Abstract
Syphilis control strategies are old, but interventions have changed and there is now a more scientific approach to evidence of effectiveness. We searched PubMed using “Syphilis control” to identify papers that measured the effectiveness of interventions. We also included novel approaches and comprehensive responses to outbreaks. Few papers used high-quality research methodology and fewer evaluated impact on prevalence or incidence; most assessed intermediate outcomes. Syphilis can often be controlled by a combination of case finding, treatment, and education. However, outbreaks are unique and ongoing evaluation is needed to see if interventions are producing intended intermediate outcomes at reasonable costs.
Syphilis is an old disease with control efforts dating back to at least 1497.1 Prevention was facilitated in the 1900’s by the identification of the causative organism (Treponema pallidum), development of antibody tests, and effective (though difficult to take) treatment.2 In 1937, Thomas Parran outlined a comprehensive syphilis control strategy with general categories of interventions that have not changed much.3 However, syphilis interventions have changed because syphilis has changed, society and technology have evolved, and there has been a more scientific approach to the collection of evidence of effectiveness.
As the prevalence and pathogenicity of infection have decreased, so has knowledge of the disease. In the 1890–1910 Oslo cohort of persons with untreated syphilis, syphilis was the cause of death for 15.1% of the men and 8.3% of the women.4 By the 1930’s syphilis was a more benign disease than it was in the 1490’s.2 Still, the death rate from syphilis among adults in the United States was 16 per 100,0002 which is equal to the diagnosis rate in 2012 (16.0 for all ages, all stages)5 and the diagnosis rate for HIV in 2011 (15.8 for all ages).6 Syphilis (dementia paralytica) was a common cause for admission to mental hospitals in 1912–1934.7 A study from Philadelphia General Hospital in 1927–1937 found evidence of syphilitic heart disease in 6.9% of 15,000 autopsies.8 Syphilis deaths among adults are now rare. The most common complication in adults appears to be acute symptomatic neurosyphilis which has been estimated to affect 1.7% of HIV-infected men who have sex with men (MSM) who acquire syphilis.8 This decrease in disease may be due to inadvertent cure of syphilis when antibiotics are used for other conditions, or an evolution of the organism.2 In contrast, congenital syphilis continues to be a major problem in many areas, and may equal HIV as a threat to newborn health and wellness.10 Even in developed countries, stillbirths due to congenital syphilis may rapidly follow the emergence of syphilis among adults. The control of congenital syphilis is discussed elsewhere in this special issue.11
Some characteristics of syphilis have a major impact on the effectiveness of control efforts. Following infection, there is an incubation period of at least 3 weeks before infection can be detected or sexually transmitted. Sexual transmission occurs mainly during the primary and secondary stages, which limits the duration of infectiousness. Lesions are usually painless and often unnoticed, so one-half to three-quarters of infections are diagnosed in the latent stage.5 Syphilis is transmitted to 15.9%–30.3% of partners of patients with early syphilis (based on the development of antibody during follow-up of untreated sexual contacts from the preceding 30 days);12, 13 this is slightly less infectious than gonorrhea, where the risk is 19% after one exposure and about 80% after several,14 and more than HIV which is 0.04%–0.08% per exposure for heterosexual discordant couples.15 Persons who are cured of syphilis are susceptible to re-infection. Re-infection is difficult to diagnose in the latent stage because diagnosis requires demonstration of an increased nontreponemal antibody titer compared to previous titers. This adds additional complexity to syphilis control because it requires maintaining readily retrievable records of past nontreponemal test titers.
Syphilis control efforts should be adjusted to fit the epidemiology of infection. In some areas syphilis has never been controlled. Those areas tend to have low access to medical care and limited ability to measure the contribution of syphilis to ill health. Some areas experience occasional outbreaks among heterosexuals that can be controlled by emphasis on the usual syphilis-control interventions.16 Other areas are experiencing high and increasing rates among men who have sex with men (MSM), and often these rates have been increasing for 10–14 years despite intensive intervention efforts.17–20 Some areas have combinations of these.21 Here, we review the published literature on syphilis control to help inform syphilis prevention and control activities.
Methods:
We conducted a systematic search using PubMed for English language articles under the heading “Syphilis control”. There were 3,509 articles on June 20, 2014. We were most interested in papers that measured the effectiveness of interventions, but also included novel approaches, papers that described comprehensive responses to outbreaks, and especially papers that were published around the time of the previous surge in syphilis rates among MSM (1969–1982). Our search was supplemented by papers referenced in the papers we read.
Results:
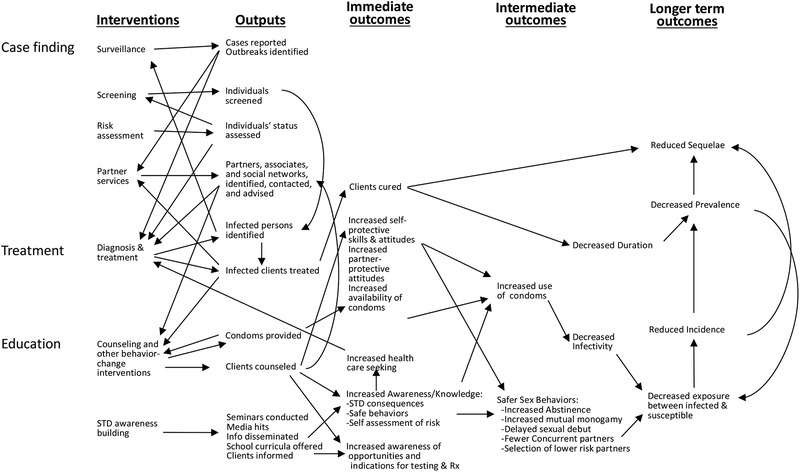
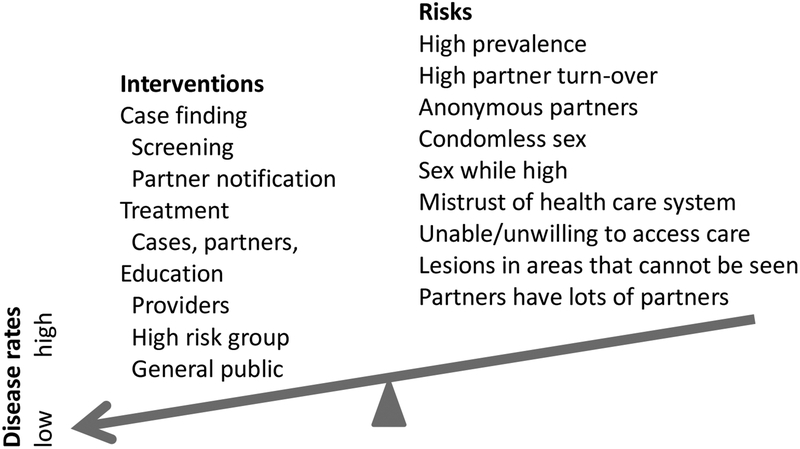
Hundreds of papers have been written about the control of syphilis; some are research studies,22, 23 but most describe the use of multiple interventions.17, 24–29 Most of the papers we found did not use high-quality research methodology.30 Very few intervention studies evaluated the effect of the intervention on the prevalence or incidence of syphilis; most evaluated the ability to influence intermediate outcomes that should ultimately influence incidence (Figure 1). For example, studies of partner notification services often measure the number of partners cured but do not assess the impact on incidence or prevalence of syphilis in the population. Many factors influence the incidence and prevalence of syphilis (Figure 2). Usually a variety of interventions are occurring with varying intensity while the population-at-risk is changing. Without randomized controlled trials, it is difficult to measure the impact of an intervention. Rising rates do not mean a control program is ineffective, because we do not know what rates would be without the program. Furthermore, effective programs often increase the detection of latent infections that would otherwise go undetected. Even if there are good randomized trials, it is difficult to generalize results to other settings.
Figure 1.
STD Prevention System. Most interventions are evaluated for their effects on immediate outcomes such as clients cured. The impact on incidence is influenced by the stage of syphilis and the number of partners that the infected person has, factors not shown on this already complicated schema.
Figure 2.
Balancing interventions and risks. Multiple interventions usually have a greater effect than any single intervention. The impact of a particular intervention is hard to measure because disease rates are influenced by many factors that are continually changing.
Our goal was to describe efforts to control syphilis, with an emphasis on evidence of effectiveness. Therefore, our findings are organized around the types of interventions, rather than the types of evidence. Intervention categories are similar to those used by Parran.3 Case finding may involve: 1) screening (widespread or targeted); 2) tracing sex partners; and sometimes 3) tracing social contacts of infected persons so they can be examined and treated. Education may involve: 1) clinicians so they test patients and examine them for lesions; 2) persons-at-risk so they seek screening, and check themselves and their partners for ulcers or rashes; and 3) the general public so they support disease control measures. Finally, treatment may include: 1) persons who seek care for symptoms; 2) infections identified by screening; 3) sex partners or others who were potentially exposed; and 4) occasionally all persons considered at-risk in a particular area.
Case finding
Screening
We did not identify any trials that assessed the effectiveness of screening to prevent transmission of syphilis at the population level. Many papers report the number tested, the percent with positive tests, or the percent with a newly positive test, however there is no benefit if infections are untreated.31, 32 Usually screening identifies latent infections and only prevents onward transmission if early latent infections are treated before they lapse or relapse into secondary syphilis. Treating late latent infections does not contribute much to preventing onward transmission.33 Screening occasionally identifies persons with primary or secondary syphilis who had not noticed or mentioned the signs of disease.34 Transmission is more likely to be prevented when persons with high numbers of partners are treated compared to persons with one steady partner who are easier to find.33 Thus, transmission is most likely prevented when an infected person with multiple partners is treated before progressing to primary syphilis, but these characteristics are rarely measured.
Parran recommended screening by insurance companies, law enforcement agencies, hospitals, and before marriage or employment.3 Most of these routine screenings have since been stopped due to low numbers of new infections identified, and the U.S. Preventive Services Task Force specifically recommends not screening low-risk persons.35 When the prevalence is low, understanding the epidemiology will help target screening to persons at highest risk. Target areas may include neighborhoods,36 bathhouses,37 jails,38–40 or HIV care clinics.34 For example, in 2011 in Seattle, it was estimated that 4% of HIV-infected MSM had acquired syphilis that year,41 so screening MSM in HIV care settings in Seattle would be a priority. Monthly syphilis testing was recommended for MSM at highest risk during the 1980’s epidemic, but compliance with the recommendation was not measured.42 In 2012, in San Francisco, screening of MSM was recommended every 3 months, and 37.5% of MSM surveyed reported compliance with that recommendation.43 Interventions to increase screening in clinics work best if they involve a structural intervention such an electronic reminder or automatic testing for syphilis when certain other tests are ordered.44–46 A clinic in Melbourne implemented a policy to do syphilis serology whenever a blood test was performed as part of HIV monitoring, and the average time between the last syphilis serology and the diagnosis of syphilis dropped from a median of 214 days to 90 days,34 which could significantly impact transmission. HIV care programs that institute opt-out syphilis testing have had higher coverage of syphilis testing (87%) compared to clinics that use opt-in testing (74%) or risk based screening (22%).47 Large increases in screening for one group often necessitate decreases in screening for other groups which might have negative consequences. For example, legislation in Victoria, Australia, required monthly screening of sex workers for certain STI and 3-monthly testing for syphilis and HIV, and compromised accessibility to services by MSM and other clients who were more likely to be infected than the sex workers.48
The cost of screening depends on the infrastructure needed to support it. Programs have sent mobile vans to neighborhoods,49 community events,50 or tried co-sponsoring parties that included testing for a variety of infections51 but these special efforts have generally identified few infections.52 For example, one program set up 16 screening events in areas of high disease rates, tested 240 persons and 45 had positive treponemal tests, of which 3 also had newly recognized positive non-treponemal tests suggesting latent infection, but none could be re-located for treatment.32 The cost of setting up a stand-alone syphilis testing service is likely to be too high compared to other approaches. Costs are reduced when syphilis testing is added to other screening programs such as HIV outreach testing in areas where MSM are at high risk for both infections. Screening is most efficiently done in medical care settings where blood is drawn for other reasons. Of course, the trade-off is that clinic-associated screening only reaches persons who are accessing care.
Rapid syphilis tests are now available in some areas,53 though in the United States none have been approved by the Food and Drug Administration for use outside of a laboratory. Rapid tests can eliminate the need to return for results and thereby facilitate immediate treatment of an infection (avoiding loss-to-follow-up and stopping onward transmission).31, 54 The weakness of current rapid tests is their inability identify re-infections in persons who were treated for syphilis in the past. This is because treponemal tests usually remain positive for life following successful treatment, and non-treponemal tests can also remain positive, especially if the infection was treated in the late latent stage. Rapid tests are most valuable in certain settings, such as screening of pregnant women in developing countries, where overtreatment is much less of an issue than undertreatment.54, 55 Also, in areas where there has not been much of a syphilis control effort, rapid tests will be valuable for a while as they identify untreated old infections, but eventually most positive tests will be from treated old infections, and treatment decisions will need to be modified because repeated retreatment will be an issue.53 For example, in Bangalore, India, point-of-care testing and immediate treatment increased the appropriate treatment of sex workers from 44.8% to 68.3%, because women often did not return for their results.56 However, the future of this screening approach is limited because these women will continue to test positive after they are treated. In one report, 94 (18.6%) of 505 persons tested reported they had been previously treated for syphilis,37 so a treponemal screening test would not be helpful for them. In another report of screening in correctional facilities, over 5% of persons tested had positive treponemal and non-treponemal tests, but only about 5% of those positives were new positive tests.52 At present, in many developed countries, rapid tests would be very useful for new outbreaks among heterosexual populations, but of limited value in addressing ongoing high rates of syphilis among MSM.
Some new approaches to screening have been tried. Online access to a lab slip good for free syphilis testing at different venues, with online availability of results, led to identification and treatment of 6 new syphilis cases within a year in San Francisco in 2003.57 A clinic in Melbourne used automated text messages and e-mail reminders that MSM could choose to remind themselves to get tested for STD. Those who used it were more likely to have early latent syphilis detected (1.7%) compared to non-users (0.4%).58
Partner Notification
Tracing and treating partners can interrupt transmission of syphilis if exposed partners are identified and treated before they transmit. Because transmission occurs during primary and secondary stages, transmission is much more likely to be interrupted by tracing partners of someone with early syphilis. Partners of persons with late latent infection may benefit from treatment so that they do not develop tertiary syphilis, but it is usually too late to interrupt transmission. Intensive partner notification efforts are a classic part of an intensive syphilis control effort,36 and a mainstay of syphilis control in general, but the net effect on transmission or prevalence in the community has not been measured.59 A review of 18 reports of partner notification for syphilis found an average of 0.22 new infections brought to treatment for every index case interviewed.59 The number of new cases found per patient interviewed has fallen in recent years. An analysis from 4 STD programs found only 5%–14% of the index case interviews led to the identification of a new infection.60 Some MSM have been reluctant to talk to the health department, and many have no way of re-contacting anonymous partners. Anonymous partners have been a problem for partner notification in the past, during outbreaks associated with exchanging sex for drugs or money.61 In one study, the numbers of contacts notified were similar for MSM and heterosexual men, though the MSM had more total partners and therefore more partners who were not notified.62
There is one good, but small, randomized controlled trial that assessed the benefit of using health department personnel to notify partners instead of relying on the patient, and it was partner notification for HIV infection, not syphilis.63 In that study, 35 patients were randomized to notify their own partners; 10 were notified and 1 new infection was discovered. For the 39 patients randomized to have the health department notify their partners, 78 were notified and 9 infections were diagnosed. More infected contacts are identified when tracing partners of persons with early syphilis compared to tracing partners of persons with late latent, but otherwise it has been difficult to predict which patients should be prioritized for partner notification interviews.60, 64 Interviewing techniques have been identified that improve recall of partners not initially named in an interview.65
In the past, unnamed sex workers might be located based on where they were met and some characteristics of their appearance, now the challenge is figuring out how to find partners met via the internet or smartphone geosocial networking applications.66 Some health departments have tried to keep up with these technologies. One investigation of 53 patients who had only e-mail addresses for partners obtained 177 e-mail addresses, notified 89 partners, evaluated 71 and found 3 new infections.67 Another investigation of 27 patients with only e-mail addresses led to the examination of 53 partners of whom 6 were treated for a new infection, and 110 others responded to the e-mail of whom 48 did not provide identifying information but reported that they had been examined.68 Social media such as Facebook have also been used to identify partners.69, 70 Thus, while new technology has provided the opportunity to meet partners who cannot be traced, it also increases the ability to trace partners in a hurry.
Contact tracing is sometimes extended to include non-sexual contacts who are thought by the patients, or their partners to possibly benefit from syphilis screening. In one report, patients’ sex partners were more likely to have syphilis (11% of 984) than other persons from the patients’ social networks (3% of 1146), but prophylactic treatment was given to more social contacts (836) than sex partners (547) and that might have played a role in stopping the outbreak.71
Partner notification alone is unlikely to control an outbreak of syphilis, particularly when some persons have dozens of partners who they cannot identify.61 For example, in Madagascar 565 index cases led to notification of 99 infected partners, but the cases also reported 2891 other partners who could not be contacted.72 Screening and partner notification are synergistic. During an outbreak, interviews of cases can lead to screening at places where cases had met their sex partners. In one such investigation, outreach screening of 69 persons identified 3 new cases.73 Occasionally resources have been concentrated to increase both screening and partner notification. In Alabama in 1965, five such “blitzes” traced the contacts of 196 patients with early syphilis, finding 68 primary, 33 secondary, and 12 early latent cases.36
Education
Providers
When syphilis emerges in a new population, there are likely to be many clinicians who have not seen it before, and some aspects of syphilis treatment and control may be unfamiliar to them. Although ulcers look painful, they are usually painless, and are likely to go unrecognized by patients if they are in areas that they cannot directly observed such as in their mouth or anus. Patients may be reluctant to mention them if they are not comfortable talking to their clinician. Even if clinicians know about a resurgence of syphilis, some may be reluctant to ask patients about potential risks. We found no studies designed to evaluate the impact of alerting providers and teaching them about syphilis, but most outbreak responses encourage providers to look for syphilis and to screen persons at risk for infection.
Public
Many now consider other STD to be of greater concern than syphilis, especially the incurable viruses: HIV, HSV, HBV, and HPV. MSM are now more familiar with HIV than syphilis, and some are unaware of the risk of syphilis via oral sex,74 or that lesions are usually painless. If persons at high risk are aware of the signs and symptoms, they may be more likely to seek medical care during these highly infectious stages. In one pilot study, MSM who read materials describing syphilis were slightly more likely to check themselves and their partners compared to men who had not (aPR 1.3, 95% CI 1.15–1.52).75 Social marketing campaigns have tried to increase awareness of syphilis, decrease risky sexual practices, and increase testing among MSM. Results are mixed. One evaluation in San Francisco found MSM with unaided awareness of the “Healthy Penis” campaign were more likely to have had a test (60%) than men who were unaware of the campaign (35%).76 An evaluation of the “Stop the Drama Downunder” campaign in Australia found it increased awareness of syphilis and increased testing at STD clinics by 19%.77 However, evaluation of a marketing campaign in Miami found limited awareness of the campaign, and no impact on knowledge, clinic visits, or testing.78 A subsequent campaign in San Francisco also found no relationship between awareness of the “Dogs are Talking” campaign and testing.79 A $812,651 campaign to increase syphilis screening among MSM in Los Angeles had unaided awareness of 20.7% and aided awareness of 67.5% but only 30.4% of those who were aware knew that the campaign was about syphilis, and total awareness was not significantly associated with testing.80
Broader interventions have been tested, mostly as a part of HIV interventions. They have had limited impact. A intervention to empower female sex workers and MSM in India led to similar changes in condom use and syphilis rates after 3 years in the 24 intervention and comparison communities.81 A behavior change intervention that used popular opinion leaders was successful in some settings, but when tested in a randomized-controlled trial with 68 community pairs around the world there was no significant impact on a composite rate of 6 STI.82
Treatment
Treating cases
The benefits of making treatment available have been demonstrated in a community randomized controlled trial in Mwanza, Tanzania where improved syndromic control of STDs lowered the prevalence of high-titer syphilis from 6.2% to 5.0% while control areas saw a slight increase from 6.2% to 7%, an adjusted relative risk of 0.71.83 There have been great declines in syphilis in some parts of Africa which might be partly due to syndromic treatment,84 though the contribution of AIDS mortality to decreases in syphilis is another factor.85 Clearly, treatment is a fundamental aspect of nearly all interventions (Figure 1).
Treating Contacts
Studies that followed untreated recent (one month) sex partners of persons with primary or secondary syphilis found 15.9–30.3% developed positive antibody tests.12,13 Once that high rate of seronegative infection was demonstrated, most clinicians started treating recent contacts presumptively because the risk of undiagnosed infection was so high, the cost of treatment was so low, presumptive treatment removed the need for return visits (or searching for persons who do not return), and it prevented any onward transmission that might otherwise occur while waiting for definitive proof of infection. Treatment of all recently exposed partners of persons with early syphilis is important. In some outbreak settings there may be a benefit to empirically treating non-sexual contacts from the same social network as syphilis cases.71
Mass treatment
Mass treatment was tried for a syphilis outbreak in Vancouver in 2000 that largely involved drug users and sex workers.86 Participants were given azithromycin (1.8 g) to take themselves and to give to others who might benefit, ultimately reaching an estimated 4036 persons. Cases decreased for 6 months, but then returned to preintervention rates, leading investigators to conclude that there was not much benefit. A randomized community trial of three rounds of mass treatment starting in 1994 in Rakaii, Uganda, lowered the antibody titers of persons with prevalent syphilis infections, but did not lower the incidence of new syphilis (1.5 per 100 person-years in both groups).87
It is possible that mass treatment would work better in other settings or when used in small outbreaks. In an RCT in Kenya, sex workers who were treated monthly with 1 g of azithromycin had a lower incidence of gonorrhea and chlamydia compared to untreated sex workers; however syphilis rates were similar (relative risk 1.02, with large 95% confidence intervals, 0.54–1.95).88 Other studies have had weak (or no) comparison groups. Periodic presumptive treatment of high-risk women in a South African mining community lowered their prevalence of gonorrhea or chlamydia from 10.9% to 6.2%, and genital ulcer disease from 5.8% to 1.3%, and also decreased rates of symptomatic disease among miners.89 A syphilis epidemic among farm workers and sex workers in Fresno, California in 1976 was addressed by asking sex workers to visit a clinic every 6–10 weeks for testing and treatment (with benzathine penicillin G).90 512 women were tested and treated 1180 times, with 30 having syphilis diagnosed at their initial visit and 6 at a subsequent visit, and the number of new syphilis cases declined by 51.3% among sex workers and 26.8% among farm workers. An outbreak associated with crack cocaine was similarly addressed in Pennsylvania over 6 days in 1989. Using a mobile van, 136 persons were tested and empirically treated with benzathine penicillin G, of whom 15 (11%) were infected.49 Case rates subsequently declined, but it is hard to know why. An outbreak in an Alabama prison was addressed by treating all 1,076 prisoners with benzathine penicillin G (or erythromycin) of whom 82 were diagnosed with syphilis.36 Theoretically, the benefits of mass treatment could be reduced if treatment caused a compensatory increase in risk behavior. One study assessed this by contacting 125 persons 1–4 months after empiric antibiotic treatment and found no evidence of an increase in sexual risk behavior, and 81% reported they would be willing to take benzathine penicillin or azithromycin monthly.91 Treatment failures and reports of Increasing resistance now limit the prospects for mass treatment using azithromycin.92 However, many MSM in Australia have reported they would be willing to take doxycycline to prevent syphilis.93
Other interventions and combination approaches
The People’s Republic of China virtually eliminated syphilis and other STDs in the late 1950’s, moving from a prevalence of 10% to nearly zero by a combination approach that included the usual training health care workers, screening, and public education, but added major efforts to eliminate sex work. Brothels were closed, sex workers were moved to re-education facilities, and there were also major changes in the status of women in the society.94 A few other large multi-component interventions have been reported. A behavioral and STI services intervention in Masaka, Uganda, lowered the incidence of high-titer syphilis compared to a control community (0.3 vs 0.6 per 100 person years).95 A multicomponent community RCT in Peru reduced (slightly) the over-all risk of STD (RR 0.84, 95% CI 0.69–1.02) by: improving syndromic treatment; promoting condoms; and mobile outreach to sex workers for STI screening, presumptive trichomoniasis treatment and condom promotion.96 Randomized controlled trials have found that male circumcision reduced the risk of HIV, HSV, and HPV, but there was no impact on syphilis (2.4% of 2083 in the intervention group vs 2.1% of 2143 controls).97
Barriers
Syphilis control efforts have identified many successful interventions, but some barriers remain unresolved. There are no reliable tests for diagnosing latent infection with Treponema pallidum.98 Instead, diagnosis is based on a combination of clinical information plus past and present antibody test results. Antibody tests are insensitive and nonspecific for infection. They may be negative during primary infection or revert to negative during late stage infection. Tests often remain positive after infection has cleared, making it difficult to monitor response to therapy, or to tell if a person who was once infected is still infected. Simple tests that detect infection would be helpful. Treatment with penicillin is highly effective, but it would be helpful to have oral, single dose, alternatives that could be administered in the field. Past studies in Tuskegee and Guatemala have led to improvements in guidelines for conducting research but they left a legacy of distrust in government-based STD control programs.99 Control programs have many strategies that they can use to intervene, but measuring the success of a given intervention is not straightforward, so it is difficult to know which interventions to use and which ones to stop.
Conclusion
Syphilis can be often controlled by a combination of case finding, treatment, and education. Evidence from past control efforts can help decide which combinations are likely to influence current outbreaks. Innovative twists on old approaches will be needed as populations change and the factors that contribute to syphilis continue to evolve. Evaluations of the costs and the effectiveness of those innovations will be helpful. However, outbreaks are unique. All programs will need ongoing evaluation of their approaches to see if they are producing the intended intermediate outcomes at a reasonable cost.
References
- 1.Parascondola John. Sex, Sin, and Science: A History of Syphilis in America. Westport CT: Praeger 2008, p7. [Google Scholar]
- 2.Moore JE. An Evaluation of public-health measures for the control of syphilis: an epidemiological study. Lancet 1951. 699–711. [DOI] [PubMed] [Google Scholar]
- 3.Parran T Shadow on the Land: Syphilis. (New York: Reynal and Hitchcock, 1937). [Google Scholar]
- 4.Gjestland T The Oslo study of untreated syphilis: an epidemiologic investigation of the natural course of the syphilitic infection based upon a re-study of the Boeck-Bruusgaard Material. Acta Dermato-Venereologica 1955;35 Supp 34:364. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance, 2012. Atlanta: U.S. Department of Health and Human Services; 2013. p 80 [Google Scholar]
- 6.Centers for Disease Control and Prevention. HIV Surveillance Report, 2011; vol. 23 http://www.cdc.gov/hiv/pdf/statistics_2011_HIV_Surveillance_Report_vol_23.pdf Page 18 Published February 2013 Accessed July 24, 2014. [Google Scholar]
- 7.Moore M, Merritt HH. Role of syphilis of the nervous system in the production of mental disease: a survey of the various forms of neurosyphilis occurring at the Boston Psychopathic Hospital from 1912 to 1934. JAMA 1936;107:1292–1293. [Google Scholar]
- 8.Welty JW. A necropsy survey of cardiovascular syphilis with particular reference to its decreasing incidence. Am J Med Sci 1939;197:782–793. [Google Scholar]
- 9.Centers for Disease Control and Prevention. Symptomatic Early Neurosyphilis Among HIV-Positive Men Who Have Sex with Men --- Four Cities, United States, January 2002--June 2004. MMWR Morbid Mortal Weekly Rep 2007;56:625–628. [PMC free article] [PubMed] [Google Scholar]
- 10.Klausner JD. The sound of silence: missing the opportunity to save lives at birth. Bull World Health Organ 2013;91:158–158a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Congenital syphilis. Sexual Health Special issue [Google Scholar]
- 12.Moore MBJr Price EV, Knox JM Elgin LW. Epidemiologic treatment of contacts to infectious syphilis. Public Health Rep 1963;78:966–970. [PMC free article] [PubMed] [Google Scholar]
- 13.Schroeter AL, Turner RH, Lucas JB, Brown WJ. Therapy for incubating syphilis: effectiveness of gonorrhea treatment. JAMA 1971;218:711–713. [PubMed] [Google Scholar]
- 14.Hooper RR, Reynolds GH, Jones OG, et al. Cohort study of venereal disease. I: The risk of gonorrhea transmission from infected women to men. Am J Epidemiol 1978;108:136–144. [DOI] [PubMed] [Google Scholar]
- 15.Patel P, Borkowf CB, Brooks JT, Lasry A, Lansky A, Mermin J. Estimating per-act HIV transmission risk: a systematic review. AIDS 2014;28:1509–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finelli L, Levine WC, Valentine J, St Louis ME. Syphilis outbreak assessment. Sex Transm Dis 2001;28:131–135. [DOI] [PubMed] [Google Scholar]
- 17.Thomas DDR, Cann KF, Evans MR, et al. The public health response to the re-emergence of syphilis in Wales, UK. Int J STD AIDS; 2011;22:488–492. [DOI] [PubMed] [Google Scholar]
- 18.Bernstein KT, Stephens SC, Strona FV, Kohn RP, Philip SS. Epidemiologic characteristics of an ongoing syphilis epidemic among men who have sex with men, San Francisco. Sex Transm Dis 2013;40:11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Z, Xu J, Liu E, et al. HIV and syphilis prevalence among men who have sex with men: a cross-sectional survey of 61 cities in China. Clin Infect Dis 2013;57:298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang HT, Tang W, Xiao ZP, et al. Worsening epidemic of HIV and syphilis among men who have sex with men in Jiangsu, China. Clin Infect Dis 2014. Mar 18 EPub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ward JS, Guy RJ, Akre SP, et al. Epidemiology of syphilis in Australia: moving toward elimination of infectious syphilis form remote Aboriginal and Torres Strait Islander communities? Med J Aust 2011;194:525–529. [DOI] [PubMed] [Google Scholar]
- 22.Wetmore CM, Manhart LE, Wasserheit JN. Randomized controlled trials of interventions to prevent sexually transmitted infections: learning from the past to plan the future. Epidemiol Rev 2010;32:121–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steen R, Chersich M, Gerbase A, et al. Periodic presumptive treatment of curable sexually transmitted infections among sex workers: a systematic review. AIDS 2012;26:437–445. [DOI] [PubMed] [Google Scholar]
- 24.Gabel H, Foust E, Ogburn D, et al. Outbreak of primary and secondary syphilis—Guilford County, North Carolina, 1996–1997. Morbid Mortal Wkly Rep 1998;47:1070–1073. [PubMed] [Google Scholar]
- 25.Gunn RA, Harper SL, Borntrager DE, Gonzales PE, St Louis ME. Implementing a syphilis elimination and importation control strategy in a low-incidence urban area: San Diego County, California, 1997–1998. Am J Public Health 2000;90:1540–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mak DB, Johnson GH, Plant AJ. A syphilis outbreak in remote Australia: epidemiology and strategies for control. Epidemiol Infect 2004;132:805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lomax N, Wheeler H, Anaraki S, Anderson H, Goh B. Management of a syphilis outbreak in street sex workers in east London. Sex Transm Infect 2006;82:437–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tucker JD, Cohen MS. China’s syphilis epidemic: epidemiology, proximate determinants of spread, and control responses. Curr Opin Infect Dis 2011;24:50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwan KSH, Giele CM, Greville HS, Reeve CA, Lyttle PH, Mak DB. Syphilis epidemiology and public health interventions in Western Australia from 1991 to 2009. Sexual Health 2012;9:272–279. [DOI] [PubMed] [Google Scholar]
- 30.Balshem H, Helfand M, Schϋnemann HJ, et al. GRADE guidelines: 3. rating the quality of evidence. J Clin Epidemiol 2011;64:401–406. [DOI] [PubMed] [Google Scholar]
- 31.Blank S, McDonnell DD, Rubin SR, et al. New approaches to syphilis control: finding opportunities for syphilis treatment and congenital syphilis prevention in a women’s correctional setting. Sex Transm Dis 1997;24:218–226. [DOI] [PubMed] [Google Scholar]
- 32.Goswami ND, Hecker EJ, Vickery C, et al. Geographic information system-based screening for TB, HIV, and syphilis (GIS-THIS): a cross-sectional study. PLoS One 2012;7:e46029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kahn RH, Peterman TA, Arno J, Coursey EJ, Berman SM. Identifying likely syphilis transmitters: implications for control and evaluation. SEx Transm Dis 2006;33:630–635. [DOI] [PubMed] [Google Scholar]
- 34.Bissessor M, Fairley CK, Leslie D, Howley K, Chen MY. Frequent screening for syphilis as part of HIV monitoring increases the detection of early asymptomatic syphilis among HIV-positive homosexual men. J Acquir Immune Defic Syndr 2010;55:211–216. [DOI] [PubMed] [Google Scholar]
- 35.U.S. Preventive Services Task Force. Screening for syphilis infection: recommendation statement http://www.uspreventiveservicestaskforce.org/3rduspstf/syphilis/syphilrs.pdf Accessed 27 June 2014. [Google Scholar]
- 36.Smith WHY. Blitz on syphilis in Alabama. Public Health Rep 1966;9:835–841. [PMC free article] [PubMed] [Google Scholar]
- 37.Wolf FC, Judson FN. Intensive screening for gonorrhea, syphilis, and hepatitis B in a gay bathhouse does not lower the prevalence of infection. Sex Transm Dis 1980;7:49–52. [DOI] [PubMed] [Google Scholar]
- 38.Blank S, Sternberg M, Neylans LL, Rubin SR, Weisfuse IB, St Louis ME. Incident syphilis among women with multiple admissions to jail in New York City. J Infect Dis 1999;180:1159–1163. [DOI] [PubMed] [Google Scholar]
- 39.Kahn RH, Voigt RF, Swint E, Weinstock H. Early syphilis in the United States identified in corrections facilities, 1999–2002. Sex Transm Dis 2004;31:360–364. [DOI] [PubMed] [Google Scholar]
- 40.Spaulding AD, Miller J, Trigg BG, et al. Screening for sexually transmitted diseases in short-term correctional institutions: summary of evidence reviewed for the 2010 Centers for Disease Control and Prevention Sexually Transmitted Diseases Treatment Guidelines. Sex Transm Dis 2013;40:679–684. [DOI] [PubMed] [Google Scholar]
- 41.Kerani RP, Golden MR. 2011 King county sexually transmitted diseases epidemiology report. p 28 http://www.kingcounty.gov/healthservices/health/communicable/std/statistics.aspx Accessed April 7, 2014.
- 42.Bolan RK. Sexually transmitted diseases in homosexuals: focusing the attack. Sex Transm Dis 1981;8:293–297. [PubMed] [Google Scholar]
- 43.Katz KA, Raymond HF, Bernstein KT, Klausner JD. Knowledge, attitudes, and practices regarding syphilis screening among men who have sex with men in San Francisco. Sex Transm Dis 2013;40:318–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cohen CE, Winston A, Asboe D, et al. Increasing detection of asymptomatic syphilis in HIV patients. Sex Transm Infect 2005;81:217–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bissessor M, Fairley CK, Leslie D, Chen MY. Use of a computer alert increases detection of early, asymptomatic syphilis among higher-risk men who have sex with men. CID 2011:53:57–58. [DOI] [PubMed] [Google Scholar]
- 46.Taylor M, et al. Special issue journal. Sex Transm Dis. In preparation. [Google Scholar]
- 47.Guy R, El-Hayek C, Fairley CK, et al. Opt-out and opt-in testing increases syphilis screening of HIV-positive men who have sex with men in Australia. PLoS One 2013:8(8):e71436. doi: 10.1371/journal.pone.0071436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chow EPF, Fehler G, Chen MY, et al. Testing commercial sex workers for sexually transmitted infections in Victoria, Australia: an evaluation of the impact of reducing the frequency of testing. PLoS ONE 2014:9:e103081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hibbs JR, Gunn RA. Public health intervention in a cocaine-related syphilis outbreak. Am J Public health 1991;81:1259–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Read PJ, Knight V, Bourne C, et al. Community event-based outreach screening for syphilis and other sexually transmissible infections among gay men in Sydney, Australia. Sex Health 2013;10:357–362. [DOI] [PubMed] [Google Scholar]
- 51.Blank S, Gallagher K, Washburn K, Rogers M. Reaching out to boys at bars: utilizing community partnerships to employ a wellness strategy for syphilis control among men who have sex with men in New York City. Sex Transm Dis 2005;32 (Suppl):S65–S72. [DOI] [PubMed] [Google Scholar]
- 52.Lewis FM, Schillinger JA, Taylor M, et al. Needle in a haystack: the yield of syphilis outreach screening at 5 US sites—2000–2007. J Public Health Manag Pract 2011;17:513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tucker JD, Bu J, Brown LB, Yue-Pin Y, Chen X-S, Cohen MS. Accelerating worldwide syphilis screening through rapid testing: a systematic review. Lancet Infect Dis 2010;10:381–386. [DOI] [PubMed] [Google Scholar]
- 54.Strasser S, Bitarakwate E, Gill M, et al. Introduction of rapid syphilis testing within prevention of mother-to-child transmission of HIV programs in Uganda and Zambia: a field acceptability and feasibility study. J Acquir Immune Defic Syndr 2012;61:e40–e46. [DOI] [PubMed] [Google Scholar]
- 55.Tucker JD, Bien CH, Peeling RW. Point-of-care testing for sexually transmitted infections: recent advances and implications for disease control. Curr Opin Infect Dis 2013;26:73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mishra S, Naik B, Venugopal B, et al. Syphilis screening among female sex workers in Bangalore, India: comparison of point-of-care testing and traditional serological approaches. Sex Transm Infect 2010;86:193–198. [DOI] [PubMed] [Google Scholar]
- 57.Levine DK, Scott KC, Klausner JD. Online syphilis testing—confidential and convenient. Sex Transm Dis 2005;32:139–141. [DOI] [PubMed] [Google Scholar]
- 58.Zou H, Fairley CK, Guy R, et al. Automated, computer generated reminders and increased detection of gonorrhoea, chlamydia and syphilis in men who have sex with men. PLoS One 2013;8:e61972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brewer DD. Case-finding effectiveness of partner notification and cluster investigation for sexually transmitted diseases/HIV. Sex Transm Dis 2005;32:78–83. [DOI] [PubMed] [Google Scholar]
- 60.Hoots BE, Lewis FMT, Anschuetz G, et al. Would targeting increase efficiency of syphilis partner services programs?—Data from New York City, Philadelphia, Texas, and Virginia. Sex Transm Dis 2014;41:407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Andrus JK, Fleming DW, Harger DR, et al. Partner notification: can it control epidemic syphilis? Ann Intern Med 1990;112:539–543. [DOI] [PubMed] [Google Scholar]
- 62.Samoff E, Koumans EH, Katkowsky S, Shouse RL, Markowitz LE, Fulton County Disease Investigation Working Group. Contact-tracing outcomes among male syphilis patients in Fulton County, Georgia, 2003. Sex Transm Dis 2007;34:456–460. [DOI] [PubMed] [Google Scholar]
- 63.Landis SE, Schoenbach VJ, Weber DJ, et al. Results of a randomized trial of partner notification in cases of HIV infection in North Carolina. N Engl J Med 1992;326:101–106. [DOI] [PubMed] [Google Scholar]
- 64.Marcus JL, Katz MH, Katz KA, Bernstein KT, Wolf W, Klausner JD. Prediction model to maximize impact of syphilis partner notification—San Francisco, 2004–2008. Sex Transm Dis 2010;37:109–114. [DOI] [PubMed] [Google Scholar]
- 65.Brewer DD, Potterat JJ, Muth SQ, et al. Randomized trial of supplementary interviewing techniques to enhance recall of sexual partners in contact interviews. Sex Transm Dis 2005;32:189–193. [DOI] [PubMed] [Google Scholar]
- 66.Rice E, Holloway I, Winetrobe, et al. Sex risk among young men who have sex with men who use Grindr, a smartphone geosocial networking application. J AIDS Clinic Res 2112, S4:005. doi: 10.4172/2155-6113.S4-005. [DOI] [Google Scholar]
- 67.Vest JR, Valadez AM, Hanner A, Lee JH, Harris PB. Using e-mail to notify pseudonymous e-mail sexual partners. Sex Transm Dis 2007;34:840–845. [DOI] [PubMed] [Google Scholar]
- 68.Ehlman DC, Jackson M, Saenz G, et al. Evaluation of an innovative internet-based partner notification program for early syphilis case management, Washington, DC, January 2007–June 2008. Sex Transm Dis 2010;37:478–485. [DOI] [PubMed] [Google Scholar]
- 69.Klausner JD, Wolf W, Fischer-Ponce L, Zolt I, Katz MH. Tracing a syphilis outbreak through cyberspace. JAMA 2000;284:447–449. [DOI] [PubMed] [Google Scholar]
- 70.Hunter P, Oyervides O, Grande KM, et al. Facebook-augmented partner notification in a cluster of syphilis cases in Milwaukee. Public Health Rep 2014;129 Suppl 1:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Engelgau MM, Wornle CH, Rolfs RT, Greenspan JR, O’Cain M, Gorsky RD. Control of epidemic early syphilis: the results of an intervention campaign using social networks. Sex Transm Dis 1995;22:203–209. [PubMed] [Google Scholar]
- 72.Khan MR, Ravelomanana N, Van Damme K, et al. Notifying partners of patients with early syphilis in Madagascar: case-finding effectiveness and public health implications. Trop Med Int Health 2010;15:1090–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Michaud JM, Ellen J, Johnson SM, Rompalo A. Responding to a community outbreak of syphilis by targeting sex partner meeting location: an example of a risk-space intervention. Sex Transm Dis 2003;30:533–538. [DOI] [PubMed] [Google Scholar]
- 74.Centers for Disease Control and Prevention. Transmission of primary and secondary syphilis by oral sex—Chicago Illinois, 1998–2002. MMWR Morb Mortal Wkly Rep 2004;53:966–968. [PubMed] [Google Scholar]
- 75.Surie D, Furness BW, Hernandez-Kline P, et al. Examining self and partners for syphilis among men who have sex with men: five US cities, 2009–2011. Int J STD AIDS 2012;23:859–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ahrens K, Kent CK, Montoya JA, et al. Healthy Penis: San Francisco’s social marketing campaign to increase syphilis testing among gay and bisexual men. PLoS Medicine 2006;3:e474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pedrana A, Hellard M, Guy R, et al. Stop the drama Downunder: a social marketing campaign increases HIV/sexually transmitted infection knowledge and testing in Australian gay men. Sex Transm Dis 2012;39:651–658. [DOI] [PubMed] [Google Scholar]
- 78.Darrow WW, Biersteker S. Short-term impact evaluation of a social marketing campaign to prevent syphilis among men who have sex with men. Am J Public Health 2008;98:337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stephens SC, Bernstein KT, McCright JE, Klausner JD. Dogs are talking: San Francisco’s social marketing campaign to increase syphilis screening. Sex Transm Dis 2010;37:173–176. [DOI] [PubMed] [Google Scholar]
- 80.Plant A, Javanbakht M, Montoya JA, Rotblatt H, O’Leary C, Kerndt PR. Check yourself: a social marketing campaign to increase syphilis screening in Los Angeles County. Sex Transm Dis 2014;41:50–57. [DOI] [PubMed] [Google Scholar]
- 81.Guiterrez JP, McPherson S, Fakoya A, Matheou A, Bertozzi SM. Community-based prevention leads to an increase in condom use and a reduction in sexually transmitted infections (STIs) among men who have sex with men (MSM) and female sex workers (FSW): the Frontiers Prevention Project (FPP) evaluation results. BMC Public health 2010;10:497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.The NIMH Collaborative HIV/STD Prevention Trial Group. Results of the NIMH collaborative HIV/sexually transmitted disease prevention trial of a community popular opinion leader intervention. J Acquir Immune Defic Syndr 2010;54:204–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mayaud P, Mosha F, Todd J, et al. Improved treatment services significantly reduce the prevalence of sexually transmitted diseases in rural Tanzania: results of a randomized controlled trial. AIDS 1997;11:1873–1880. [DOI] [PubMed] [Google Scholar]
- 84.Nagot N, Meda N, Ouangre A, et al. Review of STI and HIV epidemiological data from 1990 to 2001 in urban Burkina Faso: implications for STI and HIV control. Sex Transm Infect 2004;880:124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chesson HW, Dee TS, Aral SO. AIDS mortality may have contributed to the decline in syphilis rates in the United States in the 1990s. Sex Transm Dis 2003;30:419–424. [DOI] [PubMed] [Google Scholar]
- 86.Rekart ML, Patrick DM, Chakraborty B, et al. Targeted mass treatment for syphilis with oral azithromycin. Lancet 2003;361:313–314. [DOI] [PubMed] [Google Scholar]
- 87.Wawer MJ, Sewankambo NK, Serwadda D, et al. Control of sexually transmitted diseases for AIDS prevention in Uganda: a randomised community trial. Lancet 1999;353:525–535. [DOI] [PubMed] [Google Scholar]
- 88.Kaul R, Kimani J, Nagelkerke NJ, et al. Monthly antibiotic chemoprophylaxis and incidence of sexually transmitted infections and HIV-1 infection in Kenyan sex workers: a randomized controlled trial. JAMA 2004;291:2555–2562. [DOI] [PubMed] [Google Scholar]
- 89.Steen R, Vuylsteke B, DeCoito T, et al. Evidence of declining STD prevalence in a South African mining community following a core-group intervention. Sex Transm Dis 2000;27:1–8. [DOI] [PubMed] [Google Scholar]
- 90.Jaffe HW, Rice DT, Voigt R, Fowler J, St John RK. Selective mass treatment in a venereal disease control program. Am J Public Health 1979;69:1181–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Farley TA, Cohen DA, Kahn RH, Lolis S, Johnson G, Martin DH. The acceptability and behavioral effects of antibiotic prophylaxis for syphilis prevention. Sex Transm Dis 2003;30:844–849. [DOI] [PubMed] [Google Scholar]
- 92.Holman KM, Hook EW III. Clinical management of early syphilis. Expert Rev Anti Infect Ther. 2013;11:839–843. [DOI] [PubMed] [Google Scholar]
- 93.Wilson DP, Prestage GP, Gray RT, et al. Chemoprophylaxis is likely to be acceptable and could mitigate syphilis epidemics among populations of gay men. Sex Transm Dis 2011;38:573–579. [DOI] [PubMed] [Google Scholar]
- 94.Cohen MS, Henderson GE, Aiello P, Zheng H. Successful eradication of sexually transmitted diseases in the People’s Republic of China: Implications for the 21st century. JID 1996;174(Suppl2):S223–229. [DOI] [PubMed] [Google Scholar]
- 95.Kamali A, Quigley M, Nakiyingi J, et al. Syndromic management of sexually-transmitted infections and behavior change interventions on transmission of HIV-1 in rural Uganda: a community randomized trial. Lancet 2003;361:645–652. [DOI] [PubMed] [Google Scholar]
- 96.Garcia PJ, Holmes KK, Cárcamo CP, et al. Prevention of sexually transmitted infections in urban communities (Peru PREVEN): a multicomponent community-randomised controlled trial. Lancet 2012;379:1120–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tobian AAR, Serwadda D, Quinn TC, et al. Male circumcision for the prevention of HSV-2 and HPV infections and syphilis. N Engl J Med 2009;360:1298–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Larsen SA, Steiner BM, Rudolph AH. Laboratory diagnosis and interpretation of tests for syphilis. Clin Microbiol Rev. 1995;8:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Reverby SM. The art of medicine: listening to narratives from the Tuskegee syphilis study. Lancet 2011;377:1646–1647. [DOI] [PubMed] [Google Scholar]