Abstract
The success of chimeric antigen receptor (CAR)-T cell therapy with impressive response rates in hematologic malignancies but also promising data in solid tumors came along with the cognition of unexpected, potentially life-threatening immune-mediated toxicities, namely the cytokine release syndrome (CRS) and neurotoxicity recently referred to as “immune effector cell-associated neurotoxicity syndrome” (ICANS). These toxicities require urgent diagnostic and therapeutic interventions and targeted modulation of key cytokine pathways represents the mainstay of CRS treatment. However, as the underlying mechanisms of ICANS are not well understood, treatment options remain limited and further investigation is warranted.
Importantly, after the recent market approval of 2 CAR-T cell constructs, the application of CAR-T cells will expand to nonacademic centers with limited experience in the management of CAR-T cell-associated toxicities.
Here, we review the current evidence of CRS and ICANS pathophysiology, diagnostics, and treatment.
Introduction
Impressive response rates including long-lasting remissions even in chemorefractory hematologic malignancies using CD19-redirected T cells incorporating a chimeric antigen receptor (CAR) led to breakthrough therapy approval by both the Food and Drug Administration and European Medicines Agency. The CAR-T cell principle is based on genetically engineered autologous or allogenic T cells that express a CAR which consists of an epitope-specific binding domain (most commonly an antibody-derived single-chain variable fragment), a hinge and transmembrane domain and finally signaling domains of the T cell receptor (TCR) (mostly consisting of the CD3ζ chain). Current second-generation CAR-T constructs are combined with additional costimulatory domains such as CD28, 4-1BB, and OX40. This enables a strong antigen-specific T cell activation without the need of TCR-MHC interactions that are prone to coinhibitory signals.1
Two CD19-targeting constructs have recently been approved for B lymphoblastic leukemia (B-ALL)2,3 and aggressive lymphoma (diffuse large B cell lymphoma [DLBCL], transformed follicular lymphoma [FL], primary mediastinal B cell lymphoma [PMBCL]).4,5 Early clinical data in multiple myeloma6 and glioblastoma7 are also promising. However, especially in B-ALL and aggressive lymphoma, severe toxicities with even fatal outcome have occurred, mainly due to 2 common CAR-T cell-mediated toxicities: the cytokine release syndrome (CRS) and CAR-T cell-related neurotoxicity, before referred to as CAR-T cell-related encephalopathy syndrome (CRES) and since the publication of consensus recommendations supported by the American Society for Blood and Marrow Transplantation (ASBMT) termed immune effector cell-associated neurotoxicity syndrome (ICANS). In addition to the above-mentioned CRS and ICANS, other severe toxicities like prolonged cytopenia, B cell depletion and febrile neutropenia have been frequently reported. Here we focus, review, and discuss the current understanding of CRS and ICANS disease pathophysiology, clinical presentation, and the toxicity management of CAR-T cell therapy.
History and epidemiology of CRS
CRS represents an immune-mediated toxicity characterized by an excessive immune reaction caused by immune-modulating drugs. Recently, T cell engaging therapies like T cell redirecting antibodies (eg, blinatumomab), immune-mobilizing monoclonal TCRs against cancer (ImmTAC), or other TCR-based strategies8,9 and to an even larger extent CAR-T cell therapy have resulted in high CRS rates. With growing experience with CAR-T cell treatment, the rates of severe CRS have decreased in the more recent studies due to earlier intervention. Table 1 shows the reported incidences of CRS and ICANS in recent clinical trials.
Table 1.
Reported Data for CRS and ICANS in Recent Clinical Trials
Apart from CAR-T cell therapy, CRS has also been described with many T and B lymphocyte engaging therapies, namely muromonab-CD3 (OKT3),10,11 antithymocyte globulin (ATG),12 CD28 superagonist TGN1412,13 rituximab,14 obinutuzumab,15 alemtuzumab,16 brentuximab,17 nivolumab,18 CD40 agonists19 but also in overwhelming viral infections like influenza20,21 and in the hematopoietic stem cell transplant setting.22,23 Unlike in CAR-T cells peak CRS symptoms occurring after these therapies usually start to develop shortly after antibody administration.
CRS pathophysiology
CRS is thought to be mediated by an initial release of proinflammatory cytokines like interferon gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α) by activated or lysed effector cells including T cells activated by the tumor antigen recognizing CAR or T cell redirecting antibodies (eg, blinatumomab), lysed T and B lymphocytes in the case of, for example, ATG and rituximab. These cytokines lead to an activation of bystander immune cells and endothelial cells which in turn activate more immune cells culminating in a cytokine storm.24
Macrophages seem to play an important role in CRS pathophysiology. When activated by secreted IFN-γ,25 they produce excessive amounts of cytokines such as interleukin 6 (IL-6), TNF-α, and interleukin 10 (IL-10). Depletion of macrophages in a humanized xenotolerant mouse model abated CRS. Macrophages were shown to be the mayor source of IL-1 and IL-6 production in this model while CAR-T cells only produced negligible levels.26 In addition, IL-6 seems to be central to CRS disease pathology since it is highly elevated in CRS patients14,27–29 and CRS mouse models.26,30,31 These findings were recently emphasized by a study showing that an early increase in IL-6 and angiopoetin 2: angiopoetin 1 (Ang-2:Ang-1) ratio are associated with very severe CRS in patients treated with CAR-T cells.32 Moreover, blockade of the IL-6 pathway leads to rapid resolution of CRS symptoms in mice26,31 and humans.33,34 IL-6 has also been implicated in key pathogenetic aspects of CRS like vascular leakage, disseminated intravascular coagulation (DIC),29,35 and cardiomyopathy.36 Interestingly, blockade of IL-1 terminated CRS and ICANS symptoms in a mouse model underscoring the role of IL-1 in CRS pathogenesis.26,31 Another hallmark of CRS and ICANS is the activation of endothelial cells. Serum levels of activation markers like Ang-2, von Willebrand factor and intercellular adhesion molecule were shown to be elevated in CRS patients and might lead to capillary leakage, hypotension, and coagulopathy.37 Moreover, the endothelium has been shown to be a major producer of IL-6 in a postmortem analysis of a patient who succumbed to CRS.38Figure 1 shows the proposed pathomechanism of CRS.
Figure 1.
Proposed pathomechanism of cytokine release syndrome. Activation of manly T cells or lysis of immune cells induces a release of interferon gamma (IFN-γ) or tumor necrosis factor alpha (TNF-α). This leads to the activation of macrophages, dendritic cells, other immune cells, and endothelial cells. These cells further release proinflammatory cytokines. Importantly, macrophages and endothelial cells produce large amounts of interleukin 6 (IL-6) which in a positive feedback loop manner activates T cells and other immune cells leading to a cytokine storm. CAR = chimeric antigen receptor, FiO2 = fraction of inspired oxygen, IFN-γ = interferon gamma, IL-6 = interleukin 6, TNF-α = tumor necrosis factor alpha.
In many aspects, severe CRS resembles hemophagocytic lymphohistiocytosis (HLH) or macrophage activation syndrome (MAS).39 Clinical and laboratory findings and the cytokine profile including IL-18, IL-8, IP10, MCP1, MIG, and MIP1β representing a Th1 and myeloid-driven inflammatory responses are closely related between HLH/MAS and severe CRS40 and hemophagocytosis has been evident in clinical trials.27 Moreover, IL-6 can lead to dysfunctional cytotoxic activity in T and NK cells, a key driver of the dysregulated immune response in HLH/MAS.41 However, so far no link to genetic alterations found in familial HLH (eg, PRF1, STX11, STXBP2, and MUNC13-4) or MAS that trigger the inappropriate release of cytotoxic molecules (perforin, syntaxin) could be seen in a blinatumomab trial.27 Thus, CRS and MAS/HLH likely represent different syndromes characterized by a dysregulated immune response ending in a common pathway of hyperactivation.
The incidence of immunotoxicities also seems to differ between the different CAR constructs. The CD28 costimulatory domain appears to confer a stronger antigen-specific stimulation of T cells due to a lower activation threshold thus potentially leading to higher rates of CRS.42 However, comparisons of the CRS rates between the pivotal NHL trials JULIET, ZUMA-1, and TRANSCEND NHL 001 are hampered by the differences in the patient population, dosing and cell composition, and the use of different grading systems (Table 1).5,43,44 The newly published consensus grading might help to compare the toxicity rates in future studies with different constructs.
Brudno et al compared the toxicities of 2 CAR constructs that only differed in the hinge and transmembrane domain as well as the origin of the CD19-binding moiety (CD8 alpha/human vs CD28/murine) and found a significantly lower rate of cytokine production and toxicity (neurotoxicity 5% vs 55%) in the 20 patients treated with the CD8 alpha/murine CAR-T cells with retained antilymphoma activity.45 Thus besides the costimulatory domain other parts of the CAR construct might play a role in the induction of immunotoxicities.
Different target antigens might also result in differences of the extent of toxicities and initial reports on BCMA-directed CAR-T cell constructs implied a lower rate of CRS and ICANS compared with anti-CD 19 CAR-T cells. However, in a recent BCMA CAR-T cell trial of 16 multiple myeloma patients receiving the target dose of 9 × 106 CAR-T cell rates of severe CRS cases were comparable to the CD19-targeting constructs with 36% of the patients requiring vasopressor support but patients experienced less and milder neurotoxicity.6
New CAR constructs might mitigate the immunotoxic effects, for example, by targeted disruption of GM-cerebrospinal fluid (CSF) through gene editing.46 Moreover, the development of dedicated suicide or elimination switches (OFF), drug-controlled ON-switch CARs, adaptor-mediated CAR constructs that are, for example, dependent on the presence of short-lived adaptor molecules or the integration of more sophisticated regulatory feedback loops for autoregulation of the CAR-T cells in response to systemic inflammation might lead to safer CAR constructs.47
Disease burden has most consistently been associated with CRS severity after CAR-T cell therapy as shown in B-ALL,48–50 CLL,51 multiple myeloma,6 and B-NHL5 as well as for blinatumomab in B-ALL52 but also rituximab in CLL.14 Another important factor is the administered dose of the acting agent.50,53,54 This, along with the fact that a more intense lymphodepletion was associated with CRS in the CAR-T cell setting55 probably reflects the notion that the strength of T cell activation and the degree of T cell expansion seem to correlate with the severity of CRS.55 However, a recent updated subgroup analysis of the Memorial Sloan Kettering Cancer Center trial analyzing 14 B-ALL patients suffering from severe CRS showed no correlation of CRS severity and the lymphodepletion or peak CAR-T cell expansion.3
Although there are limited data available concerning the treatment of elderly patients with CAR-T cells, a subgroup analysis of the ZUMA-1 trial indicates that age is not a risk factor for the development of severe CRS and that the treatment of elderly patients is safe.56 Preexisting inflammation and endothelial activation seem to be associated with the severity of CRS and CAR-T cell administration is strongly discouraged in patients with preexisting inflammation and infection.55
Clinical presentation of CRS
Early CRS presents with flu-like symptoms progressing to a condition resembling sepsis ultimately resulting in multiorgan failure. Fever is a hallmark and early sign of CRS, other mild symptoms include headache, rash, arthralgia, and myalgia. Within hours symptoms can progress to hypotension, vascular leakage, DIC, and respiratory failure ultimately affecting nearly every organ system. Typically, the first symptoms occur within hours up to 14 days after CAR-T cell therapy, probably depending on the administered cell dose, tumor burden, and costimulatory domain of the applied CAR-T cell construct. Of note, severe CRS has been linked to delayed hematologic recovery.55Table 2 shows the common symptoms of CRS.
Table 2.
Symptoms of Cytokine Release Syndrome
Differential diagnoses of CRS
It is vitally important to exclude sepsis and possibly treat infections prophylactically as cancer patients in general and CAR-T cell patients after lymphodepleting chemotherapy specifically are at great risk of infectious diseases, mostly bacterial infections.57 Moreover, severe CRS was associated with higher risk for infections,57,58 probably reflecting a state of immune paralysis after severe CRS. However, CRS cannot be safely distinguished from sepsis using established diagnostic criteria for sepsis (Sepsis-3).59 This underscores the necessity to perform a thorough infectiology workup and prophylactically treat infections using broad spectrum antibiotics and/or other anti-infective drugs.
As mentioned above, CRS shares many pathogenetic and clinical similarities with HLH/MAS like high fever, very high ferritin levels, cytokine profiles, and the importance of macrophages in the pathogenesis.60 Whereas HLH can be triggered by virus infections, malignancy, autoimmune disease, and acquired immune deficiencies,61 MAS is most often associated with autoimmune and autoinflammatory diseases.60 Both conditions are characterized by a dysregulated immune response resulting in a cytokine storm. Hence, HLH/MAS are regarded as a consequence of CRS by some authors regarding CAR-T cells as a trigger of HLH/MAS. However, dysfunctional NK and T cell cytotoxic activity due to homozygous defects in cytolytic pathway genes (HLH) or acquired mutations in these genes (MAS) are critical to these conditions,62,63 but have not been shown for CRS27 suggesting that CRS and HLH/MAS are different pathologies. Nevertheless, CAR-T cells might trigger underlying MAS/HLH.
Tumor lysis syndrome might present with typical CRS symptoms such as fever, acute renal failure, cardiac arrhythmia, and seizures but is usually distinguishable by hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia64 but might occur concurrent with CRS.
Hypersensitivity reactions typically present with rash and urticaria, fever, dyspnea, hypotension, and gastrointestinal symptoms and eventually cardiorespiratory failure. Unlike in CRS symptoms of true type I reactions occur after repeated exposure to the causative agent65,66 and often require antihistamine or epinephrine therapy.67
CRS management
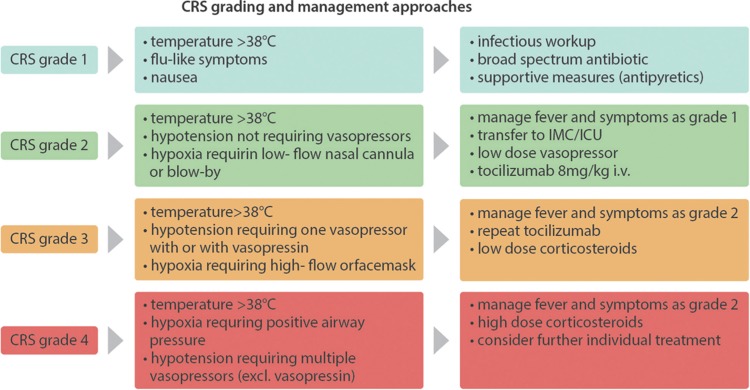
The management of CRS follows a grading and risk-adapted approach.33,68 Low-grade CRS is treated symptomatically with antipyretics and fluids. Care should be taken with regard to excessive intravenous fluid replacement in light of the risk of vascular leakage with consecutive pulmonary edema. Screening for infections and antibiotic treatment is mandatory.
Due to its role in disease pathology, the mainstay of CRS therapy has been the selective blockade of IL-6 signaling by the IL-6 receptor antagonist tocilizumab or anti-IL-6 antibody siltuximab,33,48,50,69 leading to rapid resolution of CRS symptoms, typically within a few hours. Tocilizumab gained European Medicines Agency approval for the treatment of severe CRS in June 2018 and should be considered in moderate and definitively administered in severe CRS cases.
Of note, IL-6 blockade does not seem to negatively influence CAR-T cell function in mice26 and humans,70,71 nor does it influence prognosis.4,39,53,72–75 In a safety management study of the ZUMA-1 trial, prophylactic administration of tocilizumab at day 2 after CAR-T cells lowered grade ≥ 3 incidence of CRS in the 34 patients studied compared to the 101 patients in the main trial (3% vs 13%) while not influencing peak CAR-T cell numbers. However, the patients of the safety management cohort were younger and of lower disease stage and international prognostic index (IPI).76 Nevertheless, the use of tocilizumab could result in higher IL-6 levels and increase the risk of severe neurotoxicity. In addition, tocilizumab might increase the degree of long-term immunosuppression. Moreover, in rheumatic diseases, a higher incidence of lower intestinal perforations was reported after repetitive administration of tocilizumab77 which probably does not occur in the acute setting.
Corticosteroids should be reserved to severe, tocilizumab refractory cases since early studies showed reduced response rates in early case series,49,50,78 whereas it did not seem to impact response rates in more recent clinical trials.4,53,74
In refractory cases, TNF-α blocker, T cell depleting alemtuzumab and ATG, IL-1R-based inhibitors (anakinra)26 or cyclophosphamide,54 ibrutinib,79 and GM-CSF inhibition46 or cytokine adsorption as in the setting of severe HLH80,81 might be of benefit, although evidence is limited to single patient reports. Interestingly, a recent case report showed that hemofiltration could rapidly alleviate symptoms and decrease inflammatory parameters in a 10-year-old B-ALL patient with severe CRS and concurring neurotoxicity refractory to tocilizumab, high-dose steroids, and immunoglobulins. However, tocilizumab was just given once and half of the recommended dose.82Figure 2 shows a grading-guided approach for the management of CRS.
Figure 2.
Grading and management of CRS. Considerations and approaches for the grading and management of CRS. The symptoms are divided into grade 1 to grade 4 according to the ASBMT consensus grading.83 Tocilizumab: a maximum of 800 mg per dose is recommended; a maximum of 3 doses in 24 hours are recommended. Low-dose corticosteroids: ie, 10 mg dexamethasone every 6 hours or equivalent. High-dose corticosteroids: 1000 mg methylprednisolone every 24 hours or equivalent. CRS = cytokine release syndrome. ASBMT = American Society for Blood and Marrow Transplantation.
ICANS epidemiology and clinical features
Neurotoxicity is one of the most common symptoms in patients treated with CAR-T cells. It was initially frequently referred to as CAR-T CRES. Since the publication of a new consensus grading system with the support of the ASBMT, it is recommended to refer to it as ICANS.83 The aim of the consensus was to define and summarize the symptoms and establish an easier grading system which can be applied to all patients regardless of the CAR-T cell product used in the treatment. The symptoms and the presentation of ICANS are varied and can progress from subtle signs as headaches, fatigue, and mild aphasia to more severe and potentially life-threatening presentations including seizures, raised intracranial pressure with cerebral edema, and coma. The onset of ICANS is usually later than CRS (in the ZUMA-1 trial—using CD19-CAR-T cells in DLBCL, PMBCL, transformed FL—the median onset was day 4 after transfusion84) and the symptoms usually last longer than CRS symptoms (in the ZUMA-1 trial, the median time of duration was 11 days84). The symptoms have been usually graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03.4,85 Since the publication of the consensus grading, we recommend to use the new ASBMT grading83 to facilitate the comparison between different clinical centers and to develop standardized treatment procedures for patients developing ICANS throughout all clinical centers.
In the ZUMA-1 trial, 65 patients (64%) developed any neurological event and 37 (37%) developed grade 1 to 2 ICANS graded according to the CTC-AE criteria version 4.03.4 These findings are similar to the ones reported from another trial including patients with acute lymphoblastic leukemia where 33 patients (62%) developed any kind of neurological event and 11 (21%) patients had grade 1 to 2 ICANS (CTC-AE criteria version 4.03). In a trial including patients with relapsed/refractory DLBCL, CLL, and ALL, 53 patients (40%) had any grade of ICANS and 25 (19%) had grade 1 to 2 ICANS (CTC-AE criteria version 4.03).37 The primary analysis of the JULIET trial, which included patients with relapsed or refractory (r/r) DLBCL and transformed FL reported a lower event rate for any neurological events with just 21 patients (21%) developing any grade of ICANS and just 9 (9%) grade 1 to 2 ICANS (CTC-AE criteria version 4.03).85 Throughout all clinical trials we also find a high number of patients who developed grade 3 to 4 ICANS. The reported incidences for grade 3 to 4 ICANS are 28% in the ZUMA1 trial,4 12% in the JULIET trial85 and 42% in the patients analyzed by Santomasso et al.84 A trial including patients with r/r DLBCL, B-ALL, and CLL reported 28 (21%) patients with grade 3 to 4 ICANS all of them graded according to the CTC-AE criteria version 4.03.37 These patients with more severe ICANS showed symptoms like aphasia, hallucinations, depressed level of consciousness, cranial nerve palsies, seizures, and coma requiring mechanical ventilation.37,68,84 Although almost all patients show a full resolution of the symptoms, some trials reported ICANS-associated fatalities.4,37 Data showing that patients with a DLBCL and FL developed less severe ICANS than patients with B-ALL indicates that neurotoxicity might differ with the used CAR construct and among different entities. For instance, patients treated with BCMA CAR-T cells for refractory multiple myeloma developed signs of CRS but only few patients showed mild ICANS.6 Moreover, earlier experiences with blinatumomab also showed significant rates of neurotoxicity indicating that targeting CD19 might be one of the major factors in the development of neurotoxicity. Thus further investigation with CARs targeting epitopes like BCMA or CD20 will be needed to underpin this theory. Patients who develop more severe and potentially life-threatening ICANS usually also show signs of severe CRS and there seems to be a strong correlation between the severity of CRS and the severity of ICANS.37,84,86 Nonetheless patients can develop ICANS in the absence of CRS. The tumor burden prior to CAR-T cell therapy also seems to be a risk factor for a severe ICANS26,51,86 as well as higher CAR-T cell doses86 and higher CAR-T cell peak expansion values.3,51 A correlation between a systemic inflammation with elevated levels of cytokines and severe ICANS has also been shown.37,51,84 The presence of neurologic comorbidities in patients is also associated with a higher risk for ICANS.37 Nonetheless, no validated algorithm has been established in order to predict patients who will develop potentially life-threatening ICANS and benefit from an early intervention.
MRI of the brain in some of the patients with severe ICANS showed acute abnormalities like T2/FLAIR changes indicating vasogenic edema and sometimes contrast enhancement pointing toward blood-brain barrier (BBB) disruption. These abnormalities were only found in patients suffering from severe and not mild ICANS. These findings were reversible after resolution of the symptoms indicating that early imaging might be helpful to identify patients at risk for severe ICANS. Nonetheless, 64% of patients with severe ICANS showed normal MRI scans and therefore MRI should just be used as an additional tool to identify patients at risk for severe ICANS.37,84
Continuous EEG (cEEG) showed generalized periodic discharges in patients who developed mild and severe ICANS. The clinical deterioration correlated with peak intensity of GDPs. These patients showed transient electroclinical improvement after treatment with antiepileptic drugs and especially dexamethasone.87 However, more robust data are needed to implement routine cEEG monitoring for the identification of patients at risk for severe ICANS.
Pathophysiology of ICANS
The pathophysiology of neurologic symptoms in CAR-T cell therapy is poorly understood, but the lack of a strict temporal association with CRS indicates that it might be independent from CRS. In addition, considering IL-6 pathway blockade does not seem to be beneficial in the treatment of neurologic symptoms unlike in CRS indicates a different pathomechanism.
The evaluation of larger patient collectives showed that a high tumor burden and a more severe CRS lead to a more severe ICANS.37,84 An analysis showed higher levels for cytokines which are usually associated with a systemic inflammation (ie, IL-6, IL-10, and IFN-γ) in patients who develop severe ICANS indicating a correlation between systemic inflammation and ICANS.37,51,84 A recently reported trial using a CAR with an incorporated CD8 alpha hinge as compared to a CD28-derived hinge an transmembrane domain showed similar response rates but markedly less grade 3 or 4 ICANS (5% vs 55% in a previous trial) and significantly lower levels of proinflammatory effectors (IL-2, IL-15, INFγ, Granzyme A & B) indicating a major role of CAR design for the induced cytokine profile and the pathophysiology of ICANS.45
Another important mechanism for ICANS could be an IL-1 triggered activation of by-standing monocytes which then produce IL-6 and lead to the systemic inflammation mentioned above. This possible mechanism was shown using a xenograft mouse model which also demonstrated amelioration of ICANS symptoms through blockade of IL-1 with anakinra but not through blockade of the IL-6 pathway by administration of tocilizumab. These results support the importance of IL-1 and monocytes in the pathogenesis of ICANS.26 The role of monocytes was also shown using a similar xenograft model in which the abrogation of GM-CSF signaling either using lenzilumab or GM-CSF knockout mice resulted in a significant reduction of ICANS while the antitumor effect was not suppressed.46
Other findings strongly indicate a disruption of the BBB as a possible pathomechanism of ICANS. Higher protein levels in the CSF were found in patients with severe neurotoxicity indicating a dysfunction of the BBB.37,84 Some analyses also demonstrated higher CSF/blood ratios for cytokines like IL-6, IFN-γ, and GM-CSF, indicating either a severe disruption of the BBB with consecutive trespassing of cytokines and leukocytes into the central nervous system (CNS) or intrathecal production of these cytokines which can lead to inflammation of the CNS.37,84,88 Early studies already reported that CAR-T cells could be detected in the CSF after infusion and expansion.50,53,89 A significant correlation with ICANS could just be found in one of these studies which showed higher levels of CAR-T cells in CSF in patients who developed ICANS when compared to patients without neurological symptoms.53 Nevertheless, the studies which did not show a significant correlation had a very small number of patients.50,89
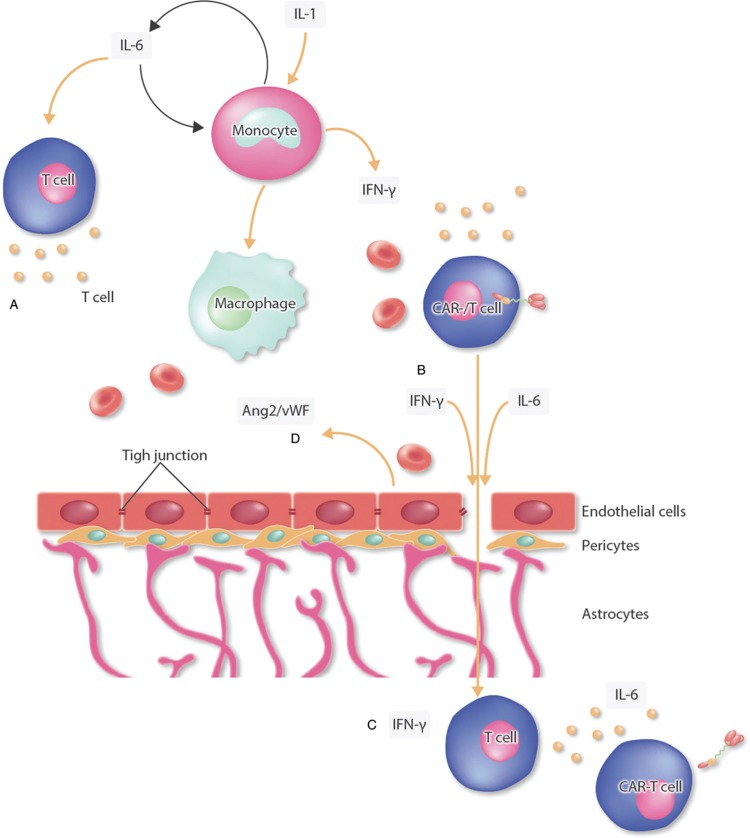
The disruption of the BBB and consequent neuroinflammation and infiltration of T cells into the CNS could also be shown through histopathological findings in a nonhuman primate model88 but also in an autopsy of a patient who died after developing severe ICANS.37Figure 3 shows the proposed pathogenesis of ICANS.
Figure 3.
Pathomechanism of ICANS. Shown are some of the discussed pathomechanism for ICANS. (A) Systemic inflammation and expression of IL-1 leads to activation of by-standing monocytes and an expression of different cytokines leading to an aggravation of the systemic inflammation by activation of T cells and macrophages.26 (B-C) A disruption of the blood-brain barrier (BBB) leads to migration of T cells (including CAR-T cells) in the brain parenchyma, and to elevated levels of cytokines and protein in the cerebrospinal fluid leading to an inflammation of the CNS.37,50,53,84,88,89 (D) Endothelial activation shown by elevated Ang-2:Ang-1 ratio aggravates the systemic inflammation and the BBB disruption.37,84 Ang-2 = angiopoetin, BBB = blood-brain barrier, CAR = chimeric antigen receptor, CNS = central nervous system, ICANS = immune effector cell-associated neurotoxicity syndrome, IFN-γ = interferon gamma, IL-1 = interleukin 1, IL-6 = interleukin 6, vWF = von Willebrand factor.
A single-cell analysis of the preinfusion CD19 CAR-T cell products demonstrated a variety of polyfunctional T cell subsets. Applying a T cell polyfunctionality strength index (PSI) showed a statistically significant association of IL-17A PSI and high peaks of CAR-T cell expansion in patients who developed grade ≥ 3 ICANS. These findings suggest that a high number of CAR Th17 cells could lead to a higher incidence of ICANS underscoring the role of different T cell subsets in the response and toxicity mechanisms of CAR-T cell treatment.90
Another frequently discussed mechanism that might lead to ICANS is the endothelial activation which might aggravate the BBB disruption. Several studies showed an increase in the Ang-2:Ang1 ratio in patients who develop severe ICANS.37,84
ICANS management
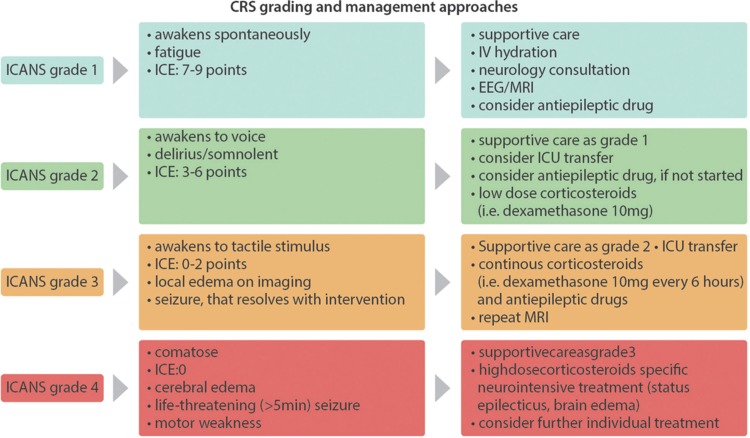
The management of ICANS is based on a grading system. Similar to the management of CRS, patients with mild (grade ≤ 1) ICANS should be closely monitored and receive supportive measures, if needed. For patients who develop more severe ICANS (grade ≥ 2) the transfer to an intermediate care or intensive care unit should be considered. The consultation of a neurologist or neurointensivist as well as a lumbar puncture and radiological imaging is crucial for the detection of other causes of neurological symptoms including CNS involvement of the underlying malignancy, infection, intracranial hemorrhage, stroke, and others. As some patients showed GDPs during cEEG monitoring87 an early cEEG monitoring might be helpful.
The treatment is based on corticosteroids with escalating doses depending on the severity of the ICANS. In most cases, patients respond to steroids, although the median time to the resolution of symptoms is longer for ICANS (median 9 days, range 4–21 days)84 than it is for CRS. The recommended treatment consists of 10 mg dexamethasone every 6 hours until the symptoms stop. In cases of grade 4 toxicities, high doses of methylprednisolone (eg, 1000 mg every 24 hours) should be given.
A treatment with tocilizumab is definitely recommended if a concurrent CRS is present. Since many patients with severe ICANS also show symptoms of CRS,4,84,86 a treatment with steroids in combination with tocilizumab should be evaluated especially keeping in mind that the severity of CRS correlates with the presence of ICANS.84 Nonetheless, the treatment with tocilizumab alone was shown to be ineffective in ICANS in clinical studies as well as in animal models.26,37,84,88 Prophylactic treatment with tocilizumab had no effect on the incidence of a CRS but does seem to increase the incidence and severity of ICANS as shown in cohort 3 of the ZUMA-1 trial.76 This effect might be due to the higher blood levels of IL-6 caused by the blockade of the IL-6 receptor.
A prophylactic treatment with antiepileptic drugs can be considered, since some patients showed pathological EEG findings with no clinical signs of a seizure.84 However, the analysis of cohort 3 of the ZUMA-1 trial did not show any significant benefit for patients who received a prophylactic treatment with levetiracetam.76
At this point there is no good clinical evidence for recommendations of treatments for patients unresponsive to high-dose steroids. Treatments with siltuximab (anti-IL-6 chimeric monoclonal antibody) might be effective due to its direct effect on circulating IL-6.24 Some authors also suggest the use of anakinra (recombinant human interleukin 1 receptor antagonist) since IL-1 seems to play an important role in the development of ICANS and anakinra was shown to be effective in a mouse model.26 A similar approach could be the inhibition of GM-CSF using lenzilumab to prevent a systemic inflammation caused by monocyte activation.46 Natalizumab, a humanized monoclonal antibody against the cell adhesion molecule α4-integrin that inhibits the infiltration of T cells into the CNS used in the treatment of multiple sclerosis has been suggested but efficacy data in the human setting is still lacking.88Figure 4 shows a grading and management algorithm for ICANS.
Figure 4.
ICANS grading and management. Considerations and approaches for the grading and management of ICANS. The symptoms are graded according to the ASBMT consensus grading83 Immune Effector Cell-Associated Encephalopathy (ICE) score, is a nonvalidated neurological scoring system to easily asses the neurological status of CAR-T cell patients (Items: orientation, naming 3 objects, following easy commands, writing, counting backwards; a maximum score of 10 points indicates no neurological impairment).83 Low-dose corticosteroids: ie, 10 mg dexamethasone every 6 hours or equivalent. High-dose corticosteroids: 1000 mg methylprednisolone every 24 hours or equivalent. A concurrent CRS should be treated additionally as shown in Figure 2. Further treatment should be evaluated individually, that is, anakinra26 or other experimental approaches as reported previously. ASBMT = American Society for Blood and Marrow Transplantation, CAR = chimeric antigen receptor, CRS = cytokine release syndrome, ICANS = immune effector cell-associated neurotoxicity syndrome.
Conclusion
Since CAR-T cells are rapidly entering clinical practice, a profound knowledge of the clinical presentation, early identification of patients at risk with appropriate monitoring as well as prompt therapeutic action is an essential requisite of the medical network surrounding cancer patients.
Although remarkable progress in the understanding of CAR-T cell-mediated immunotoxicities has been achieved within the last years, there still is a lack of knowledge especially in the field of CAR-T cell-related neurotoxicity. This is in part reflected by the scarcity of therapeutic alternatives in corticosteroid refractory ICANS cases and consecutively occurring mortality in clinical trials. Thus, clinical and preclinical studies are needed to further deepen our understanding of CAR-T cell-mediated immunotoxicities.
Footnotes
Citation: Garcia Borrega J, Gödel P, Rüger MA, Onur ÖA, Shimabukuro-Vornhagen A, Kochanek M, Böll B. In the Eye of the Storm: Immune-mediated Toxicities Associated With CAR-T Cell Therapy. HemaSphere, 2019;00:00. http://dx.doi.org/10.1097/HS9.0000000000000191
Funding/support: None.
Disclosure: The authors have indicated they have no potential conflicts of interest to disclose.
JGB and PG have contributed equally to this work.
Authors’ contributions: BB, JGB, and PG drafted the manuscript. AS-V, MAR, MK, and ÖO reviewed and edited the manuscript.
References
- 1.June CH, O’Connor RS, Kawalekar OU, et al. CAR T cell immunotherapy for human cancer. Science. 2018;359:1361–1365. [DOI] [PubMed] [Google Scholar]
- 2.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park JH, Rivière I, Gonen M, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378:449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380:45–56. [DOI] [PubMed] [Google Scholar]
- 6.Brudno JN, Maric I, Hartman SD, et al. T cells genetically modified to express an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of poor-prognosis relapsed multiple myeloma. J Clin Oncol. 2018;36:2267–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown CE, Alizadeh D, Starr R, et al. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med. 2016;375:2561–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharpe M, Mount N. Genetically modified T cells in cancer therapy: opportunities and challenges. Dis Model Mech. 2015;8:337–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carter PJ, Lazar GA. Next generation antibody drugs: pursuit of the “high-hanging fruit”. Nat Rev Drug Discov. 2018;17:197–223. [DOI] [PubMed] [Google Scholar]
- 10.Chatenoud L, Ferran C, Reuter A, et al. Systemic reaction to the anti-T-cell monoclonal antibody OKT3 in relation to serum levels of tumor necrosis factor and interferon-gamma [corrected]. N Engl J Med. 1989;320:1420–1421. [DOI] [PubMed] [Google Scholar]
- 11.Chatenoud L, Ferran C, Legendre C, et al. In vivo cell activation following OKT3 administration. Systemic cytokine release and modulation by corticosteroids. Transplantation. 1990;49:697–702. [DOI] [PubMed] [Google Scholar]
- 12.Pihusch R, Holler E, Mühlbayer D, et al. The impact of antithymocyte globulin on short-term toxicity after allogeneic stem cell transplantation. Bone Marrow Transplant. 2002;30:347–354. [DOI] [PubMed] [Google Scholar]
- 13.Suntharalingam G, Perry MR, Ward S, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355:1018–1028. [DOI] [PubMed] [Google Scholar]
- 14.Winkler U, Jensen M, Manzke O, et al. Cytokine-release syndrome in patients with B-cell chronic lymphocytic leukemia and high lymphocyte counts after treatment with an anti-CD20 monoclonal antibody (Rituximab, IDEC-C2B8). Blood. 1999;94:2217–2224. [PubMed] [Google Scholar]
- 15.Freeman C, Morschhauser F, Sehn L, et al. Cytokine release in patients with CLL treated with obinutuzumab and possible relationship with infusion related reactions. Blood. 2015;126:2646–2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wing MG, Moreau T, Greenwood J, et al. Mechanism of first-dose cytokine-release syndrome by CAMPATH 1-H: involvement of CD16 (FcgammaRIII) and CD11a/CD18 (LFA-1) on NK cells. J Clin Invest. 1996;98:2819–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alig SK, Dreyling M, Seppi B, et al. Severe cytokine release syndrome after the first dose of Brentuximab Vedotin in a patient with relapsed systemic anaplastic large cell lymphoma (sALCL): a case report and review of literature. Eur J Haematol. 2015;94:554–557. [DOI] [PubMed] [Google Scholar]
- 18.Rotz SJ, Leino D, Szabo S, et al. Severe cytokine release syndrome in a patient receiving PD-1-directed therapy. Pediatr Blood Cancer. 2017;64:e26642. [DOI] [PubMed] [Google Scholar]
- 19.Bajor DL, Mick R, Riese MJ, et al. Long-term outcomes of a phase I study of agonist CD40 antibody and CTLA-4 blockade in patients with metastatic melanoma. Oncoimmunology. 2018;7:e1468956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tisoncik JR, Korth MJ, Simmons CP, et al. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76:16–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Jong MD, Simmons CP, Thanh TT, et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med. 2006;12:1203–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abboud R, Keller J, Slade M, et al. Severe cytokine-release syndrome after T cell-replete peripheral blood haploidentical donor transplantation is associated with poor survival and anti-IL-6 therapy is safe and well tolerated. Biol Blood Marrow Transplant. 2016;22:1851–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho C, Perales MA. Rapid identification of cytokine release syndrome after haploidentical PBSC transplantation and successful therapy with tocilizumab. Bone Marrow Transplant. 2016;51:1620–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimabukuro-Vornhagen A, Gödel P, Subklewe M, et al. Cytokine release syndrome. J Immunother Cancer. 2018;6:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matthys P, Dillen C, Proost P, et al. Modification of the anti-CD3-induced cytokine release syndrome by anti-interferon-gamma or anti-interleukin-6 antibody treatment: protective effects and biphasic changes in blood cytokine levels. Eur J Immunol. 1993;23:2209–2216. [DOI] [PubMed] [Google Scholar]
- 26.Norelli M, Camisa B, Barbiera G, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med. 2018;24:739–748. [DOI] [PubMed] [Google Scholar]
- 27.Teachey DT, Rheingold SR, Maude SL, et al. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood. 2013;121:5154–5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5:177ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanaka T, Narazaki M, Kishimoto T. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy. 2016;8:959–970. [DOI] [PubMed] [Google Scholar]
- 30.van der Stegen SJC, Hamieh M, Sadelain M. The pharmacology of second-generation chimeric antigen receptors. Nat Rev Drug Discov. 2015;14:499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giavridis T, van der Stegen SJC, Eyquem J, et al. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med. 2018;24:731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faramand R, Kotani H, Morrissey D, et al. Prediction of CAR T-related toxicities in R/R DLBCL patients treated with axicabtagene ciloleucel using point of care cytokine measurements. Blood. 2019;132 suppl 1:95. [Google Scholar]
- 33.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turtle CJ, Hay KA, Gust J, et al. Cytokine release syndrome (CRS) and neurotoxicity (NT) after CD19-specific chimeric antigen receptor- (CAR-)modified T cells. J Clin Oncol. 2017;35 suppl:3020. [Google Scholar]
- 35.Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16:448–457. [DOI] [PubMed] [Google Scholar]
- 36.Pathan N, Hemingway CA, Alizadeh AA, et al. Role of interleukin 6 in myocardial dysfunction of meningococcal septic shock. Lancet (London, England). 2004;363:203–209. [DOI] [PubMed] [Google Scholar]
- 37.Gust J, Hay KA, Hanafi LA, et al. Endothelial activation and blood–brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 2017;7:1404–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Obstfeld AE, Frey NV, Mansfield K, et al. Cytokine release syndrome associated with chimeric-antigen receptor T-cell therapy; clinicopathological insights. Blood. 2017;130:2569–2572. [DOI] [PubMed] [Google Scholar]
- 39.Locke FL, Neelapu SS, Bartlett NL, et al. Phase 1 results of ZUMA-1: a multicenter study of KTE-C19 anti-CD19 CAR T cell therapy in refractory aggressive lymphoma. Mol Ther. 2017;25:285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teachey DT, Lacey SF, Shaw PA, et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov. 2016;6:664–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cifaldi L, Prencipe G, Caiello I, et al. Inhibition of natural killer cell cytotoxicity by interleukin-6: implications for the pathogenesis of macrophage activation syndrome. Arthritis Rheumatol (Hoboken, NJ). 2015;67:3037–3046. [DOI] [PubMed] [Google Scholar]
- 42.Posey AD, Fraietta J, Lee J, et al. (CARs) containing CD28 signaling domain versus 4-1BB in primary human T cells. Blood. 2013;122:2902. [Google Scholar]
- 43.Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and efficacy of axicabtagene ciloleucel (anti-CD19 CAR T) in refractory large B-cell lymphoma: a multicenter, single arm, phase 1-2 trial. Lancet Oncol. 2018;20:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abramson JS, Siddiqi T, Palomba ML, et al. High durable CR rates and preliminary safety profile for JCAR017 in R/R aggressive b-NHL (TRANSCEND NHL 001 study): a defined composition CD19-directed CAR T-cell product with potential for outpatient administration. J Clin Oncol. 2018;36 suppl:120. [Google Scholar]
- 45.Brudno J, Hartman S, Lam N, et al. Low levels of neurologic toxicity with retained anti-lymphoma activity in a phase I clinical trial of T cells expressing a novel anti-CD19 CAR. Blood. 2018;132 suppl 1:697. [Google Scholar]
- 46.Sterner RM, Sakemura R, Cox MJ, et al. GM-CSF inhibition reduces cytokine release syndrome and neuroinflammation but enhances CAR-T cell function in xenografts. Blood. 2019;133:697–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lim WA, June CH. The principles of engineering immune cells to treat cancer. Cell. 2017;168:724–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127:3321–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maude SL, Teachey DT, Porter DL, et al. CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood. 2015;125:4017–4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6:224ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turtle CJ, Hay KA, Hanafi L-A, et al. Durable molecular remissions in chronic lymphocytic leukemia treated with CD19-specific chimeric antigen receptor-modified T cells after failure of ibrutinib. J Clin Oncol. 2017;35:3010–3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Topp MS, Gökbuget N, Zugmaier G, et al. Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J Clin Oncol. 2014;32:4134–4140. [DOI] [PubMed] [Google Scholar]
- 53.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turtle CJ, Hanafi L, Berger C, et al. CD19 CAR-T cells of defined CD4+: CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;1:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hay KA, Hanafi LA, Li D, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood. 2017;130:2295–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sano D, Nastoupil LJ, Fowler NH, et al. Safety of axicabtagene ciloleucel CD19 CAR T-cell therapy in elderly patients with relapsed or refractory large B-cell lymphoma. Blood. 2018;132 suppl 1:96. [Google Scholar]
- 57.Hill JA, Li D, Hay KA, et al. Infectious complications of CD19-targeted chimeric antigen receptor-modified T cell immunotherapy. Blood. 2017;131:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park JH, Romero FA, Taur Y, et al. Cytokine release syndrome grade as a predictive marker for infections in patients with relapsed or refractory B-cell acute lymphoblastic leukemia treated with chimeric antigen receptor T cells. Clin Infect Dis. 2018;10065:25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315:801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ravelli A, Minoia F, Davì S, et al. 2016 Classification Criteria for Macrophage Activation Syndrome Complicating Systemic Juvenile Idiopathic Arthritis: A European League Against Rheumatism/American College of Rheumatology/Paediatric Rheumatology International Trials Organisation Collaborative Initiative. Ann Rheum Dis. 2016;75:481–489. [DOI] [PubMed] [Google Scholar]
- 61.Usmani GN, Woda BA, Newburger PE. Advances in understanding the pathogenesis of HLH. Br J Haematol. 2013;161:609–622. [DOI] [PubMed] [Google Scholar]
- 62.Zhang M, Behrens EM, Atkinson TP, et al. Genetic defects in cytolysis in macrophage activation syndrome. Curr Rheumatol Rep. 2014;16:439. [DOI] [PubMed] [Google Scholar]
- 63.Rigante D, Emmi G, Fastiggi M, et al. Macrophage activation syndrome in the course of monogenic autoinflammatory disorders. Clin Rheumatol. 2015;34:1333–1339. [DOI] [PubMed] [Google Scholar]
- 64.Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med. 2011;364:1844–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lenz H-J. Management and preparedness for infusion and hypersensitivity reactions. Oncologist. 2007;12:601–609. [DOI] [PubMed] [Google Scholar]
- 66.Demoly P, Adkinson NF, Brockow K, et al. International consensus on drug allergy. Allergy Eur J Allergy Clin Immunol. 2014;69:420–437. [DOI] [PubMed] [Google Scholar]
- 67.Castells M. Diagnosis and management of anaphylaxis in precision medicine. J Allergy Clin Immunol. 2017;140:321–333. [DOI] [PubMed] [Google Scholar]
- 68.Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy: assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15:47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bonifant CL, Jackson HJ, Brentjens RJ, et al. Toxicity and management in CAR T-cell therapy. Mol Ther Oncolytics. 2016;3:16011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barrett DM, Singh N, Hofmann TJ, et al. Interleukin 6 is not made by chimeric antigen receptor T cells and does not impact their function. Blood. 2016;128:654. [Google Scholar]
- 71.Singh N, Hofmann TJ, Gershenson Z, et al. Monocyte lineage-derived IL-6 does not affect chimeric antigen receptor T-cell function. Cytotherapy. 2017;19:867–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mei H, Jiang H, Wu Y, et al. Neurological toxicities and coagulation disorders in the cytokine release syndrome during CAR-T therapy. Br J Haematol. 2018;181:689–692. [DOI] [PubMed] [Google Scholar]
- 73.Ali SA, Shi V, Maric I, et al. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood. 2016;128:1688–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gardner RA, Finney O, Annesley C, et al. Intent to treat leukemia remission by CD19CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129:3322–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schuster SJ, Svoboda J, Chong EA, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 2017;377:2545–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Locke FL, Neelapu SS, Bartlett NL, et al. Preliminary results of prophylactic tocilizumab after axicabtageneciloleucel (axi-cel; KTE-C19) treatment for patients with refractory, aggressive non-Hodgkin lymphoma (NHL). Blood. 2017;130 suppl 1:1547. [Google Scholar]
- 77.Strangfeld A, Richter A, Siegmund B, et al. Risk for lower intestinal perforations in patients with rheumatoid arthritis treated with tocilizumab in comparison to treatment with other biologic or conventional synthetic DMARDs. Ann Rheum Dis. 2017;76:504–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kalos M, Levine BL, Porter DL, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ruella M, Kenderian SS, Shestova O, et al. Kinase inhibitor ibrutinib to prevent cytokine-release syndrome after anti-CD19 chimeric antigen receptor T cells (CART) for B cell neoplasms. Leukemia. 2016;31:246–248. [DOI] [PubMed] [Google Scholar]
- 80.Frimmel S, Schipper J, Henschel J, et al. First description of single-pass albumin dialysis combined with cytokine adsorption in fulminant liver failure and hemophagocytic syndrome resulting from generalized herpes simplex virus 1 infection. Liver Transplant. 2014;20:1523–1524. [DOI] [PubMed] [Google Scholar]
- 81.Greil C, Roether F, La Rosée P, et al. Rescue of cytokine storm due to HLH by hemoadsorption in a CTLA4-deficient patient. J Clin Immunol. 2017;37:273–276. [DOI] [PubMed] [Google Scholar]
- 82.Liu Y, Chen X, Wang D, et al. Hemofiltration successfully eliminates severe cytokine release syndrome following CD19 CAR-T-cell therapy. J Immunother. 2018;41:406–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee DW, Santomasso BD, Locke FL, et al. ASBMT consensus grading for cytokine release syndrome and neurological toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2018. [DOI] [PubMed] [Google Scholar]
- 84.Santomasso BD, Park JH, Salloum D, et al. Clinical and biological correlates of neurotoxicity associated with CAR T-cell therapy in patients with B-cell acute lymphoblastic leukemia. Cancer Discov. 2018;8:958–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schuster S, Bishop MR, Tam C, et al. Global pivotal phase 2 trial of the CD19-targeted therapy CTL019 in adult patients with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL)—an interim analysis. Clin Lymphoma Myeloma Leuk. 2017;17 suppl 1:S373–S374. [Google Scholar]
- 86.Gauthier J, Turtle CJ. Insights into cytokine release syndrome and neurotoxicity after CD19-specific CAR-T cell therapy. Curr Res Transl Med. 2018;66:50–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Herlopian A, Dietrich J, Abramson JS, et al. EEG findings in CAR T-cell therapy-related encephalopathy. Neurology. 2018;91:227–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Taraseviciute A, Tkachev V, Ponce R, et al. Chimeric antigen receptor T cell-mediated neurotoxicity in nonhuman primates. Cancer Discov. 2018;8:750–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rossi J, Paczkowski P, Shen Y, et al. Preinfusion polyfunctional anti-CD19 chimeric antigen receptor T cells are associated with clinical outcomes in NHL. Blood. 2018;132:804–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Porter D, Frey N, Wood PA, et al. Grading of cytokine release syndrome associated with the CAR T cell therapy tisagenlecleucel. J Hematol Oncol. 2018;11:35. [DOI] [PMC free article] [PubMed] [Google Scholar]