Abstract
Surgical resection and radiotherapy are an effective treatment in many head and neck squamous cell carcinomas (HNSCC), but in others, the development of radiotherapy resistance limits treatment efficacy and permits disease progression. We developed a novel multiwell radiation dosing method to increase the throughput of our investigation of the activity of a novel podophyllotoxin SU093 in acting as a radiosensitizer in the HNSCC models FaDu and SCC-25. These in vitro studies showed that combining SU093 with 5 Grays ionizing radiation acted synergistically to increase HNSCC apoptosis and decrease its proliferation via inhibition of Nuclear factor, erythroid 2 like 2 (Nrf2), a key effector of the DNA damage response induced by ionizing radiation. Combined treatment reduced in vitro migration in a simulated wounding model while also promoting cell cycle arrest at the G2/M phase. These findings validate the potential of SU093 as a synergistic radiosensitizing agent for use in combination with localized radiotherapy in treatment resistant HNSCC.
Keywords: Radiosensitizer, 4-azapodophyllotoxin, head and neck squamous cell carcinoma, combination therapy
Head and neck squamous cell carcinoma (HNSCC) may afflict the nasal cavity, oral mucosa, tongue, salivary glands, larynx, pharynx, or hypopharynx, often with regional spread, nodal involvement, and compromised tissue function. HNSCC is the third most common type of cancer worldwide, representing 8% of all new cases and 10% of all cancer deaths per year.1 Multimodal strategies that combine resection, extirpation of involved nodes, and reconstructive surgeries with localized radiotherapy or combination chemoradiotherapy achieve disease control and 5 year survival rates in >70% of early cases where advanced disease at diagnosis and relapse drive the majority of HNSCC-associated mortality.2−5 Radiotherapies are administered locally at doses up to 70 Grays (Gy), which may compromise organ function and is too often insufficient to control radioresistant HNSCC. While incorporation of chemoradiotherapies with cisplatin or taxanes have improved this, their combined use often produces debilitating side effects and does not prevent the emergence of chemo- or radio-resistance.6,7 The development of effective radiosensitizing agents will be necessary to realize further improvement in the treatment of HNSCC to boost the duration and potency of radio-treatment and potentially even help realize greater treatment effects from lower doses.
Previous work with derivatives of the natural compound podophyllotoxin has resulted in the development of potent anticancer drugs such as etoposide,8 which is commonly used effectively in refractory Hodgkin’s lymphoma and other refractory hematological cancers, albeit with common severe side effects and limitations in other types of cancer that have motivated the search for more potent and less toxic etoposide derivatives.9 Structure based-modeling and medicinal chemistry approaches have subsequently revealed that etoposide’s C4-sugar moiety is not essential for its role in the inhibition of topoisomerase II.10,11 Incorporation of a nitrogen heteroatom group at the C-4 position of nonsugar etoposide derivatives was then used to create a 4-azapodophyllotoxin compound library that is under ongoing investigation for improved anticancer properties.12−17
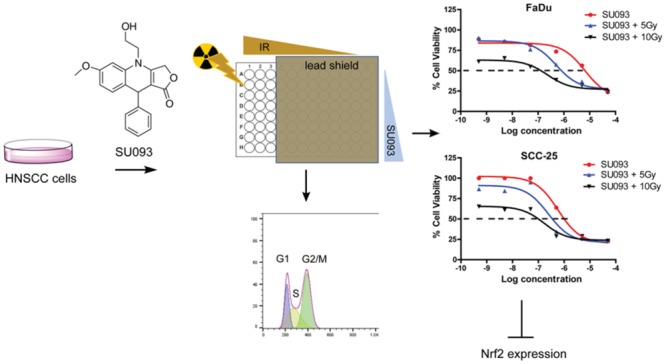
In previous work, we reported the synthesis and functional screening of novel etoposide derivatives in the National Cancer Institute’s 60 human tumor cell line panel (NCI-60).18 Using single-step, one-pot, multicomponent synthesis reactions, we explored the activity of etoposide analogs concomitantly modified at the A, C, and/or E rings (Figure 1A), which led to the identification of SU093 as a uniquely active compound in a wide variety of cancer types. The SU093 analog is structurally similar to the podophyllotoxin ring structure but differs significantly at the A-ring, which we have replaced with a methoxy group and an alkyl alcohol group that act to increase the analog’s polar character and to permit further modification, respectively. Herein, we report the activity of the 4-azapodophyllotoxin analog SU093 in the human HNSCC cell lines FaDu and SCC-25 alone and in combination with ionizing radiation (IR) to test SU093’s potential as a radiosensitizing agent. In this work, we also developed a novel method to increase the throughput of in vitro investigation of how small molecule cotreatment alters IR efficacy and restores radiosensitivity.
Figure 1.
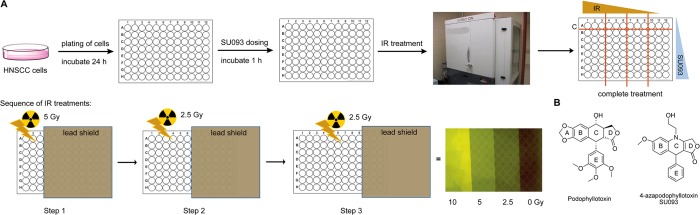
Overview of multiwell concomitant treatment of HNSCC cells with SU093 and ionizing radiation. (A) Workflow for sequence of events for conducting the multiwell treatments of combined SU093 and ionizing radiation doses. (B) Chemical structures of the natural product podophyllotoxin and its derivatized 4-azapdophyllotoxin SU093.
Results and Discussion
SU093 Sensitizes HNSCC Cells to Ionizing Radiation
Studies of radiosensitization in vitro have previously been limited by the necessity of using a different plate for each dose of ionizing radiation (IR) tested, which is labor-intensive and slow. To investigate the effect of SU093 on the radiation sensitivity of FaDu and SCC-25 HNSCC cell lines, we developed a new approach using partial coverage of each 96-well plate with a lead shield to perform IR dose curves within a single 96-well plate (Figure 1A). Twenty-four hours after plating, HNSCC cells were treated with various doses of SU093 (Figure 1B) and incubated for 1 h. Then the lead shield was used for partial irradiation of the plate at IR doses of either 2.5 or 5 Gy in an irradiation chamber. Assays were then performed 24 h after treatment.
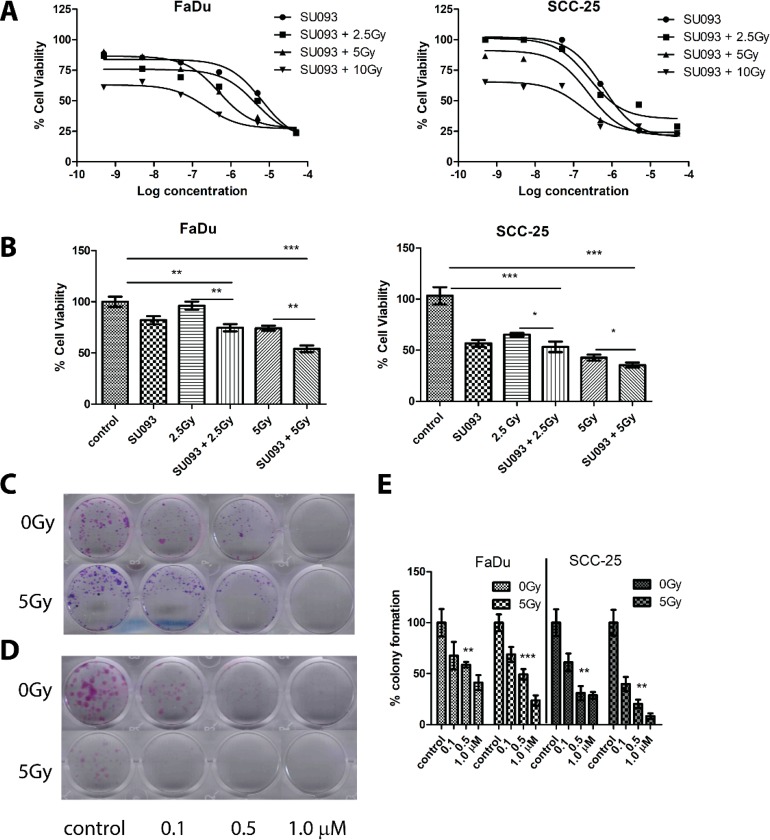
In FaDu cells, SU093 treatment resulted in a 20% decrease in viability, whereas no decrease in viability was observed at 2.5 Gy IR treatment (Figure 2A,B left). However, combined SU093 and 2.5 Gy IR treatment resulted in an additive 30% decrease in viability that increased to 48% with SU093 and 5 Gy IR treatments (Figure 2A,B left). In SCC-25 cells, 2.5 Gy IR and SU093 treatment exerted only a modest effect on cell viability when used individually but demonstrated a combined additive effect that was most extreme at 5 Gy IR and SU093 treatment where a 65% decrease in cell viability was observed (Figure 2A,B right). A similar trend was observed using the clonogenic assay to quantify HNSCC cell line capacity to proliferate after treatment in which both SU093 and IR showed dose-dependent and synergistic effects (Figure 2C,D) in both FaDu and SCC-25 models. In cotreatment, IR improved the inhibitory concentration (IC-50) by 13- and 2-fold for FaDu and SCC-25 cell lines, respectively, where 5 Gy IR with 0.496 μM SU093 inhibited FaDu colony formation to the same degree as 6.5 μM SU093 treatment alone.
Figure 2.
(A) Left and right panel, cell viability of FaDu or SCC-25 with log doses (−9.3 to −4.3) of SU093 in the absence or presence IR. (B) Left and right panels, cell viability assay of HSNCC FaDu and SCC-25, respectively, cells were treated with different doses of radiation (2.5 or 5 Gy) with or without SU093 (1 μM). On day 7 of post-treatment, viable cells were quantified using the MTT reagent, and absorbance was read at 570 nm. Cell viability was calculated by normalizing the response of each condition to the control (absence of radiation and SU093). (C,D) Colony formation assay of FaDu and SCC-25 with SU093 with or without 5 Gy of ionizing radiation. Control group received vehicle only. (E) Colony formation quantification of FaDu and SCC-25 with SU093 or combined (5 Gy) treatment. Asterisks indicate t test comparison between each treatment and control, where ***P < 0.001, **P < 0.01, *P < 0.05.
To quantify the degree of synergism, we used the Chou–Talalay method of combined median effect calculation to obtain a combination index (CI) in which CI < 1 shows synergistic effects (mutual enhancement of treatment effects), CI = 1 shows additive effects (independent, cumulative treatment effects), and CI > 1 shows antagonistic effects (treatments interfering).19
Combined SU093 and IR treatment showed an average synergistic CI = 0.686 in FaDu cells and an additive CI = 0.919 in SCC-25 (Table 1). This pattern of synergism in FaDu cells and additive effects in SCC-25 scaled with dose for both SU093 and IR, where 5 μM SU093 and 5 Gy IR showed CI = 0.314 for FaDu cells and CI = 1.017 for SCC-25 cells. These results show that SU093 and IR act in a complementary manner to increase the cytotoxic activity of one another, showing that SU093 acts as a potent radiosensitizer in HNSCC.
Table 1. Combination Index (CI) of Various Doses of SU093 Combined with Three Doses of Ionizing Radiationa.
| FaDu cells | fractional
inhibition |
combination index
(CI) |
|||||
|---|---|---|---|---|---|---|---|
| SU093 (μM) | IR: 0Gy | 2.5 | 5 | 10 | 2.5 Gy | 5 Gy | 10 Gy |
| 0 | - | 0.131 | 0.097 | 0.388 | - | - | - |
| 0.005 | 0.145 | 0.236 | 0.136 | 0.346 | 0.406 | 1.822 | 0.856 |
| 0.05 | 0.186 | 0.383 | 0.239 | 0.452 | 0.234 | 1.180 | 0.572 |
| 0.5 | 0.264 | 0.443 | 0.425 | 0.613 | 0.377 | 0.603 | 0.319 |
| 5 | 0.437 | 0.501 | 0.633 | 0.681 | 1.225 | 0.324 | 0.314 |
| SCC-25 cells | fractional
inhibition |
combination index (CI) |
|||||
|---|---|---|---|---|---|---|---|
| SU093 (μM) | IR: 0Gy | 2.5 | 5 | 10 | 2.5 Gy | 5 Gy | 10 Gy |
| 0 | - | 0.011 | 0.133 | 0.347 | - | - | - |
| 0.005 | 0.009 | 0.015 | 0.157 | 0.388 | 1.216 | 0.801 | 1.021 |
| 0.05 | 0.005 | 0.74 | 0.050 | 0.379 | 0.835 | 1.753 | 1.056 |
| 0.5 | 0.361 | 0.452 | 0.658 | 0.713 | 0.386 | 0.395 | 0.663 |
| 5 | 0.744 | 0.531 | 0.728 | 0.710 | 1.244 | 0.652 | 1.017 |
Fractional inhibition = 1 – fraction of surviving cells.
Combined SU093 and Ionizing Radiation Promotes G2/M Phase Arrest and Induces Apoptosis
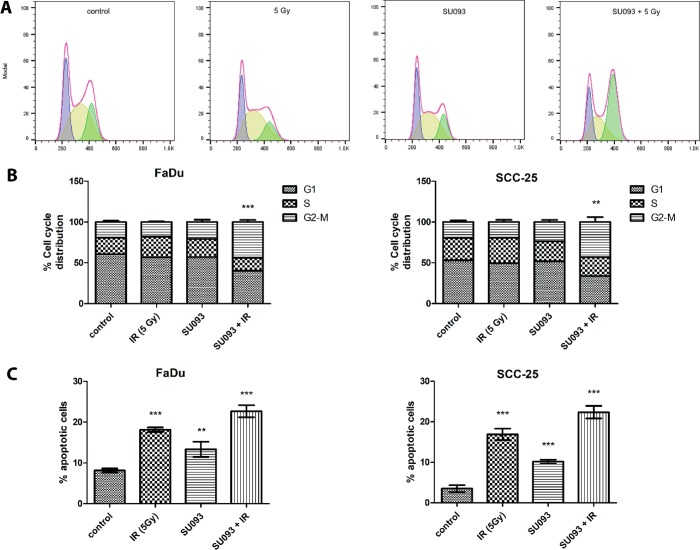
To probe the mechanism of synergism between SU093 and IR in HNSCC, we examined how treatment changed cell cycle distribution via flow cytometry and propidium iodide staining. SU093 treatment alone produced no significant shift in cell cycle distribution, whereas 5 Gy or IR alone caused a small increase of cells in S-phase, indicative of response to DNA damage. Combination treatment did not show any significant effect in cell cycle distribution at 6 h after treatment but did show an effect at 24 h with a significant decrease of FaDu and SCC-25 cells in G0/G1 or S phase and a concomitant 2-fold increase in cells in the G2/M phase (22%, p < 0.05, Figure 3A,B). This time-dependent synergism in SU093 and IR cotreatment was also seen in a 3- and 7-fold increase in apoptotic FaDu and SCC-25 cells, respectively, at 48 h post-treatment, showing that cotreatment not only decreases clonogenic proliferation via increased cell cycle arrest but also induces greater apoptosis than either treatment alone.
Figure 3.
(A) Representative cell cycle distribution of FaDu cells for each condition from left to right; control, 5 Gy, SU093, and combined 0.5 μM SU093 and 5 Gy of ionizing radiation. (B) Cell cycle distribution of FaDu and SCC-25, respectively, for each condition. Asterisks indicate t test comparison between combined treatment and control for G2/M distribution, where P < 0.001 or P < 0.01. (C) Percentage of apoptotic cells post 48 h treatments of FaDu and SCC-25 in each conditions control, 5 Gy of IR, 0.5 μM SU093, and combined treatment. Asterisks indicate t test comparison between each treatment and control, where ***P < 0.001 or **P < 0.01.
Combined Treatment Inhibitions of HNSCC Migration
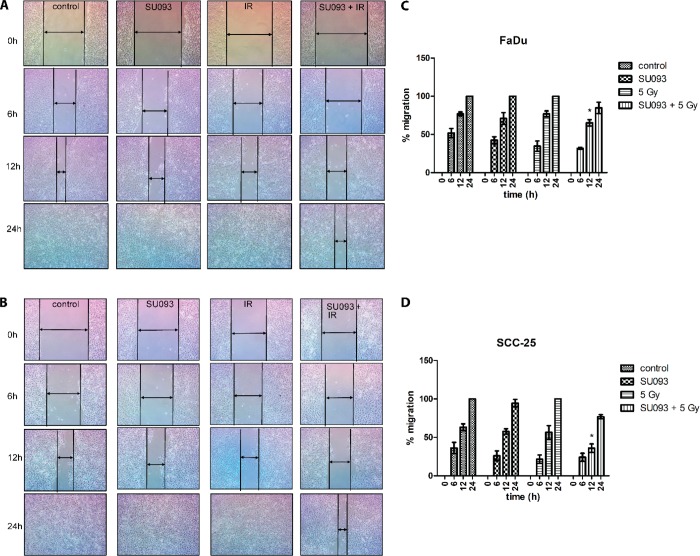
To quantify how cotreatment affected HNSCC motility and migration, we used the scratch migration assay and microscopic imaging of “wound” closure at 6, 12, and 24 h. We employed a standard protocol with mitomycin C for migration assay,20 to arrest cell proliferation and observe their migration property. Briefly, 105 cells were seeded in a 12-well plate and allowed to grow to full confluency, after which cells were treated with SU093 for 24 h and/or 5 Gy IR (Figure 4A,B). Each well was scratched with a pipet tip to induce a “wound” and washed in 1× PBS to remove detached cells and cultured in fresh media and imaged over the next 24 h to measure the decrease in “wound” thickness and infer migratory activity. Compared to untreated control, individual SU093 and IR treatments modestly restricted migration at 12 h with full recovery of the “wound” at 24 h. However, the combination of SU093 and IR showed a significant decrease in postwounding migration at 12 h in which only 65% and 36% FaDu and SCC-25 cell migration was observed, respectively. These results show that combination treatment acts to decrease HNSCC migration in addition to its effects on cell viability, proliferation, and apoptosis.
Figure 4.
Representative images of the migratory effect of FaDu (A) and SCC-25 (B) after wounding following a 24 h treatment of 0.5 μM SU093 or combined 0.5 μM SU093 and 5 Gy of IR. Wound closure was followed and recorded for three time points (0–24 h). (C,D) Quantitative assessment of wound closure for FaDu and SCC-25 followed by 24 h treatment; percent migration was calculated by measuring the gap decrease for each condition with respect to its initial wound gap at 0 h. Asterisk indicates P < 0.05 of combined SU093 and 5 Gy compared to IR treatment at 12 h postwounding.
Immunoblot Analysis of Combined SU093 and Ionizing Radiation Treatment
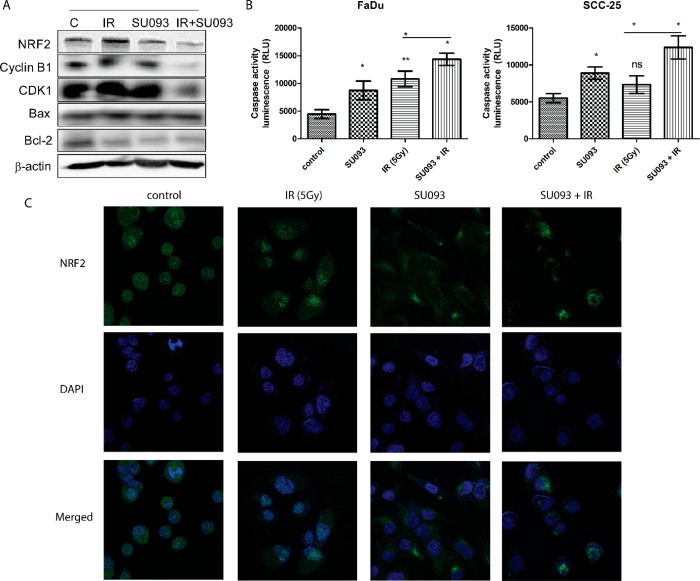
To further probe the treatment effects observed above, we used immunoblots and immunofluorescence microscopy to quantify the activity of several proteins of interest. In the treatment conditions above, this showed that individual treatment of either IR or SU093 modestly decreased Cyclin B1 and CDK1, indicative of cell cycle arrest, as well as Bcl-2, indicative of increased apoptotic sensitivity (Figure 5A), and that combination treatment exerted further synergistic reduction in each (Figure 5A). Conversely, levels of Bax, a marker of apoptotic activity, were increased in the same pattern with a synergistic cotreatment effect.
Figure 5.
Immunoblot and immunofluorescence analysis of HNSCC FaDu. (A) Immunoblot analysis of NRF2, cyclin B1, CDK1, Bax, and Bcl-2 of FaDu cell line. FaDu cells were treated with 0.5 μM SU093, 5 Gy of IR, or combined SU093 and 5 Gy. At the end of 48 h treatments, lysates were collected and analyzed using specific primary antibodies for NRF2, cyclin B1, CDK1, Bax, and Bcl-2. Membranes were stripped and pre-probed with antibeta actin to ensure equal protein loading. (B) Caspase activity of FaDu and SCC-25. (C) Immunofluorescence analysis of Nrf2 expression in FaDu cells after 48 h of respective treatments. Asterisks indicate t test comparison between each treatment and control, where **P < 0.01, *P < 0.05.
The combinatorial synergism of SU093 and IR treatment on apoptosis was confirmed by luminescent caspase activity assay, a marker of active apoptosis, which was significantly increased in both FaDu and SCC-25 cells (Figure 5B). Finally, we also examined the change in the levels of nuclear factor, erythroid 2 like 2 (Nrf2), a short-lived Phase-II cell stress response element involved in cell survival of oxidative DNA damage and implicated in radioresistance in HNSCC,21,22 via immunofluorescence microscopy, showing that both SU093 and IR treatments individually decreased Nrf2 activity while combination treatment exhibited synergistic increased Nrf2 inhibition (Figure 5C). Collectively, these results show that combination SU093 and IR treatment modulates HNSCC cell cycle, apoptosis, and DNA damage response, rendering HNSCC cells less viable, less proliferative, less migratory, and more apoptotic in a potent and synergistic manner.
Conclusions
The cellular response to the DNA double-strand breaks and reactive oxygen species generated by ionizing radiation (IR) exposure is evolutionarily conserved and mobilizes DNA damage repair, cell stress, antioxidant, and Phase-II detoxifying metabolic enzymes to mediate cell cycle arrest in G1 or S phase where repair systems are maximally effective outside of the telomere fusion and epigenetic restriction inherent to G2 and M phases.23 These pathways operate in both normal and cancerous cells, where, in the latter, use of IR as a treatment potently activates various endogenous damage response pathways that interact with oncogenic signaling in the development of treatment resistance and subsequent ability of a given clonal population to survive treatment. The emergence of treatment resistance in head and neck squamous cell carcinoma (HNSCC) and other cancers mediates disease progression, relapse, and mortality in an arms race, underscoring the urgent unmet need for treatments that restore treatment sensitivity and/or increase the cytotoxic effect of radio- and chemo-therapies in refractory disease. We have demonstrated the potency of a novel etoposide derivative, SU093, in modulating HNSCC sensitivity to radiotherapy, demonstrating a synergistic increase in the apoptotic, antiproliferative effect of radiotherapy in both FaDu and SCC-25 models of human HNSCC. Both ionizing radiation and SU093 each exerted modest antiproliferative effects in HNSCC, but this effect was magnified when used in combination, showing a synergistic combination index (CI) for FaDu cells and an additive CI for SCC-25 cells, showing potent dose- and time-dependent radiosensitization with the potential to advance the clinical treatment of refractory HNSCC. This is borne out in our findings that combination treatment significantly increased G2/M cell cycle arrest, thus rendering cells less able to induce DNA damage and antioxidant responses, inhibition of Bcl-2, an antiapoptotic factor, and decreased Nrf2, a short-lived effector crucial for survival of radiotherapy and implicated in radioresistance in HNSCC.24 SU093 treatment alone showed a modest decrease in Nrf2 levels, showing basal inhibition thereof, whereas combination treatment demonstrated a significant decrease of Nrf2 activation, which implies a decreased capacity for HNSCC cells to overcome and adapt to the effects of IR treatment. This was confirmed via significantly increased caspase activation, which signifies increased apoptotic activity, and Bax, a marker of apoptotic signaling, in both FaDu and SCC-25 cells with a concomitant significant reduction in cell viability and clonogenic proliferation. These profound alterations of HNSCC cell phenotype were also accompanied by decreased migratory capacity in SU093 and IR treated cells in a scratch “wound” assay, implying that cotreatment may even decrease HNSCC invasion and progression. In sum, the results shown here demonstrate the capacity of SU093 to act as a potent radiosensitizer that acts synergistically and/or additively with ionizing radiation treatment against HNSCC proliferation, viability, and treatment resistance via a Nrf2-mediated pathway. Further study of SU093 in vivo and with other treatments commonly used for HNSCC, such as cisplatin, will yield further insight into its mechanism of action, degree of cotreatment synergy, and clinical potential for the reversal of treatment resistance in HNSCC and other cancers.
Experimental Procedures
Synthesis of Radiosensitizer 4-Azapodophyllotoxin SU093
SU093 was synthesized through a multicomponent reaction according to a previously published procedure and used with purity up to 98% (see Supporting Information for additional details).
Cell lines and Reagents
Head and neck cancer cell line FaDu and SCC-25 were obtained from American Type Culture Collection (Manassas, VA, USA). Both cell lines were cultured in and maintained in Dubelcco’s Modified Eagle’s Medium (DMEM) supplemented with 10% Fetal Bovine Serum (FBS, v/v%) and 1% penicillin–streptomycin–amphotericin B at 37 °C with 5% CO2. Once 80% confluence was reached, cells were harvested, counted, and plated for each assay. SU093 stock solutions were prepared freshly on the day of the assay experiment in filtered DMSO and diluted in complete media.
Multiwell Combination Treatment of SU093 and Ionizing Radiation
Cells were seeded at 1000 cells per well (100 μL) in a 96-well plate (Corning #3598). After incubation for 24 h, cells received either 100 μL of SU093 in fresh medium containing different concentrations (0.1, 0.5, and 1 μM final concentration in a well) as 2-fold or media only containing vehicle (control wells) and subsequently were treated with IR (2.5, 5, or 10 Gy) after a short incubation period of 1 h. Radiation doses were administered in three sequences using an X-Rad SmART (Precision X-ray Inc., North Branford, CT), and a Cu-filter source was used to irradiate cells in multiwell plates through an additive fashion. Using a 5 mm thick lead shield, three columns were exposed to 5 Gy, then the lead shield was moved to expose the following six columns, and 2.5 Gy was provided. Finally, nine columns were exposed by moving the lead shield, and 2.5 Gy was administered. The remaining three columns are covered the entire time and protected from radiation, and represent the control or SU093 only treated cells (0 Gy) (see Figure 1A or Figure SX for detailed workflow of treatments). After radiation treatment is complete, the first three columns received a total of 10 Gy, followed by 5 Gy for the next three columns and 2.5 Gy for the next set of three. Plates were allowed to incubate for 6 days, and viable cells were quantified using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reagent (Acros Organics, Thermo Fisher Scientific, Massachusetts, USA). After the incubation period, 50 μL of MTT (0.5 mg/mL, in 1× PBS) was added to each well and allowed to incubate for 45 min. After incubation, the media solution was removed, and 100 μL of DMSO was added to each well and incubated for 5 min before the absorbance was read at 570 nm using a Synergy H1 multimode microplate reader (BioTecK, USA).
Combination Index Analysis
Combined IR and SU093 treatments were conducted with the Chou–Talalay method using CompuSyn software that is freely available for download. Data from viability assay was used for the combination index (CI) calculations using the values of CI < 1, CI = 1, and CI > 1 indicated synergistic, additive, or antagonistic effects.
Colony Formation Assay
Cell Cycle Analysis
Annexin V Apoptosis Assay
Scratch Migration Assay
Immunoblotting
Immunofluorescence
Statistics
Acknowledgments
This work was supported in part by the Stanford Cancer Institute. The authors would also like to acknowledge support by PA-14-015, Grant Number T32 CA 121940, awarded by Ruth L. Kirschstein National Research Service Award (NRSA).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.9b00270.
Detailed synthesis and experimental methodology, descriptions for combinatorial treatments for other assays conducted, the CompuSyn data, and H and C NMR spectra for SU093 (PDF)
Author Contributions
‡ The authors contributed equally to this work. D.T. and S.V.M. designed the studies. D.T. and A.R. performed all experiments. E.G. helped with radiation studies. S.V.M. supervised the entire work. A.R., D.T., and S.V.M. prepared the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Bray F.; Ferlay J.; Soerjomataram I.; Siegel R. L.; Torre L. A.; Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca-Cancer J. Clin. 2018, 68 (6), 394–424. 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Colevas A. D. Chemotherapy options for patients with metastatic or recurrent squamous cell carcinoma of the head and neck. J. Clin. Oncol. 2006, 24 (17), 2644–2652. 10.1200/JCO.2005.05.3348. [DOI] [PubMed] [Google Scholar]
- FDA Drugs Approved for Head and Neck Cancer. https://www.cancer.gov/about-cancer/treatment/drugs/head-neck (accessed April 16th, 2019).
- Boman B. M.; Wicha M. S. Cancer stem cells: A step toward the cure. J. Clin. Oncol. 2008, 26 (17), 2795–2799. 10.1200/JCO.2008.17.7436. [DOI] [PubMed] [Google Scholar]
- Naghavi A. O.; Echevarria M. I.; Strom T. J.; Abuodeh Y. A.; Ahmed K. A.; Venkat P. S.; Trotti A.; Harrison L. B.; Green B. L.; Yamoah K.; Caudell J. J. Treatment delays, race, and outcomes in head and neck cancer. Cancer Epidemiol. 2016, 45, 18–25. 10.1016/j.canep.2016.09.005. [DOI] [PubMed] [Google Scholar]
- Al-Sarraf M. Treatment of Locally Advanced Head and Neck Cancer: Historical and Critical Review. Cancer Control 2002, 9 (5), 387–399. 10.1177/107327480200900504. [DOI] [PubMed] [Google Scholar]
- Pezzuto F.; Buonaguro L.; Caponigro F.; Ionna F.; Starita N.; Annunziata C.; Buonaguro F. M.; Tornesello M. L. Update on Head and Neck Cancer: Current Knowledge on Epidemiology, Risk Factors, Molecular Features and Novel Therapies. Oncology 2015, 89 (3), 125–136. 10.1159/000381717. [DOI] [PubMed] [Google Scholar]
- Bohlin L.; Rosen B. Podophyllotoxin derivatives: Drug discovery and development. Drug Discovery Today 1996, 1 (8), 343–351. 10.1016/1359-6446(96)10028-3. [DOI] [Google Scholar]
- Kobayashi K.; Ratain M. J. Pharmacodynamics and long-term toxicity of etoposide. Cancer Chemother. Pharmacol. 1994, 34, S64–S68. 10.1007/BF00684866. [DOI] [PubMed] [Google Scholar]
- Ren J.; Wu L.; Xin W. Q.; Chen X.; Hu K. Synthesis and biological evaluation of novel 4 beta-(1,3,4-oxadiazole-2-amino)-podophyllotoxin derivatives. Bioorg. Med. Chem. Lett. 2012, 22 (14), 4778–4782. 10.1016/j.bmcl.2012.05.059. [DOI] [PubMed] [Google Scholar]
- Zhao Y.; Ge C. W.; Wu Z. H.; Wang C. N.; Fang J. H.; Zhu L. Synthesis and evaluation of aroylthiourea derivatives of 4-beta-amino-4 ’-O-demethyl-4-desoxypodophyllotoxin as novel topoisomerase II inhibitors. Eur. J. Med. Chem. 2011, 46 (3), 901–906. 10.1016/j.ejmech.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Kumar N. P.; Sharma P.; Reddy T. S.; Nekkanti S.; Shankaraiah N.; Lalita G.; Sujanakumari S.; Bhargava S. K.; Naidu V. G. M.; Kamal A. Synthesis of 2,3,6,7-tetramethoxyphenanthren-9-amine: An efficient precursor to access new 4-aza-2,3-dihydropyridophenanthrenes as apoptosis inducing agents. Eur. J. Med. Chem. 2017, 127, 305–317. 10.1016/j.ejmech.2017.01.001. [DOI] [PubMed] [Google Scholar]
- Kamal A.; Kumar B. A.; Suresh P.; Juvekar A.; Zingde S. Synthesis of 4 beta-carbamoyl epipodophyllotoxins as potential antitumour agents. Bioorg. Med. Chem. 2011, 19 (9), 2975–2979. 10.1016/j.bmc.2011.03.030. [DOI] [PubMed] [Google Scholar]
- Li W. Q.; Wang X. L.; Qian K. D.; Liu Y. Q.; Wang C. Y.; Yang L.; Tian J.; Morris-Natschke S. L.; Zhou X. W.; Lee K. H. Design, synthesis and potent cytotoxic activity of novel podophyllotoxin derivatives. Bioorg. Med. Chem. 2013, 21 (8), 2363–2369. 10.1016/j.bmc.2013.01.069. [DOI] [PubMed] [Google Scholar]
- Sun W. X.; Ji Y. J.; Wan Y.; Han H. W.; Lin H. Y.; Lu G. H.; Qi J. L.; Wang X. M.; Yang Y. H. Design and synthesis of piperazine acetate podophyllotoxin ester derivatives targeting tubulin depolymerization as new anticancer agents. Bioorg. Med. Chem. Lett. 2017, 27 (17), 4066–4074. 10.1016/j.bmcl.2017.07.047. [DOI] [PubMed] [Google Scholar]
- Tang Z. B.; Chen Y. Z.; Zhao J.; Guan X. W.; Bo Y. X.; Chen S. W.; Hui L. Conjugates of podophyllotoxin and norcantharidin as dual inhibitors of topoisomerasell and protein phosphatase 2A. Eur. J. Med. Chem. 2016, 123, 568–576. 10.1016/j.ejmech.2016.07.031. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Chen F.; Zhang Z. G.; Chen Y. Z.; Lin Y.; Wang J. Design, synthesis and evaluation of the multidrug resistance-reversing activity of pyridine acid esters of podophyllotoxin in human leukemia cells. Bioorg. Med. Chem. Lett. 2016, 26 (18), 4466–4471. 10.1016/j.bmcl.2016.07.072. [DOI] [PubMed] [Google Scholar]
- Kumar A.; Kumar V.; Alegria A. E.; Malhotra S. V. Synthetic and application perspectives of azapodophyllotoxins: alternative scaffolds of podophyllotoxin. Curr. Med. Chem. 2011, 18 (25), 3853–70. 10.2174/092986711803414331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou T. C.; Talalay P. quantitative-analysis of dose-effect relationships - the combined effects of multiple-drugs or enzyme-inhibitors. Adv. Enzyme Regul. 1984, 22, 27–55. 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Vang Mouritzen M.; Jenssen H. Optimized Scratch Assay for In Vitro Testing of Cell Migration with an Automated Optical Camera. J. Visualized Exp. 2018, (138), e57691. 10.3791/57691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B. C.; Li J.; Yu W. F.; Zhang G. Z.; Wang H. M.; Ma H. M. Elevated expression of Nrf2 mediates multidrug resistance in CD133(+) head and neck squamous cell carcinoma stem cells. Oncol. Lett. 2016, 12 (6), 4333–4338. 10.3892/ol.2016.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacy D. R.; Ely K.; Massion P. P.; Yarbrough W. G.; Hallahan D. E.; Sekhar K. R.; Freeman M. L. Increased expression of nuclear factor E2 P45-related factor 2 (NRF2) in head and neck squamous cell carcinomas. Head and Neck-Journal for the Sciences and Specialties of the Head and Neck 2006, 28 (9), 813–818. 10.1002/hed.20430. [DOI] [PubMed] [Google Scholar]
- Baumann M.; Krause M.; Hill R. Exploring the role of cancer stem cells in radioresistance. Nat. Rev. Cancer 2008, 8 (7), 545–554. 10.1038/nrc2419. [DOI] [PubMed] [Google Scholar]
- Kobayashi M.; Yamamoto M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv. Enzyme Regul. 2006, 46, 113–140. 10.1016/j.advenzreg.2006.01.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.