Abstract
Porous molecular solids are promising materials for gas storage and gas separation applications. However, given the relative dearth of structural information concerning these materials, additional studies are vital for further understanding their properties and developing design parameters for their optimization. Here, we examine a series of isostructural cuboctahedral, paddlewheel-based coordination cages, M24(tBu-bdc)24 (M = Cr, Mo, Ru; tBu-bdc2− = 5-tert-butylisophthalate), for high-pressure methane storage. As the decrease in crystallinity upon activation of these porous molecular materials precludes diffraction studies, we turn to a related class of pillared coordination cage-based metal-organic frameworks, M24(Me-bdc)24(dabco)6 (M = Fe, Co; Me-bdc2− = 5-methylisophthalate; dabco = 1,4-diazabicyclo[2.2.2]octane) for neutron diffraction studies. The five porous materials display BET surface areas from 1,057 – 1,937 m2/g and total methane uptake capacities of up to 143 cm3(STP)/cm3. Both the porous cages and cage-based frameworks display methane adsorption enthalpies of −15 to −22 kJ/mol. Also supported by molecular modeling, neutron diffraction studies indicate that the triangular windows of the cage are favorable methane adsorption sites with CD4-arene interactions between 3.7 and 4.1 Å. At both low and high loadings, two additional methane adsorption sites on the exterior surface of the cage are apparent for a total of 56 adsorption sites per cage. These results show that M24L24 cages are competent gas storage materials and further adsorption sites may be optimized by judicious ligand functionalization to control extra-cage pore space.
Graphical Abstract

INTRODUCTION
Three-dimensional porous materials have been widely investigated for gas storage applications. Although these have included carbon dioxide,1 oxygen,2 ammonia,3 acetylene,4 sulfur dioxide,5 ethylene oxide,6 ethylene,7 and nitrogen,8 they have most commonly been studied for the high-pressure storage of small molecules of consequence to energy.9 Indeed, the storage of hydrogen10–12 and methane13–15 in metal-organic frameworks has been the topic of recent review articles. In the case of the former, many MOFs have displayed high H2 storage capacities at cryogenic temperatures, although these are greatly diminished near 298 K. Methane storage capacities approaching DOE targets have been recently reported for a number of metal-organic frameworks.16 When considering the deliverable capacities of these materials, along with their actual densities (as opposed to crystallographic densities which are often utilized for total adsorption calculations), MOF capacities fall significantly short of targets.17 Although flexible MOFs have been touted as a means to elevate deliverable capacities of these materials,15 (i) flexible frameworks also suffer from a large discrepancy between bulk and crystallographic density and (ii) a complicated tank design is required to accommodate its dramatic expansion/collapse.18 Though, it is clear that significantly altered strategies must be employed to meet storage and delivery targets. Molecular adsorbents may show utility in this regard, as they offer novel strategies for precise pore design and enhanced control over crystallite size and morphology.19
Porous molecular solids have shown considerable promise for gas separation,20 catalysis,21 chiral separations,22 sensing,23 and as soluble designer pores for porous liquids.24 In addition to the benefits of three-dimensionally connected porous materials, molecular solids have the additional advantage of solubility and significantly increased molecular-level control. These can aid in the design, synthesis, characterization, and utilization of these adsorbents. However, understanding and optimizing porosity in these solids can be challenging as it can exist as a result of surface area intrinsic to the individual molecule or can arise from void space present from inefficient packing of molecules that need not display porosity.25 Regardless, molecular materials have displayed BET surface areas in excess of 3,500 m2/g.26 All-organic porous molecules encompass various molecular species; the organic molecule to first display porosity was Dianin’s compound (4-p-hydroxyphenyl-2,2,4-trimethylchroman).27 Similarly, calixarenes,28 cucurbiturils,29 Noria,30 phthalocyanines,31 boronic ester cages,32 alkyne-based cages,33 and imine-based cages have all shown considerable porosity.34 Hybrid metal-organic cages—also known as metal organic polyhedra35 have been shown to adopt analogous structures, such as octahedral and cuboctahedral cages (Figure 1), but have displayed relatively limited porosity.36,37,38 We aim to both expand the scope of porous metal-organic molecular materials and utilize spectroscopic, computational, and diffraction methods to better understand adsorption phenomena in them. In addition to the benefits of porous molecules mentioned above, the cation sites in hybrid metal-organic porous molecules could serve as sites for selective gas adsorption, gas storage, small molecule activation, or redox chemistry.
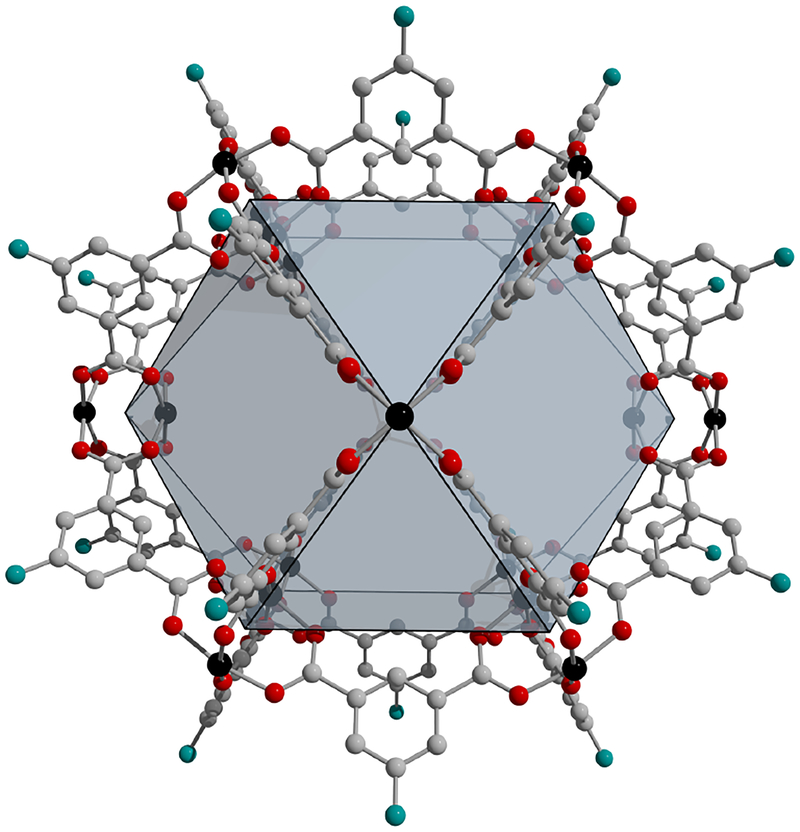
Figure 1.
M24(R-bdc)24 cuboctahedral cage featuring bimetallic paddlewheel units. This pore structure, which is isolable as molecular species, is also pervasive in isophthalic acid-based MOFs. Black, gray, and red spheres represent metal (M), carbon, and oxygen atoms, respectively. The blue spheres represent functional groups (R) that can include hydroxy, alkyl, amino, and others.
Although some coordination cages have shown moderate levels of porosity, the most widely studied porous cages have been based on bimetallic paddlewheel building blocks. This is likely a result of the relative ease with which paddlewheel-based materials assemble and their compatibility with a wide variety of ligand functional groups. Indeed, in the vast area of metal-organic frameworks, a very large number of paddlewheel-based MOFs have been reported, from one- to two- to three-dimensional frameworks.39 For porous coordination cages, paddlewheel building units often assemble into cuboctahedral and octahedral structures. These have been reported for a variety of transition metal cations and ligand functional groups. Cuboctahedral cages are an intriguing set of compounds as they have been isolated for Cr, Ni, Cu, Mo, Ru, and Rh.40–45 Although some of these materials have been reported to possess high surface areas, specifically Cr24(tBu-bdc)24 (tBu-bdc2− = 5-tert-butylisophthalic acid) with a BET surface area of 1135 m2/g,46 porosity is often overlooked in coordination cages. Relatedly, there is a lack of structural studies on porous cages upon activation or gas adsorption.
Adsorption properties of porous cages can be partially inferred from analogous metal-organic frameworks, as the cuboctahedral cage serves as the building block for many well-known MOFs. For example, one of three pore types in the canonical MOF, HKUST-1, is a cuboctahedral cage.47 Showing the versatility of paddlewheel building units, HKUST-1 analogues have been prepared using a wide variety of transition metal cations including Cr, Fe, Co, Ni, Cu, Zn, Mo, and Ru.48 Many of the members of the PCN and NOTT/MFM families of frameworks similarly contain copper paddlewheel-based cuboctahedral pores, including PCN-11,49 −12,50 and −6151 as well as NOTT/MFM-112,52 −115,53 −12254 (also known as NU-125),55 and −132.56 The high surface areas and crystallinities displayed by these materials have spurred numerous adsorption and diffraction studies.39,57 Although some of these have shown the importance of the triangular windows of the cuboctahedral pores in the MOFs as gas storage sites, the presence of additional smaller pores and ligand-based binding sites complicates structural studies. We have recently shown that a family of MOFs based on coordination cages and dabco pillars can be used to glean insight into gas adsorption sites in porous cages.58 These frameworks feature cuboctahedral cages connected in three dimensions via dabco molecules. Frameworks of this type, which have been reported for Fe, Co, Cu, and Zn, display BET surface areas in excess of 2,000 m2/g. Further, the arrangement of cuboctahedral cages in the MOFs is nearly identical to those found in M24(tBu-bdc)24 cages.
Herein, we report the synthesis and characterization of two novel dabco-pillared metal-organic frameworks based on cobalt and iron cuboctahedral cages. Detailed structural analysis of these solids suggest 5-functionalization of isophthalic acid is sterically limited to methyl substituents. The structures of these MOFs are analogous to a family of tert-butyl functionalized cuboctahedral molecules. We assess the Cr, Mo, and Ru analogs of these cages, in addition to the dabco-pillared MOFs, for high-pressure methane storage. Given the high surface areas and crystallinities of the frameworks, we investigated neutron diffraction as a means to infer the adsorption sites in the molecular solids, which display high volumetric methane uptakes and deliverable capacities. Additionally, we conducted molecular simulations of methane adsorption to confirm the analogous nature of methane binding sites in the materials.
EXPERIMENTAL SECTION
Materials and Methods.
With the exception of Ru2(OAc)4Cl,59 metal salts and organic ligands were obtained from commercial sources and used without further purification. For the syntheses of Co24(Me-bdc)24(dabco)6 and Ru24 (tBu-bdc)24Cl12 N,N-dimethylformamide (DMF), methanol (MeOH), and chloroform were obtained from commercial sources and used without further purification. Where syntheses and/or solvent exchanges are performed in a glovebox, solvents were obtained from a solvent drying system and stored in a glovebox on the appropriately sized molecular sieves.
Synthesis of Co24(Me-bdc)24(dabco)6.
In a 100 mL media bottle, CoCl2·6H2O (2.365 g, 9.941 mmol), and H2Me-bdc (1.801 g, 9.999 mmol) were dissolved in DMF (100 mL) and left to sit at room temperature for five days. In a separate 100 mL media bottle, dabco (1.116 g, 9.949 mmol), was dissolved in DMF (100 mL). The resulting cobalt/ligand stock solution (10 mL) was pipetted into five 20 mL glass scintillation vials. The dabco solution (10 mL) was layered onto the cobalt/ligand solution. The resulting solution was capped and heated at 365 K for 12 hours. The sample was allowed to cool to room temperature before removing excess solvent. Resulting dark purple cubic crystals were removed from the bottle with fresh DMF and a Pasteur pipette. Solvent exchange was completed by soaking the product in fresh DMF at 383 K for 3 days with the solvent replaced every 12 hours. The DMF washed solid was isolated by removing excess solvent with a Pasteur pipette and then soaked in chloroform at 333 K for three days with the solvent replaced every 12 hours. Activated material was obtained by evacuation at 348 K.
Synthesis of Fe24(Me-bdc)24(dabco)6.
In an N2 glovebox, FeCl2 (0.100 g, 0.789 mmol) and H2Me-bdc (0.142 g, 0.788 mmol) were dissolved in DMF (12 mL) in a capped 20 mL scintillation vial and heated at 393 K for two hours. Dabco (0.177 g, 1.578 mmol) was then dissolved in acetonitrile (4 mL) and layered on top the DMF solution. The reaction was allowed to stand for one week, after which the reaction mixture was gently swirled and decanted to remove suspended product from larger, higher-density impurities. The light-yellow solid was then isolated by centrifugation and soaked in DMF at room temperature for three days replacing the solvent with fresh DMF every 8 hours. After DMF washing the solid was washed with benzene by a similar procedure. Over the course of benzene washes, the solid turns a lime-green color. Activated material was obtained by evacuation of frozen benzene at 273 K for two days followed by heating under vacuum at 323 K for two days. The resulting light green solid is incredibly air sensitive, turning dark brown upon exposure to O2.
Synthesis of M24(tBu-bdc)24 compounds.
These compounds were prepared as previously reported.41,42,46 However, as surface areas of the Ru2+ and Mo2+ analogs were not previously reported or were low, respectively, modified solvent exchange and activation procedures were followed. For these, the solids (~200 mg) were soaked in 20 mL of anhydrous, air-free methanol for 48 hours, with the solvent replaced by fresh methanol every 8 hours. The solids were isolated by vacuum filtration and placed in gas adsorption tubes. The materials were activated under dynamic vacuum with a turbomolecular pump and a heating ramp rate of 0.1 K/min up to their final activation temperatures (Mo = 298 K, Ru = 398 K).
Optimized Synthesis of Mo24(tBu-bdc)24.
In an N2 glovebox, Mo2(OAc)4 (0.10 g, 0.234 mmol) and tert-butylisophthalic acid (0.052 g, 0.234 mmol) were dissolved in 10 mL of DMPU with 5 drops of pyridine and heated at 383 K for 1 day to yield large red crystals. The material was solvent exchanged and activated as reported above.
Gas Adsorption Measurements.
Gas adsorption measurements up to 1.1 bar were recorded on a Micromeritics 3Flex gas adsorption analyzer. Surface area measurements were recorded in a liquid nitrogen bath. 298, 308, and 318 K isotherms were recorded in an isothermal bath. Ultrahigh purity gas was used for both the free-space measurements (He) and analyses (N2 or CH4). Prior to measurements, samples were considered activated when their outgas rate under static vacuum was ≤ 2 μbar/min. High-pressure isotherms were collected on a Sieverts’ apparatus (PCT-Pro-2000 from Hy-Energy Scientific Instruments) using ultrahigh purity gases. Total adsorption was calculated using NIST Thermochemical Properties of CH4 and the pore volume of each material, as determined via 77 K N2 adsorption experiments.
Single-Crystal X-Ray Diffraction.
Crystals were mounted using viscous oil onto a plastic mesh and cooled to the data collection temperature. Data were collected on a Bruker-AXS APEX II DUO CCD diffractometer with graphite-monochromated Mo-Kα radiation (λ=0.71073 Å) for Co24(Me-bdc)24(dabco)6 and with Cu-Kα radiation (λ = 1.54178 Å) focused with Goebel mirrors for Mo24(tBu-bdc)24.60 Unit cell parameters were obtained from 36 data frames, 0.5 ° ω, from three different sections of the Ewald sphere. The polarized optical properties of the crystal, unit cell parameters, systematic absences and equivalent reflections in the diffraction data are consistent with F432 (209), F-43m (216) and Fm-3m (225) for Co24(Me-bdc)24(dabco)6; and, uniquely, with P21/n (14) for Mo24(tBu-bdc)24. The centrosymmetric space group option for Co24(Me-bdc)24(dabco)6 yielded chemically reasonable and computationally stable results of refinement. The data were treated with multi-scan absorption corrections. The structures were solved using intrinsic phasing methods61 and refined with full-matrix, least-squares procedures on F2.62
The cage in Co24(Me-bdc)24(dabco)6 is located at the intersection of two orthogonal mirror planes and a three-fold rotoinversion axis. The dabco ligand in Co24(Me-bdc)24(dabco)6 was treated as an idealized rigid group with rigid bond anisotropic restraints and equal atomic displacement parameters for chemically equivalent atoms. The cage in Mo24(tBu-bdc)24 is located at an inversion center. Although initial solutions for Mo24(tBu-bdc)24 suggested disordered DMPU molecules at the central void as well as between polyhedra, only some of the DMPU molecules coordinated on the outer surface of the cage could be modeled satisfactorily. The t-butyl groups in Mo24(tBu-bdc)24 were restrained such that the quaternary carbon to methyl carbon distance is 1.534(5) Å and the methyl carbon to methyl carbon distance is not allowed to be shorter that 2.451(5) Å. Three t-butyl groups in Mo24(tBu-bdc)24 were given additional idealized geometry with the aromatic carbon to t-butyl carbon distance restrained to 1.527(16) Å. The atoms in Mo24(tBu-bdc)24 were treated with global three-dimensional anisotropic displacement rigid bond constraints.
The protons on the water molecule at the center of the cage in Co24(Me-bdc)24(dabco)6 could neither be geometrically calculated nor located and were ignored. All other hydrogen atoms were treated as idealized contributions with geometrically calculated positions and with Uiso equal to 1.2 (or 1.5 for methyl) Ueq of the attached atom. Remaining electron density that cannot be localized as atoms was treated as a diffused contribution.63 The structures have been deposited at the Cambridge Structural Database as CCDC 1881620 and 1891146.
Neutron Diffraction.
Experiments were performed on 0.4495 g Co24(Me-bdc)24(dabco)6 and 0.7061 g Fe24(Me-bdc)24(dabco)6 at the National Institute of Standards and Technology Center for Neutron Research (NCNR). Data was collected at the high-resolution neutron powder diffractometer, BT1, utilizing a Ge(311) monochromator with an in-pile 60’ collimator, corresponding to a neutron wavelength of 2.0775 Å. Activated samples were loaded into vanadium sample cans in an He environment glovebox, and sealed with an indium o-ring onto a copper heating block containing a valved outlet for gas loading. After mounting samples onto a bottom-loaded closed cycle (CCR) refrigerator, samples were cooled to base temperature for measurement. For CD4 gas doses, the sample was connected to a fixed-volume gas manifold, heated to T = 120 K, and cooled back to base temperature for measurement. Each of the two samples were dosed with ~ 0.5 and ~1.5 CD4 per M, per formula unit. Adsorption was verified barometrically prior to cooling to avoid condensation of the vapor phase for each gas dosage, with each sample dosed twice with CD4.
Neutron powder diffraction data was analyzed using Topas Academic.64 The space group was chosen based on analogous previously reported structures.58,65 Initial Pawley refinements were first conducted to determine a background function, lattice parameters, and peak shapes.66 The position and orientation of molecules was then solved using rigid bodies with the global optimization method of simulated annealing (SA) in real space.67
Rigid bodies were refined similarly to our previous work, using a full methyl-isophthalic acid (bdc) centered and refined on a 96k (x, x, z) position (with two orientations of methyl group), and a full dabco molecule allowed to rotate along a 48i (x, 0.5, −x) position. Using trigonometric constraints, both only required one variable for rotational movement. As before, activated materials required an additional Me-bdc molecule, as well as DMF molecule, refined on a 96k (x, x, z) and a 32f (x, x, x) sites respectively, with the additional Me-bdc allowed to freely move and the DMF molecule to rotate around a fixed axis using one degree of freedom. The refined positions of these two molecules represents their disordered, occupied space, rather than absolute positions. Once a reasonable solution was found, the number of atoms and their Wyckoff positions were corrected, followed by refining occupancies and thermal parameters separately. Additionally, occupancies which refined within error of unity were fixed and thermal parameters were fixed across each rigid body.
Once activated structures were determined, SA refinements were conducted on CD4 gas loaded data. Initial positions were determined using Fourier difference maps in both Topas Academic and GSAS.64,68 This involved the addition of four CD4 molecules; one at the open metal site on a 48i (x, x, 0.5) position, one in the corners of triangular windows on a 96k (x, x, z) site, one in the corners of square windows on a 96j (0, y, z) site, and one between four triangular windows on a 32f (x, x, x) site. Initially the orientation of each molecule was fixed according to known methane interactions,69,70 which additionally mapped each CD4 molecule onto itself. Each molecule was then allowed to freely rotate, which keeping as many variables fixed as possible, then additionally allowed to refine off each Wykoff position, all while adjusting occupancies as needed. Final CD4 positions/orientations were chosen based on how each additional freedom of movement improved the refinement. Where possible the activated structure was fixed throughout this process. Once final CD4 positions were obtained occupancies, thermal parameters, and lattice parameters were refined.
The resulting refinements from this process are shown in Figures S1–6. Despite numerous attempts at deuteration on these compounds, dabco molecules contained 10–25 % hydrogen, and Me-bdc molecules contained 65–85 % hydrogen. Given that complete removal of excess DMF and Me-bdc was not possible without structural degradation, there is considerable incoherent scattering due to hydrogen. However, Weller et al.,71,72 as well as our previous MOF work,73 has shown that neutron powder diffraction is quite possible with various amounts of hydrogen in crystalline materials, with hydrogen positions readily accessible due to their negative contrast. It is understandable that due to the amount of hydrogen, as well as the large unit cell, that the patterns do not contain much information beyond Q = 2 Å−1, despite containing sharp crystalline peaks. The Rietveld analysis indicates that there is a good agreement between the data and the models (Figures S1 and 2), with the resulting activated materials being commensurate with previously reported analogous materials. The model CD4 dosed structures are additionally in exceptional agreement with the neutron data (Figures S3–S6).
Computational Methodology.
We used our open-source software for classical molecular modeling in nanoporous crystals, PorousMaterials.jl. The Julia code and input files to reproduce our calculations are available on Github.74
Molecular Model.
We approximate the material as crystalline and rigid, using the experimentally determined crystal structures (with disorder repaired to arrive at chemically valid structures) as input to our molecular simulations. We model the potential energy of a microstate of gas molecules in the material as pairwise additive interatomic interactions described by 12–6 Lennard Jones potentials. We model a methane molecule as a single, “united-atom” interaction site with parameters taken from the Transferable Potentials for Phase Equilibria (TraPPE) force field;75 molecular simulations using these parameters accurately reproduce the normal boiling point and critical properties of methane. For the atoms composing the materials, we take Lennard Jones parameters for C, H, N, and O from the Dreiding Force Field76 and for Co and Mo from the Universal Force Field77 since they are missing from Dreiding. The non-bonded Dreiding force field parameters were tuned to reproduce crystal structures and sublimation energies of a large set of compounds. Parameters for cross-atomic-species interactions follow from the Lorentz-Berthelot mixing rules. We truncate the Lennard-Jones potentials at a cutoff distance of 14 Å and apply periodic boundary conditions to the simulation box consisting of a supercell of the crystal (box in Figure 6).
Figure 6.
Binding site elucidation via molecular modeling and simulation. Visualization of the spatial probability density of methane in Mo24(tBu-bdc)24 during grand-canonical Monte Carlo simulations at 298 K and 1 bar (a), 5 bar (b), 35 bar (c), and 65 bar (d). Methane primarily adsorbs in the center of the triangular window at low pressure. At higher pressures, the corners of the triangular and square windows become populated. (e) Level surfaces of the potential energy of a methane molecule in the cage at −16 kJ/mol (orange) and −14 kJ/mol (white) highlight the triangular window and four corners of the square window as the most favorable binding sites.
Potential Energy Contours.
To generate the visualizations in Fig. 6 of the level surfaces of the potential energy V = V(x) of an isolated methane molecule at position x in the crystal, of we first superimpose on the supercell a regular grid points with less than 0.1 Å grid spacing. Then, we compute the potential energy of a methane molecule at each grid point and store it in a 3D array. We visualize the level surfaces of V(x), represented as a .cube file, using the VisIt visualization tool.78
Molecular Simulations of Methane Adsorption.
We obtained the simulated methane adsorption isotherms at 298 K in Fig. S88 by conducting a set of Markov chain Monte Carlo simulations of the grand-canonical (μVT) statistical mechanical ensemble79 at different chemical potentials. The simulation volume V is a supercell of the crystal. We relate the bulk gas pressure to a corresponding chemical potential using the Peng-Robinson equation of state for methane. As Markov chain transitions, we propose particle insertions, deletions, translations, and re-insertions with probability 0.35, 0.35, 0.25, and 0.tr05, respectively. A proposal to transition to a new microstate is accepted with probabilities governed by the Metropolis-Hastings rules so as to sample the grand-canonical statistical ensemble. Initiating the system with zero adsorbed molecules, we conducted 100,000 Monte Carlo burn-in cycles, then 100,000 Monte Carlo production cycles during which we collected statistics to compute e.g. the average number of adsorbed gas molecules in the system. A Monte Carlo cycle is defined as max(20,n) Markov chain transition proposals, where n is the number of molecules in the system immediately before a new cycle begins. Figs. S88 and S89 compare the simulated and experimental methane adsorption isotherms and heats of adsorption; the simulations over-estimate adsorption but agreement with the heats of adsorption is good.
Methane Spatial Probability Density.
We characterized the spatial probability density of methane molecules in the material during our grand-canonical Monte Carlo simulations as follows. First, we partition the simulation box (the supercell of the crystal) into a regular grid of voxels whose centers are less than 0.5 Å apart. Then, during the simulation, at the end of each Monte Carlo production cycle, we effectively count the number of methane molecules that belong to each voxel (“take a snapshot”). At the end of the simulation, we have a 3D histogram (normalized by the number of snapshots) of the positions of the methane molecules observed during the simulation. Because the voxels are too small to host two methane molecules, this gives an approximation to the integral of the spatial probability density of methane over the voxel. We visualize the spatial probability density, represented as a .cube file, using the VisIt visualization tool. We use the same scale to determine color for each pressure so the visualized densities can be readily compared across pressures. We used 100,000 burn-in cycles and 200,000 production cycles-- thus, taking 200,000 snapshots of methane molecules-- to generate the probability density plots for methane adsorption simulations at 1 bar, 5 bar, 35 bar, and 65 bar of pressure.
RESULTS AND DISCUSSION
Although metal-organic framework surface areas can often be optimized regardless of the exact nature of the synthesis, solvent exchange, and activation procedures, we have recently shown that these factors are vitally important for realizing maximal surface area in coordination cages.70 The lack of three-dimensional connectivity in molecular systems often results in significant structural rearrangement upon solvent exchange and evacuation. Cages can also display differing porosity depending on the space group into which they crystallize. For one of the coordination cages displaying the highest surface area, Cr24(tBu-bdc)24, the solid undergoes two phase changes upon activation, from P42/mnm to C2 to C2/c. In spite of this, the solid displays both high surface area and thermal stability. Encouraged by this, we sought to reinvestigate the reported M24(tBu-bdc)24 (M = Cu2+, Mo2+, Ru2+/3+) family of cages. For these adsorbents, surface areas are either entirely lacking (Ru) or reported to be significantly lower than the chromium(II)-based material (Cu = ~225 m2/g, Cr = 1135 m2/g, Mo = 437 m2/g).80
Regardless of synthesis, solvent exchange, or activation conditions, we were unable to improve upon the surface area of Cu24(tBu-bdc)24. This was similarly the case for Cu12(cdc)12 (cdc2− = carbazoledicarboxylic acid), an octahedral coordination cage that displayed significantly diminished surface areas as compared to its chromium(II) and molybdenum(II) counterparts.70 Ru24(tBu-bdc)24Cl12 displayed a comparable surface area to Cr24(tBu-bdc)24 by utilizing specific solvent exchange and activation methods. To achieve this, as-synthesized solid was thoroughly exchanged with anhydrous, air-free methanol. Upon activation at 348 K, the solid displays a BET (Langmuir) surface area of 1057 (1540) m2/g (Figure 2). This value is in relatively good agreement with the value expected based on change in formula unit mass as compared to the Cr2+ cage (962 m2/g BET, 1522 m2/g Langmuir). The infrared spectrum of the activated material indicates the absence of metal- or pore-bound DMF or methanol, confirming complete desolvation of the cage (Figure S19). Despite demonstrating good porosity, powder X-ray diffraction reveals major structural rearrangement to the point of amorphization upon solvent evacuation (Figure S20), suggesting ordered cuboctahedra cages which are rotationally disordered relative to one another.
Figure 2.
N2 adsorption isotherms collected at 77 K for the three M24L24 cages and two analogous pillared cage-based metal-organic frameworks.
In order to optimize the surface area of Mo24(tBu-bdc)24, a significantly altered strategy was employed. Solvent exchange and activation of a sample that was prepared via the previously reported route (1,2-dichlorobenzne/pyridine mixture) afforded surface areas consistently below 800 m2/g.80 For Mo12(cdc)12 we previously showed that surface area is significantly affected by synthetic conditions with higher symmetry space groups affording higher surface area materials.70 Although varying synthetic conditions, including solvent, co-solvent, acid addition, and reaction temperature typically afforded cage that crystallized in the same space group as the previously reported solid, I4/m, utilization of a pyridine/N,N’-dimethylpropyleneurea (DMPU) mixture for the synthesis afforded an optimally porous material that crystallized in P21/n. Interestingly, the cage synthesized via this route adopts the same cuboctahedral structure arranged in the same orientation as the reported structure but displays a significantly increased surface area. Here, after room temperature methanol exchanges and activation, Mo24(tBu-bdc)24 is shown to have a BET (Langmuir) surface area of 1321 (2105) m2/g. This value is significantly higher than the value predicted based on the increased formula mass of the cage as compared to Cr24(tBu-bdc)24 (BET = 977 m2/g) and, to the best of our knowledge, represents a new record value for porous coordination cages.
Close investigation of the crystal structures of all three porous M24(tBu-bdc)24 materials reveals multiple possible structural factors contributing to their high porosity (Figures S21–S40). The structures contain solvent-solvent interactions between metal-bound solvent molecules on adjacent cages. Given the ease at which solvent is removed from Cr2+, Mo2+, and Ru2+/3+ paddlewheels, and as confirmed via infrared spectroscopy, these interactions are likely no longer present in the activated phases of these solids. It has been previously posited that paddlewheel-paddlewheel interactions account for their high surface areas and stabilities.44 These types of interactions are relatively common with acetic acid-based paddlewheels. Here, the axial site of the paddlewheel cation coordinates to a carboxylate oxygen on an adjacent paddlewheel. This interaction is propagated throughout the structure to essentially form one-dimensional chains. However, examples of this are relatively rare for Cr2+ and Mo2+ paddlewheels.81 Further, the necessity of a charge balancing anion in Ru2+/3+ structures prevents this interaction. The cage-cage interactions that are most likely predominantly involved are the van der Waals contacts between tert-butyl groups on adjacent molecules. Here, each tert-butyl group interacts with two groups from neighboring cages. The body centered cubic packing arrangement of cuboctahedra facilitates this interaction. As a result, all 24 tert-butyl groups on each cage feature these interactions. In all three structures, the average distance between adjacent methyl groups on tert-butyl functionalities is ~4.0 Å. This value is consistent with the van der Waals radius of a methyl group of 2.0 Å. Ultimately, rather than solvent-solvent interactions or paddlewheel-paddlewheel interactions, it is the large number of ligand-ligand interactions that endow the solids with sufficient thermal stabilities to survive solvent removal/activation.
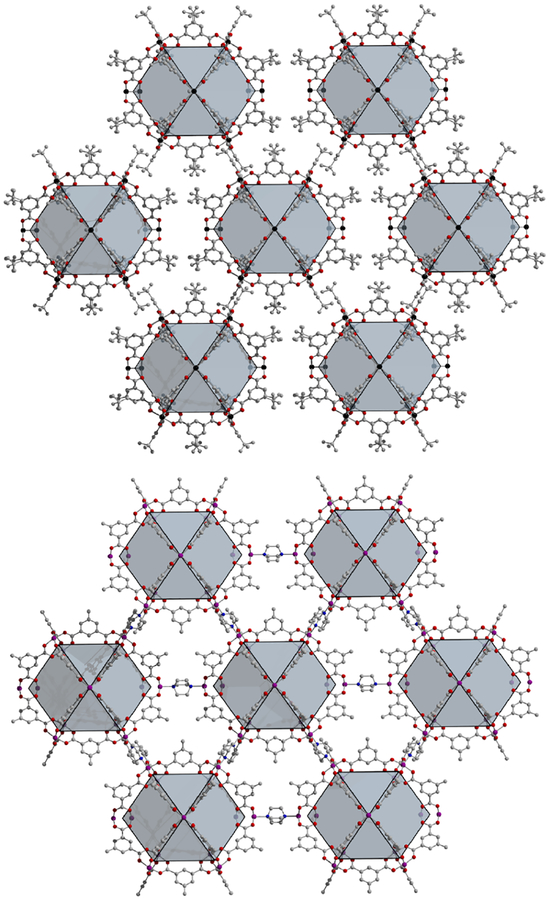
In order to better understand surface areas of tert-butyl-functionalized cuboctahedral cages, it is useful to compare them to the previously reported dabco-pillared MOFs, the structures of which are highly similar. For these, cuboctahedral cages are similarly arranged in a body centered cubic arrangement (Figure 3). Dabco molecules coordinated to the exterior sites of the cages connect them in three dimensions. Analogous to the structures of the molecular materials, the 5-position of the ligands of three cages are pointed to the same position. However, in the MOF the ligands are in much closer proximity. This is a result of the dabco pillar bringing the cages in closer contact as compared to the molecular case. The paddlewheel–paddlewheel distance in the pillared MOF is approximately 6.75 Å whereas this is expanded to 7.42 Å for the cages. This again points to the importance of ligand functionalization in designing and synthesizing these metal-organic cages. Close inspection of M24(bdc)24(dabco)6 (M = Fe, Co, Cu, Zn) suggests the structure will be incompatible with large functional groups. In order to interrogate this, we turned to the synthesis of new members of the M24(R-bdc)24(dabco)6 family with various metal cations and ligand functional groups.
Figure 3.
Structures of Mo24(tBu-bdc)24 (top) and Co24(Me-bdc)24(dabco)6 (bottom) illustrating the similarities between the cage and MOF structures, respectively. In the former, van der Waals contacts between tert-butyl groups give rise to three-dimensional pseudo-connectivity, whereas in the latter, metal-coordinated dabco ligands pillar the cages into three dimensions. Black, purple, red, gray, and blue atoms represent Mo2+, Co2+, O, C, and N, respectively. Solvent molecules and hydrogen atoms have been omitted.
Although Cu24(OH-bdc)24(dabco)6 can be isolated via the addition of dabco to a solution of pre-synthesized cage, these materials are typically prepared via one-pot solvothermal reaction as the cages that comprise them are neither soluble nor synthetically accessible. The typically soluble Cr2+- and Mo2+-based cages lack the metal acidity to strongly coordinate dabco, a result of the presence of M–M quadruple bonds for these cations. To prepare new pillared cages, we screened the reaction of various Mn, Fe, Co, Ni, Cu, Zn, and Mo salts with R-bdc (R = methyl, alkoxides, tert-butyl, hydroxy, amino, cyano, nitro, and bromo) in the presence of dabco in various amide-based solvent systems. Consistent with the lack of pore space for bulky functional groups, we were only able to isolate crystalline product for M24(Me-bdc)24(dabco)6 (M = Fe, Co, Cu) and Fe24(OH-bdc)24(dabco)6, as confirmed by powder X-ray and neutron diffraction. Of these, only M24(Me-bdc)24(dabco)6 (M = Fe, Co) displayed high levels of porosity, even after precise activation conditions, with BET (Langmuir) surface areas of 1937 (2264) and 1727 (2252) m2/g for the iron and cobalt analogs, respectively. These numbers essentially represent the upper limits of surface areas for analogous cage materials, as in the pillared MOFs both the interior and exterior surfaces of the cages are potentially accessible to probe molecules. Porous or potentially porous cages that lack directional ligand functional groups that can interact tend to close pack, exemplified by the low surface area of Cu24(bdc)24.40 When all of the pore windows are blocked off, the solids are inaccessible to nitrogen or carbon dioxide. Often these materials display low levels of porosity as only the interior surface of the cages are nitrogen assessible. However, this should be highly tunable based on judicious ligand functionalization.
Given the nearly isostructural nature of the tert-butyl-based porous cages and the dabco-pillared MOFs (Figure 3), the latter offer a unique and powerful opportunity to further understand the former in terms of the storage of small molecules. At 298 K, all five materials display similar methane uptake up to one bar, ranging from 0.4 to 0.7 mmol/g (Figures S67–S72). At low pressures and correspondingly low uptake values, the one bar gravimetric capacities do not trend with BET or Langmuir surface areas. Temperature dependence of adsorption indicates adsorption enthalpies ranging from −15 to −21 kJ/mol and following the trend Ru < Mo < Cr < Fe < Co. This range of adsorption enthalpies is well within the range of values typically seen for porous solids.82 Methane adsorption enthalpies in the two MOFs are lower as their metal cations, which have been shown to be highly polarizing adsorption sites, are blocked on the exterior surface with pillaring dabco and on the interior surface with DMF. This effectively lowers the average adsorption enthalpy of the materials.
To further evaluate the methane storage properties of all five materials, high pressure adsorption isotherms were collected at 298 K (Figures S74–S85). At both 35 and 65 bar, the total gravimetric capacities generally correlate with BET surface areas with the up-take values following the trend Ru ~ Cr < Mo < Fe < Co. The gravimetric capacities, which range from 138 to 216 cm3/g, are generally comparable to many MOFs with similar surface areas.82 Given the greatly varying crystallographic densities of the solids, the volumetric uptakes of the solids are significantly altered with Cr (88 cm3(STP)/cm3) < Ru ~ Mo < Fe < Co (144 cm3(STP)/cm3) (Figure 4). In terms of the porous cages, the gravimetric uptakes of these three materials at 35 and 65 bar are actually lower than expected based on their surface areas and as compared to our previously reported M12(cdc)12 cages (M = Cr2+, Cu2+, Mo2+; cdc2- = carbazoledicarboxylate). This is accentuated when comparing their volumetric capacities. For example, Mo12(cdc)12 displays 298 K volumetric capacities of 111.5 and 150.0 cm3(STP)/cm3 at 35 and 65 bar, respectively. Under these same conditions, Mo24(tBu-bdc)24 shows uptakes of 97 and 120 cm3(STP)/cm3, despite having a BET surface area that is 20 % higher than the carbazole-based cage. This discrepancy is likely a result of the absence of highly favorable CH4 binding sites in Mo24(tBu-bdc)24.
Figure 4.
(Top) Isosteric heats of CH4 adsorption calculated from fits to variable temperature low-pressure uptake experiments. (Bottom) High-pressure total methane adsorption in M24(tBu-bdc)24 (black = Mo, blue = Ru, green = Cr) and M24(Me-bdc)24(dabco)6 (orange = iron, purple = cobalt) at 298 K.
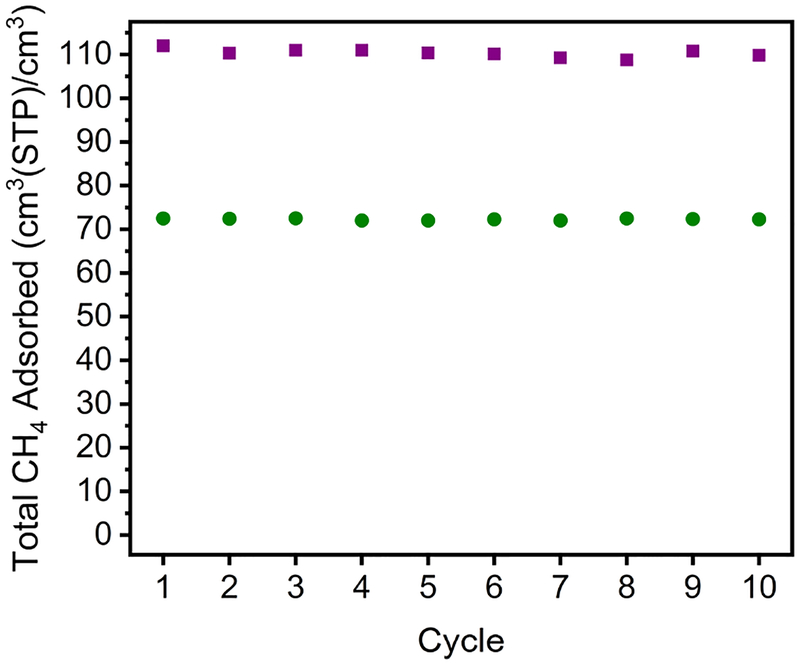
As methane storage materials, deliverable capacity, cyclability and adsorption/desorption kinetics are important considerations. As shown in Figure 5, two representative adsorbents-Cr24(tBu-bdc)24 and Co24(Me-bdc)24(dabco)6 display no loss in capacity at 298 K over 10 adsorption/desorption cycles. Under these conditions, the kinetics of both ad/desorption are very rapid, with 35 bar adsorption equilibria achieved in under two minutes. Desorption was carried out by placing the materials under dynamic vacuum at 298 K for five minutes. For a pressure swing from 35 to 5.8 bar, deliverable capacities of all five solids range from 45 (Cr) to 80 (Co) cm3(STP)/cm3. Increasing the adsorption uptake pressure to 65 bar increases the deliverable capacities to up to 102 cm3(STP)/cm3 (Full table of deliverable capacities for these materials and comparable MOFs are listed in Tables S8 and S9). Although these fall short of the record values of the best performing metal-organic frameworks (up to 200 cm3(STP)/cm3),83 they are nearly on par when taking actual bulk density (rather than crystallographic density) into consideration. With this in mind, both Mo24(tBu-bdc)24 and Co24(Me-bdc)24(dabco)6 have 65–5.8 bar working capacities of 72 cm3(STP)/cm3 as compared to 83 cm3(STP)/cm3 for HKUST-1.82 The molecular nature of the cages reported here may offer an advantage in this regard, as their solubility may afford a means to tune phase and thus bulk density.
Figure 5.
Methane cycling in Cr24(tBu-bdc)24 (green circles) and Co24(Me-bdc)24(dabco)6 (purple squares) at 298 K and a final adsorption pressure of 35 bar. Between cycles, the samples were reactivated under dynamic vacuum for 5 minutes.
We next conducted molecular simulations of methane adsorption in both the MOF Co24(Me-bdc)24(dabco)6 and the cage Mo24(tBu-bdc)24. Our aims were to (i) elucidate the methane adsorption sites in the structures and the sequence in which they are occupied as pressure increases and (ii) juxtapose the hierarchy of adsorption sites in these two structures that harbor iso-structural cuboctahedral cages. To characterize the spatial probability density of methane, we first partitioned the simulation box into a regular grid of voxels. Then, we took snapshots during grand-canonical Monte Carlo simulations of methane adsorption and counted the methane molecules that belonged to each voxel. Fig. 6a–6d visualizes the spatial probability density of methane in Mo24(tBu-bdc)24 at 298 K and 1 bar, 5 bar, 35 bar, and 65 bar. At low pressures, methane adsorbs primarily at the center of the triangular window. At higher pressures, the corners of the triangular windows and square windows are populated. Fig. 6e shows level surfaces of the computed potential energy of a methane molecule in the cage, confirming that the strongest binding sites are offered by the triangular window, followed by the corners of the square windows. The spatial probability density of methane in Co24(Me-bdc)24(dabco)6 (Fig. S86) show a similar trend, where the triangular windows are filled first, then the corners of the square windows.
To confirm the mechanism of CH4 binding in these materials, we turned to neutron powder diffraction. The lack of long-range order and low crystallinity in porous cages often precludes the use of diffraction methods for in-depth characterization. Given the structural similarities of the cages and frameworks, diffraction experiments on the porous frameworks can be used to infer information about adsorption sites, as our computational modeling confirms the similar nature of the adsorption sites between the framework and cage materials. Detailed powder neutron diffraction experiments performed on Fe24(Me-bdc)24(dabco)6 and Co24(Mebdc)24(dabco)6 allowed us to precisely determine CD4 binding sites and occupancies. For both frameworks, methane doses of 0.5/M2+ and 1.5 CD4/M2+ were utilized. In Fe24(Me-bdc)24(dabco)6 this corresponds to adsorption uptakes (pressures) of 36.8 cm3/g (4.2 bar) and 110.0 cm3/g (17.9 bar). As Co24(Me-bdc)24(dabco)6 exhibits similar methane uptakes and a similar molar mass, the loadings correspond to 36.5 cm3/g (3.5 bar) and 109.4 cm3/g (16 bar).
In both materials at both loadings, four different methane binding sites are apparent (Table 1), three of which show loading-dependent occupancies (Figures S90–S101). The highest occupancy site in both frameworks is the center of the six triangular windows in the cage (Figure 7). Methane adsorbs in the bowl that is formed by three paddlewheel sites and three Me-bdc ligands. The CD4-arene distances for the iron and cobalt frameworks are 4.12(7) and 4.15(4) Å, respectively. Together with the methyl groups of the ligand, this center site forms the basis for additional adsorption sites at the triangular window that is disordered over three positions. These sites involve CD4–CD4 distances of ~3.6 Å and CD4–methyl distances of approximately 4 Å. It is of interest to note that the pillaring dabco ligands are adjacent to this site and within van der Waals contact of the CD4 molecule. As a result, dabco displays less rotational disorder as compared to the bare structures. The metal site on the interior surface of the cuboctahedron is the second highest occupancy site in both materials (~0.28 and 0.21 for Fe and Co, respectively). The occupancy of this site, with a M-C distance of approximately 2.3 Å, is loading independent. This is likely a result of the presence of metal-coordinated DMF and unreacted ligand on the interior surface of the cage, which was also observed in the IR spectra of the material. The corner of the square windows of the cuboctahedral cages serve as the final binding sites at these loadings. At this site methane is interacting with the carboxylate group of the ligand with CD4–C distances of ~3.4 Å. These binding sites have also been observed in the cuboctahedral pore of HKUST-1 with similar ligand–CD4 and CD4–CD4 distances (Figures S102–S103).69 Between the six square windows and eight octahedral windows in the cuboctahedron, these account for 56 adsorption sites. Full population of these adsorption sites would afford gravimetric uptake capacities of 192, 165, and 154 cm3/g for Cr24(tBu-bdc)24, Mo24(tBu-bdc)24, and Ru24(tBu-bdc)24Cl12, respectively. On average, the cages achieve 90 % of these uptake values at 65 bar. Previous studies at higher methane loadings in HKUST-1 indicate additional methane binding sites on the interior surface of the cuboctahedral cage.69
Table 1.
Methane occupancies in M24(Me-bdc)24(dabco)6 (M = Fe, Co) at various loadings.
| Site | 0.5 CD4/Fe2+ |
1.5 CD4/Fe2+ |
0.5 CD4/Co2+ |
1.5 CD4/Co2+ |
|---|---|---|---|---|
| Center of triangle | 0.51(4) | 0.62(4) | 0.59(2) | 0.62(2) |
| Metal | 0.28(2) | 0.29(2) | 0.21(2) | 0.21(1) |
| Top of triangle | 0.20(2) | 0.77(2) | 0.14(2) | 0.92(1) |
| Corner of square | 0.13(1) | 0.66(2) | 0.13(1) | 0.69(1) |
Figure 7.
Methane binding sites in Co24(Me-bdc)24(dabco)6 as determined by powder neutron diffraction. At loadings up to 1.5 CD4/M2+, four binding sites are apparent: the center of the triangular window, corners of the triangular window, corners of the square window, and open metal coordination sites on the interior surface of the cage. Black, red, gray, and blue spheres represent cobalt, oxygen, and carbon, and deuterium, respectively. The pink, yellow, and teal spheres represent carbon atoms of adsorbed methane.
The higher-loading powder neutron diffraction data shed light on the large discrepancy between the gravimetric and volumetric uptakes of M24(tBu-bdc)24 and M12(cdc)12 materials. At a pressure of approximately 30 bar, the entire center of the cuboctahedral cage remains essentially empty, whereas the corresponding octahedral, carbazole-based cage displays negligible wasted volume. Further, the twelve-metal carbazole cages display minimal extra-cage pore volume. Comparison of pore size distribution plots for both cages reveals the presence of significant pore size greater than 15 Å in the cuboctahedral cage. In terms of volumetric gas storage, this wasted pore space is detrimental to the overall deliverable capacity of an adsorbent. However, the molecular nature of these cages can be utilized in this regard by judicious ligand functionalization to tune the extra-cage pore space. Further, their solubility and propensity to crystallize into a number of space groups and crystal morphologies renders them completely tunable for small molecule storage applications.
CONCLUSIONS
By optimizing synthesis, solvent exchange, and activation conditions, surface areas of porous coordination cages can approach those displayed by three-dimensional metal-organic frameworks. We have shown this to be the case for a family of tert-butyl-functionalized cuboctahedral coordination cages. The Cr2+, Mo2+, and Ru2+/3+ analogs of these molecules display high BET surface areas, with Mo24(tBu-bdc)24 having a porous cage record surface area of 1320 m2/g. Additionally, calculations demonstrated molecular gas interactions are analogous in nature to pillared cuboctahedra coordination cages. Although the methane storage properties of these materials are not remarkable when compared to flexible, or ultra-high surface area metal-organic frameworks, the fact that they are molecular renders them significantly more tunable than their MOF counterparts. Further, in terms of deliverable capacities based on bulk as opposed to crystallographic densities, Mo24(tBu-bdc)24 boasts 87 % the methane capacity of HKUST-1, despite having a surface area that is approximately 40 % lower. Moving forward, this insight will aid in the development of future porous metal-organic materials. In particular, by precisely tuning both the pore size and shape of the cage, in addition to the pore space between cages, adsorbents with true molecular level control are attainable.
Supplementary Material
ACKNOWLEDGMENT
We thank Maxwell I. Martin for assistance with crystal structure data acquisition/evaluation.
Funding Sources
We are grateful to the University of Delaware for start-up funds. This manuscript was prepared under cooperative agreement #70NANB17H302 from NIST, U.S. Department of Commerce. We acknowledge the support of the National Institute of Standards and Technology, U.S. Department of Commerce in providing the neutron research facilities used in this work. This work utilized facilities supported in part by the National Science Foundation under Agreement No. DMR-0944772. B.A.T. recognizes the National Academies/National Research Council for his Postdoctoral Fellowship. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02–06CH11357. We thank the staff of 17-BM for help with synchrotron data collection. A portion of this work was supported by the National Institutes of Health under award number P20GM104316. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank the ACS Petroleum Research Fund for support of C.A.R.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Synthetic details, neutron diffraction fits, gas adsorption plots (pdf)
Crystal structure data (cif)
The authors declare no competing financial interest.
REFERENCES
- (1).Keum Y; Park, Seonghun P; Chen Y-P; Park J Titanium-Carboxylate Metal-Organic Framework based on an Unprecedented Ti-Oxo Chain Cluster. Angew. Chem. Int. Ed 2018, 57, 14852–14856. [DOI] [PubMed] [Google Scholar]
- (2).Piscopo CG; Trapani F; Polyzoidis A; Schwarzer M; Pace A; Loebbecke S Positive Effect of the Fluorine Moiety on the Oxygen Storage Capacity of UiO-66 Metal-Organic Frameworks. New J. Chem 2016, 40, 8220–8224. [Google Scholar]
- (3).Rieth AJ; Tulchinsky Y; Dinca M High and Reversible Ammonia Uptake in Mesoporous Azolate Metal-organic Frameworks with Open Mn, Co, and Ni Sites. J. Am. Chem. Soc 2016, 138, 9401–9404. [DOI] [PubMed] [Google Scholar]
- (4).Moreau F; Silva ID; Smail NH; Easun TL; Savage M; Godfrey HGW; Parker SF; Manuel P; Yang S; Schroder M Unravelling Exceptional Acetylene and Carbon Dioxide Adsorption Within a Tetra-Amide Functionalized Metal-Organic Framework. Nat. Commun 2017, 8, 14085–14094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Savage M; Cheng Y; Easun TL; Eyley JE; Argent SP; Warren MR; Lewis W; Murray C; Tang CC; Frogley MD; Clinque G; Sun J; Rudic S; Murden RT; Benham MJ; Fitch AN; Blake AJ; Ramirez-Cuesta AJ; Yang S; Schroder M Selective Adsorption of Sulfur Dioxide in a Robust Metal-Organic Framework Material. Adv. Mater 2016, 28, 8705–8711. [DOI] [PubMed] [Google Scholar]
- (6).Barea E; Montoro C; Navarro JAR Toxic Gas Removal - Metal-Organic Frameworks for the Capture and Degradation of Toxic Gases and Vapours. Chem. Soc. Rev 2014, 43, 5419–5430. [DOI] [PubMed] [Google Scholar]
- (7).Liao Y; Zhang L; Weston MH; Morris W; Hupp JT; Farha OK Tuning Ethylene Gas Adsorption via Metal Node Modulation: Cu-MOF-74 for a High Ethylene Deliverable Capacity. Chem. Commun 2017, 53, 9376–9379. [DOI] [PubMed] [Google Scholar]
- (8).Britt D; Tranchemontagne D; Yaghi OM Metal-Organic Frameworks with High Capacity and Selectivity for Harmful Gases. Proc. Natl. Acad, Sci. U. S. A 2008, 105, 11623–11627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Zhang H; Deria P; Farha OK; Hupp JT; Snurr RQ A Thermodynamic Tank Model for Studying the Effect of Higher Hydrocarbons on Natural gas Storage on Metal-Organic Frameworks. Energy Environ. Sci 2015, 8, 1501–1510. [Google Scholar]
- (10).Pham T; Forrest KA; Furukawa H; Eckert J; Space B Hydrogen Adsorption in a Zeolitic Imidazolate Framework with Ita Topology. J. Phys. Chem. C 2018, 122, 15435–15445. [Google Scholar]
- (11).Zhang Y-B; Furukawa H; Ko N; Nie W; Park HJ; Okajima S; Cordova KE; Deng H; Kim J; Yaghi OM Introduction of Functionality, Selection of Topology, and Enhancement of Gas Adsorption in Multivariate Metal-Organic Framework-177. J. Am. Chem. Soc 2015, 137, 2641–2650. [DOI] [PubMed] [Google Scholar]
- (12).Yan Y; Silva ID; Blake AJ; Daily A; Manuel P; Yang S; Schroder M High Volumetric Hydrogen Adsorption in a Porous Anthracene-Decorated Metal–Organic Framework. Inorg. Chem 2018, 57, 12050–12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Jiang J; Furukawa H; Zhang Y-B; yaghi OM High Methane Storage Working Capacity in metal-organic Frameworks with Acrylate Links. J. Am. Chem. Soc 2016, 138, 10244–10251. [DOI] [PubMed] [Google Scholar]
- (14).Yan Y; Kolokolov DI; Silva ID; Stepanov AG; Blake AJ; Dailly A; Manuel P; Tang CC; Yang S; Schroder M Porous Metal–Organic Polyhedral Frameworks with Optimal Molecular Dynamics and Pore Geometry for Methane Storage. J. Am. Chem. Soc 2017, 139, 13349–13360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Mason JA; Oktawiec J; Taylor MK; Hudson MR; Rodriguez J; Bachman JE; Gonzalez MI; Cervellino A; Guagliardi A; Brown CM; llewellyn PL; Masciocchi N; Long JR Methane Storage in Flexible Metal-Organic Frameworks with Intrinsic Thermal Management. Nature 2015, 527, 357–361. [DOI] [PubMed] [Google Scholar]
- (16).Wen H-M; Li B; Li L; Lin R-B; Zhou W; Qian G; Chen B A Metal-Organic Framework with Optimized Porosity and Functional Sites for High Gravimetric and Volumetric Methane Storage Working Capacities. Adv. Mater 2018, 30, 1704792–1704798. [DOI] [PubMed] [Google Scholar]
- (17).Tian T; Zeng Z; Vulpe D; Casco ME; Divitini G; Midgley PA; Silvestre-Albero J; Tan J-C; Moghadam PZ; Fairen-Jimenez D A sol-gel monolithic metal-organic framework with enhanced methane up-take. Nat. Mater 2018, 17, 174–180. [DOI] [PubMed] [Google Scholar]
- (18).Connolly BM; Aragones,-Anglada M; Gandara-Loe J; Danaf NA; Lamb DC; Mehta JP; Vulpe D; Wuttke S; Silvestre-Albero J; Moghadam PZ; Wheatley AEH; Fairen-Jimenez D Tuning porosity in macroscopic monolithic metal-organic frameworks for exceptional natural gas storage. Nat. Commun 2019, 10 DOI: 10.1038/s41467-019-10185-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Tranchemontagne DJ; Ni Z; O’Keeffe M; Yaghi OM Reticular Chemistry of Metal-Organic Polyhedra. Angew. Chem. Int. Ed 2008, 47, 5136–5147. [DOI] [PubMed] [Google Scholar]
- (20).Zhao D; Yuan D; Krishna R; Van Baten JM; Zhou H-C Thermosensitive Gating Effect and Selective gas Adsorption in a Porous Coordination Nanocage. Chem. Commun 2010, 46, 7352–7354 [DOI] [PubMed] [Google Scholar]
- (21).Murase T; Nishijima Y; Fujita M Cage-Catalyzed knoevenagel Condensation Under Neutral Conditions in Water. J. Am. Chem. Soc 2012, 134, 162–164 [DOI] [PubMed] [Google Scholar]
- (22).Zhang J-H; Xie S-M; Wang B-J; He P-G; Yuan L-M Highly Selective Separation of Enantiomers Using a Chiral porous Organic Cage. J. Chromatogr. A 2015, 1426, 174–182. [DOI] [PubMed] [Google Scholar]
- (23).Jiao Y; Zhang J; Zhang L; Lin Z; He C; Duan C Metal-Organic Polyhedra Containing 36 and 24 Folds of Amide Groups For Selective luminescent Recognition of Natural Disaccharides. Chem. Commun 2012, 48, 6022–6024. [DOI] [PubMed] [Google Scholar]
- (24).Giri N; Del Popolo MG; Melaugh G; Greenaway RL; Ratzke K; Koschine T; Pison L; Costa Gomes MF; Cooper AI; James SL Liquids With Permanent Porosity. Nature 2015, 527, 216–220. [DOI] [PubMed] [Google Scholar]
- (25).Cooper AI Porous Molecular Solids and Liquids. ACS Cent. Sci 2017, 3, 544–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Mastalerz M Porous Shape-Persistent Organic Cage Compounds of Different Size, Geometry, and Function. Acc. Chem. Res 2018, 51, 2411–2422. [DOI] [PubMed] [Google Scholar]
- (27).Flippen JL; Karle J; Karle IL The Crystal Structure of a Versatile Organic Clathrate. 4-p-Hydroxyphenyl-2,2,4-trimethylchroman (Dianin’s Compound). J. Am. Chem. Soc 1970, 92, 3749–3754. [Google Scholar]
- (28).Koifman OI; Mamardashvili N. Zh.; Surov OV Porous Molecular Crystals of Calix[4]arenes. Russ. Chem. Bull. Int. Ed 2017, 66, 241–253. [Google Scholar]
- (29).Lim S; Kim H; Selvapalam N; Kim K-J; Cho SJ; Seo G; Kim K Cucurbit[6]uril: Organic Molecular Porous Material With Permanent Porosity, Exceptional Stability, and Acetylene Sorption Properties. Angew. Chem. Int. Ed 2008, 47, 3352–3355. [DOI] [PubMed] [Google Scholar]
- (30).Tian J; Thallapally PK; Dalgarno SJ; McGrail PB; Atwood JL Amorphous Molecular Organic Solids for Gas Adsorption. Angew. Chem. Int. Ed 2009, 48, 5492–5495. [DOI] [PubMed] [Google Scholar]
- (31).Bezzu CG; Hellowell M; Warren JE; Allen DR; McKeown NB heme-Like Coordination Chemistry Within Nanoporous Molecular Crystals. Science 2010, 327, 1627–1630. [DOI] [PubMed] [Google Scholar]
- (32).Christinat N; Scopelliti R; Severin K Multicomponent Assembly of Boronic Acid Based Macrocycles and Cages. Angew. Chem. Int. Ed 2008, 47, 1848–1852. [DOI] [PubMed] [Google Scholar]
- (33).Lee S; Yang A; Moneypenny TP; Moore JS Kinetically Trapped Tetrahedral Cages via Alkyne Metathesis. J. Am. Chem. Soc 2016, 138, 2182–2185. [DOI] [PubMed] [Google Scholar]
- (34).Tozawa T; Jones JTA; Swamy SL; Adams DJ; Shakespeare S; Clowes R; Bradshaw D; Hasell T; Chong SY Tang C; Thompson S; Parker J; Trewin A; Bacsa J; Slawin AMZ; Steiner A; Cooper AI Porous Organic Cages. Nat. Mater 2009, 8, 973–978. [DOI] [PubMed] [Google Scholar]
- (35).Tranchemontagne DJ; Ni Z; O’Keeffe M; Yaghi OM Reticular Chemistry of Metal–Organic Polyhedra. Angew. Chem. Int. Ed 2008, 47, 5136–5147. [DOI] [PubMed] [Google Scholar]
- (36).Ni Z; Yassar A; Antoun T; Yaghi OM Porous Metal-Organic Truncated Octahedron Constructed from Paddle-Wheel Squares and Terthiophene Links. J. Am. Chem. Soc 2005, 127, 12752–12753. [DOI] [PubMed] [Google Scholar]
- (37).Furukawa H; Kim J; Ockwig NW; O’Keeffe M; Yaghi OM Control of Vertex Geometry, Structure Dimensionality, Functionality, and Pore Metrics in the Reticular Synthesis of Crystalline Metal-Organic Frameworks and Polyhedra. J. Am. Chem. Soc 2008, 130, 11650–11661. [DOI] [PubMed] [Google Scholar]
- (38).Boer SA; White KF; Slater B; Emerson AJ; Knowles GP; Donald WA; Thornton AW; Ladewig BP; Bell TDM; Hill MR; Chaffee AL; Abrahams BF; Turner DR A Multifunctional, Charge-Neutral, Chiral Octahedral M12L12 Cage. Chem. Eur. J 2019, 25 DOI: 10.1002/chem.201901681 [DOI] [PubMed] [Google Scholar]
- (39).Yan Y; Yang S; Blake AJ; Schroder M Studies on Metal-Organic Frameworks of Cu(II) with Isophthalate Linkers for Hydrogen Storage. Acc. Chem. Res 2014, 47, 296–307. [DOI] [PubMed] [Google Scholar]
- (40).Eddaoudi M; Kim J; Wachter JB; Chae HK; O’Keeffe M; Yaghi OM Porous Metal-Organic Polyhedra: 25 Å Cuboctahedron Constructed from 12 Cu2(CO2)4 Paddle-Wheel Building Blocks. J. Am. Chem. Soc 2001, 123, 4368–4369. [DOI] [PubMed] [Google Scholar]
- (41).Ke Y; Collins DJ; Zhou H-C Synthesis and Structure of Cub-octahedral and Anticuboctahedral Cages Containing 12 Quadruply Bonded Dimolybdenum Units. Inorg. Chem 2005, 44, 4154–4156. [DOI] [PubMed] [Google Scholar]
- (42).Young MD; Zhang Q; Zhou H-C Metal-organic polyhedra constructed from dinuclear ruthenium paddlewheels. Inorg. Chim. Acta 2015, 424, 216–220. [Google Scholar]
- (43).Furukawa S; Horike N; Kondo M; Hijikata Y; Carne-Sanchez A; Larpent P; Louvain N; Diring S; Sato H; Matsuda R; Kawano R; Kitagawa S Rhodium-Organic Cuboctahedra as Porous Solids with Strong Binding Sites. Inorg. Chem 2016, 55, 10843–10846. [DOI] [PubMed] [Google Scholar]
- (44).Park J; Perry Z; Chen Y-P; Bae J; Zhou H-C Chromium(II) Metal-Organic Polyhedra as Highly Porous Materials. ACS Appl. Mater. Interfaces 2017, 9, 28064–28068. [DOI] [PubMed] [Google Scholar]
- (45).Gosselin EJ; Rowland CA; Balto KP; Yap GPA; Bloch ED Design and Synthesis of Porous Nickel(II) and Cobalt(II) Cages. Inorg. Chem 2018, 57, 11847–11850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Lorzing GR; Trump BA; Brown CM; Bloch ED Selective Gas Adsorption in Highly Porous Chromium(II)-Based Metal-Organic Polyhedra. Chem. Mater 2017, 29, 8583–8587. [Google Scholar]
- (47).Chiu SS-Y; Lo SM-F; Charmant JPH; Orpen AG; Williams IDA Chemically Functionalizable Nanoporous Material [Cu3(TMA)2(H2O)]n. Science 1999, 283, 1148–1150. [DOI] [PubMed] [Google Scholar]
- (48).Wade CR; Dincă M Investigation of the synthesis, activation, and isosteric heats of CO2 adsorption of the isostructural series of metal-organic frameworks M3(BTC)2 (M = Cr, Fe, Ni, Cu, Mo, Ru) Dalton Trans 2012, 41, 7931–7938. [DOI] [PubMed] [Google Scholar]
- (49).Wang X-S; Ma S; Rauch K; Simmons JM; Yuan D; Wang X; Yildirim T; Cole WC; Lopez JJ; de Meijere A; Zhou H-C Metal-Organic Frameworks Based on Double-Bond-Coupled Di-Isophthalate Linkers with High Hydrogen and Methane Uptakes. Chem. Mater 2008, 20, 3145–3152. [Google Scholar]
- (50).Wang X-S; Ma S; Forster PM; Yuan D; Eckert J; Lopez JJ; Murphy BJ; Parise JB; Zhou H-C Enhancing H2 Uptake by “Close-Packing” Alignment of Open Copper Sites in Metal-Organic Frameworks. Angew. Chem. Int. Ed 2008, 47, 7263–7266. [DOI] [PubMed] [Google Scholar]
- (51).Yuan D; Zhao D; Sun D; Zhou H -C. An Isoreticular Series of Metal-Organic Frameworks with Dendritic Hexacarboxylate Ligands and Exceptionally High Gas-Uptake Capacity. Angew. Chem. Int. Ed 2010, 49, 5357–5361. [DOI] [PubMed] [Google Scholar]
- (52).Yan Y; Lin X; Yang S; Blake AJ; Dailly A; Champness NR; Hubberstey P; Schröder M Exceptionally high H2 storage by a metal-organic polyhedral framework. Chem. Commun 2009, 1025–1027. [DOI] [PubMed] [Google Scholar]
- (53).Yan Y; Blake AJ; Lewis W; Barnett SA; Dailly A; Champness NR; Schröder M Modifying Cage Structures in Metal-Organic Polyhedral Frameworks for H2 Storage. Chem. Eur. J 2011, 17, 11162–11170. [DOI] [PubMed] [Google Scholar]
- (54).Yan Y; Suyetin M; Bichoutskaia E; Blake AJ; Allan DR; Barnett SA; Schröder M Modulating the packing of [Cu24(isophthalate)24] cuboctahedra in a triazole-containing metal-organic polyhedral framework. Chem. Sci 2013, 4, 1731–1736. [Google Scholar]
- (55).Wilmer CE; Farha OK; Yildirim T; Eryazici I; Krungleviciute V; Sarjeant AA; Snurr RQ; Hupp JT Gram-scale, high-yield synthesis of a robust metal-organic framework for storing methane and other gases. Energy Environ. Sci 2013, 6, 1158–1163. [Google Scholar]
- (56).Yan Y; Telepeni I; Yang S; Lin X; Kockelmann W; Dailly A; Blake AJ; Lewis M; Walker GS; Allan DR; Barnett SA; Champness NR; Schröder M Metal-Organic Polyhedral Frameworks: High H2 Adsorption Capacities and Neutron Powder Diffraction Studies. J. Am. Chem. Soc 2010, 132, 4092–4094. [DOI] [PubMed] [Google Scholar]
- (57).Yan Y; Kolokolov DI; Silva ID; Stepanov AG; Blake AJ; Dailly A; Manuel P; Tang CC; Yang S; Schroder M Porous Metal–Organic Polyhedral Frameworks with Optimal Molecular Dynamics and Pore Geometry for Methane Storage. J. Am. Chem. Soc 2017, 139, 13349–13360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Gosselin EJ; Lorzing GR; Trump BA; Brown CM; Bloch ED Gas adsorption in an isostructural series of pillared coordination cages. Chem. Commun 2018, 54, 6392–6395. [DOI] [PubMed] [Google Scholar]
- (59).Wang C-H; Das A; Gao W-Y; Powers DC Probing Substrate Diffusion in Interstitial MOF Chemistry with Kinetic Isotope Effects. Angew. Chem. Int. Ed 2018, 57, 3676–3681. [DOI] [PubMed] [Google Scholar]
- (60).Apex3; Bruker AXS Inc.: Madison, WI, 2015. [Google Scholar]
- (61).Sheldrick GM SHELXT – Integrated space-group and crystal-structure determination. Acta Cryst 2015, A71, 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Sheldrick GM Crystal structure refinement with SHELXL. Acta Cryst 2015, C71, 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Spek AL PLATON SQUEEZE: a tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Cryst 2015, C71, 9–18. [DOI] [PubMed] [Google Scholar]
- (64).Coelho A Topas Academic V6. Coelho Softw 2017. [Google Scholar]
- (65).Chun H; Jung H; Seo J Isoreticular Metal-Organic Polyhedral Networks Based on 5-Connecting Paddlewheel Motifs. Inorg. Chem 2009, 48, 2043–2047. [DOI] [PubMed] [Google Scholar]
- (66).Pawley GS Unit-Cell Refinement from Powder Diffraction Scans. J. Appl. Crystallogr 1981, 14, 357–361. [Google Scholar]
- (67).Andreev YG; MacGlashan GS; Bruce PG Ab Initio Solution of a Complex Crystal Structure from Powder-Diffraction Data Using Simulated-Annealing Method and a High Degree of Molecular Flexibility. Phys. Rev. B 1997, 55, 12011–12017. [Google Scholar]
- (68).Toby BH EXPGUI, a Graphical User Interface for GSAS. J. Appl. Crystallogr 2001, 34, 210–213. [Google Scholar]
- (69).Hulvey Z; Vlaisavljevich B; Mason JA; Tsivion E; Dougherty TP; Bloch ED; Head-Gordon M; Smit B; Long JR; Brown CM Critical Factors Driving the High Volumetric Uptake of Methane in Cu3(Btc)2. J. Am. Chem. Soc 2015, 137, 10816–10825. [DOI] [PubMed] [Google Scholar]
- (70).Rowland CA; Lorzing GR; Gosselin EJ; Trump BA; Yap GPA; Brown CM; Bloch ED Methane Storage in Paddlewheel-Based Porous Coordination Cages. J. Am. Chem. Soc 2018, 140, 11153–11157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Weller MT; Henry PF; Ting VP; Wilson CC Crystallography of Hydrogen-Containing Compounds: Realizing the Potential of Neutron Powder Diffraction. Chem. Commun 2009, 7345, 2973. [DOI] [PubMed] [Google Scholar]
- (72).Wilson CC; Henry PF; Schmidtmann M; Ting VP; Williams E; Weller MT Neutron Powder Diffraction-New Opportunities in Hydrogen Location in Molecular and Materials Structure. Crystallogr. Rev 2014, 20, 162–206. [Google Scholar]
- (73).Kapelewski MT; Geier SJ; Hudson MR; Stück D; Mason JA; Nelson JN; Xiao DJ; Hulvey Z; Gilmour E; FitzGerald SA; et al. M2(m-dobdc) (M = Mg, Mn, Fe, Co, Ni) Metal–Organic Frameworks Exhibiting Increased Charge Density and Enhanced H2 Binding at the Open Metal Sites. J. Am. Chem. Soc 2014, 136, 12119–12129. [DOI] [PubMed] [Google Scholar]
- (74). https://github.com/SimonEnsemble/BlochCages.
- (75).Martin MG; Siepmann JI Transferable Potentials for Phase Equilibria. 1. United-Atom Description of n-Alkanes. J. Phys. Chem. B 1998, 102, 2569–2577. [Google Scholar]
- (76).Mayo SL; Olafson BD; Goddard WA DREIDING: A Generic Force Field for Molecular Simulations. J. Phys. Chem 1990, 94, 8897–8909. [Google Scholar]
- (77).Rappe AK; Casewit CJ; Colwell KS; Goddard WA; Skiff WM UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J. Am. Chem. Soc 1992, 114, 10024–10035. [Google Scholar]
- (78).Childs H; Brugger E; Whitlock B; Meredith J; Ahern S; Pug-mire D; Biagas K; Miller M; Harrison C; Weber GH; Krishnan H; Fogal T; Sanderson A; Garth C; Bethel EW; Camp D; Rubel O; Durant M; Favre JM; Navratil P “VisIt: An End-User Tool for Visualizing and Analyzing Very Large Data” High Performance Visualization-Enabling Extreme-Scale Scientific Insight 2012, 357–372. [Google Scholar]
- (79).Frenkel D; Smit B Understanding Molecular Simulation: From Algorithms to Applications; Academic Press: Harcourt, 1996. [Google Scholar]
- (80).Li J-R; Yakovenko AA; Lu W; Timmons DJ; Zhuang W; Yuan D; Zhou H-C Ligand Bridging-Angle-Driven Assembly of Molecular Architectures Based on Quadruply Bonded Mo–Mo Dimers. J. Am. Chem. Soc 2010, 132, 17599–17610. [DOI] [PubMed] [Google Scholar]
- (81).Cotton FA; Hillard EA; Murillo CA; Zhou H-C After 155 Years, A Crystalline Chromium Carboxylate with a Supershort Cr–Cr Bond. J. Am. Chem. Soc 2000, 122, 416–417. [Google Scholar]
- (82).Mason JA; Veenstra M; Long JR Evaluating metal-organic frameworks for natural gas storage. Chem. Sci 2014, 5, 32–51. [Google Scholar]
- (83).Tian T; Zeng Z; Vulpe D; Casco ME; Divitini G; Midgley PA; Silvestre-Albero J; Tan J-C; Moghadam PZ; Fairen-Jimenez D A Sol-Gel Monolithic Metal-Organic Framework with Enhanced Methane Uptake Nat. Mater 2018, 17, 174–179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.