Abstract
Chemoimmunotherapy has been the standard of care for patients with chronic lymphocytic leukemia (CLL) over the last decade. Advances in monoclonal antibody technology have resulted in the development of newer generations of anti-CD20 antibodies with improved therapeutic effectiveness. In parallel, our knowledge about the distinctive biological characteristics of CLL has progressively deepened and has revealed the importance of B-cell receptor (BCR) signaling and upregulated antiapoptotic proteins for survival and expansion of malignant cell clones. This knowledge provided the basis for development of novel targeted agents that revolutionized treatment of CLL. Ibrutinib and idelalisib inhibit the Bruton tyrosine kinase (BTK) and phosphoinositide 3-kinase (PI3K) delta, respectively, thus interfering with supportive signals coming from the microenvironment via the BCR. These drugs induce egress of CLL cells from secondary lymphoid organs and remarkably improve clinical outcomes, especially for patients with unmutated immunoglobulin heavy-chain genes or with p53 abnormalities that do not benefit from classical treatment schemes. Latest clinical trial results have established ibrutinib with or without anti-CD20 antibodies as the preferred first-line treatment for most CLL patients, which will reduce the use of chemoimmunotherapy in the imminent future. Further advances are achieved with venetoclax, a BH3-mimetic that specifically inhibits the antiapoptotic B-cell lymphoma 2 protein and thus causes rapid apoptosis of CLL cells, which translates into deep and prolonged clinical responses including high rates of minimal residual disease negativity. This review summarizes recent advances in the development of targeted CLL therapies, including new combination schemes, novel BTK and PI3K inhibitors, spleen tyrosine kinase inhibitors, immunomodulatory drugs, and cellular immunotherapy.
Introduction
Chronic lymphocytic leukemia (CLL) is the most common leukemia in the Western world and affects mainly elderly patients.1 It is characterized by accumulation of small B lymphocytes with a mature appearance in blood, bone marrow, lymph nodes, or other lymphoid tissues.2 The biological heterogeneity of the disease (hypermutation status of the immunoglobulin heavy-chain genes [IGHV], presence of specific genomic aberrations and/or recurrent mutations in oncogenes and tumor suppressor genes) determines its variable clinical manifestation.3–5
Allogeneic stem cell transplantation (allo-SCT) is still the only known curative therapy but is limited to a small fraction of young patients, while CLL is mainly a disease of the elderly.1,6 Chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab (FCR) has been the standard of care for the past decade but its use is limited by the patient's age, comorbidities, and performance status.7–9 Moreover, patients with high-risk aberrations like del(17p) or TP53 mutation have poor outcomes with standard chemoimmunotherapy.4
Recent developments overcome some of these challenges or limit their effect. Improved understanding of CLL has resulted in the development of new therapeutic approaches that have dramatically improved patient outcomes.10,11 Ongoing preclinical and clinical research continues to refine the use of these novel therapies while evolving biological knowledge keeps on identifying promising treatment targets.
Advances in understanding the biology of CLL
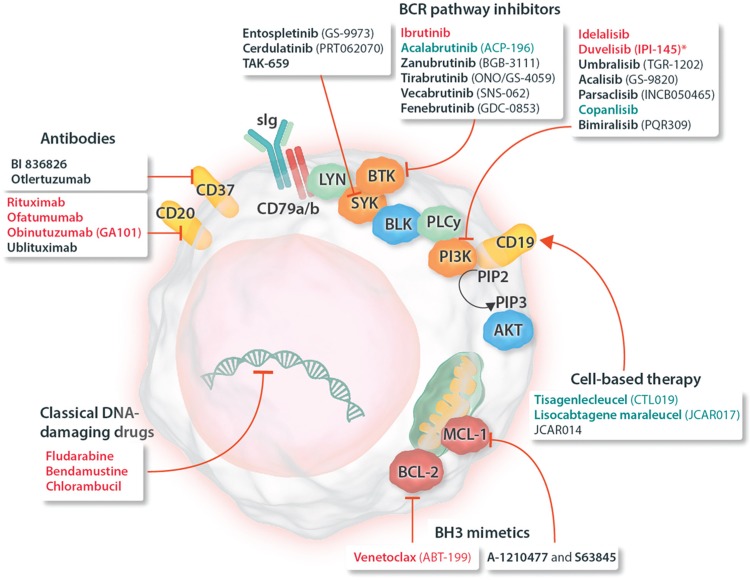
CD20 is a nonglycosylated phosphoprotein expressed on the surface of B-lineage cells, as well as on most B-cell malignancies, including CLL.12,13 CD20 has no known natural ligand and its exact functions are not yet clear but there is evidence that it colocalizes with the B-cell receptor (BCR) and that it acts as a calcium channel participating in BCR activation and signaling.12,13 In CLL cells, constitutive BCR signaling is involved in expansion and maintenance of the cell clone and thus plays a key role for the pathogenesis of the disease.14,15 Upon antigen engagement of the BCR, associated adapter protein tyrosine kinases including spleen tyrosine kinase (SYK) and LCK/YES novel kinase (LYN) are recruited and become phosphorylated. The activated kinases in turn activate the downstream targets Bruton tyrosine kinase (BTK) and phosphoinositol-3-kinases (PI3Ks), which then initiate downstream cascades resulting in activation of protein kinase B (AKT), extracellular signal-regulated kinases ERK1 and 2, nuclear factor (NF)-κB, and nuclear factor of activated T-cells (NFAT).15–18 Hence, key components of the BCR signaling pathway such as BTK and PI3K attracted significant attention as potential therapeutic targets in CLL and other B-cell malignancies, and selective inhibitors were developed (Fig. 1).19
Figure 1.
Schematic representation of a CLL cell with established and experimental drug targets, as well as a classification of respective drugs (approved and experimental). Names of drugs with approval for use in CLL are given in red; drugs approved for use in other indications are shown in blue; drugs in various stages of clinical development are shown in black. ∗Duvelisib has been approved for treatment of CLL by the FDA but not yet by the EMA. AKT = protein kinase B, BCL-2 = B-cell lymphoma 2, BCL-XL = B-cell lymphoma-extra large, BCR = B-cell receptor, BLK = B lymphocyte kinase, BTK = Bruton tyrosine kinase, CLL = chronic lymphocytic leukemia, EMA = European Medicines Agency, FDA = Food and Drug Administration, LYN = LCK/YES novel tyrosine kinase, MCL-1 = induced myeloid leukemia cell differentiation protein Mcl-1, PD-1 = programmed cell death protein 1, PI3K = phosphatidylinositol-4,5-bisphosphate 3-kinase, PIP2 = phosphatidylinositol (4,5)-bisphosphate, PIP3 = phosphatidylinositol (3,4,5)-trisphosphate, PLC = phospholipase C, sIg = surface immunoglobulin, SYK = spleen tyrosine kinase.
CLL is also characterized by high levels of B-cell lymphoma 2 (BCL-2) protein as well as by hypomethylation of the BCL2 promoter.20,21 BCL-2 overexpression in CLL is not completely understood and only in some cases (≈10%) it is caused by gene translocation to immunoglobulin loci.22 For the remaining cases, a deletion or down-regulation of MIR15A and MIR16-1 could be the cause, as these miRNAs are known to negatively down-regulate BCL-2.23,24 In general, overexpressed BCL-2 or other antiapoptotic proteins (eg, BCL-XL and MCL-1) sequester activator BH3-only proteins (BIM and/or BID) and cells thereby become “primed for death,” that is, very sensitive to so-called “sensitizer BH3-proteins” (BAD, BIK, NOXA, HRK, PUMA, and BMF), which can then very quickly trigger apoptosis.25 This provides an opportunity to selectively induce apoptosis in primed cancer cells, for example, it could be shown that survival of CLL cells is dependent on BCL-2 sequestering BIM, hence displacing BIM from BCL-2's BH3-binding pocket activates BAX and quickly induces mitochondrial permeabilization and cell death.26 These observations led to the development of efficient BH3-mimetics that induce apoptosis in CLL and other BCL-2-dependent cancers.
Established treatments
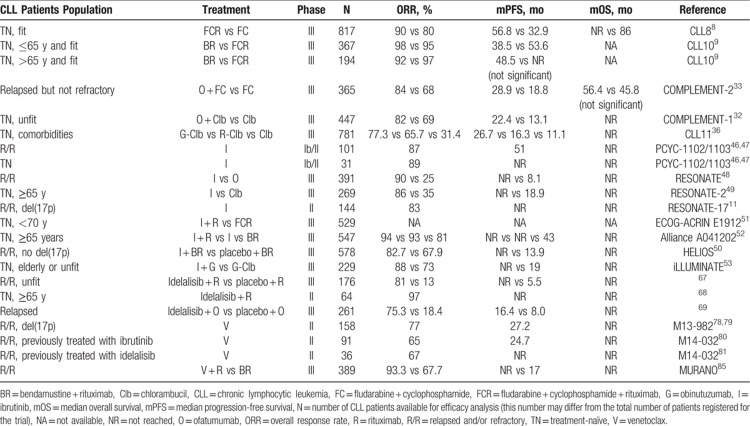
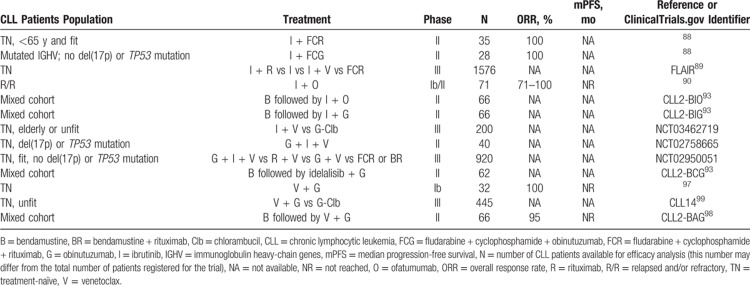
Clinical trials that led to the establishment of current CLL therapy are summarized in Table 1 and Figure 2 shows a timeline with the regulatory approval of major drugs and in parallel the improving survival of CLL patients. The choice of the available treatment options is guided by biomarkers and prognostic subgroups. Table 2 attempts to summarize and update current guidelines for first-line treatment of CLL based on latest data from clinical trials (see also the section on novel combination strategies further below).
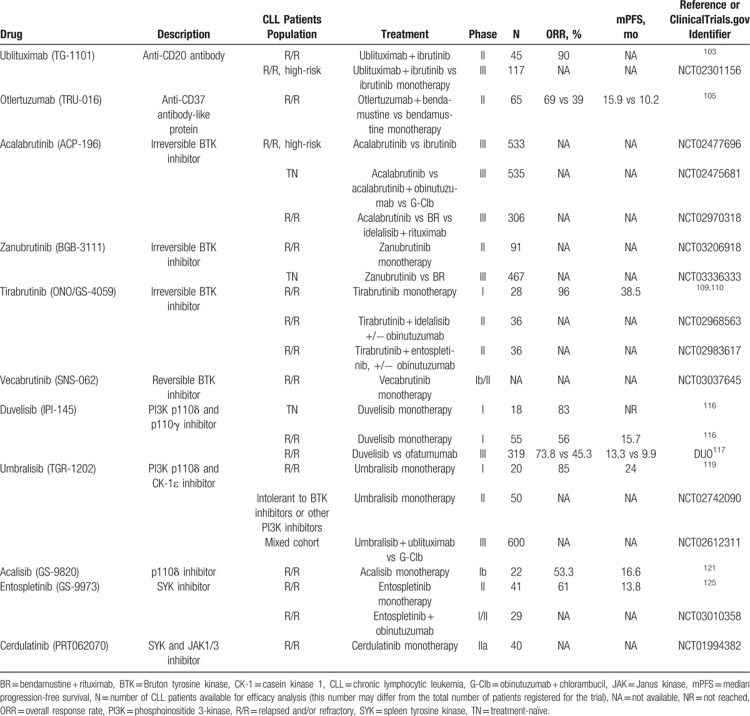
Table 1.
Clinical Trials That Led to the Establishment of Current CLL Therapy
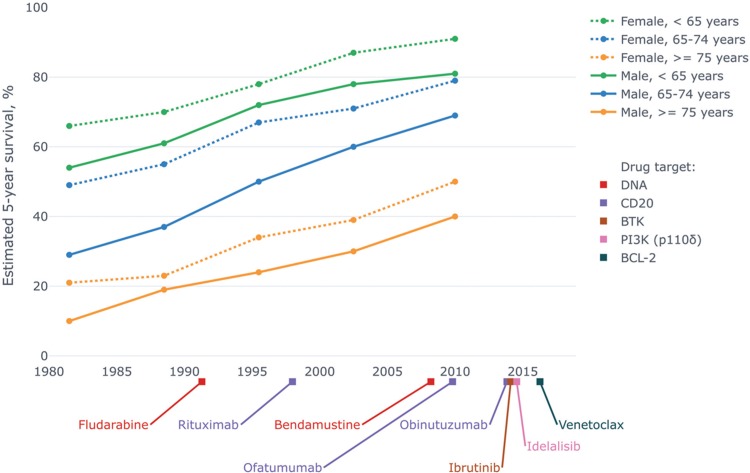
Figure 2.
Timeline of regulatory approval of major drugs for treatment of CLL paralleled by improving survival of CLL patients. Survival data are from a Danish population-based study150 and drug approval dates are taken from the website of the FDA. Chlorambucil and cyclophosphamide, which were introduced in the 1950s, are omitted from the diagram for space reasons. CLL = chronic lymphocytic leukemia, FDA = Food and Drug Administration.
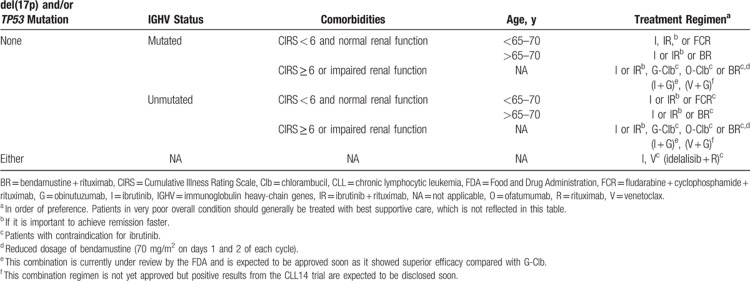
Table 2.
Preferred and Alternative First-Line Therapy of Symptomatic CLL According to Prognostic and Clinic Subgroups
Chemoimmunotherapy
The success story of chemoimmunotherapy in CLL started with the introduction of the chimeric anti-CD20 antibody rituximab culminating in the establishment of the FCR regimen as the gold standard for first-line treatment of fit patients,7,27 although this role is now more and more being taken over by ibrutinib-containing regimens (see below). Rituximab induces reorganization of CD20 molecules into lipid rafts and efficiently activates the classical pathway of the complement system, effects that are characteristic of so-called type I anti-CD20 antibodies.12 Rituximab depletes B cells also by triggering antibody-dependent cell-mediated cytotoxicity (ADCC), whereas its ability to induce apoptosis is less relevant.12 The latest follow-up data from the pivotal CLL8 trial showed that patients in the FCR group had a median progression-free survival (PFS) of 56.8 months while the median overall survival (OS) was not reached (compared to median PFS of 32.9 months and OS of 86 months in the FC arm).8 Patients with IGHV-mutated CLL benefited most. The bendamustine-rituximab (BR) schedule is less effective than FCR in younger patients (median PFS of 38.5 months vs 53.6 months in the CLL10 study) but noninferior in the age group above 65 years, in which it is preferred because of the lower frequency of adverse events (AEs), especially neutropenia and infections.9 Rituximab-based chemoimmunotherapy is less effective in the presence of some factors with strong negative prognostic impact on both PFS and OS: unmutated IGHV, mutated TP53, del(17p), and del(11q).8,9,27 Moreover, it appears that FCR is not superior to FC in patients harboring NOTCH1 mutation.8,28
Ofatumumab is a fully human type I monoclonal anti-CD20 antibody that targets an epitope closer to the membrane,29 and ofatumumab-opsonized cells bind more C1q even when the concentration of C1q in the medium is low.30 These characteristics are thought to explain the stronger complement-dependent cytotoxicity (CDC) of ofatumumab compared with rituximab, whereas ADCC induction by the two antibodies is similar.31 Ofatumumab is approved for treatment of CLL refractory to fludarabine and alemtuzumab, as well as in combination with fludarabine and cyclophosphamide for relapsed CLL, and in combination with chlorambucil or bendamustine for treatment-naïve (TN) patients who cannot be treated with fludarabine. In the COMPLEMENT-1 study, performed in patients who are elderly or have comorbidities, median PFS was 22.4 months with ofatumumab and chlorambucil versus 13.1 months with chlorambucil only and the combination was well tolerated.32 Good tolerability was also observed in the COMPLEMENT-2 study, in which addition of ofatumumab to the FC regimen increased the median PFS of relapsed patients from 18.8 months to 28.9 months.33 Unmutated IGHV, mutated TP53 and del(17p) were independent negative prognostic factors for both PFS and OS in this trial.34 Patients with mutated NOTCH1 also benefited less from the addition of ofatumumab.34 In addition, a retrospective, phase IV observational study of ofatumumab-based therapy in heavily pretreated patients with poor-prognosis CLL demonstrated limited efficacy with an overall response rate (ORR) of only 22%.35 This suggests that ofatumumab is better used earlier in the course of the disease.
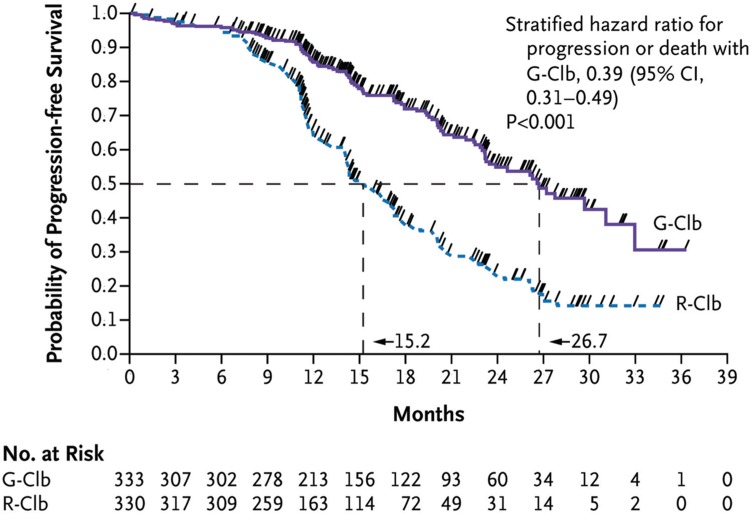
In contrast to type I, type II anti-CD20 monoclonal antibodies can induce direct cell death upon binding to CD20 but are weak CDC inducers.12 Obinutuzumab (GA101) is a type II antibody, the Fc region of which is glycoengineered to provide better binding to FcγRIIIa and thus to enhance ADCC.12 Obinutuzumab was approved by the Food and Drug Administration (FDA) in November 2013 and by the European Medicines Agency (EMA) in July 2014 in combination with chlorambucil for untreated CLL patients who are not eligible for more intensive therapy. Basis for this approval was the CLL11 study, which demonstrated increased response rates and prolonged median PFS (26.7 months with obinutuzumab-chlorambucil vs 16.3 months with rituximab-chlorambucil vs 11.1 months with chlorambucil alone; Fig. 3).36 OS was prolonged with obinutuzumab-chlorambucil compared with chlorambucil alone. More grade ≥3 infusion-related reactions were observed with obinutuzumab compared with rituximab (20% vs 4%).36 Cytopenias were also increased but the incidence of infections was not different. Unmutated IGHV, mutated TP53 and del(17p) remained independent negative prognostic factors also for patients treated with obinutuzumab-chlorambucil, whereas mutated NOTCH1 was not predictive of lack of benefit for the addition of obinutuzumab to chlorambucil therapy.37
Figure 3.
Progression-free survival with obinutuzumab-chlorambucil versus rituximab-chlorambucil in the CLL11 study. G-Clb = obinutuzumab-chlorambucil, R-Clb = rituximab-chlorambucil. Reprinted from Goede et al36 with permission from the Massachusetts Medical Society.
Currently, all three approved anti-CD20 antibodies have their place in CLL treatment. Although obinutuzumab is more clinically effective in previously untreated patients, it is also associated with a higher rate of AEs and this can be a reason to prefer the older antibodies in frail patients. Moreover, as obinutuzumab relies to a great extent on ADCC, it has been hypothesized that its effectiveness might be reduced in patients with deficient effector response (eg, multiply pretreated or with refractory disease).38 On the other hand, rituximab is currently the only antibody that has been approved for use in combinations with some of the newer agents (see below) and as such its use might even expand in short term, unless ongoing studies demonstrate superiority of the newer antibodies in this setting. Additionally, following the recent expiry of patents on rituximab, several biosimilars have now been approved and their lower cost might make them an attractive alternative, especially in resource-limited health systems.
Drugs targeting BCR signaling
Ibrutinib
An orally available small molecule, binds covalently to Cys481 in the active site of BTK and thereby inhibits activation of downstream survival pathways that involve MAP kinases, PI3K and NF-κB.39,40 Ibrutinib has been shown to inhibit other kinases (albeit with less potency), for example, interleukin-2-inducible kinase (ITK) which has a significant homology to BTK and is involved in activation of natural killer and T cells.40,41 Nevertheless, irreversible binding to BTK combined with the short half-life of the drug ensures good selectivity for BTK in vivo.40 Ibrutinib inhibits CLL-cell survival and migration in response to physiologically relevant stimuli and blocks secretion of chemokines by CLL cells.42 In an adoptive transfer mouse model of CLL, ibrutinib led to a transient early lymphocytosis and inhibition of disease progression.42 Similar effects are observed in patients, as ibrutinib induces rapid shrinkage of lymph nodes and spleen, which is accompanied by a transient increase of leukemia cells in the circulation. This lymphocytosis is: (i) most often asymptomatic and usually resolves during the first 8 months of therapy43; (ii) not associated with inferior PFS43; (iii) not due to disease progression but rather reflects redistribution of cells, as confirmed by measuring CLL-cell birth and death rates in patients by labeling with heavy water44; and (iv) common to all BCR signaling inhibitors.45
Ibrutinib was initially approved by the FDA in February 2014 and by the EMA in October 2014 for previously treated patients. The decision was based on a phase Ib/II study (PCYC-1102) in 85 patients with relapsed or refractory (R/R) CLL given a daily dose of 420 or 840 mg ibrutinib.46 The ORR was 71% in both dose groups. In addition, 20% (420 mg) and 15% (840 mg) of patients had a partial response (PR) with persistent lymphocytosis. The response was independent of clinical or genomic risk factors including number of prior therapies, disease stage, and presence of del(17p). Follow-up data on the extended cohort of PCYC-1102 and additional patients with TN CLL showed that continuous treatment with ibrutinib improves responses and leads to durable remissions.47 At a median follow-up of 5 years, the ORR was 87% in TN patients and 89% in R/R patients, with increasing complete response (CR) rates over time (29% and 10%, respectively). The median PFS was not reached in TN patients and was 51 months in R/R patients; the 5-year PFS rate was 92% or 44%, respectively, and the 5-year OS rate was 92% or 60%, respectively.47
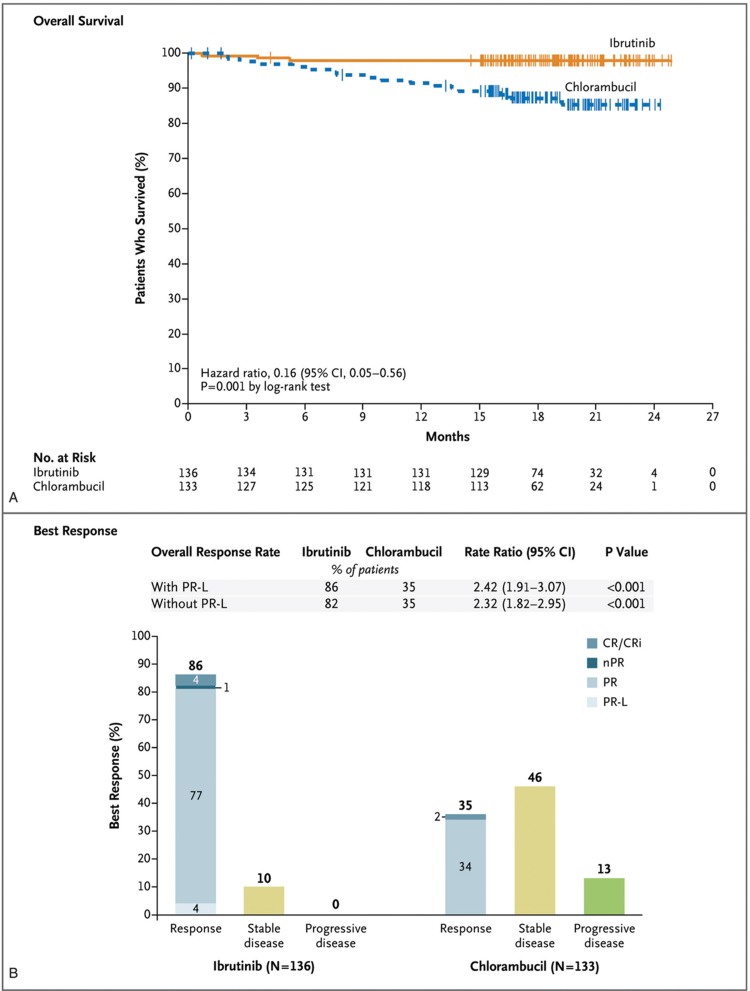
Ibrutinib is currently approved in the European Union as monotherapy for all adult CLL patients on the basis of the results of the RESONATE and RESONATE-2 trials as well as in combination with BR for R/R patients on the basis of the HELIOS study. RESONATE (PCYC-1112) is a phase III study comparing ibrutinib with ofatumumab in patients with R/R CLL or small lymphocytic lymphoma (SLL).48 PFS was significantly longer in the ibrutinib arm (median not reached vs 8.1 months, at a median follow-up of 19 months).48 The 24-month PFS rate and the 18-month OS rate in the ibrutinib arm were 74% and 86%, respectively, and the risk of death was reduced with approximately 64%. The phase III RESONATE-2 (PCYC-1115) study compared ibrutinib to chlorambucil in elderly patients (≥65 years) with TN CLL or SLL.49 Ibrutinib demonstrated superior ORR (86% vs 35%), PFS (median not reached vs 18.9 months), and OS rate at 24 months (98% vs 85%) compared with chlorambucil (Fig. 4). The observation that ibrutinib improved bone marrow function has clinical relevance because bone marrow failure is a common cause of complications in CLL patients.49 In the phase III HELIOS (CLL3001) trial, PFS at 18 months was 79% in the BR plus ibrutinib group and 24% in the BR plus placebo group.50 The RESONATE-17 (PCYC-1117) trial showed that ibrutinib is also effective in patients with R/R CLL and del(17p).11 After a median follow-up of 27.6 months, the ORR was 83% and the 24-month PFS and OS rates were 63% and 75%, respectively. Ibrutinib is thus a primary consideration for first-line therapy of CLL patients with del(17p).11 Del(11q) and mutations in NOTCH1, SF3B1, BIRC3, and ATM had no prognostic impact in ibrutinib-treated patients.47,48
Figure 4.
Overall survival and response rates with ibrutinib versus chlorambucil in the RESONATE-2 trial. Shown are overall survival with ibrutinib versus chlorambucil (A) and the best response to treatment as assessed by an independent review committee (B). The tick marks indicate patients with censored data. CR = complete response, CRi = complete response with incomplete blood count recovery, nPR = nodular partial response, PR = partial response, PR-L = partial response with lymphocytosis. Reprinted from Burger et al49 with permission from the Massachusetts Medical Society.
Most recently, results from the ongoing phase III ECOG-ACRIN E1912, Alliance A041202 and iLLUMINATE trials have shown superiority of ibrutinib-based regimens over chemoimmunotherapy in the front-line setting.51–53 Interim results from the E1912 trial demonstrated better PFS with ibrutinib-rituximab (IR) than with FCR in fit patients (hazard ratio [HR] 0.352), and this superiority was independent of age, sex, performance status, disease stage, or the presence/absence of del(11q23).51 Importantly, OS was also improved (HR 0.17) and the incidence of grade ≥3 treatment-related AEs was lower with IR than with FCR (58.5% vs 72.1%, respectively). In difference to the subgroup with unmutated IGHV, the increase of the PFS in the IGHV-mutated group was not statistically significant but this might be attributed to the low number of events (14) in that subgroup so far.51 Based on these results, which are soon to be published in full, ibrutinib (with or without rituximab) should become the preferred first-line treatment for younger CLL patients with unmutated IGHV and can be used as an alternative to FCR in patients with mutated IGHV who would value a less intensive but continuous oral therapy more than an intensive time-limited treatment. Unfortunately, the E1912 trial did not include a patient group treated with ibrutinib alone, which makes it difficult to assess the relative contribution of rituximab to outcomes in the IR arm. However, trials in other patient populations showed similar efficacy of IR and ibrutinib alone (see below),52,54 so it might be justified to leave rituximab out of the treatment protocol also for younger patients with TN CLL. The A041202 trial is comparing IR or ibrutinib alone against the BR regimen in older (≥65 years) patients with TN CLL. The estimated 2-year PFS rates in this trial were 74%, 87%, and 88% with BR, ibrutinib alone, or IR, respectively.52 The HR for progression was 0.39 for ibrutinib and 0.38 for IR compared with BR, whereas there was no significant difference between the 2 ibrutinib-containing regimens. The ORR was lower with BR (81%) than with the ibrutinib-containing regimens (93–94%); however, the CR rate and the percentage of patients with undetectable minimal residual disease (MRD) were higher with BR.52 The rate of grade ≥3 hematologic AEs was higher with BR (61%) than with ibrutinib or IR (41% and 39%, respectively), whereas the rate of grade ≥3 nonhematologic AEs was lower with BR (63%) than with the ibrutinib-containing regimens (74% with each regimen). The iLLUMINATE trial is comparing ibrutinib-obinutuzumab to the standard chlorambucil-obinutuzumab regimen in elderly or unfit patients with TN CLL. The ibrutinib-containing regimen achieved significantly higher 30-month PFS rates (79% vs 31% for the standard) and reduced the risk of progression or death with 77% in the whole population and with 85% in the subgroup of high-risk patients (del17p, del11q, TP53 mutation or unmutated IGHV).53 Median PFS was not reached in the ibrutinib-obinutuzumab arm and was 19 months in the chlorambucil-obinutuzumab arm. More patients in the ibrutinib-obinutuzumab group achieved CRs (19% vs 8%) and MRD-negativity (35% vs 25%). Taken together, the A041202 and iLLUMINATE trials establish ibrutinib-based regimens as the preferred first-line treatment for older/unfit patients. The role of anti-CD20 antibodies as combination partners in this setting is for now debatable. Similar to the E1912 trial, the iLLUMINATE trial lacked an ibrutinib-only treatment arm, which makes it difficult to directly estimate the contribution of obinutuzumab to treatment responses, whereas a cross-trial comparison with the RESONATE-2 study suggests that OR and PFS rates are similar for ibrutinib and ibrutinib-obinutuzumab.55 The A041202 trial and a phase II trial in a cohort of patients with relapsed or high-risk CLL showed that the addition of rituximab to ibrutinib did not improve PFS or ORR, although patients in the IR arm of the latter study reached CR faster and achieved lower residual disease levels.54 It was concluded that ibrutinib monotherapy should remain the standard of care, but that addition of rituximab might be justified in cases in which it is desirable to achieve remission faster.
Ibrutinib is well-tolerated as shown by the extensive safety data from the 5-year follow-up of the PCYC-1102/1103 studies.47 The most common cumulative AE was diarrhea, mostly transient, occurring in 58% of patients and thought to result from off-target blockade of the epithelial growth factor receptor (EGFR).56 Infections were the most frequent grade ≥3 AEs, observed more often in the R/R population (58%) compared with TN patients (13%), and most commonly involving the respiratory tract.47 However, infections are more probably a consequence of immune dysregulation by the underlying disease56 and prior chemotherapy might also play a role. Other common grade ≥3 AEs were hypertension (32% TN, 25% R/R), neutropenia (3% TN, 21% R/R), thrombocytopenia (3% TN, 11% R/R), and atrial fibrillation (6% TN, 9% R/R).47 Of note, more than two-thirds of patients with grade ≥3 hypertension had pre-existing hypertension. Inhibition of BTK and TEC kinases by ibrutinib impairs platelet aggregation and increases the risk of bleeding.56,57 Drug discontinuations and dose reductions due to AEs were more frequent during the first year and subsequently decreased.47
While ibrutinib is an effective therapy leading to durable responses, some patients acquire resistance and relapse. Most often this is due to C481S mutation in BTK or mutations in the downstream phospholipase C gamma 2 (PLCγ2).58–60 The mutation BTK C481S prevents covalent binding of ibrutinib, and in combination with the short half-life of ibrutinib, this results in only transient inhibition of BTK and poor therapeutic outcome.61 PLCγ2 mutants identified in resistant patients are strikingly hypersensitive to activation by RAC2 and no longer depend on BTK for activation.62 An analysis of 4 clinical trials with a median follow-up of 3.4 years demonstrated that 19% of patients relapsed and among them 85% had acquired PLCγ2 or BTK mutations.60 Resistant cell clones could be detected at median 9.4 months before clinical relapse. The emergence of del(8p) clones harboring driver mutations in EP300, MLL2, and/or EIF2A has been identified as an additional mechanism of resistance development.63 CLL progression on ibrutinib tends to occur later in therapy (after 12 months of attaining a response), in contrast to Richter transformations (RTs), which mostly occur during the first 1 to 2 years of treatment.59,61 Relapsed disease is usually progressing rapidly, and salvage therapy should be started within 2 weeks of discontinuing ibrutinib. Clinical data support the efficacy of idelalisib and in particular venetoclax in the setting of ibrutinib resistance or intolerance (see below).
Overall, ibrutinib achieves considerably better outcomes than chemoimmunotherapy and is now the preferred first-line therapy for most patients. However, the major disadvantage of ibrutinib therapy is the low proportion of MRD-negative CRs and the current practice of continuous application of ibrutinib to prevent relapses leads to significant expenses and AEs. Ongoing and future clinical trials are expected to rationalize treatment strategies by identifying combination regimens with deeper responses and by clarifying whether and under which circumstances discontinuation of ibrutinib is feasible without negatively affecting patient outcomes. Studies should also elucidate whether early treatment with ibrutinib would ultimately lead to better outcomes. For example, the phase III CLL12 trial aims to clarify whether ibrutinib can prolong event-free and OS of patients with asymptomatic Binet stage A CLL and intermediate or higher risk of disease progression.64
Idelalisib
Is an orally available inhibitor of PI3K with selectivity for the delta isoform of the catalytic subunit of PI3K (p110δ), which is preferentially expressed in leukocytes.65 Similar to ibrutinib, idelalisib interferes with interactions between CLL cells and protective tissue microenvironments, mobilizing CLL cells, and thereby causing transient lymphocytosis and rapid lymph node shrinking.66 Idelalisib was initially approved by the FDA (July 2014) and then by the EMA (September 2014) in combination with rituximab for treatment of CLL patients who have relapsed or who have del(17p) or TP53 mutation.67,68 The decision was based on the results of two trials. The first one compared idelalisib + rituximab versus placebo + rituximab in patients with relapsed CLL who were unable to receive cytotoxic agents. Idelalisib-treated patients had significantly better ORR (81% vs 13%), median PFS (unreached at 14 months vs 5.5 months), and OS at 12 months (90% vs 80%).67 In the second trial, the ORR in a population of TN elderly patients treated with idelalisib-rituximab was 97% (100% in patients with del(17p)/TP53 mutations) and the PFS and OS rates at 36 months were 83% and 90%, respectively.68 In 2016, idelalisib was approved in combination with ofatumumab based on a phase III trial comparing ofatumumab + idelalisib versus ofatumumab alone, in which the combination achieved a superior ORR (75.3% vs 18.4%) and median PFS (16.4 vs 8.0 months), as well as longer OS in the subgroup with del(17p) (25.8 vs 19.3 months).69 However, the indication for first-line therapy of CLL with del(17p)/TP53 mutation was restricted only to patients not eligible for any other therapies, as alternative treatments have better benefit/risk ratio.
Overall, idelalisib combined with rituximab demonstrated acceptable tolerability in R/R CLL with the most common grade ≥3 AEs being neutropenia (34%), thrombocytopenia (10%), anemia (5%), ALT/AST elevation (5%), and diarrhea (4%).67 However, toxicity was considerably higher in the front-line setting, with the most common grade ≥3 AEs being diarrhea and/or colitis (42%), neutropenia (28%), serum ALT/AST elevation (23%), pneumonia (19%), rash (13%), and urinary tract infection (6%).68 Infiltration of T lymphocytes was noted in colonoscopic biopsies of patients with diarrhea. As T-cell levels are typically normal in TN CLL but quite low in patients with R/R disease, and as PI3Kδ activity seems important for maintenance of self-tolerance,70 it is plausible that infiltrating T cells could have caused the symptoms.68 Immune-mediated AEs (colitis, pneumonitis, transaminitis) often had delayed onset and were more common in younger patients and in patients with mutated IGHV.56,71 These conditions were typically resolved by withholding idelalisib and applying corticosteroids.69,71 Another problem with idelalisib is an increased rate of serious infections, including deaths due to sepsis, Pneumocystis jirovecii pneumonia (PJP) and cytomegalovirus infection. This necessitates careful monitoring of patients and prophylaxis of PJP during treatment with idelalisib and for 2 to 6 months thereafter.69
Importantly, clinical effectiveness of idelalisib combined with anti-CD20 antibodies is not influenced by IGHV mutational status or by presence of del(17p) and/or TP53 mutation.68,69 Acquired resistance to idelalisib has been described in patients but could not be attributed to specific recurrent mutations, especially not in PI3K or related pathways.72 Relapsed patients might be successfully salvaged with ibrutinib or venetoclax.
Targeting BCL-2 with BH3-mimetics
As it became clear that CLL cells overexpress BCL-2,21 efforts were started to develop inhibitors of this antiapoptotic protein. Success came with the development of the so-called BH3-mimetics, small molecules that bind to the hydrophobic groove of prosurvival BCL-2 proteins, thus inhibiting their functional activity and inducing apoptosis.73 The prototypic drug of this class was ABT-737 that binds with high affinity to BCL-2, BCL-XL, and BCL-W, but not MCL-1 or A1.74 In vitro experiments with ABT-737 revealed that CLL cells are especially sensitive to BCL-2 inhibition, as almost all BIM in CLL cells is sequestered by BCL-2.26 A natural therapeutic window was thus predicted to exist between CLL cells bearing primed BCL-2 and normal cells with BCL-2 that is relatively unoccupied by activators. Navitoclax (ABT-263), an orally bioavailable derivative of ABT-737 showed promising activity in patients with CLL75 but its further development was hampered by dose-limiting thrombocytopenia, a consequence of the drug's action on platelets, which rely on BCL-XL for survival.76 This led to the design and development of venetoclax (ABT-199), a highly BCL-2-selective BH3-mimetic that spares platelets.77
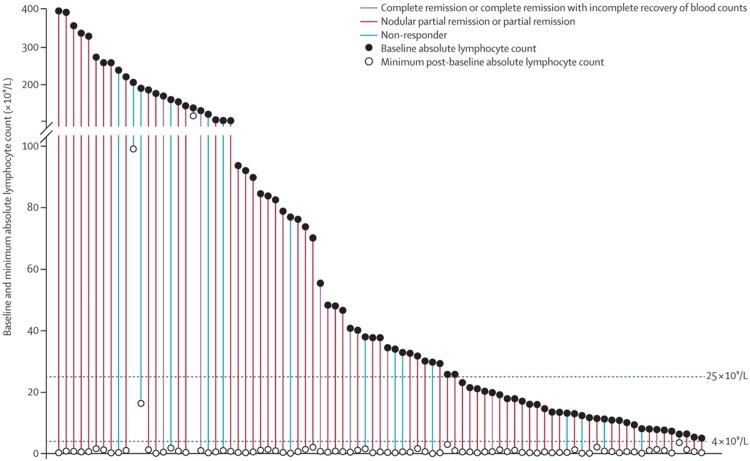
Venetoclax was approved by the EMA in December 2016 as monotherapy for CLL patients with del(17p) or TP53 mutation who have relapsed or are otherwise not suitable for treatment with BCR pathway inhibitors, as well as for patients who do not have del(17p) or TP53 mutation and are refractory to chemoimmunotherapy and BCR pathway inhibitors. This approval was based on the phase II M13-982 and M14-032 trials in R/R CLL. Patients in the M13-982 trial had 17p13 (TP53 locus) deletion.78 At a median follow-up of 27.8 months, 77% of them had achieved an OR and the 24-month estimates for PFS and OS were 54% and 73%, respectively (see also Fig. 5).79 Patients in the M14-032 trial were previously treated with ibrutinib or idelalisib. The ORR in the first group was 65%, the median PFS was 24.7 months, and the estimated 12-month PFS was 75%.80 In patients previously treated with idelalisib, the ORR was 67% and the estimated 12-month PFS was 79%.81 In difference to BCR pathway inhibitors, venetoclax induced deep remissions with a high rate of MRD negativity in blood (30% in the M13-982 trial and about 40–42% in the M14-032 trial).79–81 The most common grade 3 to 4 AEs in the above studies were neutropenia (39–51% of patients), anemia (15–29%), thrombocytopenia (15–29%), and infection (13–25%).78–81 Application of venetoclax is followed by a dramatic reduction of CLL cells within 12 hours, which has resulted in tumor lysis syndrome (TLS) and several deaths in the clinical trials. Amendments to treatment protocols showed that the risk for TLS can be effectively reduced by initiating treatment with low dose of venetoclax (20 mg once daily) and gradually increasing it in the course of 5 weeks until reaching the recommended daily dose of 400 mg.78 The risk for TLS is greatest in patients with high tumor burden (any lymph node with a diameter ≥5 cm or absolute lymphocyte count ≥25 × 109/L). A recent study in patients with R/R CLL identified refractoriness to fludarabine and complex karyotype as the dominant risk factors for CLL progression or RT during treatment with venetoclax and concluded that patients with these risk factors should have occult RT excluded before initiating venetoclax therapy.82 Interestingly, while del(17p) and/or TP53 mutation are not associated with reduced responsiveness to venetoclax10,82 they are associated with decreased remission duration.83 Acquired resistance to venetoclax has been observed and shown to be in about half of the cases a consequence of a novel mutation in BCL2 (Gly101Val) that reduces the affinity of venetoclax for BCL-2 by ∼180-fold.84 Increased BCL-XL expression seems to be an additional resistance mechanism.84
Figure 5.
Absolute change from baseline in peripheral absolute lymphocyte count in patients with a baseline lymphocyte count ≥5 × 109 cells/L and treated with venetoclax in the M13-982 trial (N = 87). The threshold of 4 × 109 cells/L corresponds to requirements for complete remission. Line length indicates absolute best change from baseline; each line represents one patient with patients arranged in descending order of baseline measurement. Reprinted from Stilgenbauer et al78 with permission from Elsevier.
In June 2018, the FDA extended the approval of venetoclax, making it available as second-line treatment for all CLL patients regardless of their del(17p) status. The EMA followed suit in October 2018 for the combination of venetoclax with rituximab (VR). These decisions were based on the results of the phase III MURANO trial that compared the efficacy and safety of a fixed-duration VR regimen (2 years of venetoclax plus rituximab during the first 6 cycles) against 6 cycles of BR in patients with R/R CLL.85 This study showed a profound improvement of PFS in the VR arm (median not reached vs 17 months; HR 0.17). Two-year PFS estimates were 84.9% versus 36.3%, respectively. Key secondary endpoints, including OS, ORR, and CR rate, also showed consistent improvements with remarkable rates of peripheral blood MRD-negativity at 9 months (62.4% vs 13.3%). Grade 3 to 4 neutropenia was more frequent in the VR arm (57.7% vs 38.8%) but the incidences of grade 3 to 4 febrile neutropenia and infections were lower.85 A pooled analysis of 323 patients from 3 other clinical studies concluded that the VR combination is synergistic,86 thus serving as supportive evidence. The 3-year follow-up of the MURANO trial demonstrated sustained benefit of VR against BR in terms of PFS rates (71.4% vs 15.2%) and OS rates (87.9% vs 79.5%)87; 64% of the patients who completed venetoclax treatment were MRD-free and with 10 months of follow-up after treatment cessation 70% of them remained MRD-negative and only 2 patients (2.4%) had disease progression. MRD levels correlated with PFS and were prognostic for remission duration.87
Venetoclax-based regimens have huge potential to become the new gold standard in CLL treatment as the high rate of MRD-negativity allows achievement of durable responses with time-limited treatment and this should reduce both the risk of resistance development and expenses compared with regimens based on BCR signaling inhibitors.
Emerging therapies
A number of new therapeutic approaches are currently being investigated in CLL, including new combination regimens (Table 3), improved versions of successful drugs, and innovative therapies based on the evolving knowledge about the pathobiology of CLL (Table 4).
Table 3.
Selected List of Novel Combination Regimens in Clinical Development for CLL
Table 4.
Selected List of Novel Drugs in Clinical Development for CLL
Novel combination strategies
Ibrutinib plus chemoimmunotherapy
An ongoing phase II trial is testing whether combined treatment with ibrutinib and FCR (iFCR) will lead to deep and durable remissions and possibly cure in younger TN CLL patients.88 Patients are initially treated with ibrutinib for 1 week to mobilize CLL cells out of the lymph nodes into the blood, where they are more vulnerable, and then they receive 6 months of iFCR followed by 2 years of ibrutinib maintenance. Preliminary results are promising, as iFCR induced deep responses even though a significant part of the patients had adverse prognostic markers.88 Another phase II trial (NCT02629809) is investigating the possibility to limit chemotherapy to 3 cycles, potentially reducing short- and long-term toxicity, while maintaining efficacy through replacing rituximab with obinutuzumab.88 This trial includes only patients with mutated IGHV and no del(17p) or TP53 mutation. If the patients achieve CR with undetectable MRD in the bone marrow after 3 courses of iFCG, they receive ibrutinib with obinutuzumab (iG) for cycles 4 to 6, then ibrutinib only for cycles 7 to 12. Patients not achieving the primary endpoint receive iG for cycles 4 to 12. All patients with undetectable MRD at 1 year stop all therapy. Promising preliminary results have already been disclosed.88
Ibrutinib plus anti-CD20 antibodies alone
Apart from the practice-changing ECOG-ACRIN E1912, A041202, and iLLUMINATE trials (see above in the main ibrutinib section), there are other ongoing studies that aim to assess the effectiveness of combined treatment with ibrutinib and anti-CD20 antibodies. The phase III FLAIR trial comparing IR with FCR is evaluating an MRD-based rule for stopping therapy, as the current standard practice of continuous use of ibrutinib imposes a significant financial and toxicological cost.89 The protocol of the trial was recently amended to include two additional treatment arms: ibrutinib monotherapy and ibrutinib plus venetoclax. Ibrutinib was also combined with ofatumumab in a phase Ib/II trial with CLL patients who had failed ≥2 prior therapies and most of whom had high-risk disease.90 Three different administration schedules were tested: (1) ibrutinib lead-in, (2) concurrent start, and (3) ofatumumab lead-in. The ORRs were 100%, 79%, and 71%, and the estimated 12-month PFS was 89%, 85%, and 75% in groups 1, 2, and 3, respectively. Ibrutinib-induced lymphocytosis resolved fast, an effect also observed with IR.91 However, ibrutinib increased the occurrence of mild neuropathy previously associated with ofatumumab.90 The phase II CLL2-BIO and CLL2-BIG trials follow the so-called “sequential triple-T” concept92: initial debulking with bendamustine (to decrease the risk of infusion-related reactions and to help achieve remissions faster) followed by induction and maintenance with ibrutinib-ofatumumab or ibrutinib-obinutuzumab.93 Maintenance treatment is terminated in case of a CR and MRD-negativity. Similar to the FLAIR trial, these trials will elucidate whether ibrutinib can be stopped in case of a deep remission.93
Ibrutinib plus venetoclax
Pretreating CLL cells with ibrutinib increases their dependence on BCL-2, thus enhancing apoptosis in response to venetoclax.76 The 2 drugs have different mechanisms of action and toxicity profiles, and they complement each other regarding their activity on disease compartments (ibrutinib is particularly effective at clearing nodal disease and less so at clearing blood/marrow, whereas the opposite is true for venetoclax). Also, venetoclax is able to induce MRD-negativity, and combined treatment of CLL with ibrutinib and venetoclax thus seems very promising.76 The ongoing phase II CLARITY, CAPTIVATE, and NCT02756897 trials investigate the safety and efficacy of this combination. Preliminary results are encouraging (eg, 100% CR rate and 82% MRD-negativity in peripheral blood after 6 cycles of the combination in the CAPTIVATE trial94) and further data should clarify whether therapy can be safely stopped in patients achieving deep remission.88 Other phase II clinical trials will evaluate the combination in patients with R/R CLL (NCT03226301 and NCT03045328), high-risk CLL (NCT03128879), or CLL with ibrutinib resistance mutations (NCT03513562). Additionally, a phase III study is planned to compare ibrutinib-venetoclax against chlorambucil-obinutuzumab in elderly or otherwise unfit patients with TN CLL (NCT03462719). A strategy to achieve even deeper remissions is triple therapy with ibrutinib, venetoclax, and obinutuzumab, and this regimen is currently being evaluated in TN CLL (trials NCT02427451, NCT02758665/CLL2-GIVe, and NCT02950051/CLL13).
Idelalisib combination therapy
In a phase III trial with R/R CLL patients, addition of idelalisib to the standard BR regimen improved the ORR from 45% to 70% and increased the median PFS from 11.1 to 20.8 months.95 However, increased rate of serious AEs and efficacy similar to that of idelalisib-rituximab96 reduce the practical value of the triple combination.
The ongoing phase II CLL2-BCG trial is using initial debulking with bendamustine followed by induction and maintenance with idelalisib-obinutuzumab.93 This study should elucidate whether treatment with idelalisib can be terminated in case of a deep remission.
Venetoclax plus obinutuzumab
Deep and durable responses with unprecedented MRD-negativity rates were observed in a phase Ib trial testing the combination of venetoclax with obinutuzumab in patients with TN CLL: 100% ORR, 56.3% CR, 100% MRD-negativity in blood, 100% PFS rate at 12 months, and 90.5% PFS rate at 18 months.97 The high effectiveness of this combination is confirmed by the recently published primary endpoint analysis of the phase II CLL2-BAG trial (initial debulking with bendamustine followed by induction and maintenance with venetoclax-obinutuzumab according to the sequential triple-T concept92).98 The ORR at the end of the induction phase of this study was 95% (100% and 90% in the TN and R/R cohorts, respectively) and the MRD-negativity rate in peripheral blood was 87% (91% and 83%, respectively). The trial did not reveal unexpected or cumulative toxicities. The venetoclax-obinutuzumab combination induced good responses also in elderly patients with TN CLL and coexisting medical conditions and is currently being tested in the phase III CLL14 trial against the standard chlorambucil-obinutuzumab regimen.99 Cumulative evidence suggests that venetoclax-obinutuzumab might be one of the most efficacious treatment regimens for CLL but completion of the ongoing phase III trials will be necessary before more definitive statements about its future role in CLL therapy can be made.
New antibodies
Ublituximab (TG-1101) is a type I chimeric monoclonal anti-CD20 antibody.100 It targets a unique epitope on the CD20 antigen and is glycoengineered to enhance affinity for FcγRIIIa. Accordingly, ublituximab demonstrated greater antibody-dependent cellular phagocytosis and enhanced ADCC against CLL cells compared to rituximab, whereas CDC induction was weaker.101 Ublituximab rapidly depleted circulating lymphocytes and induced PRs in R/R CLL patients previously treated with rituximab.102 A phase II study combining ublituximab with ibrutinib demonstrated an impressive ORR of 90% in R/R CLL patients, a significant number of whom had high-risk disease.103 The most common AEs were infusion-related reactions (53% of all patients; 7% had a grade 3/4 reaction) and they often necessitated dose interruptions.103 The ublituximab-ibrutinib combination is now being compared in the phase III GENUINE study against ibrutinib monotherapy in patients with R/R high-risk CLL (NCT02301156).
BI 836826 is a chimeric monoclonal anti-CD37 antibody.104 CD37 is a member of the transmembrane 4 superfamily of tetraspanin proteins and is expressed on developing B cells but not on plasma cells. BI 836826 is glycoengineered to enhance its affinity for FcγRIIIa and to strengthen its ADCC potential.104 It is currently being evaluated in combination with ibrutinib in R/R CLL (NCT02759016).
Otlertuzumab (TRU-016) is a fully humanized homodimeric therapeutic protein consisting of antibody-derived single-chain variable fragments (scFv) specific for CD37 that are linked to immunoglobulin constant domains.105 Its binding to CD37 leads to ADCC and apoptosis induction through upregulation of BIM.106 Otlertuzumab does not induce CDC. In a phase II study, addition of otlertuzumab led to higher ORR (69%) and longer median PFS (15.9 months) compared to bendamustine alone (39% and 10.2 months, respectively) in patients with R/R CLL.105 An ongoing phase Ib study (NCT01644253) is evaluating the efficacy and safety of otlertuzumab in combination with either rituximab, obinutuzumab, ibrutinib, or idelalisib-rituximab.
New BTK inhibitors
Acalabrutinib (ACP-196) is a second-generation irreversible BTK inhibitor with higher selectivity than ibrutinib.56 It also binds to the C481 residue in BTK but is only a weak inhibitor of TEC and does not inhibit EGFR and ITK, thus having fewer adverse effects.57,107 Acalabrutinib was recently approved by the FDA for treatment of mantle cell lymphoma. Some of the ongoing clinical trials with acalabrutinib in CLL include: a phase II study evaluating its safety and efficacy in patients with R/R CLL who do not tolerate ibrutinib (NCT02717611); a phase III trial of acalabrutinib versus ibrutinib in patients with high-risk R/R CLL (NCT02477696); a phase III trial comparing acalabrutinib versus acalabrutinib-obinutuzumab versus chlorambucil-obinutuzumab in TN older patients (NCT02475681); a phase III trial comparing acalabrutinib versus idelalisib-rituximab versus BR in patients with R/R CLL (NCT02970318).45
Zanubrutinib (BGB-3111) is another irreversible BTK inhibitor that binds covalently to C481 and that is more selective than ibrutinib.45 Preliminary data from phase I trials suggest that zanubrutinib has favorable safety profile and clinical activity, alone or in combination with obinutuzumab, in patients with B-cell lymphoid malignancies, including CLL.108 A phase II clinical study in patients with R/R CLL is ongoing (NCT03206918) and a recently launched phase III trial will compare zanubrutinib versus BR as first-line therapy for CLL (NCT03336333).
Tirabrutinib (ONO/GS-4059) is yet another irreversible BTK inhibitor that is more selective than ibrutinib, although it can also inhibit the TEC kinase.45 Data from a phase I study show efficacy of tirabrutinib in patients with R/R CLL, with estimated median PFS and OS of 38.5 and 44.9 months, respectively.109,110 The most frequent grade ≥3 AES were infections (42.9%), neutropenia (25%), thrombocytopenia (14.3%), anemia (10.7%), and diarrhea (7.1%). As with all other BTK inhibitors, most patients (82%) exhibited transient lymphocytosis.109 Two newly launched phase II studies in patients with R/R CLL will evaluate the safety and efficacy of tirabrutinib combined with either idelalisib or entospletinib, with or without additional obinutuzumab (NCT02968563 and NCT02983617).
Vecabrutinib (SNS-062) binds to BTK in a noncovalent manner and inhibits it regardless of the presence of the C481S mutation, thus differing from the previously mentioned compounds.45 This drug shows potential for treating ibrutinib-resistant patients with the BTK C481S mutation, although it would not be expected to overcome resistance due to activating mutations in PLCγ2.45 A phase Ib/II study was recently initiated in patients with advanced B-cell malignancies, including CLL, with or without BTK mutation (NCT03037645).
Fenebrutinib (GDC-0853) is the most selective of all BTK inhibitors currently in clinical development.111 Like vecabrutinib, it also binds to BTK in a reversible noncovalent manner regardless of the presence of C481S substitution.111 Fenebrutinib did not induce any grade ≥3 AEs in healthy subjects.112 A phase I study in patients with R/R CLL and non-Hodgkin lymphoma also showed a favorable safety profile with the most frequent grade ≥3 AEs being infections (16.7%), anemia (12.5%), and hemorrhage (8.3%).113 Despite promising results also in cases with BTK C481S, Genentech decided to stop the trial prematurely and to develop fenebrutinib for other indications.111-113
Results from clinical trials are eagerly awaited to ultimately elucidate how selectivity profiles and binding mode of BTK inhibitors influence their safety and effectiveness.
New PI3K inhibitors
Duvelisib (IPI-145) is an orally bioavailable, highly potent small-molecule inhibitor of the p110δ and p110γ isoforms of the catalytic subunit of PI3K.114 Both isoforms are expressed mostly in leukocytes but have distinct roles115 and a dual inhibitor would be expected to have a better effect against CLL.114 A phase I study of duvelisib demonstrated an ORR of 56% and 83% in patients with R/R and TN CLL, respectively.116 As with ibrutinib and idelalisib, the vast majority of responses were partial. The median PFS was 15.7 months in the R/R CLL group and was not reached in the TN group. The most common grade 3 to 4 AEs were neutropenia (20%), increased ALT (19.5%), increased AST (15.3%), anemia (14.3%), thrombocytopenia (14.3%), diarrhea (11.4%) and pneumonia (9.5%).116 Toxicity was mostly manageable and there were differences in AE incidence between R/R and TN CLL patients, as with idelalisib. In TN patients, addition of duvelisib to FCR substantially increased MRD-negativity rate, a strong surrogate for long-term outcome in CLL.76 The phase III DUO study comparing duvelisib with ofatumumab in R/R CLL patients met its primary endpoint, showing significantly longer median PFS in the duvelisib arm (13.3 vs 9.9 months, HR 0.52), also in patients with del(17p) and/or TP53 mutation.117 The ORR was significantly higher with duvelisib (74% vs 45%). Based on these results, duvelisib was approved by the FDA in September 2018 for treatment of R/R CLL/SLL after at least 2 prior therapies. Another trial is evaluating the safety and efficacy of duvelisib in CLL patients previously treated with a BTK inhibitor (NCT03370185).
Umbralisib (TGR-1202) is a highly selective dual inhibitor of PI3Kδ and casein kinase-1ε (CK-1ε).118 It has a reduced inhibitory activity on regulatory T cells and is less hepatotoxic compared to other PI3Kδ inhibitors.108 A phase I study of umbralisib demonstrated an ORR of 85% and median PFS of 2 years in patients with R/R CLL.119 The most common grade 3 to 4 AEs were neutropenia (13%), anemia (9%), thrombocytopenia (7%), and pneumonia (6%). Occurrences of grade ≥3 increased transaminases (in 3% patients) or colitis (2%) were less frequent compared to reported with idelalisib and duvelisib, suggesting together with other observations that PI3Kδ inhibition is not invariably associated with immune-mediated toxicities and that CK-1ε inhibition has a protective effect.119 Some of the ongoing clinical trials with umbralisib include: a phase II study in CLL patients intolerant to BTK inhibitors or other PI3K inhibitors (NCT02742090); a phase III trial comparing umbralisib-ublituximab versus chlorambucil-obinutuzumab (NCT02612311); several phase I/II studies evaluating the efficacy and safety of umbralisib in various double and triple combinations with ublituximab, obinutuzumab, ibrutinib, bendamustine, and pembrolizumab (NCT02006485, NCT02535286, NCT03283137, NCT02100852, and NCT02268851). A study is planned to test umbralisib in combination with ublituximab and venetoclax in R/R CLL (NCT03379051).
Acalisib (GS-9820) and parsaclisib (INCB050465) are the most selective inhibitors of the p110δ isoform.120 Acalisib was tested in a phase Ib trial in patients with R/R lymphoid malignancies and the ORR and median PFS of CLL patients were 53.3% and 16.6 months, respectively.121 Its safety profile was similar to that of other PI3K inhibitors and the most common grade ≥3 AEs were diarrhea (10.5%), rash (10.5%), and neutropenia (7.9%).
Copanlisib is a highly potent pan-class I PI3K inhibitor with a slight preference for the p110α and p110δ isoforms and has been recently approved by the FDA for treatment of follicular lymphoma.118 In a phase II study, copanlisib demonstrated efficacy in patients with R/R CLL (38.5% ORR) and a distinct toxicity profile.122 Hepatic and gastrointestinal toxicity were less pronounced compared with idelalisib and duvelisib but postinfusion hyperglycemia and hypertension were typical AEs.122 No further trials of copanlisib in CLL have been initiated so far.
Bimiralisib (PQR309) is a dual pan-PI3K/mTOR inhibitor with preference for the p110α isoform.123 Differently from most PI3K and mTOR inhibitors, it is able to cross the blood-brain barrier. Clinical trials in patients with R/R lymphoma, including CLL, have been initiated (NCT03127020 and NCT02249429).
Results from ongoing clinical trials should define the merits of inhibiting specific isoforms of class I PI3Ks versus inhibiting all isoforms and possibly other kinases, too.
SYK inhibitors
Entospletinib (GS-9973) is a selective and orally bioavailable inhibitor of SYK.124 Similar to the other drug classes that inhibit BCR signaling, entospletinib disrupts microenvironmental interactions and causes CLL-cell redistribution that manifests clinically as lymph node reduction with associated transient lymphocytosis.124 In a phase II study of entospletinib, patients with R/R CLL had an ORR of 61% (all PRs) and median PFS of 13.8 months.125 The most common grade ≥3 AEs in this study, including also patients with other R/R hematological malignancies, were neutropenia (14.5%), ALT/AST elevations (13.4%), fatigue (10.2%), anemia (8.1%), and dyspnea (6.5%).125 Entospletinib was found to synergize with idelalisib in vitro126 and a phase II study of this combination was initiated. Despite efficacy (60% ORR), the trial was terminated because 12 patients (18%) developed pneumonitis (grade ≥3 in 11 patients, 2 fatalities).127 This high rate of pneumonitis was probably due to excessive mTOR inhibition by the combination of entospletinib and idelalisib, causing an increase in cytokines/chemokines related to immune cell recruitment and Th1-type responses.127 A phase I/II clinical trial of entospletinib in combination with obinutuzumab in R/R CLL was recently initiated (NCT03010358).
Cerdulatinib (PRT062070) is a dual inhibitor of SYK and JAK1/3 and has been shown to inhibit BCR- and IL4-induced downstream signaling in CLL cells, to overcome stromal support, and to synergize with venetoclax.124 An ongoing phase IIa study will evaluate efficacy and tolerability of cerdulatinib in patients with R/R B-cell malignancies, including CLL (NCT01994382).
TAK-659 is a dual SYK and FLT3 inhibitor that synergizes with ibrutinib against CLL cells in vitro.124 A phase I trial of TAK-659 in patients with advanced malignancies, including CLL, is ongoing (NCT02000934).
MCL-1 inhibitors
MCL-1 is constitutively overexpressed in CLL and is related to apoptosis inhibition and worse patient outcomes.128,129 Resistance to venetoclax is also largely driven by MCL-1, thus this antiapoptotic protein is a promising target in CLL.76 Inhibitors of BCR signaling tend to decrease MCL-1 levels in CLL cells, thus providing a rationale for simultaneous use with venetoclax.130,131 More specific approaches to antagonize MCL-1 function are by using the novel MCL-1 selective BH3-mimetics (A-1210477 and S63845) that are at preclinical stage of development,132,133 or by transcriptional repression via inhibition of cyclin-dependent kinase 9 (CDK9).76 Pan-CDK inhibitors like alvocidib and dinaciclib have already been tested in clinical trials in CLL and encouraging responses were observed.134,135
Immunomodulatory drugs
CLL patients develop progressive immunodeficiency, in part due to the ability of leukemia cells to induce immune suppression as a strategy to evade immune control.136 Two main classes of drugs are able to enhance immune responses against CLL cells: thalidomide analogues, also known as IMiDs, and PD-1 checkpoint inhibitors.
Lenalidomide is the most extensively studied IMiD in CLL. Its main target is the protein cereblon that as part of an E3-ubiquitin ligase complex is able to induce degradation of several target proteins.137 Through stimulating this process, lenalidomide causes a multitude of effects: upregulation of ligands and receptors on CLL cells and enhanced immune recognition; activation of T and NK cells; increase of immunoglobulins; downregulation of inhibitory ligands on T and CLL cells; changes in the microenvironment that reduce support for CLL cells.136 Two phase III trials (CLLM1 and CONTINUUM) explored lenalidomide as maintenance treatment for CLL.138,139 The CLLM1 trial was performed in patients with high-risk CLL (high MRD levels or intermediate levels combined with an unmutated IGHV or TP53 alterations) after at least a PR to first-line chemoimmunotherapy. Median PFS was 13.3 months in the placebo group and not reached with lenalidomide maintenance after 17.9 months (HR 0.168).139 The CONTINUUM trial was done in patients with at least a PR after second-line therapy. Median PFS was significantly longer in the lenalidomide group than in the placebo group (33.9 vs 9.2 months, HR 0.46) and lenalidomide maintenance did not adversely affect response to subsequent therapy.138 However, attempts to use lenalidomide as first-line therapy for CLL have not been particularly successful, as exemplified by the phase III ORIGIN trial performed in elderly patients. In this trial, lenalidomide did not prolong PFS and was associated with a lower response rate, a higher incidence of grade ≥3 AEs, and a higher number of deaths compared with chlorambucil.140 Overall, tolerability of lenalidomide in CLL is problematic and apart from the potentially fatal TLS and tumor flare reaction that can be mitigated by slow dose escalation, it also causes a high incidence of myelosuppression (grade 3–4 neutropenia was reported in up to 80% of patients and was the main cause of dose interruption), as well as venous thromboembolism.136 As BCR signaling inhibitors and venetoclax have better benefit/risk ratios, it seems that lenalidomide can find a potential use only in particular subgroups of patients, for example, as maintenance treatment after chemoimmunotherapy in high-risk patients who are not eligible or do not have access to BCR signaling inhibitors or venetoclax, or after failure of these novel drugs if clinical trials prove lenalidomide effective in this setting.
T cells from CLL patients have elevated expression of the immune checkpoint receptor PD-1 and can exhibit a pseudo-exhaustion phenotype.141 CLL cells can also express PD-1 and/or its ligands PD-L1/2. These findings as well as encouraging preclinical data142,143 provided the rationale for clinical trials of PD-1 checkpoint inhibitors in CLL. Pembrolizumab was ineffective as monotherapy in a phase II trial in R/R CLL (ORR 0%, median PFS 2.4 months) but it benefited patients with RT (ORR 44%, median PFS 5.4 months).144 Based on these results, the protocol of the trial was amended to add a focused RT cohort and a second study with RT patients was initiated (NCT02576990). Several phase I/II studies in CLL are evaluating combinations of pembrolizumab with ibrutinib (NCT03153202), fludarabine and ibrutinib (NCT03204188), umbralisib (NCT03283137), or ublituximab and umbralisib (NCT02535286). Nivolumab is another anti-PD-1 monoclonal antibody under clinical investigation in CLL as a combination partner for ibrutinib (NCT02420912).
Cell-based immunotherapy
A promising treatment option is cellular immunotherapy and especially the use of chimeric antigen receptor (CAR) T cells that are genetically modified to target specific antigens on malignant cells.145 The target most focused on is CD19, as it is expressed only on B-lineage cells.145 CTL019 cells are autologous T cells that are engineered to express a CD19-targeting CAR that has an intracellular activation domain from the CD3-zeta chain and a costimulatory domain from CD137 (4-1BB).146 In a pilot phase I study, CTL019 cells were infused in 14 patients with R/R CLL and 8 of them (57%) responded (4 CRs).147 No patient with a CR relapsed after a median follow-up of 40 months. The median PFS and OS for all treated patients were 7 and 29 months, respectively. Clinical responses correlated with in vivo expansion of the CTL019 cells.147 CTL019 cells were recently approved under the name tisagenlecleucel by the FDA for treatment of R/R B-cell acute lymphoblastic leukemia and R/R diffuse large B-cell lymphoma and thus became the first approved gene therapy. Multiple trials with tisagenlecleucel in CLL are currently ongoing. In difference to CTL019 cells, JCAR014 and JCAR017 cells are manufactured as fixed-ratio (1:1) compositions of CD4+ and CD8+ T cells.148 In addition to the CAR, JCAR014 and JCAR017 cells express also a truncated form of EGFR that can improve safety by allowing eradication of the CAR T-cell clone using anti-EGFR monoclonal antibodies like cetuximab.148 JCAR014 cells were highly effective in high-risk R/R CLL patients experiencing progression while on ibrutinib.149 At 4 weeks after infusion, the ORR in this cohort was 71%. The malignant clone could not be detected in the marrow of 58% of tested patients. The ongoing TRANSCEND-CLL-004 trial is evaluating the safety and efficacy of JCAR017 cells (under the name lisocabtagene maraleucel) in patients with R/R CLL/SLL (NCT03331198).
One of the most important adverse effects of therapy with CAR T cells is the potentially fatal cytokine release syndrome (CRS).145 CRS is often accompanied by macrophage activation syndrome that represents a hyper-inflammatory status with hemophagocytosis and very high levels of ferritin and C-reactive protein.148 Management strategies for these syndromes include supportive care and immunosuppressive therapy with anti-IL6 antibody and/or corticosteroids for more severe cases.145,148 Another common adverse effect of CAR T-cell therapy is persisting B-cell aplasia resulting in hypogammaglobulinemia that has to be managed by intravenous immunoglobulin repletion.145,147
CARs against other proteins expressed by CLL cells are also being developed. Promising targets include CD20, CD22, CD38, CD70, CD123, the kappa light chain, and ROR-1.148 Novel generations of CARs are being developed by including 2 costimulatory domains or by adding a safety mechanism (eg, suicide genes) or constitutive production of cytokines.148
Conclusions
The past two decades brought enormous advancements in understanding of CLL biology that incited numerous drug development programs. Novel classes of drugs (BTK inhibitors, PI3K inhibitors, and BH3-mimetics) were introduced which fundamentally changed management of CLL and considerably improved patient outcomes, especially in subgroups that previously had very poor prognosis. In parallel, new and improved anti-CD20 antibodies were developed which not only increased effectiveness of chemoimmunotherapy but are proving in multiple ongoing clinical trials as valuable combination partners of the novel BCR pathway inhibitors and BH3-mimetics. Considerable efforts are currently focused not only on further improvement of the already successful approaches, but also on making use of recent major advances in immunology like the technology to generate tumor-targeting CAR T cells. Current exciting developments promise to further limit the use of conventional broadly cytotoxic chemotherapeutic drugs and to bring curative therapy for CLL closer to reality.
Footnotes
Citation: Yosifov DY, Wolf C, Stilgenbauer S, Mertens D. From Biology to Therapy: The CLL Success Story. HemaSphere, 2019;00:00. http://dx.doi.org/10.1097/HS9.0000000000000175
Funding/support: Our work is supported by the Deutsche José Carreras Leukämie-Stiftung (DJCLS 14R/2016), the ERA-NET TRANSCAN-2 program (FIRE-CLL), the Deutsche Forschungsgemeinschaft (D-A-CH ME 3667/3-1 and SFB1074B1/B2), and the Bundesministerium für Bildung und Forschung (e:Bio “PRECiSe”).
Disclosure: SS receives research funding and is an advisory board member for AbbVie, Amgen, Boehringer Ingelheim, Celgene, Genentech, Genzyme, Gilead, GSK, Janssen, Mundipharma, Novartis, Pharmacyclics, Hoffmann La-Roche, and Sanofi. DYY, CW, and DM have no potential conflicts of interest to disclose.
References
- 1.Tausch E, Mertens D, Stilgenbauer S. Advances in treating chronic lymphocytic leukemia. F1000Prime Rep. 2014;6:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zenz T, Mertens D, Kuppers R, et al. From pathogenesis to treatment of chronic lymphocytic leukaemia. Nat Rev Cancer. 2010;10:37–50. [DOI] [PubMed] [Google Scholar]
- 3.Dohner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343:1910–1916. [DOI] [PubMed] [Google Scholar]
- 4.Zenz T, Eichhorst B, Busch R, et al. TP53 mutation and survival in chronic lymphocytic leukemia. J Clin Oncol. 2010;28:4473–4479. [DOI] [PubMed] [Google Scholar]
- 5.Nadeu F, Clot G, Delgado J, et al. Clinical impact of the subclonal architecture and mutational complexity in chronic lymphocytic leukemia. Leukemia. 2018;32:645–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woyach JA, Johnson AJ. Targeted therapies in CLL: mechanisms of resistance and strategies for management. Blood. 2015;126:471–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robak T, Dmoszynska A, Solal-Celigny P, et al. Rituximab plus fludarabine and cyclophosphamide prolongs progression-free survival compared with fludarabine and cyclophosphamide alone in previously treated chronic lymphocytic leukemia. J Clin Oncol. 2010;28:1756–1765. [DOI] [PubMed] [Google Scholar]
- 8.Fischer K, Bahlo J, Fink AM, et al. Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: updated results of the CLL8 trial. Blood. 2016;127:208–215. [DOI] [PubMed] [Google Scholar]
- 9.Eichhorst B, Fink AM, Bahlo J, et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2016;17:928–942. [DOI] [PubMed] [Google Scholar]
- 10.Freise KJ, Jones AK, Eckert D, et al. Impact of venetoclax exposure on clinical efficacy and safety in patients with relapsed or refractory chronic lymphocytic leukemia. Clin Pharmacokinet. 2017;56:515–523. [DOI] [PubMed] [Google Scholar]
- 11.O’Brien S, Jones JA, Coutre SE, et al. Ibrutinib for patients with relapsed or refractory chronic lymphocytic leukaemia with 17p deletion (RESONATE-17): a phase 2, open-label, multicentre study. Lancet Oncol. 2016;17:1409–1418. [DOI] [PubMed] [Google Scholar]
- 12.Boross P, Leusen JH. Mechanisms of action of CD20 antibodies. Am J Cancer Res. 2012;2:676–690. [PMC free article] [PubMed] [Google Scholar]
- 13.Cragg MS, Walshe CA, Ivanov AO, et al. The biology of CD20 and its potential as a target for mAb therapy. Curr Dir Autoimmun. 2005;8:140–174. [DOI] [PubMed] [Google Scholar]
- 14.Herishanu Y, Perez-Galan P, Liu D, et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood. 2011;117:563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stevenson FK, Krysov S, Davies AJ, et al. B-cell receptor signaling in chronic lymphocytic leukemia. Blood. 2011;118:4313–4320. [DOI] [PubMed] [Google Scholar]
- 16.Beitz LO, Fruman DA, Kurosaki T, et al. SYK is upstream of phosphoinositide 3-kinase in B cell receptor signaling. J Biol Chem. 1999;274:32662–32666. [DOI] [PubMed] [Google Scholar]
- 17.Le Roy C, Deglesne PA, Chevallier N, et al. The degree of BCR and NFAT activation predicts clinical outcomes in chronic lymphocytic leukemia. Blood. 2012;120:356–365. [DOI] [PubMed] [Google Scholar]
- 18.Wolf C, Garding A, Filarsky K, et al. NFATC1 activation by DNA hypomethylation in chronic lymphocytic leukemia correlates with clinical staging and can be inhibited by ibrutinib. Int J Cancer. 2018;142:322–333. [DOI] [PubMed] [Google Scholar]
- 19.Burger JA, Chiorazzi N. B cell receptor signaling in chronic lymphocytic leukemia. Trends Immunol. 2013;34:592–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Filarsky K, Garding A, Becker N, et al. Kruppel-like factor 4 (KLF4) inactivation in chronic lymphocytic leukemia correlates with promoter DNA-methylation and can be reversed by inhibition of NOTCH signaling. Haematologica. 2016;101:e249–e253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanada M, Delia D, Aiello A, et al. bcl-2 gene hypomethylation and high-level expression in B-cell chronic lymphocytic leukemia. Blood. 1993;82:1820–1828. [PubMed] [Google Scholar]
- 22.Adachi M, Tefferi A, Greipp PR, et al. Preferential linkage of bcl-2 to immunoglobulin light chain gene in chronic lymphocytic leukemia. J Exp Med. 1990;171:559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allegra D, Bilan V, Garding A, et al. Defective DROSHA processing contributes to downregulation of MiR-15/-16 in chronic lymphocytic leukemia. Leukemia. 2014;28:98–107. [DOI] [PubMed] [Google Scholar]
- 24.Cimmino A, Calin GA, Fabbri M, et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci USA. 2005;102:13944–13949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Certo M, Del Gaizo Moore V, Nishino M, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–365. [DOI] [PubMed] [Google Scholar]
- 26.Del Gaizo Moore V, Brown JR, Certo M, et al. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J Clin Invest. 2007;117:112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376:1164–1174. [DOI] [PubMed] [Google Scholar]
- 28.Stilgenbauer S, Schnaiter A, Paschka P, et al. Gene mutations and treatment outcome in chronic lymphocytic leukemia: results from the CLL8 trial. Blood. 2014;123:3247–3254. [DOI] [PubMed] [Google Scholar]
- 29.Teeling JL, Mackus WJ, Wiegman LJ, et al. The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. J Immunol. 2006;177:362–371. [DOI] [PubMed] [Google Scholar]
- 30.Pawluczkowycz AW, Beurskens FJ, Beum PV, et al. Binding of submaximal C1q promotes complement-dependent cytotoxicity (CDC) of B cells opsonized with anti-CD20 mAbs ofatumumab (OFA) or rituximab (RTX): considerably higher levels of CDC are induced by OFA than by RTX. J Immunol. 2009;183:749–758. [DOI] [PubMed] [Google Scholar]
- 31.Gupta IV, Jewell RC. Ofatumumab, the first human anti-CD20 monoclonal antibody for the treatment of B cell hematologic malignancies. Ann N Y Acad Sci. 2012;1263:43–56. [DOI] [PubMed] [Google Scholar]
- 32.Hillmen P, Robak T, Janssens A, et al. Chlorambucil plus ofatumumab versus chlorambucil alone in previously untreated patients with chronic lymphocytic leukaemia (COMPLEMENT 1): a randomised, multicentre, open-label phase 3 trial. Lancet. 2015;385:1873–1883. [DOI] [PubMed] [Google Scholar]
- 33.Robak T, Warzocha K, Govind Babu K, et al. Ofatumumab plus fludarabine and cyclophosphamide in relapsed chronic lymphocytic leukemia: results from the COMPLEMENT 2 trial. Leuk Lymphoma. 2017;58:1084–1093. [DOI] [PubMed] [Google Scholar]
- 34.Tausch E, Dolnik A, Estenfelder S, et al. Predictive and prognostic impact of gene mutations in the context of fludarabine and cyclophosphamide (FC) with or without ofatumumab treatment in patients with rel/ref CLL. Abstract presented at: EHA22. 2017. [Google Scholar]
- 35.Moreno C, Montillo M, Panayiotidis P, et al. Ofatumumab in poor-prognosis chronic lymphocytic leukemia: a phase IV, non-interventional, observational study from the European Research Initiative on Chronic Lymphocytic Leukemia. Haematologica. 2015;100:511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370:1101–1110. [DOI] [PubMed] [Google Scholar]
- 37.Estenfelder S, Tausch E, Robrecht S, et al. Gene mutations and treatment outcome in the context of chlorambucil (Clb) without or with the addition of rituximab (R) or obinutuzumab (GA-101, G)—results of an extensive analysis of the phase III study CLL11 of the German CLL Study Group. Blood. 2016;128:3227. [Google Scholar]
- 38.Freeman CL, Sehn LH. A tale of two antibodies: obinutuzumab versus rituximab. Br J Haematol. 2018;182:29–45. [DOI] [PubMed] [Google Scholar]
- 39.Herman SE, Mustafa RZ, Gyamfi JA, et al. Ibrutinib inhibits BCR and NF-kappaB signaling and reduces tumor proliferation in tissue-resident cells of patients with CLL. Blood. 2014;123:3286–3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Honigberg LA, Smith AM, Sirisawad M, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci USA. 2010;107:13075–13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dubovsky JA, Beckwith KA, Natarajan G, et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood. 2013;122:2539–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ponader S, Chen SS, Buggy JJ, et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012;119:1182–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woyach JA, Smucker K, Smith LL, et al. Prolonged lymphocytosis during ibrutinib therapy is associated with distinct molecular characteristics and does not indicate a suboptimal response to therapy. Blood. 2014;123:1810–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burger JA, Li KW, Keating MJ, et al. Leukemia cell proliferation and death in chronic lymphocytic leukemia patients on therapy with the BTK inhibitor ibrutinib. JCI Insight. 2017;2:e89904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thompson PA, Burger JA. Bruton's tyrosine kinase inhibitors: first and second generation agents for patients with chronic lymphocytic leukemia (CLL). Expert Opin Investig Drugs. 2018;27:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Brien S, Furman RR, Coutre S, et al. Single-agent ibrutinib in treatment-naive and relapsed/refractory chronic lymphocytic leukemia: a 5-year experience. Blood. 2018;131:1910–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown JR, Hillmen P, O’Brien S, et al. Extended follow-up and impact of high-risk prognostic factors from the phase 3 RESONATE study in patients with previously treated CLL/SLL. Leukemia. 2018;32:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373:2425–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chanan-Khan A, Cramer P, Demirkan F, et al. Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): a randomised, double-blind, phase 3 study. Lancet Oncol. 2016;17:200–211. [DOI] [PubMed] [Google Scholar]
- 51.Shanafelt TD, Wang V, Kay NE, et al. A randomized phase III study of ibrutinib (PCI-32765)-based therapy vs. standard fludarabine, cyclophosphamide, and rituximab (FCR) chemoimmunotherapy in untreated younger patients with chronic lymphocytic leukemia (CLL): a trial of the ECOG-ACRIN Cancer Research Group (E1912). Blood. 2018;132:LBA-4. [Google Scholar]
- 52.Woyach JA, Ruppert AS, Heerema NA, et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med. 2018;379:2517–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moreno C, Greil R, Demirkan F, et al. Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:43–56. [DOI] [PubMed] [Google Scholar]
- 54.Burger JA, Sivina M, Jain N, et al. Randomized trial of ibrutinib versus ibrutinib plus rituximab in patients with chronic lymphocytic leukemia. Blood. 2019;doi: 10.1182/blood-2018-10-879429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tedeschi A, Greil R, Demirkan F, et al. Single-agent ibrutinib versus chlorambucil-obinutuzumab as first-line treatment in patients with chronic lymphocytic leukemia or small lymphocytic lymphoma (CLL/SLL): results of a cross-trial comparison. Blood. 2018;132:5565–15565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pettijohn EM, Ma S. Targeted therapy in chronic lymphocytic leukemia (CLL). Curr Hematol Malig Rep. 2017;12:20–28. [DOI] [PubMed] [Google Scholar]
- 57.Barf T, Covey T, Izumi R, et al. Acalabrutinib (ACP-196): a covalent Bruton tyrosine kinase inhibitor with a differentiated selectivity and in vivo potency profile. J Pharmacol Exp Ther. 2017;363:240–252. [DOI] [PubMed] [Google Scholar]
- 58.Ahn IE, Underbayev C, Albitar A, et al. Clonal evolution leading to ibrutinib resistance in chronic lymphocytic leukemia. Blood. 2017;129:1469–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maddocks KJ, Ruppert AS, Lozanski G, et al. Etiology of ibrutinib therapy discontinuation and outcomes in patients with chronic lymphocytic leukemia. JAMA Oncol. 2015;1:80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Woyach JA, Ruppert AS, Guinn D, et al. BTK(C481S)-mediated resistance to ibrutinib in chronic lymphocytic leukemia. J Clin Oncol. 2017;35:1437–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Woyach JA. Patterns of resistance to B cell-receptor pathway antagonists in chronic lymphocytic leukemia and strategies for management. Hematology Am Soc Hematol Educ Program. 2015;2015:355–360. [DOI] [PubMed] [Google Scholar]
- 62.Walliser C, Hermkes E, Schade A, et al. The phospholipase Cγ2 mutants R665W and L845F identified in ibrutinib-resistant chronic lymphocytic leukemia patients are hypersensitive to the Rho GTPase Rac2 protein. J Biol Chem. 2016;291:22136–22148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burger JA, Landau DA, Taylor-Weiner A, et al. Clonal evolution in patients with chronic lymphocytic leukaemia developing resistance to BTK inhibition. Nat Commun. 2016;7:11589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Langerbeins P, Bahlo J, Rhein C, et al. The CLL12 trial protocol: a placebo-controlled double-blind phase III study of ibrutinib in the treatment of early-stage chronic lymphocytic leukemia patients with risk of early disease progression. Future Oncol. 2015;11:1895–1903. [DOI] [PubMed] [Google Scholar]
- 65.Brown JR, Byrd JC, Coutre SE, et al. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110δ, for relapsed/refractory chronic lymphocytic leukemia. Blood. 2014;123:3390–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fiorcari S, Brown WS, McIntyre BW, et al. The PI3-kinase delta inhibitor idelalisib (GS-1101) targets integrin-mediated adhesion of chronic lymphocytic leukemia (CLL) cell to endothelial and marrow stromal cells. PLoS ONE. 2013;8:e83830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370:997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.O’Brien SM, Lamanna N, Kipps TJ, et al. A phase 2 study of idelalisib plus rituximab in treatment-naive older patients with chronic lymphocytic leukemia. Blood. 2015;126:2686–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jones JA, Robak T, Brown JR, et al. Efficacy and safety of idelalisib in combination with ofatumumab for previously treated chronic lymphocytic leukaemia: an open-label, randomised phase 3 trial. Lancet Haematol. 2017;4:e114–e126. [DOI] [PubMed] [Google Scholar]
- 70.Patton DT, Garden OA, Pearce WP, et al. Cutting edge: the phosphoinositide 3-kinase p110 delta is critical for the function of CD4+CD25+Foxp3+ regulatory T cells. J Immunol. 2006;177:6598–6602. [DOI] [PubMed] [Google Scholar]
- 71.Lampson BL, Kasar SN, Matos TR, et al. Idelalisib given front-line for treatment of chronic lymphocytic leukemia causes frequent immune-mediated hepatotoxicity. Blood. 2016;128:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arnason JE, Brown JR. Targeting B cell signaling in chronic lymphocytic leukemia. Curr Oncol Rep. 2017;19:61. [DOI] [PubMed] [Google Scholar]
- 73.Billard C. BH3 mimetics: status of the field and new developments. Mol Cancer Ther. 2013;12:1691–1700. [DOI] [PubMed] [Google Scholar]
- 74.van Delft MF, Wei AH, Mason KD, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kipps TJ, Eradat H, Grosicki S, et al. A phase 2 study of the BH3 mimetic BCL2 inhibitor navitoclax (ABT-263) with or without rituximab, in previously untreated B-cell chronic lymphocytic leukemia. Leuk Lymphoma. 2015;56:2826–2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bose P, Gandhi V. Recent therapeutic advances in chronic lymphocytic leukemia. F1000Res. 2017;6:1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Souers AJ, Leverson JD, Boghaert ER, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19:202–208. [DOI] [PubMed] [Google Scholar]
- 78.Stilgenbauer S, Eichhorst B, Schetelig J, et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: a multicentre, open-label, phase 2 study. Lancet Oncol. 2016;17:768–778. [DOI] [PubMed] [Google Scholar]
- 79.Stilgenbauer S, Eichhorst B, Schetelig J, et al. Venetoclax for patients with chronic lymphocytic leukemia with 17p deletion: results from the full population of a phase ii pivotal trial. J Clin Oncol. 2018;36:1973–1980. [DOI] [PubMed] [Google Scholar]
- 80.Jones JA, Mato AR, Wierda WG, et al. Venetoclax for chronic lymphocytic leukaemia progressing after ibrutinib: an interim analysis of a multicentre, open-label, phase 2 trial. Lancet Oncol. 2018;19:65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Coutre S, Choi M, Furman RR, et al. Venetoclax for patients with chronic lymphocytic leukemia who progressed during or after idelalisib therapy. Blood. 2018;131:1704–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Anderson MA, Tam C, Lew TE, et al. Clinicopathological features and outcomes of progression of CLL on the BCL2 inhibitor venetoclax. Blood. 2017;129:3362–3370. [DOI] [PubMed] [Google Scholar]
- 83.Dreger P, Ghia P, Schetelig J, et al. High-risk chronic lymphocytic leukemia in the era of pathway inhibitors: integrating molecular and cellular therapies. Blood. 2018;132:892–902. [DOI] [PubMed] [Google Scholar]
- 84.Blombery P, Anderson MA, Gong JN, et al. Acquisition of the recurrent Gly101Val mutation in BCL2 confers resistance to venetoclax in patients with progressive chronic lymphocytic leukemia. Cancer Discov. 2019;doi: 10.1158/2159-8290.CD-18-1119. [DOI] [PubMed] [Google Scholar]
- 85.Seymour JF, Kipps TJ, Eichhorst B, et al. Venetoclax-rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2018;378:1107–1120. [DOI] [PubMed] [Google Scholar]
- 86.Freise KJ, Jones AK, Menon RM, et al. Relationship between venetoclax exposure, rituximab coadministration, and progression-free survival in patients with relapsed or refractory chronic lymphocytic leukemia: demonstration of synergy. Hematol Oncol. 2017;35:679–684. [DOI] [PubMed] [Google Scholar]
- 87.Kater AP, Seymour JF, Hillmen P, et al. Fixed duration of venetoclax-rituximab in relapsed/refractory chronic lymphocytic leukemia eradicates minimal residual disease and prolongs survival: post-treatment follow-up of the MURANO phase III study. J Clin Oncol. 2019;doi: 10.1200/JCO.18.01580. [DOI] [PubMed] [Google Scholar]
- 88.Owen C, Toze C, Christofides A. Updates from the 2017 American Society of Hematology annual meeting: practice-changing studies in untreated chronic lymphocytic leukemia. Curr Oncol. 2018;25:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Collett L, Howard DR, Munir T, et al. Assessment of ibrutinib plus rituximab in front-line CLL (FLAIR trial): study protocol for a phase III randomised controlled trial. Trials. 2017;18:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jaglowski SM, Jones JA, Nagar V, et al. Safety and activity of BTK inhibitor ibrutinib combined with ofatumumab in chronic lymphocytic leukemia: a phase 1b/2 study. Blood. 2015;126:842–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Burger JA, Keating MJ, Wierda WG, et al. Safety and activity of ibrutinib plus rituximab for patients with high-risk chronic lymphocytic leukaemia: a single-arm, phase 2 study. Lancet Oncol. 2014;15:1090–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hallek M. Signaling the end of chronic lymphocytic leukemia: new frontline treatment strategies. Blood. 2013;122:3723–3734. [DOI] [PubMed] [Google Scholar]
- 93.Cramer P, von Tresckow J, Bahlo J, et al. CLL2-BXX phase II trials: sequential, targeted treatment for eradication of minimal residual disease in chronic lymphocytic leukemia. Future Oncol. 2018;14:499–513. [DOI] [PubMed] [Google Scholar]
- 94.Wierda WG, Siddiqi T, Flinn I, et al. Phase 2 CAPTIVATE results of ibrutinib (ibr) plus venetoclax (ven) in first-line chronic lymphocytic leukemia (CLL). J Clin Oncol. 2018;36:7502. [Google Scholar]
- 95.Zelenetz AD, Barrientos JC, Brown JR, et al. Idelalisib or placebo in combination with bendamustine and rituximab in patients with relapsed or refractory chronic lymphocytic leukaemia: interim results from a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2017;18:297–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mauro FR. Another treatment option for relapsed or refractory chronic lymphocytic leukaemia. Lancet Oncol. 2017;18:270–271. [DOI] [PubMed] [Google Scholar]
- 97.Flinn IW, Gribben JG, Dyer MJS, et al. Safety, efficacy and MRD negativity of a combination of venetoclax and obinutuzumab in patients with previously untreated chronic lymphocytic leukemia—results from a phase 1b study (GP28331). Blood. 2017;130:430. [Google Scholar]
- 98.Cramer P, von Tresckow J, Bahlo J, et al. Bendamustine followed by obinutuzumab and venetoclax in chronic lymphocytic leukaemia (CLL2-BAG): primary endpoint analysis of a multicentre, open-label, phase 2 trial. Lancet Oncol. 2018;19:1215–1228. [DOI] [PubMed] [Google Scholar]
- 99.Fischer K, Al-Sawaf O, Fink AM, et al. Venetoclax and obinutuzumab in chronic lymphocytic leukemia. Blood. 2017;129:2702–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Babiker HM, Glode AE, Cooke LS, et al. Ublituximab for the treatment of CD20 positive B-cell malignancies. Expert Opin Investig Drugs. 2018;27:407–412. [DOI] [PubMed] [Google Scholar]
- 101.Butler LA, Tam CS, Seymour JF. Dancing partners at the ball: rational selection of next generation anti-CD20 antibodies for combination therapy of chronic lymphocytic leukemia in the novel agents era. Blood Rev. 2017;31:318–327. [DOI] [PubMed] [Google Scholar]
- 102.Sawas A, Farber CM, Schreeder MT, et al. A phase 1/2 trial of ublituximab, a novel anti-CD20 monoclonal antibody, in patients with B-cell non-Hodgkin lymphoma or chronic lymphocytic leukaemia previously exposed to rituximab. Br J Haematol. 2017;177:243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sharman JP, Farber CM, Mahadevan D, et al. Ublituximab (TG-1101), a novel glycoengineered anti-CD20 antibody, in combination with ibrutinib is safe and highly active in patients with relapsed and/or refractory chronic lymphocytic leukaemia: results of a phase 2 trial. Br J Haematol. 2017;176:412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Witkowska M, Smolewski P, Robak T. Investigational therapies targeting CD37 for the treatment of B-cell lymphoid malignancies. Expert Opin Investig Drugs. 2018;27:171–177. [DOI] [PubMed] [Google Scholar]
- 105.Robak T, Hellmann A, Kloczko J, et al. Randomized phase 2 study of otlertuzumab and bendamustine versus bendamustine in patients with relapsed chronic lymphocytic leukaemia. Br J Haematol. 2017;176:618–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lapalombella R, Yeh YY, Wang L, et al. Tetraspanin CD37 directly mediates transduction of survival and apoptotic signals. Cancer Cell. 2012;21:694–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Byrd JC, Harrington B, O’Brien S, et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374:323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Robak P, Robak T. Novel synthetic drugs currently in clinical development for chronic lymphocytic leukemia. Expert Opin Investig Drugs. 2017;26:1249–1265. [DOI] [PubMed] [Google Scholar]
- 109.Walter HS, Jayne S, Rule SA, et al. Long-term follow-up of patients with CLL treated with the selective Bruton's tyrosine kinase inhibitor ONO/GS-4059. Blood. 2017;129:2808–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Walter HS, Rule SA, Dyer MJ, et al. A phase 1 clinical trial of the selective BTK inhibitor ONO/GS-4059 in relapsed and refractory mature B-cell malignancies. Blood. 2016;127:411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Crawford JJ, Johnson AR, Misner DL, et al. Discovery of GDC-0853: a potent, selective, and noncovalent Bruton's tyrosine kinase inhibitor in early clinical development. J Med Chem. 2018;61:2227–2245. [DOI] [PubMed] [Google Scholar]
- 112.Herman AE, Chinn LW, Kotwal SG, et al. Safety, pharmacokinetics, and pharmacodynamics in healthy volunteers treated with GDC-0853, a selective reversible Bruton's tyrosine kinase inhibitor. Clin Pharmacol Ther. 2018;103:1020–1028. [DOI] [PubMed] [Google Scholar]
- 113.Byrd JC, Smith S, Wagner-Johnston N, et al. First-in-human phase 1 study of the BTK inhibitor GDC-0853 in relapsed or refractory B-cell NHL and CLL. Oncotarget. 2018;9:13023–13035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Balakrishnan K, Peluso M, Fu M, et al. The phosphoinositide-3-kinase (PI3K)-delta and gamma inhibitor, IPI-145 (Duvelisib), overcomes signals from the PI3K/AKT/S6 pathway and promotes apoptosis in CLL. Leukemia. 2015;29:1811–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ali AY, Wu X, Eissa N, et al. Distinct roles for phosphoinositide 3-kinases gamma and delta in malignant B cell migration. Leukemia. 2018;32:1958–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Flinn IW, O’Brien S, Kahl B, et al. Duvelisib, a novel oral dual inhibitor of PI3K-δ,γ, is clinically active in advanced hematologic malignancies. Blood. 2018;131:877–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Flinn IW, Hillmen P, Montillo M, et al. The phase 3 DUO trial: duvelisib vs ofatumumab in relapsed and refractory CLL/SLL. Blood. 2018;132:2446–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lampson BL, Brown JR. PI3Kδ-selective and PI3Kα/δ-combinatorial inhibitors in clinical development for B-cell non-Hodgkin lymphoma. Expert Opin Investig Drugs. 2017;26:1267–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Burris HA, III, Flinn IW, Patel MR, et al. Umbralisib, a novel PI3Kδ and casein kinase-1ε inhibitor, in relapsed or refractory chronic lymphocytic leukaemia and lymphoma: an open-label, phase 1, dose-escalation, first-in-human study. Lancet Oncol. 2018;19:486–496. [DOI] [PubMed] [Google Scholar]
- 120.Shugg RP, Thomson A, Tanabe N, et al. Effects of isoform-selective phosphatidylinositol 3-kinase inhibitors on osteoclasts: actions on cytoskeletal organization, survival, and resorption. J Biol Chem. 2013;288:35346–35357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kater AP, Tonino SH, Spiering M, et al. Final results of a phase 1b study of the safety and efficacy of the PI3Kδ inhibitor acalisib (GS-9820) in relapsed/refractory lymphoid malignancies. Blood Cancer J. 2018;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dreyling M, Morschhauser F, Bouabdallah K, et al. Phase II study of copanlisib, a PI3K inhibitor, in relapsed or refractory, indolent or aggressive lymphoma. Ann Oncol. 2017;28:2169–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Beaufils F, Cmiljanovic N, Cmiljanovic V, et al. 5-(4,6-Dimorpholino-1,3,5-triazin-2-yl)-4-(trifluoromethyl)pyridin-2-amine (PQR309), a potent, brain-penetrant, orally bioavailable, pan-class I PI3K/mTOR inhibitor as clinical candidate in oncology. J Med Chem. 2017;60:7524–7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu D, Mamorska-Dyga A. Syk inhibitors in clinical development for hematological malignancies. J Hematol Oncol. 2017;10:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sharman J, Hawkins M, Kolibaba K, et al. An open-label phase 2 trial of entospletinib (GS-9973), a selective spleen tyrosine kinase inhibitor, in chronic lymphocytic leukemia. Blood. 2015;125:2336–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Burke RT, Meadows S, Loriaux MM, et al. A potential therapeutic strategy for chronic lymphocytic leukemia by combining Idelalisib and GS-9973, a novel spleen tyrosine kinase (Syk) inhibitor. Oncotarget. 2014;5:908–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Barr PM, Saylors GB, Spurgeon SE, et al. Phase 2 study of idelalisib and entospletinib: pneumonitis limits combination therapy in relapsed refractory CLL and NHL. Blood. 2016;127:2411–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Awan FT, Kay NE, Davis ME, et al. Mcl-1 expression predicts progression-free survival in chronic lymphocytic leukemia patients treated with pentostatin, cyclophosphamide, and rituximab. Blood. 2009;113:535–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hussain SR, Cheney CM, Johnson AJ, et al. Mcl-1 is a relevant therapeutic target in acute and chronic lymphoid malignancies: down-regulation enhances rituximab-mediated apoptosis and complement-dependent cytotoxicity. Clin Cancer Res. 2007;13:2144–2150. [DOI] [PubMed] [Google Scholar]
- 130.Cervantes-Gomez F, Lamothe B, Woyach JA, et al. Pharmacological and protein profiling suggests venetoclax (ABT-199) as optimal partner with ibrutinib in chronic lymphocytic leukemia. Clin Cancer Res. 2015;21:3705–3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Patel VM, Balakrishnan K, Douglas M, et al. Duvelisib treatment is associated with altered expression of apoptotic regulators that helps in sensitization of chronic lymphocytic leukemia cells to venetoclax (ABT-199). Leukemia. 2017;31:1872–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kotschy A, Szlavik Z, Murray J, et al. The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature. 2016;538:477–482. [DOI] [PubMed] [Google Scholar]
- 133.Leverson JD, Zhang H, Chen J, et al. Potent and selective small-molecule MCL-1 inhibitors demonstrate on-target cancer cell killing activity as single agents and in combination with ABT-263 (navitoclax). Cell Death Dis. 2015;6:e1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Blachly JS, Byrd JC, Grever M. Cyclin-dependent kinase inhibitors for the treatment of chronic lymphocytic leukemia. Semin Oncol. 2016;43:265–273. [DOI] [PubMed] [Google Scholar]
- 135.Ghia P, Scarfo L, Perez S, et al. Efficacy and safety of dinaciclib vs ofatumumab in patients with relapsed/refractory chronic lymphocytic leukemia. Blood. 2017;129:1876–1878. [DOI] [PubMed] [Google Scholar]
- 136.Itchaki G, Brown JR. Lenalidomide in the treatment of chronic lymphocytic leukemia. Expert Opin Investig Drugs. 2017;26:633–650. [DOI] [PubMed] [Google Scholar]
- 137.Zhu YX, Braggio E, Shi CX, et al. Identification of cereblon-binding proteins and relationship with response and survival after IMiDs in multiple myeloma. Blood. 2014;124:536–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chanan-Khan AA, Zaritskey A, Egyed M, et al. Lenalidomide maintenance therapy in previously treated chronic lymphocytic leukaemia (CONTINUUM): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Haematol. 2017;4:e534–e543. [DOI] [PubMed] [Google Scholar]
- 139.Fink AM, Bahlo J, Robrecht S, et al. Lenalidomide maintenance after first-line therapy for high-risk chronic lymphocytic leukaemia (CLLM1): final results from a randomised, double-blind, phase 3 study. Lancet Haematol. 2017;4:e475–e486. [DOI] [PubMed] [Google Scholar]
- 140.Chanan-Khan A, Egyed M, Robak T, et al. Randomized phase 3 study of lenalidomide versus chlorambucil as first-line therapy for older patients with chronic lymphocytic leukemia (the ORIGIN trial). Leukemia. 2017;31:1240–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xu-Monette ZY, Zhou J, Young KH. PD-1 expression and clinical PD-1 blockade in B-cell lymphomas. Blood. 2018;131:68–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.McClanahan F, Hanna B, Miller S, et al. PD-L1 checkpoint blockade prevents immune dysfunction and leukemia development in a mouse model of chronic lymphocytic leukemia. Blood. 2015;126:203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.McClanahan F, Riches JC, Miller S, et al. Mechanisms of PD-L1/PD-1-mediated CD8 T-cell dysfunction in the context of aging-related immune defects in the Emicro-TCL1 CLL mouse model. Blood. 2015;126:212–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ding W, LaPlant BR, Call TG, et al. Pembrolizumab in patients with CLL and Richter transformation or with relapsed CLL. Blood. 2017;129:3419–3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mato AR, Thompson MC, Nabhan C, et al. Chimeric antigen receptor T-cell therapy for chronic lymphocytic leukemia: a narrative review. Clin Lymphoma Myeloma Leuk. 2017;17:852–856. [DOI] [PubMed] [Google Scholar]
- 146.Porter DL, Kalos M, Zheng Z, et al. Chimeric antigen receptor therapy for B-cell malignancies. J Cancer. 2011;2:331–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Porter DL, Hwang WT, Frey NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7:303ra139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ruella M, June CH. Chimeric antigen receptor T cells for B cell neoplasms: choose the right CAR for you. Curr Hematol Malig Rep. 2016;11:368–384. [DOI] [PubMed] [Google Scholar]
- 149.Turtle CJ, Hay KA, Hanafi LA, et al. Durable molecular remissions in chronic lymphocytic leukemia treated with CD19-specific chimeric antigen receptor-modified T cells after failure of ibrutinib. J Clin Oncol. 2017;35:3010–3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.da Cunha-Bang C, Simonsen J, Rostgaard K, et al. Improved survival for patients diagnosed with chronic lymphocytic leukemia in the era of chemo-immunotherapy: a Danish population-based study of 10455 patients. Blood Cancer J. 2016;6:e499. [DOI] [PMC free article] [PubMed] [Google Scholar]