the protective effect of antibodies and their removal did not efficiently prevent parasite infection of new RBC generations and parasite multiplication rate. These findings demonstrate that parasite-specific antibodies act synergistically with splenic macrophages and dendritic cells to restrict the transition of parasites from one to the following RBC generation (Fig. 1). This process does not involve complement-mediated mechanisms. Whereas antibodies in immune sera have a poor ability to bind antigens on the surface of infected RBCs, they show a relevant capacity to bind multiple internal structures in permeabilized infected RBCs. Therefore upon late-stage pRBC rupture, antibodies might be able to recognize those parasite structures, giving rise to a protective immune response (Fig. 1). This study highlights how targeting pRBC surface with antibody is not required for parasite control, but recognition of free parasite structures is. Finally, the authors showed the validity of their findings for different Plasmodium strains, including Plasmodium yoelii and Plasmodium chabaudi chabaudi.
Figure 1.
Parasite-specific antibodies reduce parasitemia and infected RBC number. Schematic representation of the effect of immune sera without (A) or with (B) parasite-specific antibodies on parasitemia and pRBC number. During plasmodium infection, a naturally acquired humoral immunity develops and gives rise to antimalarial antibodies with the ability to significantly reduce parasitemia and infected RBC number (B). These antibodies act by recognizing specific parasite structures released upon infected RBC rupture and start an immune response that involves the role of phagocytes. This mechanism does not imply infected RBC clearance but rather the prevention of parasite transition to a new generation of yet uninfected RBCs. Overall this process leads to a drastic reduction of infected RBC number, with beneficial effect in infected individuals.
Overall the model proposed in the present study supports a framework where infection-induced antibodies target specific parasite structures upon pRBC rupture and work in concert with phagocytes to avoid parasite transition from one to another RBC4 (Fig. 1). Moreover, these observations indicate that naturally acquired antibodies do not mediate pRBC clearance but rather prevent parasite transition from one pRBC generation to the next. These in vivo data provide further insight into antibody functions that might protect naturally exposed and immunized humans against malaria. Finally, these findings suggest that antibody-mediated immunity might be boosted in a rational manner to achieve protection against malaria infection. Increasing the plasma concentration of antibodies that recognize pRBC is likely to be of primary importance to reduce the risk and severity of malaria and studies aimed at improving our ability of inducing robust, long-lived antibody responses will help to reach this goal.
Footnotes
Citation: Vinchi F, Lobo CA. Stopping Plasmodium Parasite Invasion by Boosting Humoral Immunity. HemaSphere, 2019;0:0. http://dx.doi.org/10.1097/HS9.0000000000000206
Funding/support: None.
Disclosure: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Teo A, Feng G, Brown GV, et al. Functional antibodies and protection against blood-stage malaria. Trends Parasitol. 2016;32:887–898. [DOI] [PubMed] [Google Scholar]
- 2.Dobbs KR, Dent AE. Plasmodium malaria and antimalarial antibodies in the first year of life. Parasitology. 2916;143:129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen S, Mc GI, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961;192:733–737. [DOI] [PubMed] [Google Scholar]
- 4.Akter J, Khoury DS, Aogo R, et al. Plasmodium-specific antibodies block in vivo parasite growth without clearing infected red blood cells. PLoS Pathog. 2019;15:e1007599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khoury DS, Cromer D, Akter J, et al. Host-mediated impairment of parasite maturation during blood-stage Plasmodium infection. Proc Natl Acad Sci USA. 2017;114:7701–7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khoury DS, Cromer D, Best SE, et al. Reduced erythrocyte susceptibility and increased host clearance of young parasites slows Plasmodium growth in a murine model of severe malaria. Sci Rep. 2015;5:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]