Abstract

Peptide-based subunit vaccines require an immunostimulant (adjuvant) and/or delivery system to protect the antigenic peptide from degradation and induce the desired immunity. Currently available adjuvants are either too toxic for human use (experimental adjuvants) or they are limited for use in particular vaccines or licensed countries (commercial adjuvants). Therefore, there is an immediate need for novel adjuvants that are both safe and effective. Herein, we assessed the ability of cholic acid (a major bile acid) as a nontoxic, biodegradable, human-derived, potent vaccine delivery system. An antigenic peptide derived from Group A Streptococcus was conjugated to hydrophobic cholic acid via solid phase peptide synthesis to produce lipopeptide that self-assembled into rod-like nanoparticles under aqueous conditions. Following intranasal immunization in mice, this lipopeptide was capable of inducing the production of opsonic epitope-specific antibodies on its own and in liposomal formulation. The cholic acid-based conjugate induced significantly stronger humoral immune responses than cholera toxin-based adjuvant. Thus, we demonstrated, for the first time, capability of the human-derived lipid to act as a built-in immunoadjuvant for vaccines.
Keywords: Cholic acid, adjuvant, subunit vaccine, liposomes, intranasal delivery, bile salt
Vaccination is one of the most effective medical treatments, and the practice of immunization can be traced back for centuries.1 Although vaccinology has advanced greatly, traditional vaccination strategies based on attenuated or inactivated whole microorganisms are still relevant and widely used. These forms of vaccination can produce long-lasting immunity; however, they can also elicit autoimmunity, inflammation, or strong allergic responses, leading to concerns about their safety. Whole microorganism-based vaccines are often difficult to manufacture, due to the limited technology available for the production and purification of human pathogens (e.g., malaria parasite) in large quantities.2 They are also unstable in storage conditions, and especially during transportation, as consistent cold temperature is required to maintain vaccine quality and efficacy. Thus, the emergence of subunit vaccines is an appealing alternative to the traditional approaches.
Subunit vaccines utilize minimal microbial components (polysaccharides, proteins, and peptides) to stimulate adaptive immunity against a pathogen. As a result, many of the adverse effects of traditional vaccines are eliminated.3,4 Moreover, peptide-based subunit vaccines are usually produced by chemical synthesis, which eliminates the possibility for potentially harmful biological contamination. Peptide antigen is chemically fully defined, making its production customizable, simple, reproducible, fast and cost-effective. The vaccine produced is usually water-soluble and more stable, and it can be freeze-dried, making storage easier. However, subunit vaccines, especially peptide-based vaccines, require immunostimulants (adjuvants), and/or delivery systems to induce effective immune responses.
Despite extensive research on adjuvants, there is still only a limited selection of safe and potent adjuvants available for use, which has hindered the development of subunit vaccines. Available experimental adjuvants (e.g., complete Freund’s adjuvant (CFA), incomplete Freund’s adjuvant (IFA), and lipid A) show excellent immunostimulating ability, but they also cause adverse effects.5,6 However, commercial adjuvants (aluminum salt, liposome-based adjuvant (virosome and AS01), recombinant CTB, emulsions (MF59, AS03, and AF03), RC-529 synthetic monophosphoryl lipid A, and Montanide ISA-51) are not only limited in number, but they are also approved only for specific vaccines and often only for use in certain countries.6 Therefore, there is an urgent demand for the discovery of novel, universal adjuvants that are both effective and safe for human use. The ideal adjuvant needs to be inexpensive, stable under storage and in vivo conditions, nontoxic, and should help to induce a protective response even against weak antigenic molecules, such as peptides.3,6
Most adjuvants are administered as a physical mixture with an antigen to boost immune responses against the targeted pathogen. However, it has been observed that the incorporation of adjuvant and antigen in the same construct can greatly improve the efficacy.5,7,8 The conjugation ensures that both adjuvant and antigen are taken up by the same antigen presenting cells (APCs), thus inducing stronger immune responses. For instance, the conjugation of acrylic polymer or lipids to peptide antigens, in contrast to a physical mixture, provided “self-adjuvanting” properties and the generation of strong immune responses.9,10 Double copies of 2-amino-D,L-hexadecanoic acid (C16) conjugated to B-cell peptide epitopes have been shown to enhance epitope uptake by APCs (e.g., dendritic cells (DCs)), their maturation, as well as the stimulation of antigen-specific antibody responses.11−15 In addition, the lipidation of antigenic peptide can protect the antigen from enzymatic degradation, give the antigen the ability to self-assemble to form nanoparticles, and enable the antigen to be anchored to liposomes.16−18 A liposomal delivery system was approved for the protein-based vaccine against malaria.19,20 The incorporation of self-adjuvanting lipopeptide into liposome delivery systems has also been proven to generate long-lasting immunity.21
Herein, we assessed the ability of cholic acid, a major bile acid produced from cholesterol in the liver,22 as a liposomal anchoring moiety and potential adjuvant for vaccine delivery. We selected cholic acid as potential immunostimulating agent because bile salts are known to have immunomodulatory activity. The ability of bile salts to activate neutrophils, enhance inflammatory monocyte recruitment, and modulate the phagocytic capability of monocytes has been reported.23 Cholic acid has been used for delivery of anticancer drugs to overcome drug resistance in tumor cells.22−24 In recent studies, cholic acid was able to enhance antimicrobial activity of caragenin (bactericidal agent) against Gram-positive and Gram-negative bacteria.25 In all of these studies, cholic acid was found to be safe and well-tolerated by animals. Cholic acid has not previously been used for antigen delivery despite it can be easily conjugated to an antigen via its carboxylic group24,26 and incorporated into a liposome, serving as an anchoring moiety for peptide antigens.
Vaccine candidates (Figure 1) were designed based on PADRE universal T-helper and J8 conserved B-cell epitope derived from Group A Streptococcus (GAS) M protein. GAS is a Gram-positive, cocci bacterium that can cause various diseases, ranging from noninvasive (e.g., pharyngitis and pyoderma), to invasive (e.g., cellulitis, erysipelas, and Streptococcal toxic shock syndrome) and lethal postinfectious diseases (e.g., rheumatic fever and rheumatic heart disease).27−29 Peptide 1 bearing PADRE and J8 epitopes was conjugated to cholic acid to produce lipopeptide 2, which was then incorporated into cationic liposomes (L2). This system was compared to classic self-adjuvanting C16-based lipopeptide alone (3) and in liposome formulation (L3). The compounds 1–3 and encapsulated liposomes L1–L3 were evaluated for their ability to trigger humoral immune responses following intranasal administration in mice.
Figure 1.
Structures of peptide 1 and lipopeptides 2–3, encapsulated into liposome delivery systems L1–L3.
Results
Preparation and Characterization of Vaccine Candidates
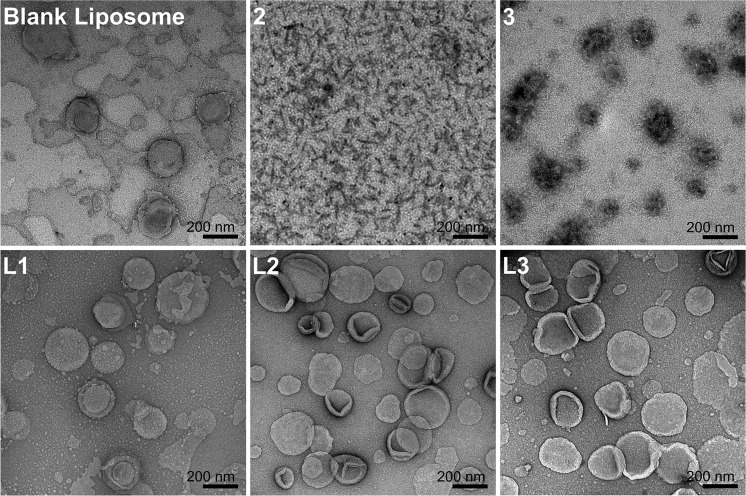
Vaccine candidates were designed to incorporate PADRE universal T-helper and J8 conserved B-cell epitopes, conjugated to cholic acid (2) and C16 lipid moiety (3) (Figure 1). The epitopes were linked through Lys-Lys spacers. An additional Ser-Ser spacer was included between the peptide sequence and the two C16 lipid moieties, following a previously published design.30 Compounds 1–3 were successfully synthesized using Fmoc-SPPS (Figure S1) and self-assembled to form nanoparticles (2 and 3) in water. In addition, compounds 1–3 were also incorporated into liposomes composed of DPPC:CH:DDAB to produce liposomes L1–L3, respectively. The liposomes were extruded via a 100 nm membrane to form uniform unilamellar vesicles. The size, size distribution (PDI), surface charge, morphology, and stability of compounds 2 and 3 and liposomes L1–L3 were characterized using DLS and TEM (Tables 1 and S1, Figures 2 and S2).
Table 1. Physicochemical Characterization of the Compounds 2, 3, and Liposomes L1–L3 by Dynamic Light Scattering (DLS).
| Vaccine construct | Particle size (nm) | PDI | Zeta potential (mV) |
|---|---|---|---|
| 2 | 128 ± 2 and 9 ± 1 | 0.328 ± 0.002 | 20.8 ± 3.1 |
| 3 | 312 ± 8 | 0.116 ± 0.105 | 46.1 ± 2.0 |
| L1 | 159 ± 2 | 0.035 ± 0.017 | 47.7 ± 0.8 |
| L2 | 163 ± 1 | 0.034 ± 0.022 | 46.9 ± 0.3 |
| L3 | 165 ± 2 | 0.033 ± 0.017 | 45.9 ± 0.9 |
| Blank liposome | 161 ± 1 | 0.035 ± 0.008 | 43.6 ± 0.5 |
Figure 2.
Particle-imaging and morphology of lipopeptides 2 and 3, blank liposomes, and encapsulated liposomes L1–L3, captured by TEM (bar 200 nm).
Compound 2 self-assembled in water, and DLS size distribution analysis showed two separate peaks (10 and 130 nm, Table 1). These corresponded to rod-like particles observed on TEM images, 10 nm thick and 100 nm long (Figure 2). In contrast, compound 3 produced rather spherical particles, approximately 300 nm in diameter, as confirmed by both DLS and TEM. Compounds 2 and 3 were both positively charged, but compound 2 had a lower zeta potential. However, it has to be taken into account that DLS does not provide accurate zeta potential for nonspherical particles (zetasizers measure free mobility and hydrodynamic size to convert these characteristics to zeta potential, using theoretical formulas approximating the nanoparticle as a sphere).31 Incorporating peptide 1 into the liposome did not affect the liposome size; both blank liposome and liposome L1 exhibited an average diameter of 160 nm. The anchoring of compounds 2 and 3 onto a liposomal surface (L2 and L3) resulted in a minor increase of the size of liposomes. Both anchoring and encapsulation of positively charged J8 epitope increased the zeta potential of liposomes (L1–L3 vs blank liposomes). All of the liposomes had uniform size distributions (PDI < 0.1) and unilamellar structure, as observed through TEM images. In addition, the liposomes were stable for at least 11 weeks at RT according to DLS analysis (Table S1).
Induction of Antibody Responses
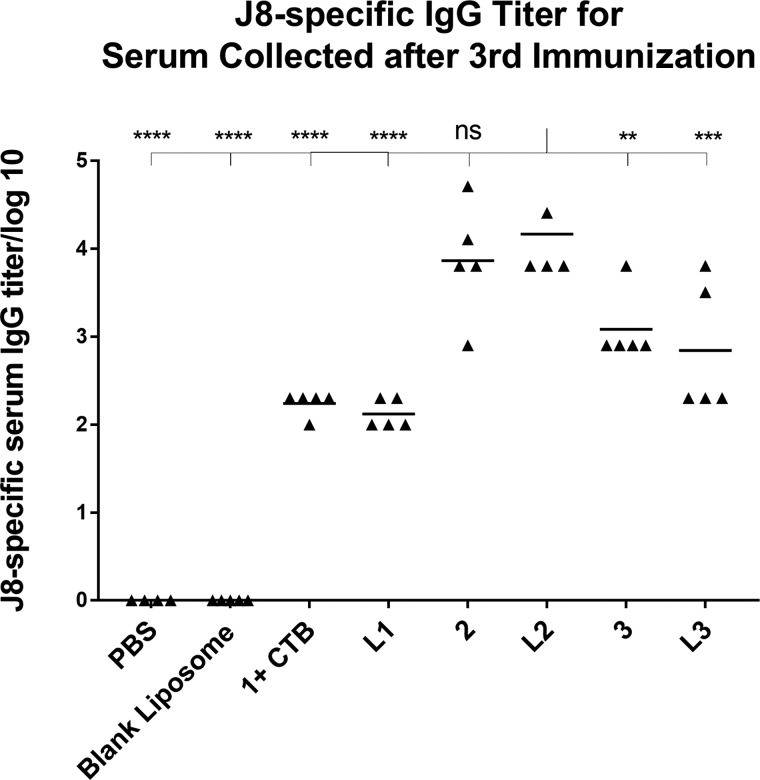
Immunological evaluation of peptide 1 + CTB (positive control), lipopeptides 2–3, and liposomes L1–L3 was performed in C57BL/6 mice using the prime-boost vaccination strategy. A negative control group was treated with PBS and blank liposome. All of the vaccine candidates were able to induce J8-specific IgG production after the final boost (Figure 3). Compound 2 and liposome L2 induced the most significant antibody titers when compared to the positive control group (p < 0.0001). Compound 3 (p < 0.01) and liposome L3 (p < 0.001) also stimulated higher antibody production than CTB-adjuvanted peptide antigen. There was no significant difference in antibody titers generated by compound 2 and L2. The vaccine candidates did not induce saliva J8-specific IgA antibody production, which was consistent with other peptide vaccine candidates bearing PADRE T-helper.32
Figure 3.
J8-specific serum IgG antibody titers from the final bleed after immunization with peptide 1 + CTB (positive control), lipopeptides 2–3, and liposomes L1–L3 in C57BL/6 mice, as analyzed by ELISA. Each point represents an individual mouse; bars represent the average antigen-specific serum IgG antibody titers. Statistical analysis was performed using one-way ANOVA with Tukey’s multiple comparison test; (*) p < 0.05, (**) p < 0.01, (***) p < 0.001, (****) p < 0.0001.
Antibody Opsonization Assay
Antibodies produced by the immunized mice were tested for their ability to opsonize different strains of GAS clinical isolates (Figure 4). Sera collected from mice immunized with compound 2 and liposome L2 showed significantly higher levels of opsonization against all GAS strains when compared to PBS. L2 induced the production of slightly more opsonic antibodies than compound 2. Sera from mice immunized with compound 3 and liposome L3 show poor opsonic activity, which might be related to the general lower level of antibody titers induced by them.
Figure 4.
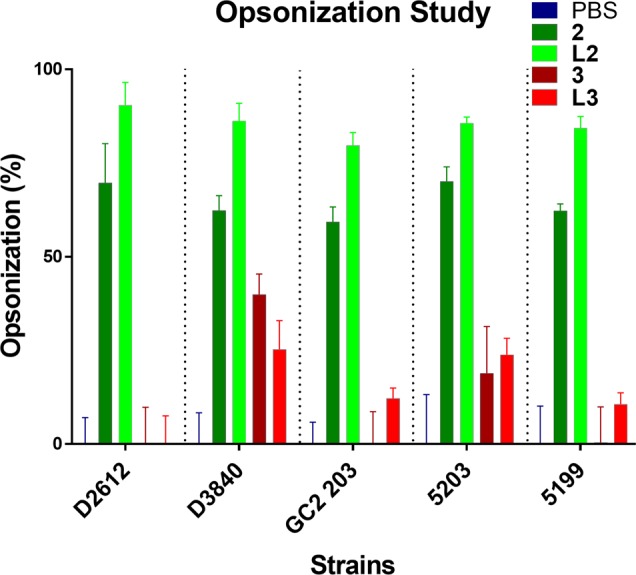
Average opsonization percentage of different GAS strains, ACM-5199, ACM-5203, GC2 203, D3840, and D2612, by serum taken on day 60 after the primary immunisation to C57BL/6 mice (n = 5).
MTT Cytotoxicity Assay
Three cell lines, HEK293, SW620, and NCIH460, were used to evaluate the cytotoxicity of lead vaccine candidates (Figure S3). In concentrations of up to 2 mg/mL, compound 2 did not reduce human cell lines viability, suggesting lack of cholic acid-associated toxicity. However, slight toxicity was observed for liposome L2 with increased concentration, which is often reported for cationic liposomes,33,34 even when used as a vaccine adjuvant.
Discussion
The peptide-based vaccines presented here were designed to stimulate humoral-mediated adaptive immunity, utilizing cholic acid as an adjuvant, against GAS through the incorporation of B-cell epitope J8 and universal T-helper cell epitope PADRE into peptide antigen 1. J8 was selected as an antigen as it is derived from the conserved region of GAS M protein and is able to provide immune protection against the majority of GAS serotypes M proteins.35,36 Vaccine candidates that incorporate J8 epitope successfully completed phase 1 clinical trials as a vaccine candidate against GAS and no complications or side effects were reported.37 Synthetic universal T-helper cell epitope PADRE was selected due to its high efficiency and safety profile in human clinical trials.3,38 PADRE was able to bind strongly with 15 of the 16 HLA-DR types, which could overcome issues associated with high polymorphism of HLA-DR in the human population.38
Peptide-based vaccines need to be incorporated into appropriate delivery systems or coadministered with an adjuvant as peptides are poor immunogens and susceptible to enzymatic degradation.3,39 Delivery systems enhance antigen recognition by mimicking pathogens based on their size, shape, surface, and morphological and physiochemical properties. Ideal adjuvants and delivery systems should be stable under storage and in vivo conditions and nontoxic. They also need to be able to help to induce strong immune responses against poor antigens such as peptides.
We designed compounds 2 and 3 and liposomes L2 and L3 to compare the potential adjuvanting properties of cholic acid with classic lipopeptide. Lipidation of antigenic peptide with Ser-Ser-C16-C16- (a well-recognized self-adjuvanting lipid moiety15) produced compounds that were able to induce strong immune responses and antibody production upon intranasal administration.40 Lipopeptides 2–3 were incorporated into liposomal delivery systems, which have self-adjuvanting properties and are often used for antigen delivery.17,21,41 Both C16 and cholic acid, as a cholesterol derivative and bile salt, can be easily incorporated into liposomes.11,42 Large liposomes (>400 nm) are less potent in inducing humoral immune responses;43 therefore, liposomes L1–L3 were extruded through a 100 nm membrane. Being a bile salt, there was a chance that cholic acid could disrupt the liposomal membrane and reduce stability; therefore, the stability of L1–L3 was monitored over 11 weeks. The anchoring of lipopeptides 2–3 did not affect liposomes stability (Table S1).
Liposomes boost protective immunity by inducing both innate and adaptive immunity against carried antigens.44−46 Liposomes also enable intranasal delivery of vaccines, relating to better patient compliance and improved safety profile in comparison to traditional injection routes.47 Intranasal vaccines are more easily administered, especially in developing countries, where medical skills and facilities are less efficient but the prevalence of GAS infection is higher.48 Therefore, intranasal administration has been chosen as a preferable delivery route for compounds 1–3 and liposomes L1–L3. Similar to our previous observations,21 liposomes bearing lipidated antigen (L3) were more effective in inducing immune responses than liposomes carrying entrapped antigen (L1). Remarkably, L2 induced significantly higher immune responses than CTB-adjuvanted antigenic peptide 1 and other liposomes (L1 and L3), proving the capability of cholic acid to act as an efficient anchoring moiety with potential self-adjuvanting properties. Moreover, cholic acid conjugate 2 stimulated similar immune responses even without the presence of liposomes. The higher antibody titers stimulated by lipopeptide 2 compared to 3 can be correlated to its ability to self-assemble into rod-like particles, compared to the larger, cluster-like nanoparticles formed by peptide 3. Cells recognize rod-like structures by their thickness, not their length,49,50 and smaller nanoparticles (20–50 nm) are generally more efficient in inducing immune responses than larger particles (>100 nm).51 Thus, compound 2 was more efficient in antigen delivery than 3, most likely because of its size/shape properties.
Finally, in opsonization assays, only the sera from mice immunized with compound 2 and liposome L2 were able to kill clinical GAS isolates. However, liposome L2 showed signs of toxicity in examined cells (Figure S3). Therefore, despite the stronger opsonic capabilities of L2, compound 2 might be a more promising vaccine candidate for further development.
Overall, the lipidation of antigenic peptide with cholic acid enhanced the immune response of targeted peptide antigen and showed stronger adjuvating capacity than traditional self-adjuvating lipids.
Conclusion
We demonstrated that cholic acid conjugated to peptide antigen can be self-assembled into nanoparticles or incorporated into liposomes. Both liposomes and conjugate alone were able to induce high antibody titers that were opsonic against GAS clinical isolates. Thus, we have shown for the first time that human-derived lipid, cholic acid, can act as a built-in immunoadjuvant for vaccines.
Acknowledgments
We acknowledge the facilities, scientific, and technical assistance provided by the Australian Microscopy & Microanalysis Research Facility at the Centre for Microscopy and Microanalysis, The University of Queensland. We also acknowledge A. D. Paterson and P. Harris (Centre for Clinical Research, The University of Queensland) for providing GAS isolates GC 2 203, D3840, and D2612.
Biographies
Dr. Mariusz Skwarczynski completed his Ph.D. in Chemistry (1999) at Wroclaw University of Technology, Poland. His postdoctoral training began in Japan (Tokushima Bunri University, Japan, under the direction of late Professor M. Nishizawa, and Kyoto Pharmaceutical University under guidance of Professor Yoshiaki Kiso). In 2008, Dr. Skwarczynski joined the group of Professor Istvan Toth at The University of Queensland, Australia, to work on drug, gene, and vaccine delivery strategies. In 2010, Dr. Skwarczynski was awarded with the Strategic Fund Research Fellowship at The University of Queensland. Since then, his research is mainly focused on nanotechnology-based synthetic vaccine delivery approaches.
Professor Istvan Toth, Chair in Biological Chemistry & Professor of Pharmacy, The University of Queensland (UQ), Brisbane, Australia, graduated with a degree in Chemical Engineering and obtained his Ph.D. from the Technical University, Budapest, Hungary. In 1994, he was awarded a DSc for his work on drug delivery. He worked at the School of Pharmacy, University of London (1985–1998) then moved to the University of Queensland, and leads a productive medicinal chemistry research group. He is an elected RACI Fellow, Fellow of the Queensland Academy of Arts and Sciences and Fellow of the Hungarian Academy of Sciences.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.9b00239.
Materials; synthesis of compound 1–3, nanoparticle formation, preparation of liposome L1–L3, DLS, TEM, MTT cytotoxicity assay, intranasal immunization in an in vivo model, collection of serum and saliva, antibody titer detection by ELISA, antibody opsonization assay, and ethic statement; Figure S1: Analysis of purified compounds 1–3 by analytical RP-HPLC and ESI-MS; Figure S2: DLS spectra of particle size, by intensity and number for peptide 1, lipopeptides 2–3, blank liposome, and encapsulated liposomes L1–L3; Figure S3: MTT cytotoxicity assay of lipopeptide 2 and encapsulated liposome L2; Table S1: Liposome size over 11 weeks at room temperature. (PDF)
Author Contributions
The manuscript was written through contributions of all authors.
This work was supported by the National Health and Medical Research Council (NHMRC; Program Grant APP1132975).
The authors declare no competing financial interest.
Supplementary Material
References
- Plotkin S. History of vaccination. Proc. Natl. Acad. Sci. U. S. A. 2014, 111, 12283. 10.1073/pnas.1400472111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallerup R. S.; Foged C.. Classification of vaccines. In Subunit Vaccine Delivery; Foged C., Rades T., Perrie Y., Hook S., Eds.; Springer New York: New York, 2015; pp 15–29. [Google Scholar]
- Skwarczynski M.; Toth I. Peptide-based synthetic vaccines. Chem. Sci. 2016, 7, 842–854. 10.1039/C5SC03892H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skwarczynski M.; Zaman M.; Toth I.. Lipo-Peptides/Saccharides for Peptide Vaccine Delivery; Elsevier, Inc., 2013. [Google Scholar]
- Azmi F.; Ahmad Fuaad A. A. H.; Skwarczynski M.; Toth I. Recent progress in adjuvant discovery for peptide-based subunit vaccines. Hum. Vaccines Immunother. 2014, 10, 778–796. 10.4161/hv.27332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevagi R. J.; Toth I.; Skwarczynski M.. Peptide-based vaccines. In Peptide Applications in Biomedicine, Biotechnology and Bioengineering; Koutsopoulos S., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp 327–358. [Google Scholar]
- Moyle P. M.; Toth I. Self-adjuvanting lipopeptide vaccines. Curr. Med. Chem. 2008, 15, 506–516. 10.2174/092986708783503249. [DOI] [PubMed] [Google Scholar]
- Myschik J.; Rades T.; Hook S. Advances in lipid-based subunit vaccine formulations. Current Immunology Reviews 2009, 5, 42–48. 10.2174/157339509787314378. [DOI] [Google Scholar]
- Skwarczynski M.; Zaman M.; Urbani C. N.; Lin I. C.; Jia Z.; Batzloff M. R.; Good M. F.; Monteiro M. J.; Toth I. Polyacrylate dendrimer nanoparticles: a self-adjuvanting vaccine delivery system. Angew. Chem., Int. Ed. 2010, 49, 5742–5745. 10.1002/anie.201002221. [DOI] [PubMed] [Google Scholar]
- Zhong W.; Skwarczynski M.; Toth I. Lipid core peptide system for gene, drug, and vaccine delivery. Aust. J. Chem. 2009, 62, 956–967. 10.1071/CH09149. [DOI] [Google Scholar]
- Skwarczynski M.; Parhiz B.; Soltani F.; Srinivasan S.; Kamaruzaman K.; Lin I.; Toth I. Lipid peptide core nanoparticles as multivalent vaccine candidates against Streptococcus pyogenes. Aust. J. Chem. 2012, 65, 35–39. 10.1071/CH11292. [DOI] [Google Scholar]
- Skwarczynski M.; Kamaruzaman K.; Srinivasan S.; Zaman M.; Lin I-C.; Batzloff M.; Good M.; Toth I. M-protein-derived conformational peptide epitope vaccine candidate against Group A Streptococcus. Curr. Drug Delivery 2013, 10, 39–45. 10.2174/1567201811310010007. [DOI] [PubMed] [Google Scholar]
- Azmi F.; Ahmad Fuaad A. A. H.; Giddam A. K.; Batzloff M. R.; Good M. F.; Skwarczynski M.; Toth I. Self-adjuvanting vaccine against Group A Streptococcus: Application of fibrillized peptide and immunostimulatory lipid as adjuvant. Bioorg. Med. Chem. 2014, 22, 6401–6408. 10.1016/j.bmc.2014.09.042. [DOI] [PubMed] [Google Scholar]
- Chan A.; Hussein W.; Ghaffar K.; Marasini N.; Mostafa A.; Eskandari S.; Batzloff M.; Good M.; Skwarczynski M.; Toth I. Structure-activity relationship of lipid core peptide-based Group A Streptococcus vaccine candidates. Bioorg. Med. Chem. 2016, 24, 3095–3101. 10.1016/j.bmc.2016.03.063. [DOI] [PubMed] [Google Scholar]
- Zaman M.; Abdel-Aal A.; Phillipps K.; Fujita Y.; Good M.; Toth I. Structure-activity relationship of lipopeptide Group A streptococcus (GAS) vaccine candidates on toll-like receptor 2. Vaccine 2010, 28, 2243–2248. 10.1016/j.vaccine.2009.12.046. [DOI] [PubMed] [Google Scholar]
- BenMohamed L.; Wechsler S. L.; Nesburn A. B. Lipopeptide vaccines-yesterday, today, and tomorrow. Lancet Infect. Dis. 2002, 2, 425–431. 10.1016/S1473-3099(02)00318-3. [DOI] [PubMed] [Google Scholar]
- Marasini N.; Ghaffar K. A.; Skwarczynski M.; Toth I.. Liposomes as a Vaccine Delivery System; William Andrew Inc: Norwich, UK, 2017. [Google Scholar]
- Cupri S.; Graziano A. C. E.; Cardile V.; Skwarczynski M.; Toth I.; Pignatello R. A study on the encapsulation of an occludin lipophilic derivative in liposomal carriers. J. Liposome Res. 2015, 25, 287–293. 10.3109/08982104.2014.992025. [DOI] [PubMed] [Google Scholar]
- Tinto H.; D’Alessandro U.; Sorgho H.; Valea I.; Tahita M.; Kaboré W.; Kiemde F.; Lompo P.; Ouédraogo S.; Derra K.; Ouédraogo F.; Ouédraogo J.-B.; Ballou W. R.; Cohen J.; Guerra Post Y.; Heerwegh D.; Jongert E.; Lapierre D.; Schellenberg D. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: Final results of a phase 3, individually randomised, controlled trial. Lancet 2015, 386, 31–45. 10.1016/S0140-6736(15)60721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didierlaurent A. M.; Laupeze B.; Di Pasquale A.; Hergli N.; Collignon C.; Garcon N. Adjuvant system AS01: Helping to overcome the challenges of modern vaccines. Expert Rev. Vaccines 2017, 16, 55–63. 10.1080/14760584.2016.1213632. [DOI] [PubMed] [Google Scholar]
- Ghaffar K. A.; Marasini N.; Giddam A. K.; Batzloff M. R.; Good M. F.; Skwarczynski M.; Toth I. Liposome-based intranasal delivery of lipopeptide vaccine candidates against Group A Streptococcus. Acta Biomater. 2016, 41, 161–168. 10.1016/j.actbio.2016.04.012. [DOI] [PubMed] [Google Scholar]
- Nurunnabi M.; Khatun Z.; Revuri V.; Nafiujjaman M.; Cha S.; Cho S.; Moo Huh K.; Lee Y.-k. Design and strategies for bile acid mediated therapy and imaging. RSC Adv. 2016, 6, 73986–74002. 10.1039/C6RA10978K. [DOI] [Google Scholar]
- Zhu C.; Fuchs C. D.; Halilbasic E.; Trauner M. Bile acids in regulation of inflammation and immunity: Friend or foe?. Clinical and Experimental Rheumatology 2016, 34, 25–31. [PubMed] [Google Scholar]
- Faustino C.; Serafim C.; Rijo P.; Reis C. P. Bile acids and bile acid derivatives: Use in drug delivery systems and as therapeutic agents. Expert Opin. Drug Delivery 2016, 13, 1133–1148. 10.1080/17425247.2016.1178233. [DOI] [PubMed] [Google Scholar]
- Lai X. Z.; Feng Y.; Pollard J.; Chin J. N.; Rybak M. J.; Bucki R.; Epand R. F.; Epand R. M.; Savage P. B. Ceragenins: Cholic acid-based mimics of antimicrobial peptides. Acc. Chem. Res. 2008, 41, 1233–1240. 10.1021/ar700270t. [DOI] [PubMed] [Google Scholar]
- Randazzo R. A.; Bucki R.; Janmey P. A.; Diamond S. L. A series of cationic sterol lipids with gene transfer and bactericidal activity. Bioorg. Med. Chem. 2009, 17, 3257–3265. 10.1016/j.bmc.2009.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J. N.; Barnett T. C.; Nizet V.; Walker M. J. Molecular insight into invasive Group A Streptococcal disease. Nat. Rev. Microbiol. 2011, 9, 724–736. 10.1038/nrmicro2648. [DOI] [PubMed] [Google Scholar]
- Ferretti J. J.; Stevens D. L.; Fischetti V. A.. Streptococcus pyogenes: Basic Biology to Clinical Manifestations; University of Oklahoma Health Sciences Center: Oklahoma City, OK, 2016; pp 1–756. [PubMed] [Google Scholar]
- WHO . The Current Evidence for the Burden of Group a Streptococcal Diseases, 2005; pp 1–52. [Google Scholar]
- Zaman M.; Chandrudu S.; Giddam A. K.; Reiman J.; Skwarczynski M.; McPhun V.; Moyle P. M.; Batzloff M. R.; Good M. F.; Toth I. Group A Streptococcal vaccine candidate: Contribution of epitope to size, antigen presenting cell interaction and immunogenicity. Nanomedicine (London, U. K.) 2014, 9, 2613–2624. 10.2217/nnm.14.190. [DOI] [PubMed] [Google Scholar]
- Park S.; Sinha N.; Hamad-Schifferli K. Effective size and zeta potential of nanorods by ferguson analysis. Langmuir 2010, 26, 13071–13075. 10.1021/la1024108. [DOI] [PubMed] [Google Scholar]
- Nevagi R. J.; Khalil Z. G.; Hussein W. M.; Powell J.; Batzloff M. R.; Capon R. J.; Good M. F.; Skwarczynski M.; Toth I. Polyglutamic acid-trimethyl chitosan-based intranasal peptide nano-vaccine induces potent immune responses against Group A Streptococcus. Acta Biomater. 2018, 80, 278–287. 10.1016/j.actbio.2018.09.037. [DOI] [PubMed] [Google Scholar]
- Christensen D.; Korsholm K. S.; Andersen P.; Agger E. M. Cationic liposomes as vaccine adjuvants. Expert Rev. Vaccines 2011, 10, 513–521. 10.1586/erv.11.17. [DOI] [PubMed] [Google Scholar]
- Nisini R.; Poerio N.; Mariotti S.; De Santis F.; Fraziano M. The multirole of liposomes in therapy and prevention of infectious diseases. Front. Immunol. 2018, 9, 00155. 10.3389/fimmu.2018.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steer A. C.; Batzloff M. R.; Mulholland K.; Carapetis J. R. Group A Streptococcal vaccines: Facts versus fantasy. Curr. Opin. Infect. Dis. 2009, 22, 544–552. 10.1097/QCO.0b013e328332bbfe. [DOI] [PubMed] [Google Scholar]
- Hayman W. A.; Brandt E. R.; Relf W. A.; Cooper J.; Saul A.; Good M. F. Mapping the minimal murine T cell and B cell epitopes within a peptide vaccine candidate from the conserved region of the M protein of Group A Streptococcus. Int. Immunol. 1997, 9, 1723–1733. 10.1093/intimm/9.11.1723. [DOI] [PubMed] [Google Scholar]
- Sekuloski S.; Batzloff M. R.; Griffin P.; Parsonage W.; Elliott S.; Hartas J.; O’Rourke P.; Marquart L.; Pandey M.; Rubin F. A.; Carapetis J.; McCarthy J.; Good M. F. Evaluation of safety and immunogenicity of a Group A Streptococcus vaccine candidate (MJ8VAX) in a randomized clinical trial. PLoS One 2018, 13, e0198658 10.1371/journal.pone.0198658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander J.; Del Guercio M.-F.; Maewal A.; Fikes J.; Chesnut R. W.; Sette A.; Qiao L.; Paulson J.; DeFrees S.; Bundle D. R. Linear PADRE T helper epitope and carbohydrate B cell epitope conjugates induce specific high titer IgG antibody responses. J. Immunol. 2000, 164, 1625–1633. 10.4049/jimmunol.164.3.1625. [DOI] [PubMed] [Google Scholar]
- Olive C.Development of subunit vaccines for Group A Streptococcus. In Molecular Vaccines: From Prophylaxis to Therapy; Giese M., Ed.; Springer Vienna: Vienna, 2013; Vol. 1, pp 207–216. [Google Scholar]
- Zaman M.; Abdel-Aal A. B. M.; Fujita Y.; Ziora Z. M.; Batzloff M. R.; Good M. F.; Toth I. Structure-activity relationship for the development of a self-adjuvanting mucosally active lipopeptide vaccine against Streptococcus pyogenes. J. Med. Chem. 2012, 55, 8515–8523. 10.1021/jm301074n. [DOI] [PubMed] [Google Scholar]
- Khongkow M.; Liu T. Y.; Bartlett S.; Hussein W. M.; Nevagi R.; Jia Z. F.; Monteiro M. J.; Wells J. W.; Ruktanonchai U. R.; Skwarczynski M.; Toth I. Liposomal formulation of polyacrylate-peptide conjugate as a new vaccine candidate against cervical cancer. Precision Nanomedicine 2018, 1, 183–193. 10.33218/prnano1(3).181003.1. [DOI] [Google Scholar]
- Marasini N.; Giddam A. K.; Ghaffar K. A.; Batzloff M. R.; Good M. F.; Skwarczynski M.; Toth I. Multilayer engineered nanoliposomes as a novel tool for oral delivery of lipopeptide-based vaccines against Group A Streptococcus. Nanomedicine (London, U. K.) 2016, 11, 1223–1236. 10.2217/nnm.16.36. [DOI] [PubMed] [Google Scholar]
- Ghaffar K. A.; Marasini N.; Giddam A. K.; Batzloff M. R.; Good M. F.; Skwarczynski M.; Toth I. The role of size in development of mucosal liposome-lipopeptide vaccine candidates against Group A Streptococcus. Med. Chem. 2016, 13, 22–27. 10.2174/1573406412666160720093138. [DOI] [PubMed] [Google Scholar]
- Ghaffar K. A.; Giddam A. K.; Zaman M.; Skwarczynski M.; Toth I. Liposomes as nanovaccine delivery systems. Curr. Top. Med. Chem. 2014, 14, 1194–1208. 10.2174/1568026614666140329232757. [DOI] [PubMed] [Google Scholar]
- Baca-Estrada M. E.; Foldvari M.; Snider M.; Harding K.; Kournikakis B.; Babiuk L. A.; Griebel P. Intranasal immunization with liposome-formulated Yersinia pestis vaccine enhances mucosal immune responses. Vaccine 2000, 18, 2203–2211. 10.1016/S0264-410X(00)00019-0. [DOI] [PubMed] [Google Scholar]
- Torchilin V. P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discovery 2005, 4, 145–160. 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- Wang S.; Liu H.; Zhang X.; Qian F. Intranasal and oral vaccination with protein-based antigens: Advantages, challenges and formulation strategies. Protein Cell 2015, 6, 480–503. 10.1007/s13238-015-0164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . Group A Streptococcal Vaccine Development: Current Status and Issues of Relevance to Less Developed Countries, 2005; pp 1–18. [Google Scholar]
- Champion J. A.; Mitragotri S. Role of target geometry in phagocytosis. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 4930–4934. 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tscheka C.; Hittinger M.; Lehr C.-M.; Schneider-Daum N.; Schneider M. Macrophage uptake of cylindrical microparticles investigated with correlative microscopy. Eur. J. Pharm. Biopharm. 2015, 95, 151–155. 10.1016/j.ejpb.2015.03.010. [DOI] [PubMed] [Google Scholar]
- Skwarczynski M.; Toth I. Recent advances in peptide-based subunit nanovaccines. Nanomedicine (London, U. K.) 2014, 9, 2657–2669. 10.2217/nnm.14.187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.