Abstract
Background:
One of the primary goals of treatment for HFrEF is to improve patients’ health status; their symptoms, function, and quality of life, which has even been proposed as a performance measure for quality. We examined whether physician-led changes in HFrEF medications improved patients’ health status to highlight the opportunity for clinicians to improve patients’ health status.
Objectives:
To describe the association between changes in patients’ medical regimens with change in the health status of outpatients with heart failure and reduced ejection fraction (HFrEF).
Methods:
Using a multi-center, observational outpatient registry of patients with HFrEF, we examined the association of any change in HFrEF medications with 3-month change in health status, as measured by the 12-item Kansas City Cardiomyopathy Questionnaire Overall Summary Scale (KCCQ-OS). Unadjusted and multivariable-adjusted (25 clinical characteristics, baseline health status) results were obtained using hierarchical linear regression models.
Results:
Among 3,313 outpatients with HFrEF from 140 centers, 21.9% had a change in their HFrEF medications during routine clinical care. At 3 months, 23.7% and 46.4% experienced clinically meaningfully worse (≥ 5-point decrease) and improved (≥ 5-point increase) KCCQ-OS scores. The 3-month median change in KCCQ-OS for patients whose HFrEF medical regimen was changed was significantly larger (7.3 points [IQR: −3.1, 20.8]) than for patients whose medications were not changed (3.1 points [IQR:−4.7, 12.5], adjusted difference = 3.0 points (95% CI: 1.4, 4.6; p<0.001)). The proportion with a very large clinical improvement (≥20 points) was 26% in those whose medications were adjusted, vs. 14% when they were not.
Conclusions:
In routine care of patients with HFrEF, changes in HFrEF medications were associated with significant improvements in patients’ health status. Health status-based performance measures can quantify the benefits of titrating medicines in HFrEF patients.
Tweet:
In routine care, titration of HF medications associated with significant improvements in patients’ health status.
Introduction:
One of the primary treatment goals for patients with heart failure and reduced ejection fraction (HFrEF) is to optimize their health status; their symptoms, function, and quality of life (1). Towards that end, regulatory agencies have increasingly supported the use of patient-reported outcomes measures (PROs), such as the Kansas City Cardiomyopathy Questionnaire (KCCQ), to support the approval and labeling of new therapies (2-4). Moreover, there has been an increasing call from entities such as the International Consortium for Health Outcomes Measurement and the Center for Medicare and Medicaid Services (5, 6) to use PROs as performance measures for quantifying the quality of HF care (7-9). Such efforts seem particularly important given the importance of symptom control, function, and quality of life to patients and the marked variability in the control of patients’ symptoms and health status across US practices (10).
The KCCQ-12 is a self- or interview-administered, disease-specific PRO that consists of 12 items that quantify four domains of patients’ health status; their physical limitations (KCCQ-PL), symptom frequency (KCCQ-SF), social limitations (KCCQ-SL), and quality of life (KCCQ-QoL) (19). These 4 domains are summarized into an Overall Summary score (KCCQ-OS) that ranges from 0 to 100, with higher scores indicating fewer symptoms, less limitations and better quality of life. The KCCQ-12 has been extensively validated and shown to be both extremely reproducible and sensitive to clinical change (21). Also, KCCQ scores are prognostic of subsequent mortality, hospitalization, and healthcare-associated cost (22).
While there has been extensive demonstration of the responsiveness of the KCCQ after interventions such as valve replacement, cardiac resynchronization therapy, and mechanical circulatory support (11-14), few data have examined the association between changes in clinicians’ treatment of patients and changes in their health status. Patient’s health status is a critical feature to ascertain whether PRO-based performance measures are actionable in clinical practice and whether providers can be held accountable for such a performance measure (15-16). Supplementing the known prognostic importance of cross-sectional (17) and serial (18, 19) PROs with evidence that patients’ health status is, in part, under the locus of control of providers is an important next step towards supporting the use of patients’ health status as a means for assessing and improving the quality of HF care. To better address this gap in knowledge, we used data from a large, prospective, multicenter registry of patients with HFrEF to examine the association between changes in HF treatment with patients’ health status (20).
Methods:
Study Design
The CHAnge the Management of Patients with HF (CHAMP-HF) study is a multicenter, prospective registry of outpatients with HFrEF conducted throughout the United States that serially documented patients’ disease-specific health status and carefully measured changes in patients’ medical treatment (20). Briefly, consecutive patients with chronic HFrEF (left ventricular ejection fraction (LVEF) ≤ 40%) that were treated with ≥1 HFrEF pharmacotherapy were enrolled at 140 outpatient centers across the US. Patients less than 18 years of age, currently enrolled or planning to participate in a clinical trial, receiving comfort care measures or hospice care, diagnosed with end-stage cardiomyopathy with planned heart transplant or left ventricular assist device implantation, and undergoing dialysis were excluded. Study coordinators recruited patients for the registry during the course of routine outpatient visits. To be included in this analysis, patients had to have completed both a “baseline” (enrollment) and follow-up (3-month) KCCQ assessment and to have been enrolled between December 2015 and October 2017. All study participants provided written informed consent, and each study center obtained site-specific institutional review board approval. Novartis Pharmaceuticals Corporation (East Hanover, NJ) sponsored CHAMP-HF, and Duke Clinical Research Institute (Durham, NC) served as the data analytic center.
Data Collection and Defining Change in Medical Therapy
Each clinical site collected baseline patient sociodemographic data, information on medical and device therapies, and administered the KCCQ at enrollment and 3 months after enrollment. Patient data was serially collected through in-person interviews at enrollment and by in-person or phone interviews at each follow-up visit. Using data from the baseline visit, we defined a change in treatment as any increase/addition or decrease/discontinuation of a HFrEF medical therapy (beta-blocker, angiotensin-receptor blocker [ARB] or angiotensin converting-enzyme inhibitor (ACEI), aldosterone antagonist, angiotensin-neprilysin inhibitor [ARNI], and diuretic) within seven days of enrollment.
Study Outcomes
Change in KCCQ-OS between enrollment and 3-month follow-up was the primary outcome of this analysis. A 5-point change in score signifies a clinically meaningful change in both individual and population-level assessments of health status (23, 24) and is associated with a ~10% change in mortality and rehospitalizations (25, 26) Large and very large clinical changes are associated with changes of 10 and 20 points, respectively, on the KCCQ.
Statistical Analysis
The baseline characteristics of the primary cohort, as well as for those experiencing a ≥10-point improvement (versus not) in KCCQ-OS were described and compared using Wilcoxon-Rank sum and chi-square tests for continuous and categorial variables, respectively. To highlight variability in change in patients’ health status, we described mean (±SD) change in KCCQ-OS scores between enrollment and 3-month follow-up. To render these differences more clinically interpretable, we further categorized health status change as: (i) ≥ 20-point decrease (very large deterioration); ii) ≥ 10 to < 20-point decrease (moderate-to-large deterioration); iii) ≥ 5 to < 10-point decrease (small-to-moderate deterioration); iv) < 5-point decrease to < 5-point increase (no clinically important change); v) ≥ 5 to < 10-point increase (small-to-moderate improvement); vi) ≥ 10 to < 20-point increase (moderate-to-large improvement); and vii) ≥ 20-point increase (very large improvement). We then described the univariate association between patient and practice-level characteristics, as well as changes in HFrEF treatment, with a ≥10-point improvement in KCCQ-OS scores as well as median (IQR) KCCQ-OS change per HFrEF treatment change.
Multivariable-adjusted hierarchical linear regression models were used to describe the independent association of treatment change in any HFrEF therapy with patients’ health status. Site was included as a random effect to account for clustering of patients within practices, and variable selection was performed based upon clinical experience and prior literature (25). Our final models adjusted for 4 sociodemographic (age, sex, race, and ethnicity), 4 socioeconomic (employment status, insurance provider, highest level of education, and total annual household income), 13 medical (atrial fibrillation, chronic obstructive pulmonary disease, coronary artery disease, depression, diabetes mellitus, hypertension, hyperlipidemia, smoking status, ventricular tachycardia/ventricular fibrillation, chronic renal insufficiency, heart failure hospitalization in the last 12 months, cardiac resynchronization therapy, and NYHA functional classification) and 4 physiologic (body mass index, systolic blood pressure, heart rate, left ventricle ejection fraction) characteristics, as well as baseline health status score.
Missing Data
Baseline or 3-month KCCQ scores were missing in 652 of 3,965 eligible patients (16.4%), and these patients were excluded. Supplemental Table 1 compares the demographic and clinical characteristics of those with and without available KCCQ scores. Missing patient characteristics (other than KCCQ) were imputed using a full conditional specification method while taking into account the joint distribution of other variables. All estimates were reported using 95% confidence intervals and a p-value ≤0.05 was considered a statistically significant finding. All analyses were performed using SAS software (version 14.3 SAS Institute, Cary, NC). Analyses were performed independently by the Duke Clinical Research Institute, and the lead author takes responsibility for guiding data analysis and interpretation.
Results:
Patient cohort.
A total of 3,313 outpatients with HFrEF were enrolled in the CHAMP-HF registry for at least 3 months between 2015 and October 2017 and had baseline and 3-month KCCQ scores available. Patient characteristics that differ between those with and without follow-up are shown in Supplemental Table 1. The average age of participants was 66.2 ± 12.5, 30.0% were women and 74.8% were of White race. Cardiac and non-cardiac comorbidities were common, with 33.4% of patients having valvular heart disease, 41.3% diabetes mellitus, 31.4% chronic obstructive lung disease/asthma, 19.4% ventricular tachycardia/fibrillation, and 20.1% with chronic renal insufficiency. Most patients were classified as NYHA II (58.5%) and NYHA III (29.2%), mean systolic blood pressure was 121 ± 18 mmHg, and mean LVEF (%) was 29 ± 8%. Evidence-based HF therapies were frequently used, including beta-blockers (78.7%), ACEI/ARB (56.4%), ARNI (10.1%), MRA (30.4%), and diuretics (48.7%). The baseline characteristics of patients whose medications were and were not changed is provided in Table 1.
Table 1:
Baseline Characteristics of those with and without a change in medications
| Table 1a: Baseline Patient Characteristics by Any Change in Medication | |||
|---|---|---|---|
| Change in Any Medication | |||
| Characteristic | Yes (N=727) |
No (N=2586) |
P- Value |
| Demographics | |||
| Age (Years) | <.001 | ||
| N | 727 | 2585 | |
| Mean (SD) | 63.3 (13.26) | 67.0 (12.09) | |
| Median (25th, 75th) | 64.0 (56.0, 73.0) | 68.0 (60.0, 76.0) | |
| Min, Max | 18.0, 96.0 | 22.0, 97.0 | |
| Female Sex | 236/727 (32.5%) | 759/2585 (29.4%) | 0.107 |
| Race | 0.408 | ||
| American Indian or Alaska Native | 9/727 (1.2%) | 19/2585 (0.7%) | |
| Asian | 8/727 (1.1%) | 45/2585 (1.7%) | |
| Black or African American | 120/727 (16.5%) | 432/2585 (16.7%) | |
| Native Hawaiian or Pacific Islander | 3/727 (0.4%) | 5/2585 (0.2%) | |
| White | 546/727 (75.1%) | 1930/2585 (74.7%) | |
| Multi-Racial (no primary race) | 11/727 (1.5%) | 27/2585 (1.0%) | |
| Other | 30/727 (4.1%) | 127/2585 (4.9%) | |
| Hispanic Ethnicity | 65/727 (8.9%) | 532/2585 (20.6%) | <.001 |
| Obese (BMI >= 30 mg/m2) | 236/727 (32.5%) | 774/2585 (29.9%) | 0.192 |
| Insurance Status | 0.006 | ||
| Managed care (HMO, PPO) | 128/727 (17.6%) | 425/2584 (16.4%) | |
| Private insurance (high-deductible health plan/health savings account) | 80/727 (11.0%) | 244/2584 (9.4%) | |
| Medicare | 383/727 (52.7%) | 1519/2584 (58.8%) | |
| Medicaid | 79/727 (10.9%) | 226/2584 (8.7%) | |
| Military health care (Tricare/VA/CHAMPUS) | 9/727 (1.2%) | 60/2584 (2.3%) | |
| Uninsured | 21/727 (2.9%) | 47/2584 (1.8%) | |
| Other | 27/727 (3.7%) | 63/2584 (2.4%) | |
| Highest Level of Education | 0.068 | ||
| Less than high school | 68/727 (9.4%) | 336/2584 (13.0%) | |
| High school/GED | 269/727 (37.0%) | 866/2584 (33.5%) | |
| Some college | 230/727 (31.6%) | 810/2584 (31.3%) | |
| Four year college (bachelor's degree) | 97/727 (13.3%) | 329/2584 (12.7%) | |
| Graduate or other professional (post-undergraduate) degree | 63/727 (8.7%) | 243/2584 (9.4%) | |
| Total Household Income | 0.594 | ||
| Less than $25,000 | 217/727 (29.8%) | 818/2585 (31.6%) | |
| $25,000 to $49,999 | 150/727 (20.6%) | 512/2585 (19.8%) | |
| $50,000 to $74,999 | 89/727 (12.2%) | 331/2585 (12.8%) | |
| $75,000 to $99,999 | 55/727 (7.6%) | 148/2585 (5.7%) | |
| $100,000 to $149,999 | 39/727 (5.4%) | 127/2585 (4.9%) | |
| $150,000 or more | 16/727 (2.2%) | 66/2585 (2.6%) | |
| Prefer not to answer | 161/727 (22.1%) | 583/2585 (22.6%) | |
| Employment Status | 0.006 | ||
| Working full-time, that is 35 hours/week or more | 125/727 (17.2%) | 333/2585 (12.9%) | |
| Working part-time, that is less than 35 hours | 56/727 (7.7%) | 181/2585 (7.0%) | |
| Disability for medical reasons | 196/727 (27.0%) | 666/2585 (25.8%) | |
| Not employed for other reasons (retired, student, etc.) | 350/727 (48.1%) | 1405/2585 (54.4%) | |
| Medical History | |||
| Diabetes Mellitus | 276/727 (38.0%) | 1093/2585 (42.3%) | 0.037 |
| Chronic Renal Insufficiency | 133/727 (18.3%) | 534/2585 (20.7%) | 0.160 |
| Asthma, bronchitis, chronic obstructive pulmonary disease (COPD) | 228/727 (31.4%) | 811/2585 (31.4%) | 0.995 |
| Depression | 180/727 (24.8%) | 681/2585 (26.3%) | 0.389 |
| Cigarette Smoking | 137/727 (18.8%) | 510/2585 (19.7%) | 0.595 |
| Atrial Fibrillation | 250/727 (34.4%) | 922/2585 (35.7%) | 0.524 |
| Coronary Artery Disease | 441/727 (60.7%) | 1662/2585 (64.3%) | 0.072 |
| Hypertension | 592/727 (81.4%) | 2176/2585 (84.2%) | 0.077 |
| Hyperlipidemia | 509/727 (70.0%) | 2039/2585 (78.9%) | <.001 |
| Ventricular Tachycardia or Ventricular Fibrillation | 134/727 (18.4%) | 507/2585 (19.6%) | 0.476 |
| Cardiac Resynchronization Therapy | 58/727 (8.0%) | 180/2585 (7.0%) | 0.349 |
| Implantable Cardioverter-Defibrillator | 264/727 (36.3%) | 1134/2585 (43.9%) | <.001 |
| Heart Failure Hospitalization in 12 Months Prior to Enrollment | 356/727 (49.0%) | 866/2585 (33.5%) | <.001 |
| Valvular Heart Disease | 251/727 (34.5%) | 855/2585 (33.1%) | 0.464 |
| Peripheral Artery Disease | 81/727 (11.1%) | 376/2585 (14.5%) | 0.019 |
| Stroke/TIA | 80/727 (11.0%) | 289/2585 (11.2%) | 0.894 |
| Obstructive Sleep Apnea | 169/727 (23.2%) | 532/2585 (20.6%) | 0.120 |
| Cancer | 73/727 (10.0%) | 308/2585 (11.9%) | 0.162 |
| NYHA Classification | 0.001 | ||
| I | 59/713 (8.3%) | 276/2522 (10.9%) | |
| II | 392/713 (55.0%) | 1500/2522 (59.5%) | |
| III | 248/713 (34.8%) | 696/2522 (27.6%) | |
| IV | 14/713 (2.0%) | 50/2522 (2.0%) | |
| KCCQ Overall Summary Scores | |||
| Vital Signs at Enrollment | |||
| Systolic blood pressure (mm/Hg) | 0.959 | ||
| N | 716 | 2455 | |
| Mean (SD) | 121.2 (18.30) | 121.2 (17.35) | |
| Median (25th, 75th) | 120.0 (110.0, 132.0) | 120.0 (110.0, 130.0) | |
| Min, Max | 68.0, 197.0 | 70.0, 195.0 | |
| Diastolic blood pressure (mm/Hg) | 0.304 | ||
| N | 716 | 2455 | |
| Mean (SD) | 73.2 (11.58) | 72.5 (10.62) | |
| Median (25th, 75th) | 72.0 (65.0, 80.0) | 72.0 (64.0, 80.0) | |
| Min, Max | 42.0, 118.0 | 33.0, 148.0 | |
| Heart Rate | <.001 | ||
| N | 701 | 2427 | |
| Mean (SD) | 76.0 (13.73) | 73.5 (11.82) | |
| Median (25th, 75th) | 75.0 (67.0, 83.0) | 72.0 (65.0, 80.0) | |
| Min, Max | 41.0, 127.0 | 30.0, 125.0 | |
| LVEF (%) | <.001 | ||
| N | 726 | 2584 | |
| Mean (SD) | 27.9 (8.09) | 29.6 (7.76) | |
| Median (25th, 75th) | 28.0 (22.0, 35.0) | 30.0 (25.0, 35.5) | |
| Min, Max | 5.0, 43.0 | 1.0, 50.0 | |
| Practice Type | |||
| Practice Type | <.001 | ||
| Cardiology | 655/727 (90.1%) | 1999/2586 (77.3%) | |
| Emergency Medicine | 5/727 (0.7%) | 43/2586 (1.7%) | |
| Family Practice/General Medicine | 12/727 (1.7%) | 187/2586 (7.2%) | |
| Internal Medicine | 27/727 (3.7%) | 265/2586 (10.2%) | |
| Other, Specify | 28/727 (3.9%) | 92/2586 (3.6%) | |
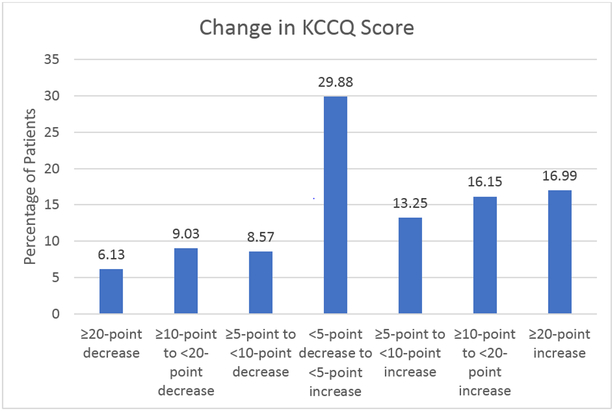
At 3 months, 23.7% and 46.4% of the primary cohort experienced clinically meaningfully worse (≥ 5-point decrease) and improved (≥ 5-point increase) KCCQ-OS scores (Figure 1), respectively; 33.1% had a ≥ 10 point increase and 15.2% a ≥ 10 point decrease. Within one week of their baseline visit, 21.9% of patients had a change in their HFrEF medical therapy (688, 20.8% with increase in medication dosing and 71, 2.1% with decrease in medication dosing). Most of these changes (72.1%) were changes in a single medication and 18.3% of patients had changes in 2 medications and 9.7% had changes in 3 or more HF medications. Among those with a change in their medications, 40% (291 of 727) and of those who did not have an initial change in their therapy, 18.9% (489 of 2586) had a change in therapy between enrollment and 3 months (p<0.001).
Figure 1:
Change in KCCQ Scores
Patient factors associated with a large improvement in health status.
Patient characteristics and changes in medical therapy associated with a moderate or greater improvements in their KCCQ-OS scores (≥10-points; 33.1% of the cohort) are shown in Supplemental Table 2. Patients with large improvements in their health status were more likely to be younger (65.6 ± 12.5 versus 66.5 ± 12.4; p = 0.01), obese (32.9% versus 29.3%; p = 0.04), diabetic (44.0% versus 40.0%; p = 0.03) and to smoke (21.9% versus 18.3%; p = 0.01). They, also, had worse baseline KCCQ scores (51.6 vs 71.3; p < 0.01) and were more likely to have been hospitalized within the preceding 12 months (44.8% versus 33.0%; p < 0.01). Those with lower systolic blood pressure (120 ± 17 versus 122 ± 18 mmHg; p < 0.01) and higher heart rate (75.8 ± 13.2 versus 73.2 ± 11.8, p<0.01) were, also, more likely to experience a 10-point improvement in their KCCQ-OS. There were few other differences between the groups.
Association of medication changes with health status.
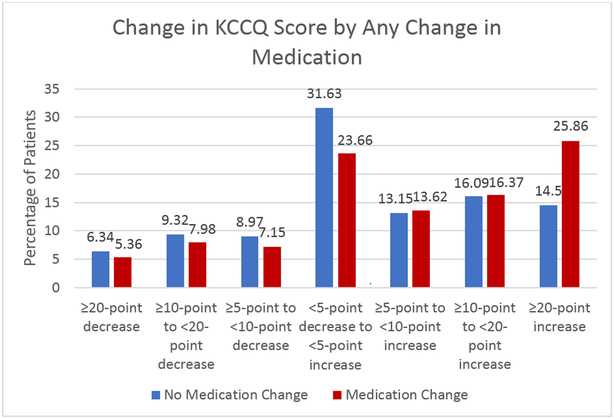
Table 2 shows the unadjusted median differences in KCCQ scores over 3 months by change in HF medications. When examining changes in KCCQ-OS scores as a continuous variable, any change in HFrEF medication was associated with statistically significant improvements in KCCQ-OS compared with no change (7.3 points [95% CI −3.1, 20.8] versus 3.1 [95% CI −4.7, 12.5] points; p < 0.001). This effect was similar in those whose medications were increased (6.8 points [95% CI −3.1, 20.4] versus 3.1 points [95% CI −4.7, 13.0]; p < 0.001) or decreased (11.5 points [95% CI −1.0, 22.9] versus 3.6 points [95% CI −4.2, 14.6]; p = 0.009). These mean differences were primarily driven by a greater proportion of patients whose medications were adjusting having experienced very large (>20 point) improvements in their HF-specific health status (25.9% versus 14.5%, p<0.01; Figure 2).
Table 2:
Treatment Interventions and KCCQ Change
| Table 2: Treatment Intervention and KCCQ Change | |||||
|---|---|---|---|---|---|
| Median KCCQ Change(Q1, Q3)[1] | |||||
| Medication Change | No Medication Change | ||||
| Intervention [2,3,4] | N | Median (Q1, Q3) | N | Median (Q1, Q3) | P- Value |
| Change in at least one medication [5] | 727 | 7.3 (−3.1, 20.8) | 2586 | 3.1 (−4.7, 12.5) | <.001 |
| Increase/addition in any medication [5] | 688 | 6.8 (−3.1, 20.4) | 2625 | 3.1 (−4.7, 13.0) | <.001 |
| De-escalation in any medication [5] | 71 | 11.5 (−1.0, 22.9) | 3242 | 3.6 (−4.2, 14.6) | 0.009 |
| Change of beta blocker | 279 | 7.3 (−3.1, 22.9) | 3034 | 3.1 (−4.5, 13.5) | <.001 |
| Increase/addition of beta blocker | 263 | 7.3 (−3.1, 22.9) | 3050 | 3.5 (−4.5, 13.9) | <.001 |
| De-escalation of beta blocker | 16 | 17.2 (−3.6, 26.6) | 3297 | 3.6 (−4.2, 14.6) | 0.136 |
| Change of ACEI/ARB | 236 | 7.0 (−3.1, 21.4) | 3077 | 3.5 (−4.2, 13.9) | <.001 |
| Increase/addition of ACEI/ARB | 211 | 6.8 (−3.1, 22.9) | 3102 | 3.6 (−4.2, 13.9) | <.001 |
| De-escalation of ACEI/ARB | 25 | 9.4 (−2.6, 16.7) | 3288 | 3.6 (−4.2, 14.6) | 0.547 |
| Change of aldosterone | 144 | 6.3 (−3.1, 23.4) | 3169 | 3.6 (−4.2, 14.1) | 0.011 |
| Increase/addition of aldosterone | 129 | 5.7 (−3.1, 24.0) | 3184 | 3.6 (−4.2, 14.3) | 0.036 |
| De-escalation of aldosterone | 15 | 13.2 (1.0, 22.9) | 3298 | 3.6 (−4.2, 14.6) | 0.200 |
| Change of ARNI | 174 | 7.3 (−2.1, 19.3) | 3139 | 3.6 (−4.2, 14.6) | 0.025 |
| Increase/addition of ARNI | 168 | 8.6 (−2.1, 19.8) | 3145 | 3.6 (−4.2, 14.6) | 0.017 |
| De-escalation of ARNI | 6 | −1.0 (−3.8, 2.1) | 3307 | 3.6 (−4.2, 14.6) | 0.565 |
| Change of diuretic | 193 | 9.9 (−1.6, 23.4) | 3120 | 3.1 (−4.3, 13.5) | <.001 |
| Increase/addition of diuretic | 178 | 9.4 (−1.6, 22.9) | 3135 | 3.3 (−4.2, 13.9) | <.001 |
| De-escalation of diuretic | 15 | 13.0 (4.2, 35.2) | 3298 | 3.6 (−4.2, 14.6) | 0.009 |
KCCQ Change is defined as KCCQ Score at 90 days - KCCQ Score at baseline.
Increase/addition of medication is defined as dose at baseline+7 > dose at baseline-7.
De-escalation of medication is defined as dose at baseline+7 < dose at baseline-7.
Change of medication is defined as dose at baseline+7 not equal to dose at baseline-7.
Because a patient could increase in one medication and decrease in a different medication, these three rows are no longer mutually exclusive.
Abbreviations: ACEI = angiotensin converting enzyme inhibitor, ARB = angiotensin II receptor blocker, ARNI = angiotensin receptor-neprilysin inhibitors
Figure 2:
Change in KCCQ Score by Any Change in Medication
The observed and adjusted, mean differences in change in health status between patients with and without an alteration in their medication regimen is shown in Table 3. After multivariable adjustment, a statistically significant 3.0-point mean improvement in KCCQ-OS scores was observed with any change in guideline-directed medical therapy (95% CI 1.43-4.60; p < 0.001). The odds ratio in unadjusted and adjusted models for predicting a 10-point improvement in KCCQ-OS scores with any change in medications were 1.57 (95%CI: 1.31, 1.87) and 1.42 (95%CI: 1.17, 1.72), respectively (Supplemental Table 3). These odds ratios for a 5-point change in KCCQ scores were 1.54 (95%CI: 1.30, 1.83) and 1.40 (95%CI: 1.16, 1.68; Supplemental Table 4).
Table 3:
Association of Treatment Intervention by 90 Day Visit with KCCQ Change
| KCCQ Change [1] | ||||
|---|---|---|---|---|
| Change in Treatment | Difference in Means (95% CI) [2] |
P-value | Adjusted Difference in Means (95% CI) [3,4] |
P-value |
| Change in Any Treatment | 4.12 (2.58, 5.66) | <.001 | 3.01 (1.43, 4.60) | <.001 |
| Beta Blocker | 5.15 (2.89, 7.41) | <.001 | 4.27 (1.95, 6.59) | <.001 |
| ACEI/ARB | 5.04 (2.59, 7.49) | <.001 | 4.06 (1.57, 6.56) | 0.001 |
| Aldosterone | 4.50 (1.42, 7.57) | 0.004 | 3.77 (0.63, 6.90) | 0.018 |
| ARNI | 2.46 (−0.38, 5.29) | 0.089 | 1.52 (−1.32, 4.35) | 0.294 |
| Diuretic | 7.46 (4.80, 10.12) | <.001 | 5.43 (2.68, 8.18) | <.001 |
KCCQ Change is defined as KCCQ Score at 90 days - KCCQ Score at baseline.
Unadjusted analysis is based occurrence of a change in treatment (Yes vs. No) using a hierarchical linear model adjusting for site.
Adjusted analysis is based on the occurrence of a change in treatment (Yes vs. No), age, gender, race, Hispanic ethnicity, employment status, insurance provider, highest level of education, total household income, body mass index, systolic blood pressure, heart rate, left ventricle ejection fraction (%), atrial fibrillation, chronic obstructive pulmonary disease, coronary artery disease, depression, diabetes mellitus, hypertension, hyperlipidemia, smoking status, ventricular tachycardia/ventricular/fibrillation, chronic renal insufficiency, heart failure hospitalization in the prior 12 months, cardiac resynchronization therapy, and NYHA classification using a hierarchical linear model adjusting for site.
Missing covariate data was imputed using a full conditional specification method taking into account the joint distribution of other variables.
Abbreviations: ACEI = angiotensin converting enzyme inhibitor, ARB = angiotensin II receptor blocker, ARNI = angiotensin receptor-neprilysin inhibitors
Discussion:
In this large, outpatient, observational registry of patients with HFrEF, we found that GDMT HF medication adjustments by healthcare providers in clinical practice were associated with an improvement in patients’ health status. The majority of medication changes involved uptitration of GDMT consistent with the minority of patients in this registry being treated with GDMT at target doses; however, we found both increases and decreases in HF medications to be associated with statistically significant and clinically relevant improvements in patients’ health status (27). Importantly, a much greater proportion of patients whose medications were changed experienced a very large improvement in their KCCQ scores (≥20 points), which has been shown in prior studies to be associated with substantial reductions in the hazard for all-cause mortality and HF hospitalizations (25). These findings provide empiric, real-world evidence that physician-led changes in HFrEF medications can be associated with meaningful improvements in patients’ health status within as early as 3 months.
Over the past decade, there have been increasing calls to use PROs as measures of healthcare quality in treating patients with HFrEF (2-9). However, to be a valuable, outcomes-based performance measure, there needs to be evidence that (1) there is variability in the outcome and that (2) changes in the outcome are modifiable in routine clinical care. We have previously shown substantial variability in KCCQ scores across practices in the CHAMP-HF registry (28), and that there are disparities in the health status of women, minorities, and those of lower socio-economic status (29). In this study, we extend this prior work to show that changes in HF therapies are associated with significant, clinically meaningful improvements in patients’ health status, supporting that patients’ health status is, in part, under the locus of control of physicians’ treatment of their patients. Collectively, these data suggest that the use of patient-reported outcomes, like the KCCQ, can be a means for qualifying and potentially improving the quality of care for patients with HF.
Our work significantly extends the prior study of patients’ health status outcomes in routine clinical care. Prior studies in outpatients with heart failure have focused on medication use alone, instead of the impact of those therapies on patients’ symptoms, function or quality of life. For example, IMPROVE-HF and OPTIMIZE-HF assessed the adherence with heart failure guidelines and found that initiation of therapies prior to hospital discharge, clinical decision support tools, structured improvement strategies, and chart audits with feedback could improve the treatment of patients with HFrEF (30-32). However, the impact of these changes in treatment with patient-centered outcomes was not assessed. Our findings suggest that adjustments made to patients’ heart failure medications is associated with improvement in health status, which further underscores the benefits of improving care through the use of GDMT.
In oncologic practice, studies have shown that the routine use of PROs in care have improved patients’ treatment, pain control and mortality (33-34). To that end, there have been recent efforts to integrate these measures into routine clinical care in HF. For example, Stehlik and colleagues have recently described the prospective collection of the KCCQ and PROMIS scales in an outpatient heart failure clinic (35). Such efforts, coupled with the findings from this study, suggest that the use of serial health status measures can help monitor patients’ responses to therapy and may also enable practitioners to quantitatively assess the impact of changes in treatment on patients’ health status. By sharing KCCQ scores with patients, it is also possible that they may gain an understanding of why their medications are being adjusted, be more compliant with their heart failure regimen, and become more engaged in their medical treatment. Future studies should examine the impact of routinely using health status measures on the care and outcomes of pateints with heart failure.
Our findings should be interpreted in the context of the following potential limitations. First, as with all observational studies, we are merely reporting an association between medical changes and improved health status, and there were certainly differences between those whose medications were and were not changed. Nevertheless, we conducted multivariable analyses to reduce some of this bias and still found statistically significant improvements in patients’ health status when medications were adjusted. However, we cannot exclude the possibility of residual confounding or even a placebo effect (e.g., patients with a medication change may be more likely to report improvement in health status). While we categorized patients by whether or not medications were changed at the initial visit, 19% of patients who did not have a change at their initial visit did have a change over the next 3 months. This would be expected to bias our results to the null and the observed improvements in health status among those classified as not having had an initial change in therapy may have been due to subsequent changes in treatment. Moreover, the CHAMP-HF registry, while including a broad distribution of outpatient practices, may not be generalizable throughout the country and only includes patients that signed informed consent and exhibited the ability to complete multiple surveys over time.
Conclusions:
In a large, outpatient, observational registry of patients with HFrEF, we found that medication adjustments by healthcare providers were associated with improvement in patients’ health status. When coupled with prior work showing marked variability in the health status of patients across practices in the US, these findings suggest that clinicians might be able to further improve their patients’ health status through careful adjustment of their medical regimens and potentially reduce the observed variability in patients’ health status. Collectively, these observations support the use of PROs as quality assessment tools, although future studies are needed to see if the prospective use of PROs in clinical care can improve patients’ health status and clinical outcomes.
Supplementary Material
Clinical Perspectives:
While improving pateints’ health status is a primary goal for heart failure treatment, the impact of changes in medications doses has not been described. In a large, multi-center cohort of patients with HFrEF, we found that changes in medications are associated with rapid improvements in patients health status, as measured by the KCCQ. This suggests that careful medication titration can improve patients’ health status and supports the use of health status as a measure of healthcare quality.
Translational Outlook:
There has been increasing interest in implementing PROs in the routine care of outpatients with HFrEF. In this study, we show that the KCCQ, a PRO, can be used to serially measure patients’ health status and can monitor responses to changes in patients’ treatments. The findings of our study strengthen the argument for incorporating these measures into clinical practice and considering their use as measures of healthcare quality.
Acknowledgments
Disclosures:
Dr. Yevgeniy Khariton is supported by the National Heart, Lung, and Blood Institutes of Health Under Aware Number T32HL110837; the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Dr. Gregg C. Fonarow reports research support from the NIH, consulting for Amgen, Janssen, Medtronic, Novartis, and St Jude Medical, and serving on the Get-With-The-Guidelines Steering Committee.
Dr. Javed Butler has received research support from the National Institutes of Health and the European Union and serves as a consultant for Amgen, Bayer, Boehringer Ingelheim, Cardiocell, CVRx, Gilead, Janssen, Medtronic, Merck, Novartis, Relypsa, and ZS Pharma.
Dr. Laine Thomas reports research funding from Novartis Pharmaceuticals Corporation.
Dr. Adam D. DeVore receives research Support from the American Heart Association, Amgen, NIH, and Novartis; he provides consulting services for Novartis.
Dr. Adrian F. Hernandez reports research support from AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Luitpold Pharmaceuticals, Merck, and Novartis as well as honoraria from Bayer, Boston Scientific, and Novartis.
Dr. Nancy M. Albert reports consulting for Novartis and Boston Scientific and receiving honoraria from Novartis.
Dr. John A. Spertus discloses grant funding from NIH, PCORI, Novartis, Abbott Vascular. He serves on a Scientific Advisory Board for United Healthcare and as a consultant for Novartis, Bayer, V-Wave, AstraZeneca, Jansssen, Corvia and Bayer. He has intellectual property rights for the Kansas City Cardiomyopathy Questionnaire and an equity interest in Health Outcomes Science.
Glossary
- HFrEF
heart failure reduced ejection fraction
- PROs
patient-reported outcomes
- KCCQ
Kansas City Cardiomyopathy Questionarre
- ACEI
angiotensin-converting enzyme ihibitor
- ARB
angiotensin receptor blocker
- ARNI
angiotensin-neprilysin inhibitor
- MRA
mineralocorticoid antagonist
- GDMT
goal-directed medical therapy
References:
- 1.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147–239. [DOI] [PubMed] [Google Scholar]
- 2.Committee on Quality of Health Care in America Institute of Medicine: Crossing the quality chasm: a new health system for the 21st century. Washington, D.C: National Academy Press; 2001. . [Google Scholar]
- 3.Voice, F. (2018). Kansas City Cardiomyopathy Questionnaire (KCCQ) ∣ FDA Voice. [online] Blogs.fda.gov. Available at: https://blogs.fda.gov/fdavoice/index.php/tag/kansas-city-cardiomyopathy-questionnaire-kccq/ [Accessed 7 Jun. 2018].
- 4.Anon, (n.d.). The 21st Century Cures Act. [online] Available at: https://www.nih.gov/research-training/medical-research-initiatives/cures [Accessed 14 Jun. 2018].
- 5.Spertus JA, Eagle KA, Krumholz HM, Mitchell KR, Normand SL, American College of C, et al. American College of Cardiology and American Heart Association methodology for the selection and creation of performance measures for quantifying the quality of cardiovascular care. Circulation. 2005;111(13):1703–12. [DOI] [PubMed] [Google Scholar]
- 6.Spertus JA, Bonow RO, Chan P, Diamond GA, Drozda JP Jr., Kaul S, et al. ACCF/AHA new insights into the methodology of performance measurement: a report of the American College of Cardiology Foundation/American Heart Association Task Force on performance measures. J Am Coll Cardiol. 2010;56(21):1767–82. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Clinical Standards and Quality, Centers for Medicare and Medicaid Services. CMS Quality Measure Development Plan: Supporting the Transition to the Quality Payment Program 2017 Annual Report. 2017.
- 8.Porter ME. A strategy for health care reform--toward a value-based system. N Engl J Med. 2009;361(2):109–12. [DOI] [PubMed] [Google Scholar]
- 9.Porter ME. What is value in health care? N Engl J Med. 2010;363(26):2477–81. [DOI] [PubMed] [Google Scholar]
- 10.Khariton Y, Hernandez AF, Fonarow GC, Sharma PP, Duffy CI, Thomas L, et al. Health Status Variation Across Practices in Outpatients With Heart Failure: Insights From the CHAMP-HF (Change the Management of Patients With Heart Failure) Registry. Circ Cardiovasc Qual Outcomes. 2018;11(4):e004668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993–1004. [DOI] [PubMed] [Google Scholar]
- 12.Baron SJ, Arnold SV, Reynolds MR, Wang K, Deeb M, Reardon MJ, et al. Durability of quality of life benefits of transcatheter aortic valve replacement: Long-term results from the CoreValve US extreme risk trial. Am Heart J. 2017;194:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cowger JA, Naka Y, Aaronson KD, Horstmanshof D, Gulati S, Rinde-Hoffman D, et al. Quality of life and functional capacity outcomes in the MOMENTUM 3 trial at 6 months: A call for new metrics for left ventricular assist device patients. J Heart Lung Transplant. 2018;37(1):15–24. [DOI] [PubMed] [Google Scholar]
- 14.Arnold SV, Spertus JA, Vemulapalli S, Li Z, Matsouaka RA, Baron SJ, et al. Quality-of-Life Outcomes After Transcatheter Aortic Valve Replacement in an Unselected Population: A Report From the STS/ACC Transcatheter Valve Therapy Registry. JAMA Cardiol. 2017;2(4):409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spertus JA, Eagle KA, Krumholz HM, et al. American College of Cardiology and American Heart Association methodology for the selection and creation of performance measures for quantifying the quality of cardiovascular care. Circulation. 2005;111(13):1703–1712. [DOI] [PubMed] [Google Scholar]
- 16.Spertus JA, Bonow RO, Chan P, et al. ACCF/AHA new insights into the methodology of performance measurement: a report of the American College of Cardiology Foundation/American Heart Association Task Force on performance measures. Circulation. 2010;122(20):2091–2106. [DOI] [PubMed] [Google Scholar]
- 17.Soto GE, Jones P, Weintraub WS, Krumholz HM, Spertus JA. Prognostic value of health status in patients with heart failure after acute myocardial infarction. Circulation. 2004;110(5):546–51. [DOI] [PubMed] [Google Scholar]
- 18.Pokharel Y, Khariton Y, Tang Y, Nassif ME, Chan PS, Arnold SV, et al. Association of Serial Kansas City Cardiomyopathy Questionnaire Assessments With Death and Hospitalization in Patients With Heart Failure With Preserved and Reduced Ejection Fraction: A Secondary Analysis of 2 Randomized Clinical Trials. JAMA Cardiol. 2017;2(12):1315–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kosiborod M, Soto GE, Jones PG, Krumholz HM, Weintraub WS, Deedwania P, et al. Identifying heart failure patients at high risk for near-term cardiovascular events with serial health status assessments. Circulation. 2007;115(15):1975–81. [DOI] [PubMed] [Google Scholar]
- 20.DeVore AD, Thomas L, Albert NM, Butler J, Hernandez AF, Patterson JH, et al. Change the management of patients with heart failure: Rationale and design of the CHAMP-HF registry. American Heart Journal. 2017;189:177–83. [DOI] [PubMed] [Google Scholar]
- 21.Spertus JA, Jones PG. Development and Validation of a Short Version of the Kansas City Cardiomyopathy Questionnaire. Circ Cardiovasc Qual Outcomes. 2015;8(5):469–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan PS, Soto G, Jones PG, Nallamothu BK, Zhang Z, Weintraub WS, et al. Patient health status and costs in heart failure: insights from the eplerenone post-acute myocardial infarction heart failure efficacy and survival study (EPHESUS). Circulation. 2009;119(3):398–407. [DOI] [PubMed] [Google Scholar]
- 23.Spertus J, Peterson E, Conard MW, Heidenreich PA, Krumholz HM, Jones P, et al. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J. 2005;150(4):707–15. [DOI] [PubMed] [Google Scholar]
- 24.Dreyer RP, Jones PG, Kutty S, Spertus JA. Quantifying clinical change: discrepancies between patients’ and providers’ perspectives. Qual Life Res. 2016;25(9):2213–20.] [DOI] [PubMed] [Google Scholar]
- 25.Pokharel Y, Khariton Y, Tang Y, Nassif ME, Chan PS, Arnold SV, Jones PG and Spertus JA. Association of Serial Kansas City Cardiomyopathy Questionnaire Assessments With Death and Hospitalization in Patients With Heart Failure With Preserved and Reduced Ejection Fraction: A Secondary Analysis of 2 Randomized Clinical Trials. JAMA Cardiol. 2017;2(12):1315–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kosiborod M, Soto GE, Jones PG, Krumholz HM, Weintraub WS, Deedwania P, Spertus JA. Identifying heart failure patients at high risk for near-term cardiovascular events with serial health status assessments. Circulation. 2007;115(15):1975–81. [DOI] [PubMed] [Google Scholar]
- 27.Greene SJ, Butler J, Albert NM, DeVore AD, Sharma PP, Duffy CI, et al. Medical Therapy for Heart Failure with Reduced Ejection Fraction. Journal of the American College of Cardiology, 2018;72(4):351–366. [DOI] [PubMed] [Google Scholar]
- 28.Khariton Y, Hernandez AF, Fonarow GC, et al. Health Status Variation Across Practices in Outpatients With Heart Failure: Insights From the CHAMP-HF (Change the Management of Patients With Heart Failure) Registry. Circ Cardiovasc Qual Outcomes. 2018;11(4):e004668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khariton Yevgeniy. “Health Status Disparities by Sex, Race/Ethnicity, and Socioeconomic Status in Outpatients with Heart Failure.” JACC.Heart Failure, vol. 6, no. 6, June/2018, pp. 465–473, doi: 10.1016/j.jchf.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fonarow GC, Albert NM, Curtis AB, et al. Associations between outpatient heart failure process-of-care measures and mortality. Circulation. 2011;123(15):1601–1610. [DOI] [PubMed] [Google Scholar]
- 31.Fonarow GC, Albert NM, Curtis AB, et al. Improving evidence-based care for heart failure in outpatient cardiology practices: primary results of the Registry to Improve the Use of Evidence-Based Heart Failure Therapies in the Outpatient Setting (IMPROVE HF). Circulation. 2010;122(6):585–596. [DOI] [PubMed] [Google Scholar]
- 32.Fonarow Gregg C. “Influence of a Performance-Improvement Initiative on Quality of Care for Patients Hospitalized with Heart Failure: Results of the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF).” Archives of Internal Medicine (1960), vol. 167, no. 14, July/2007, pp. 1493–1502, doi: 10.1001/archinte.167.14.1493. [DOI] [PubMed] [Google Scholar]
- 33.Basch Ethan. “Symptom Monitoring with Patient-Reported Outcomes during Routine Cancer Treatment: A Randomized Controlled Trial.” Journal of Clinical Oncology, vol. 34, no. 6, February/2016, pp. 557–565, doi: 10.1200/JCO.2015.63.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Basch Ethan, et al. “Overall Survival Results of a Trial Assessing Patient-Reported Outcomes for Symptom Monitoring During Routine Cancer Care.” JAMA, vol. 318, no. 2, June/2017, pp. 198–199, doi: 10.1001/jama.2017.7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stehlik J, Rodriguez-Correa C, Spertus JA, et al. Implementation of Real-Time Assessment of Patient-Reported Outcomes in a Heart Failure Clinic: A Feasibility Study. J Card Fail. 2017;23(11):813–816. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.