Abstract
Background:
Multipotent neural crest cells (NCC) contribute to the corneal endothelium and keratocytes during ocular development, but the molecular mechanisms that underlie this process remain poorly understood. We performed RNA-Seq analysis on periocular neural crest (pNC), corneal endothelium, and keratocytes and validated expression of candidate genes by in situ hybridization.
Results:
RNA-Seq profiling revealed enrichment of genes between pNC and neural crest-derived corneal cells, which correspond to pathways involved in focal adhesion, ECM-receptor interaction, cell adhesion, melanogenesis, and MAPK signaling. Comparisons of candidate NCC genes to ocular gene expression revealed that majority of the NCC genes are expressed in the pNC, but they are either differentially expressed or maintained during corneal development. Several genes involved in RA, TGFβ, and Wnt signaling pathways and their modulators are also differentially expressed. We identified differentially expressed transcription factors as potential downstream candidates that may instruct expression of genes involved in establishing corneal endothelium and keratocyte identities.
Conclusion:
Combined, our data reveal novel changes in gene expression profiles as pNC differentiate into highly specialized corneal endothelial cells and keratocytes. These data serve as platform for further analyses of the molecular networks involved in NCC differentiation into corneal cells, and provide insights into genes involved in corneal dysgenesis and adult diseases.
Keywords: RNA-Seq, neural crest cells, cornea, corneal endothelium, keratocytes
INTRODUCTION
The cornea is a multilayered transparent tissue comprised predominantly of cells derived from a multipotent embryonic cell population, the neural crest cells (NCC). Cranial NCC progenitors of the cornea originate from the neural tube region encompassing the caudal diencephalon and the rostral metencephalon (Lwigale et al., 2004; Creuzet et al., 2005b). This population of NCC forms different streams that migrate into the frontal-nasal, periocular, and maxilla-mandibular regions (Noden, 1975; Johnston et al., 1979; Serbedzija et al., 1992) where they respond to environmental cues and differentiate into region-specific tissues. Periocular neural crest cells (pNC) contribute to various ocular structures including the cornea, eyelids, and connective tissues (Lwigale et al., 2004; Creuzet et al., 2005b). Defects in pNC migration, proliferation, and differentiation are associated with a condition known as anterior segment dysgenesis (ASD) (Cook, 1989). ASD is comprised of spectrum of ocular disorders characterized by malformation of the cornea, iris, lens, and eyelids (Churchill and Booth, 1996; Sowden, 2007). Despite the significance of NCC to eye development, very little is known about the molecular underpinnings of their differentiation into ocular tissues.
During chick corneal development, pNC occupy the mesenchyme surrounding the rudimentary eye for approximately two days prior to their initial migration into the presumptive corneal region at about embryonic day (E)4.5. Migration of pNC into the presumptive corneal region occurs in two waves (Hay and Revel, 1969; Lwigale et al., 2005). The first wave forms the corneal endothelium, an interior monolayer of cells that establishes a barrier and regulates fluid movement between the anterior chamber and the cornea (Waring et al., 1982). The second wave of migration occurs at about E6, when pNC invade the acellular primary stroma and differentiate into keratocytes, which synthesize the extracellular matrix of the corneal stroma (Linsenmayer et al., 1984; Quantock and Young, 2008). The intricate behavior of pNC during chick corneal development is in part regulated by the lens vesicle, given that its ablation results in precocious migration and malformation of the cornea (Beebe and Coats, 2000; Lwigale and Bronner-Fraser, 2009). The molecular signals involved in regulating pNC migration were pinpointed to lens-derived Semaphorin3A and the spatiotemporal downregulation of its receptor, NRP1, by a subset of pNC, which permitted their migration into the presumptive corneal region (Lwigale and Bronner-Fraser, 2009). Studies in mice have identified that retinoic acid (RA) signaling regulates transcription factors expressed by pNC including Pitx2 and Foxc1 that inhibit canonical Wnt signaling via upregulation of Dkk2 (Lehmann et al., 2003; Evans and Gage, 2005; Gage et al., 2008). Knockout of any one of these genes in mice phenocopy the corneal and iridial defects observed in humans with ASD (Gage et al., 1999; Kitamura et al., 1999; Kume and Seo, 2010). Together, these studies have advanced our understanding of early corneal development, but the molecular mechanisms that transform pNC into the diverse progeny of ocular cells remain unclear.
In this study, we take advantage of the stepwise contribution of avian pNC to the nascent cornea and survey their gene expression profile during differentiation into corneal endothelium and keratocytes by RNA-Seq analysis. We evaluated changes in expression profile of candidate NCC markers following aggregation in the periocular region. We studied changes in gene expression profiles of components of the major signaling pathways (RA, TGFβ, and Wnt) associated with ocular development. We identified genes that are likely to be involved in pNC differentiation into corneal endothelium and keratocytes. Altogether, these data serve as a foundation to advance our understanding of the molecular mechanisms underlying pNC migration, proliferation and differentiation.
RESULTS AND DISCUSSION
Generation of a comprehensive transcriptome during pNC differentiation into corneal cells
During early ocular development in chick, the status of pNC differentiation can be categorized into three phases: (1) aggregation of NCC into the periocular region, which occurs between E2-E3; (2) migration of pNC into the presumptive corneal region to form the endothelial layer by E5; and (3) migration of pNC into the primary corneal extracellular matrix to form the stromal keratocytes by E7. At each of the above stages, NCC are subjected to different environmental cues that play important roles in their migration, proliferation, and differentiation (Brugmann et al., 2006; Lwigale and Bronner-Fraser, 2009). Our analysis of NCC contribution to early ocular development using the quail/chick chimera technique indicates that the periocular mesenchyme at E3 is mostly comprised of quail-derived QCPN-positive pNC that give rise to the corneal endothelium at E5, and to the keratocytes and endothelium at E7 (Fig. 1A).
Figure 1. Experimental design for tissue isolation, RNA-Seq analysis, and comparison of genes identified in pNC, En, and KEn.
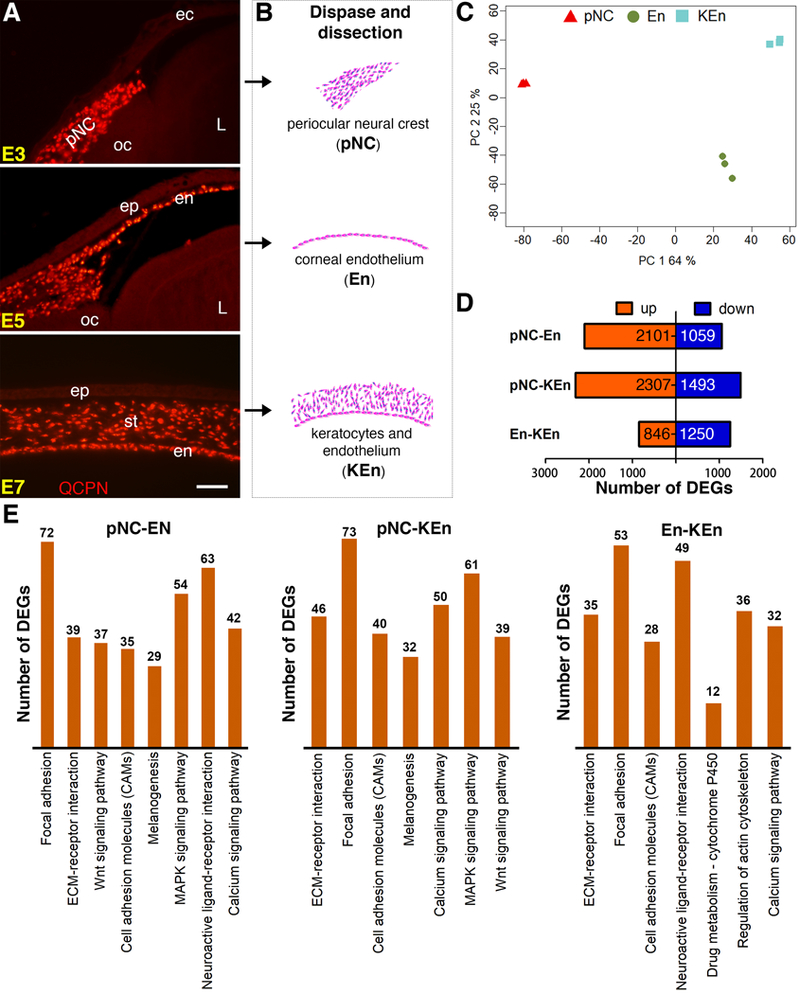
(A) Localization of neural crest cells in the periocular region and their corneal derivatives revealed by immunostaining cross sections of quail-chick chimera eyes for QCPN (red) at various stages of ocular development. (B) Periocular neural crest cells (pNC), corneal endothelium (En), or the combined keratocytes and corneal endothelium (KEn) were isolated at E3, E5, and E7, respectively. (C) PCA plot showing that the triplicate samples from pNC (Red), En (Green), and KEn (Blue) cluster together. Principal components 1 and 2 summarize 89% of the system variance. (D) Bar graph showing numbers of differentially expressed genes (red indicates upregulated and blue indicates downregulated genes). (E) Histogram representation indicating the number of DEGs between pNC-En, pNC-KEn, and En-KEn belonging to significant KEGG pathways. Abbreviations: ec, ectoderm; oc, optic cup; L, lens; ep, epithelium; en, endothelium; st, stroma. Scale bar: 100 μm.
To identify changes in gene expression during pNC formation corneal cells in chick, we isolated periocular mesenchyme from E3 (pNC), the monolayer of corneal endothelium at E5 (En), and the combined stroma and endothelium at E7 (KEn) (Fig. 1B). Three biological triplicates for each time point were prepared for RNA-Seq analysis as described in the methods section. Principal-components analysis (PCA) was applied to all mapped genes from the 9 samples to determine the reproducibility of biological repeats by Noiseq. The Scatter plots indicate good separation between pNC, En, and KEn, as well as good clustering of the three biological repeats of each sample, with system variance of 89% (Fig. 1C).
Identification of differentially expressed genes between pNC and corneal cells
To identify differentially expressed genes, we performed further analysis using a cutoff of abs(log2)≥1 and FDR<0.05 on genes that overlapped between NoiseqBio and EdgeR analyses (Supplementary Table 1). Based on these analyses, 3160 genes were differentially expressed between pNC and En (Fig. 1D). The 2101 upregulated genes represent En and the 1059 downregulated genes represent pNC. Likewise, 3800 genes were differentially expressed between pNC and KEn (Fig. 1D), of which 2307 upregulated genes represent KEn and the 1493 downregulated genes represent pNC. Given that both En and KEn contain corneal endothelial cells, we analyzed the difference between these two groups. Differentially expressed genes between En and KEn (2096) may either be involved in keratocyte differentiation or associated with further development of the corneal endothelium between E5 and E7. In this group, 846 genes are upregulated and 1250 genes are downregulated. KEGG pathway analysis indicated enrichment of similar pathways between pNC/En and pNC/KEn, with the top five corresponding to focal adhesion, ECM-receptor interaction, cell adhesion, melanogenesis, and MAPK signaling (Fig. 1E). A complete KEGG pathway list that includes the major signaling pathways during ocular development (RA metabolism, Wnt, and TGFβ signaling) is provided in Supplementary Table 2.
Changes in NCC gene expression in the periocular region and cornea
Given that NCC reside in the periocular region for approximately two days before their initial migration into the presumptive cornea (Hay and Revel, 1969), we investigated whether they change their molecular signature prior to, and during their differentiation into corneal cells. A list of 47 genes was generated from candidate chick NCC genes (Simoes-Costa et al., 2014; Simoes-Costa and Bronner, 2015), and we examined their expression in pNC, En, and KEn. From this list, 43 NCC genes were expressed in pNC, of which 20 were downregulated, 7 were upregulated, and 10 were constitutively expressed in both En and KEn. Interestingly, some genes (6) were either downregulated or maintained in only En or KEn (Fig. 2A and Supplementary Table. 3). Of the 4 NCC genes that were not expressed in the pNC (ADAM11, DLX6, ZIC3/4), ADAM11 was upregulated during corneal development. These results indicate that the molecular signature of NCC is maintained in the periocular region, and partially during early corneal development. This is supported by our previous study, showing that keratocytes isolated from E10 quail corneas were capable of differentiating into other NCC-derived tissues when grafted into the migratory stream of stage 9 chick embryos (Lwigale et al., 2005). To validate the expression of NCC genes, we chose one gene from each category based on whether they were downregulated (MSX2), maintained (SNAI2), or upregulated (RHOB) during corneal development and analyzed their spatiotemporal expression by in situ hybridization. Our results revealed that MSX2 and SNAI2 are expressed in the periocular region (Fig. 2B and 2C). Unlike MSX2, which is restricted to the periocular region, expression of SNAI2 is maintained in the corneal endothelium (Fig. 4C, arrowhead) and stroma. In mouse, Msx1 and Msx2 are involved in NCC survival, proliferation, and differentiation (Liu et al., 1999; Ishii et al., 2005), and it is possible that some of the early functions are maintained only in the chick pNC. SNAI2 is involved in the epithelial-mesenchymal transition required for NCC delamination from the neural tube (Taneyhill et al., 2007). We show that expression of SNAI2 is maintained when pNC undergo mesenchyme-endothelial transition to form the corneal endothelium, which strongly expresses N-cadherin (Reneker et al., 2000; Lwigale et al., 2005). Inhibition of E-cadherin by SNAI2 requires LMO4 (Ochoa et al., 2012), which is downregulated in our RNA-Seq data (Fig 2A and Supplementary Table. 3), suggesting that SNAI2 may play a different role that does not affect cell adhesion molecules in the corneal endothelium.
Figure 2. Changes in the molecular identity of NCC during their localization in the periocular region and differentiation into corneal cells.
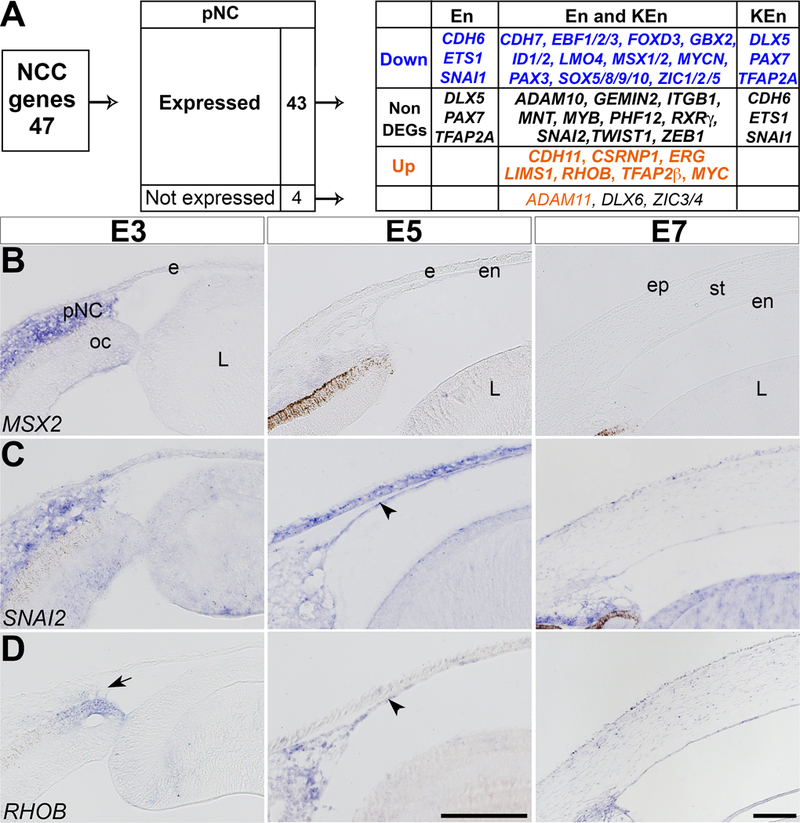
(A) Analysis based on 47 candidate NCC genes indicates that 43 genes were expressed and 4 were not expressed in the periocular region. Of the 43 expressed genes, 20 were downregulated, 10 were constitutively expressed, and 7 were upregulated during corneal development. (B-D) Section in situ hybridization of E3, E5, and E7 anterior eyes indicating that: (B) MSX2 is strongly expressed in the periocular region but undetectable in the corneal endothelium and stroma; (C) SNAI2 is maintained at all three time points; and (D) RHOB is minimal in the periocular region, but it is strongly expressed in the corneal endothelium and stroma. Arrow indicates periocular region and arrowheads indicate corneal endothelium. Abbreviations: pNC, periocular neural crest; ec, ectoderm; oc, optic cup; L, lens; ep, epithelium; en, endothelium; st, stroma. Scale bars: 100 μm.
Figure 4. TGFβ Signaling pathway.
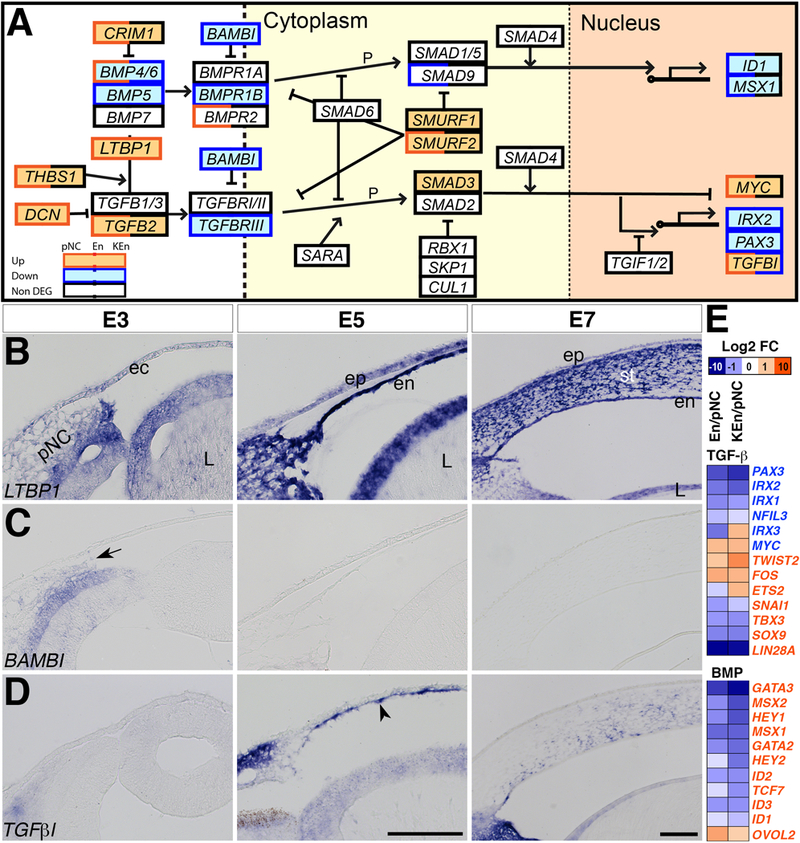
(A) Black boxes indicate constitutively expressed genes, red boxes indicate upregulated genes, and blue boxes indicate downregulated genes. Double colored boxes indicate partial upregulation, downregulation, or maintenance during corneal development. (B-D) Section in situ hybridization of E3, E5, and E7 anterior eyes showing that: (B) LTBP1 is lightly expressed in the periocular mesenchyme at E3 and it is strongly expressed in the corneal endothelium and stroma. (C) BAMBI is expressed in the optic cup and low in the periocular mesenchyme and it is not detectable in the corneal endothelium and stroma. (D) Expression of TGFβi is also low in the periocular mesenchyme at E3, but it is vividly expressed in the corneal endothelium at E5 (arrowhead) and stroma at E7. (E) Heatmap showing the relative upregulation and downregulation of TGFβ and BMP target genes between En/pNC and KEn/pNC. Font color of genes indicates TGFβ- or BMP-upregulated (red) or TGFβ- downregulated (blue) genes. Abbreviations: pNC, periocular neural crest; ec, ectoderm; oc, optic cup; L, lens; ep, epithelium; en, endothelium; st, stroma. Dotted lines represent the cell and nuclear membranes. Scale bars: 100 μm.
From our list of NCC genes, only CDH11, CSRNP1, ERG, LIMS1, RHOB, TFAP2β, and MYC are upregulated during corneal development. As an example from this group, we validated the spatiotemporal expression of RHOB. Our data indicate that RHOB is expressed by a few cells in the periocular region (Fig. 2D, arrow), then it is strongly expressed in the corneal endothelium (Fig. 2D, arrowhead), and in both the corneal endothelium and stroma at E7. During early development, RHOB is expressed in the dorsal neural tube and transiently in migratory NCC, and it is involved in the delamination process (Liu and Jessell, 1998). The function of RHOB during corneal development remains unclear, but it has been shown to play a critical role during barrier formation in vascular endothelial cells (Marcos-Ramiro et al., 2016). Our data also show upregulation of TFAP2β, which suggests its potential role during chick corneal development. Conditional knockout of TfAp2β in the NCC lineage in mice caused several defects in NCC-derived ocular tissues including malformation of the corneal endothelium and stroma (Martino et al., 2016).
Differentiation of pNC is regulated by multiple signaling pathways
Signals from the neural plate, ectoderm, and adjacent mesoderm mediate NCC formation at the neural plate border with the ectoderm. These include growth factors such as Wnt (Saint-Jeannet et al., 1997), bone morphogenetic protein (BMP; Liem et al., 1995), fibroblast growth factor (FGF; Monsoro-Burq et al., 2003), and RA signaling (Villanueva et al., 2002; Martinez-Morales et al., 2011). These signaling pathways regulate NCC formation at all embryonic axial levels, except for RA signaling, which is absent in the rostral cranial NCC streams that contribute to the eye (Maden et al., 1998). pNC experience similar signals from the adjacent ocular tissues (optic cup, lens, and ectoderm) prior to their migration into the presumptive corneal region. RA signaling is from the optic cup and ectoderm (Li et al., 2000; Matt et al., 2005; Molotkov et al., 2006), whereas TGFβ and BMP are expressed in the lens and optic cup (Trousse et al., 2001; Saika et al., 2002). Several WNTs are expressed in the ectoderm, optic cup, and lens (Jin et al., 2002; Fokina and Frolova, 2006). Interestingly, disruption of these major signaling pathways lead to dysgenesis of NCC-derived ocular tissues (Gage et al., 1999; Kitamura et al., 1999; Kume and Seo, 2010), but the mechanisms of how they are regulated and their action on pNC are not clearly understood. Our KEGG pathway analysis also indicates that the above signaling pathways change during corneal development (Supplementary Table 2), thus, we analyzed the expression of RA, TGFβ, and Wnt signaling components and their downstream targets.
RA signaling pathway
Genetic studies in mice showed that three retinaldehyde dehydrogenase enzymes (Raldh1/2/3) responsible for RA synthesis are all required for proper development (Dupe et al., 2003; Molotkov et al., 2006; Maden, 2007). Our data indicate high-level expression of components of the RA signaling pathway during pNC differentiation into corneal cells (Fig. 3A and Supplementary Table 4). These include transporter genes (STRA6, CRABP1/2 and RBP5, FABP5), enzymes (DHRS3, RDH5/12/14, and ALDH1A2), regulator (CYP26B1), and nuclear receptors (NR2F1/2, NR2C1, RXRΑ, RARΑ, and RARΒ, PPARD). Of these genes, ALDH1A2, DHRS3, and CYP26B1, are significantly upregulated in En and KEn compared to pNC (Fig. 3A and Supplementary Table 4). Upregulation of ALDH1A2 and DHRS3 was confirmed by in situ hybridization to determine the specific localization of RA synthesis and inhibition, respectively, in the cornea. Although ALDH1A2 is strongly expressed in the optic cup at E3, it is not expressed in the pNC. Consistent with our RNA-Seq data, ALDH1A2 is vividly expressed in the corneal endothelium at E5 and E7 (Fig. 3C, arrowheads), with sparse staining in the stroma. Similarly, expression of DHRS3 is minimal in the pNC at E3, but robust in the corneal endothelium at E5 and E7 (Fig. 3D, arrowheads), and also expressed in the stroma. Our data show for the first time that the chick corneal endothelium expresses ALDH1A2 and thus, acts as a potential source for RA signaling, which may act in either an autocrine or paracrine fashion to regulate corneal development. Interestingly, our results also suggest that the RA inhibitors DHRS3 and CYP26B1 (Sakai et al., 2001; Feng et al., 2010; Kam et al., 2013) may act at the same time to modulate the levels of signaling in the corneal endothelium and stroma. This is in conjunction with previous observation that RA signaling intensified in various ocular tissues including the cornea of Cyp26a1 null mice (Sakai et al., 2004).
Figure 3. RA signaling pathway.
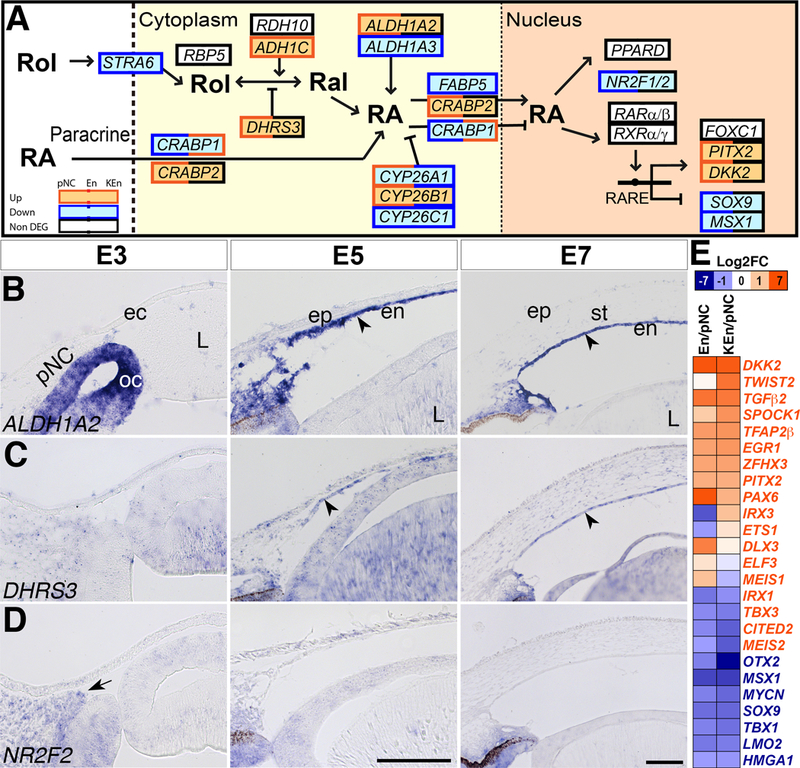
(A) Black boxes indicate constitutively expressed genes, red boxes indicate upregulated genes, and blue boxes indicate downregulated genes. Double colored boxes indicate partial upregulation, downregulation, or maintenance during corneal development. (B-D) Section in situ hybridization of E3, E5, and E7 anterior eyes showing that: (B) ALDH1A2 is expressed in the optic cup but not the periocular mesenchyme at E3, but it is vividly expressed in the corneal endothelium (arrowhead), and sparsely expressed in the stroma at E7. (C) Expression of DHRS3 is ubiquitous but low at E3, but becomes strong in the corneal endothelium (arrowhead) and stroma. (D) NR2F2 is expressed in the periocular mesenchyme at E3 (arrow), but it is not detectable in the corneal endothelium and stroma at E5 and E7. (E) Heatmap showing the relative upregulation and downregulation of RA target genes between En/pNC and KEn/pNC. Font color of genes indicates RA-upregulated (red) or RA-downregulated (blue) genes. Abbreviations: pNC, periocular neural crest; ec, ectoderm; oc, optic cup; L, lens; ep, epithelium; en, endothelium; st, stroma. Dotted lines represent the cell and nuclear membranes. Scale bars: 100 μm.
Downregulated RA genes in En and KEn include CRABP1, NR2F1 and NR2F2 (Fig. 3A and Supplementary Table 4). Downregulation of CRABP1, which promotes RA degradation (Dong et al., 1999; Michalik and Wahli, 2007), may cause an increase in nuclear RA mediated by CRABP2 that is constitutively expressed. NR2F1 and NR2F2 (also known as COUP-TF1 and COUP-TF2) are transcription factors that function as RA nuclear receptors (Kruse et al., 2008; Pickens et al., 2013), but they have also been shown to inhibit RA signaling by binding to retinoic acid response elements (RAREs) of downstream genes (Kliewer et al., 1992; Tran et al., 1992). Our in situ hybridization analysis confirms that NR2F2 is expressed in the pNC (Fig. 3E, arrow), but it is not detectable in the corneal endothelium or stroma (Fig. 3E). A similar pattern of downregulation is expected for the expression of NR2F1, which may indicate a potential increase in expression of RA-target genes in the corneal endothelium and stroma.
Activation of RA signaling leads to upregulation or downregulation of target genes (Balmer and Blomhoff, 2002; Matt et al., 2005). Examples of RA-target genes in the cornea include transcription factors that are either upregulated (PITX2, DKK2, PAX6, TFAP2β) or downregulated (SOX9, MSX1, OTX2, LMO2) (Fig. 3A and 3E, and Supplementary Table 4). Pitx2, Foxc1, Pax6 play important roles during mouse corneal development (Smith et al., 2000; Kanakubo et al., 2006; Gage et al., 2014), and they are among the ASD genes linked to dysgenesis of the human cornea (Sowden, 2007).
TGFβ pathway
The TGFβ superfamily comprises of TGFβs, BMPs, and other ligands, which regulate differentiation, proliferation, migration, and apoptosis in numerous cell types including the NCC (Shah et al., 1996; Chai et al., 2003; Wurdak et al., 2005). Comparison of En and KEn to pNC indicated that TGFβ1/2/3 and BMP4/7 are expressed during corneal development. TGFβ2 was upregulated and BMP5 was the only gene downregulated in En and KEn (Fig. 4A and Supplementary Table 5). In mice TGFβ2 signaling from the lens induces expression of Pitx2 and Foxc1 in the NCC mesenchyme of the presumptive cornea, and its absence causes severe ocular defects including corneal thinning and absence of the corneal endothelium (Saika et al., 2001; Ittner et al., 2005). Ectopic expression of BMP5 in the chick neural retina transforms it into retinal pigment epithelium (Steinfeld et al., 2017), but its function in the pNC remains unclear.
The following regulators of TGFβ (CRM1, THBS1, LTBP1/2, DCN, TGIF1/2, SMURF1/2, RBX1, SKP1 AND CUL1) are expressed during corneal development. Of these, THBS1, LTBP1/2, and DCN are upregulated in En and KEn (Fig. 4A and Supplementary Table 5), indicating their potential function during corneal development. Thbs1 activates TGFβ and inhibits angiogenesis (Good et al., 1990; Schultz-Cherry et al., 1994; Tolsma et al., 1997). In the adult cornea, THBS1 is expressed in the corneal endothelium and stroma (Hiscott et al., 2006), where it plays a role as an antiangiogenic agent during wound healing (Hiscott et al., 1999; Matsuba et al., 2011; Blanco-Mezquita et al., 2013). LTBP1 is expressed in a few cells in the periocular region, but it is robust in the corneal endothelium and stroma (Fig. 4B). LTBP1 plays an important role during secretion, deposition, and activation of TGFβ in glioma cells (Tritschler et al., 2009) and it is possible that it plays similar roles during corneal development. DCN inhibits TGFβ signaling by binding directly to its active form (Yamaguchi et al., 1990; Border et al., 1992), and it has also been shown to prevent fibrillogenesis of corneal collagen and to cause fibrosis during wound healing (Rada et al., 1993; Mohan et al., 2010). Therefore, upregulation of DCN in En and KEn indicates potential modulation of TGFβ signaling, which might be required for activation of specific downstream genes during corneal development.
Our data indicate that BAMBI is the only TGFβ regulator that is downregulated in En and KEn (Fig. 4A). BAMBI is a pseudo-receptor with similar structure to TGFβR1 that inhibits TGFβ signaling and activates canonical Wnt signaling (Onichtchouk et al., 1999; Lin et al., 2008). We confirmed that BAMBI is expressed in the pNC (Fig. 4C, arrowhead) where it may play a role in Wnt signaling, but it is not detectable in the corneal endothelium and stroma, which correspond with the upregulation of TGFβ signaling in the cornea.
The TGFβ superfamily receptors TGFβR1/2, BMPR1A/2, and downstream components including SMAD1/2/4/5/6 are all constitutively expressed in pNC, En, and KEn (Fig. 4A and Supplementary Table 5). Several transcription factors downstream of TGFβ signaling are either upregulated (MYC, TWIST2, FOS) or downregulated (SOX9, LIN28A). Surprisingly, some TGFβ activated transcription factors such as SNAI1, TBX3, SOX9, and LIN28A are downregulated, whereas some that are inhibited by TGFβ (IRX3 and MYC) are upregulated (Fig. 4E and Supplementary Table 5). Our data also indicate that majority of the transcription factors activated by BMP signaling such as ID1/2/3, MSX1, and HEY1 are downregulated during corneal development (Fig. 4A and 4E and Supplementary Table 5). An example of a TGFβ downstream target that is upregulated in En and KEn is TGFβ-induced (TGFβI, also known as BIGH3), which was confirmed to be expressed in the corneal endothelium (Fig. 4D, arrowhead) and stroma. TGFβI encodes an ECM protein induced by TGFβ that interacts with collagen (Skonier et al., 1992; Hashimoto et al., 1997). Recent studies have shown that mutations in TGFβI in humans cause a condition known as granular corneal dystrophy, which is characterized by opaque deposits in the corneal stroma (Kattan et al., 2017; Nielsen et al., 2017; Chao-Shern et al., 2018). Therefore its upregulation in our data suggests that TGFβI may play a role in organizing collagen fibrils synthesized by the corneal endothelium and keratocytes during early development.
Wnt signaling
Defects in Wnt signaling pathway cause dysgenesis of the anterior ocular tissues in humans and mice (Ittner et al., 2005; Reis and Semina, 2011; Bankhead et al., 2015). Analysis of the components of the Wnt signaling pathway indicates that despite the expression of several ligands in chick ocular tissues (Fokina and Frolova, 2006), only WNT2B, WNT4, WNT5A, WNT6, WNT9A, and WNT9B are highly expressed in the pNC and its corneal derivatives during development (Fig. 5A and Supplementary Table 6). Genes representing regulators of Wnt signaling at multiple levels including FRZB, DACT1/2, DKK3, SFRP1/2, BAMBI, AXIN1/2, NLK, GROUCHO, WLS, AES, and CTBP1 are also constitutively expressed in pNC, En and KEn. In this group, only WLS and BAMBI mediate Wnt signaling and the rest are inhibitors. We confirmed by in situ hybridization that both WLS and AES are constitutively expressed in the pNC, corneal endothelium, and stroma (Fig. 5B and 5C). Wnt receptors and co-receptors including FRZ1/2/6/7/9 and LRP5/6 are also constitutively expressed. FRZ8/10 are upregulated in En but downregulated in KEn, and FRZ4 is downregulated during corneal development (Fig. 5A and Supplementary Table 6). Downstream components of Wnt signaling such as DVL1/3, GSK3Β, and CTNNB1 are either upregulated or constitutively expressed, whereas TCF7, TCF7L1 and LEF1 are downregulated. Combined, our data suggest that the pNC, corneal endothelium and keratocytes have the potential for canonical Wnt signaling. However, the expression of multiple inhibitors including the upregulation of DKK2 suggest that Wnt signaling is regulated at multiple levels during corneal development. We therefore examined the expression of Wnt target genes in En and KEn and observed that some Wnt-upregulated genes are downregulated (SOX17, FST, JAG1, STRA6, SALL4, RET, TBX3), whereas others are upregulated (NOS2, MYC, AXIN2, FN1, CYR61, PITX2, MMP2, LBH, WISP1) in our data (Fig. 5E and Supplementary Table 6). However, CLDN1 is upregulated in En but downregulated in KEn, and SP5 is downregulated in En but upregulated KEn. Our data also show that some Wnt-downregulated genes (TNFRSF1, CDH1) are upregulated during corneal development. As an example of a Wnt target gene, we confirmed the expression of PITX2 and observed vivid expression in the pNC, En, and KEn (Fig. 5D). Mouse studies have shown that Pitx2 is activated by Wnt signaling (Kioussi et al., 2002; Briata et al., 2003), but also indirectly acts as a Wnt inhibitor via upregulation of Dkk2 (Gage et al., 2008).
Figure 5. Wnt Signaling pathway.
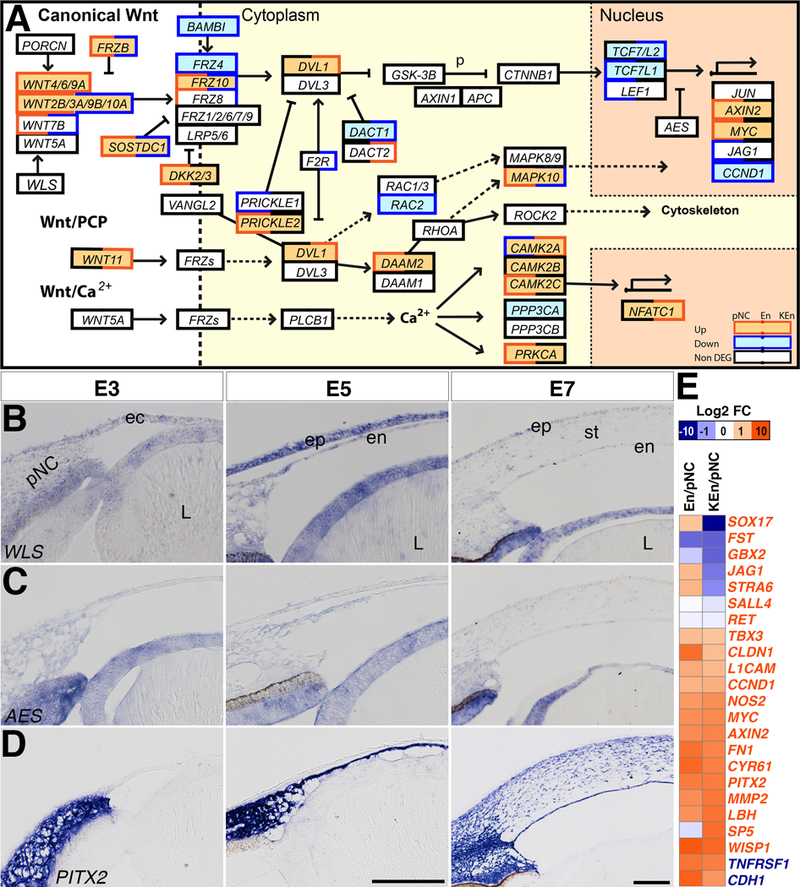
(A) Black boxes indicate constitutively expressed genes, red boxes indicate upregulated genes, and blue boxes indicate downregulated genes. Double colored boxes indicate partial upregulation, downregulation, or maintenance during corneal development. (B-D) Section in situ hybridization of E3, E5, and E7 anterior eyes showing that: (B) WLS and (C) AES are both expressed in the periocular mesenchyme at E3, and in the corneal endothelium and stroma. (D) PITX2 is strongly expressed in the periocular mesenchyme, corneal endothelium, and stroma. (E) Heatmap showing the relative upregulation and downregulation of Wnt target genes between En/pNC and KEn/pNC. Font color of genes indicates Wnt-upregulated (red) or Wnt-downregulated (blue) genes. Abbreviations: pNC, periocular neural crest; ec, ectoderm; oc, optic cup; L, lens; ep, epithelium; en, endothelium; st, stroma. Dotted arrows indicate implied connection in the literature. Scale bars: 100 μm.
WNT11, the main ligand for the Wnt/PCP pathway, is upregulated in KEn, and WNT5A, which functions in both the Wnt/calcium and Wnt/PCP pathways (Yamanaka et al., 2002; Sato et al., 2010), is maintained at all stages of corneal development. Components of the Wnt/PCP pathway (DAMM2, MAPK10, ROCK2, PRICKLE2, RHOA, RAC1, CDC42) and Wnt/calcium pathway (NFACTC1, PRKCA, CAMK2D, RYK, CAMK2B) are either upregulated or constitutively expressed. These results raise the possibility that non-canonical Wnt signaling occurs during corneal development and it may be involved in inducing cell migration and polarity.
Differential expression of transcription factors during early corneal development
Our data indicate that differential expression of components of the major signaling pathways (RA, Wnt, TGFβ) correspond with the transformation of pNC into corneal endothelium and keratocytes. To identify the genes involved in the process of pNC differentiation into corneal cells, we examined the changes in expression of transcription factors (Table 1 and Supplementary Table 7). Upregulated genes with known ocular functions include PAX6 and ZNF469. PAX6, which is induced by RA signaling, is expressed in various ocular tissues and it has been shown to play a major role in eye development (Hill et al., 1991; Fujiwara et al., 1994; Baulmann et al., 2002). Analysis of NCC-derived cells in Pax6sey/+ mice showed abnormal migration into the eye (Kanakubo et al., 2006). ZNF469 is involved in ECM synthesis and defects in this gene cause connective tissue disorders in humans including an ocular defect known as brittle cornea syndrome (Zlotogora et al., 1990; Al-Hussain et al., 2004; Vincent et al., 2014). Based on these examples, the upregulated genes may represent transcription factors involved in key processes of corneal development including cell migration, proliferation, and differentiation.
Table 1.
Top 20 differentially expressed transcription factors in En/pNC and KEn/pNC
| Gene ID | Symbol | Log2FC (En/pNC) |
q-value | Gene ID | Name | Log2FC (KEn/pNC) |
q-value |
|---|---|---|---|---|---|---|---|
| Upregulated | Upregulated | ||||||
| 101750712 | ZNF750 | 9.86 | 2E-18 | 100857422 | POU3F4 | 8.77 | 1E-121 |
| 418669 | SHOX | 8.22 | 2E-224 | 100858556 | DMRT2 | 7.09 | 2E-135 |
| 396546 | HMX1 | 8.03 | 2E-19 | 426886 | TWIST3 | 6.69 | 5E-142 |
| 396346 | SOHO-1 | 7.85 | 5E-20 | 418669 | SHOX | 6.67 | 5E-128 |
| 100858556 | DMRT2 | 7.59 | 3E-160 | 101748749 | ZNF469 | 6.12 | 6E-108 |
| 419863 | IRF6 | 6.99 | 2E-30 | 101750712 | ZNF750 | 5.46 | 7E-05 |
| 395943 | PAX6 | 6.69 | 4E-14 | 107051987 | POU3F3 | 5.29 | 3E-36 |
| 100857422 | POU3F4 | 5.65 | 2E-58 | 396158 | MYBL1 | 5.28 | 2E-226 |
| 424652 | GLIS1 | 5.34 | 1E-167 | 395405 | TWIST2 | 5.12 | 0 |
| 777244 | SHOX2 | 5.30 | 1E-91 | 396346 | SOHO-1 | 4.86 | 1E-08 |
| 395714 | BARX2 | 4.69 | 9E-19 | 423315 | NPAS3 | 4.56 | 2E-156 |
| 101748749 | ZNF469 | 4.66 | 3E-71 | 777244 | SHOX2 | 4.34 | 2E-58 |
| 395590 | DLX3 | 4.53 | 6E-19 | 373990 | TBX2 | 4.10 | 1E-240 |
| 421416 | FOSL2 | 4.45 | 6E-61 | 768789 | FOXE1 | 4.08 | 1E-43 |
| 374101 | SCX | 4.05 | 2E-194 | 100859610 | CREB3L1 | 3.86 | 2E-120 |
| 429506 | GRHL2 | 3.98 | 3E-22 | 396210 | NFIA | 3.79 | 3E-159 |
| 373990 | TBX2 | 3.97 | 8E-228 | 419631 | TFAP2E | 3.61 | 3E-82 |
| 428651 | GRHL1 | 3.71 | 3E-41 | 395713 | TFAP2B | 3.50 | 2E-240 |
| 423923 | EMX2 | 3.64 | 9E-114 | 427171 | PIK3R1 | 3.44 | 3E-113 |
| 419631 | TFAP2E | 3.42 | 1E-74 | 374101 | SCX | 3.41 | 1E-139 |
| Downregulated | Downregulated | ||||||
| 418313 | TBX15 | −10.53 | 7E-38 | 418313 | TBX15 | −10.53 | 2E-41 |
| 373932 | NKX3–2 | −8.69 | 6E-65 | 107053847 | DLX2 | −10.44 | 5E-65 |
| 396383 | ISL1 | −7.84 | 2E-15 | 428534 | SOX17 | −10.25 | 4E-37 |
| 396391 | GATA5 | −7.35 | 8E-12 | 419106 | GATA3 | −9.68 | 6E-123 |
| 396129 | SIM1 | −7.33 | 2E-30 | 724086 | SIX2 | −9.55 | 3E-137 |
| 396109 | FOXD1 | −7.00 | 2E-119 | 428021 | ZIC2 | −9.30 | 4E-94 |
| 419106 | GATA3 | −6.89 | 2E-100 | 395573 | SOX10 | −8.93 | 5E-147 |
| 503512 | FOXL2 | −6.75 | 1E-90 | 396109 | FOXD1 | −8.18 | 1E-138 |
| 395752 | NKX6–1 | −6.60 | 9E-50 | 395191 | OTX2 | −7.86 | 2E-19 |
| 395176 | EBF2 | −6.12 | 5E-72 | 395633 | MYF5 | −7.81 | 4E-16 |
| 395794 | FOXD3 | −5.98 | 3E-49 | 395794 | FOXD3 | −7.69 | 1E-61 |
| 374137 | MEOX2 | −5.93 | 2E-36 | 421786 | LIN28B | −7.65 | 0 |
| 374127 | PAX3 | −5.74 | 2E-81 | 374127 | PAX3 | −7.46 | 7E-103 |
| 107053847 | DLX2 | −5.68 | 3E-47 | 503512 | FOXL2 | −7.46 | 4E-102 |
| 395795 | FOXD2 | −5.57 | 5E-133 | 396391 | GATA5 | −7.35 | 1E-13 |
| 419388 | PRDM16 | −5.56 | 7E-155 | 395752 | NKX6–1 | −7.19 | 5E-57 |
| 100857760 | FOXF2 | −5.54 | 5E-100 | 395596 | EPAS1 | −7.05 | 8E-174 |
| 395961 | NR5A2 | −5.42 | 5E-25 | 373932 | NKX3–2 | −6.98 | 8E-67 |
| 724086 | SIX2 | −5.35 | 4E-94 | 395977 | GFI1B | −6.97 | 1E-25 |
| 428021 | ZIC2 | −4.68 | 4E-57 | 374137 | MEOX2 | −6.93 | 8E-44 |
Bold values indicate differentially expressed genes between the two groups (abs(log2)≥1 and q < 0.05). Minus signs indicate downregulation. The individual TPM and standard deviations for the RNAseq replicates are listed in Supplementary Table 7.
The downregulated genes represent transcription factors that are only expressed in the pNC (Table 1 and Supplementary Table 7). Among these are some of the bona fide NCC genes such as FOXD3, SOX9, and SOX10 (Fig. 2A). FOXD3 is required for self-renewal and maintenance of NCC multipotency (Labosky and Kaestner, 1998; Dottori et al., 2001; Mundell and Labosky, 2011), thus its downregulation may be required during pNC differentiation into corneal cells. SOX9 plays an early role during NCC induction (McKeown et al., 2005), but it is later required for chondrogenesis (Zhao et al., 1997; Sahar et al., 2005). Therefore, it is possible that the expression of SOX9 in the pNC may promote the formation of orbital bones, whereas its downregulation in En and KEn might be involved in preventing ossification of the cornea. Similarly, SOX10 is required during NCC induction, but it is later involved in their differentiation into non-mesenchymal derivatives including melanocytes and peripheral neurons (Southard-Smith et al., 1998; Dutton et al., 2001). Downregulation of SOX10 in En and KEn raises the possibility that both the melanocyte and neural lineages are prevented in the cornea.
Also, among the downregulated genes are representatives of transcription factors not expressed by cranial NCC, therefore we considered them specific for the periocular mesenchyme. pNC that do not form the cornea give rise to various skeletomuscular derivatives and also contribute pericytes to the ocular vasculature (Creuzet et al., 2005a). This is reflected in the pNC transcription factors that play important roles in osteogenesis, myogenesis, and vasculogenesis. For example, HEY1 is a downstream target of Notch signaling that is involved in vasculogenesis and osteoblastic differentiation (Fischer et al., 2004; Salie et al., 2010). DACH2 is required during eye and limb development in drosophila (Mardon et al., 1994), and it synergizes with EYA2 during myogenesis in chick (Heanue et al., 1999). CITED2 is a negative regulator of Hif-1 that is required for normal lens development and regression of the hyaloid vasculature (Chen et al., 2009).
Identification of genes involved in pNC differentiation into corneal endothelium
The corneal endothelium is comprised of polarized cells that form tight junctions and transport fluids between the cornea and anterior chamber, which is critical for maintaining stromal deturgescence and transparency (Waring et al., 1982). Our data indicate that most genes involved in membrane transport, cell polarity, and cell junctions are upregulated in both En and KEn (Table 2 and Supplementary Table 8). Examples of membrane transporter genes that are upregulated in the corneal endothelium include AQP1 and ABCB5. AQP1 is a bi-directional water transporter (Sui et al., 2001), which indicates potential increase in fluid absorption during pNC differentiation into corneal cells. ABCB5 is an ATP-dependent transporter that is also a well-known marker for limbal stem cells (Chen et al., 2005; Ksander et al., 2014).
Table 2.
Differential expression of corneal endothelium related genes in En/pNC and KEn/pNC.
| Gene ID | Symbol | Log2FC (En/pNC) |
q-value | Log2FC (KEn/pNC) |
q-value |
|---|---|---|---|---|---|
| Membrane transporters | |||||
| 420384 | AQP1 | 9.24 | 0 | 10.34 | 0 |
| 420606 | ABCB5 | 8.04 | 8E-114 | 7.12 | 5E-89 |
| 417849 | BEST3 | 7.29 | 4E-34 | 6.78 | 1E-27 |
| 776258 | SLC35F4 | 6.21 | 4E-60 | 7.21 | 1E-91 |
| 769904 | FAM26E | 5.65 | 3E-167 | 7.19 | 2E-268 |
| 395443 | SLC1A3 | −5.16 | 7E-135 | −4.38 | 7E-121 |
| 428653 | KCNS3 | −4.43 | 2E-11 | −4.76 | 7E-14 |
| 396532 | SLC4A1 | −4.34 | 1E-19 | −7.32 | 5E-35 |
| 770954 | KCNK2 | −4.26 | 1E-70 | −5.07 | 1E-90 |
| 414871 | KCNH6 | −3.27 | 4E-29 | −0.78 | 0.0012 |
| Cell polarity | |||||
| 378897 | THY1 | 3.70 | 6E-180 | 4.87 | 1E-293 |
| Tight junction | |||||
| 395144 | CLDN4 | 7.16 | 6E-20 | 2.57 | 0.0308 |
| 374029 | CLDN3 | 5.76 | 1E-18 | 0.72 | 0.3202 |
| 429506 | GRHL2 | 3.98 | 3E-22 | −0.13 | 0.8131 |
| 424910 | CLDN1 | 3.00 | 1E-05 | −1.79 | 0.0112 |
| 769245 | CLDN19 | 2.93 | 1E-07 | 2.80 | 2E-07 |
| 420022 | RAMP2 | −3.27 | 2E-32 | −5.47 | 3E-59 |
| 374028 | CLDN5 | −2.73 | 2E-29 | −3.35 | 3E-41 |
| 396047 | SNAI1 | −1.61 | 9E-10 | −0.85 | 0.0011 |
| 422858 | PPP2R2C | −1.38 | 6E-05 | 3.56 | 2E-54 |
| 420481 | MPP7 | −1.23 | 7E-18 | −1.49 | 7E-26 |
| Gap junction | |||||
| 374196 | PDGFA | 4.78 | 7E-35 | 3.18 | 5E-17 |
| 395771 | GJB6 | 3.61 | 9E-11 | 0.53 | 0.4574 |
| 374128 | PDGFB | 1.93 | 9E-12 | 0.86 | 0.0035 |
| 395581 | HTR2B | 1.81 | 0.001 | 3.40 | 6E-11 |
| 408035 | EGF | 1.75 | 5E-04 | −2.32 | 0.0024 |
| 430991 | ADRB3 | −3.71 | 3E-11 | −3.08 | 1E-09 |
| 771678 | GUCY1A2 | −3.47 | 8E-26 | −4.19 | 6E-35 |
| 396502 | GJA5 | −3.04 | 2E-11 | −7.27 | 2E-35 |
| 404529 | GJA4 | −2.11 | 1E-12 | −5.78 | 2E-43 |
| 422407 | GUCY1A3 | −2.08 | 1E-14 | −5.46 | 2E-50 |
| Adherens junction | |||||
| 414745 | CDH2 | 3.31 | 1E-100 | 1.59 | 4E-27 |
| 414849 | CDH13 | 3.14 | 0.444 | 3.61 | 2E-15 |
| 423718 | CDH23 | 2.37 | 0.107 | 1.68 | 3E-10 |
| 419222 | CDH4 | 1.90 | 9E-13 | −0.45 | 5E-09 |
| 415797 | CDH11 | 1.49 | 5E-36 | 0.01 | 1E-36 |
| 374068 | CDH5 | −8.37 | 4E-32 | −4.73 | 3E-97 |
| 374007 | CDH7 | −6.90 | 9E-61 | −1.72 | 1E-79 |
| Expressed in adult corneal endothelium* | |||||
| 770869 | MGARP | 6.35 | 0 | 5.75 | 0 |
| 424391 | MYOC | 5.00 | 5E-23 | 1.07 | 0.0282 |
| 428221 | COL8A2 | 3.12 | 2E-69 | 3.69 | 1E-92 |
| 396221 | LDHA | 1.88 | 2E-24 | 3.26 | 1E-65 |
| 418425 | CD200 | −3.08 | 3E-42 | −4.47 | 3E-71 |
| 396092 | ALCAM | −2.00 | 1E-41 | −1.57 | 5E-28 |
| 374110 | PTGDS | −1.44 | 0.009 | −0.41 | 0.4664 |
Bold values indicate differentially expressed genes between the two groups (abs(log2)≥1 and q < 0.05). Minus signs indicate downregulation. The individual TPM and standard deviations for the RNAseq replicates are listed in Supplementary Table 8.
The characteristic hexagonal shape of the corneal endothelium is due to forces that straighten the actomyosin fibers on the apical surface that faces the anterior chamber. The apical surface is also marked by ZO-1, whereas the basal surface that is in contact with the Descemet’s membrane is labeled by α3β1 (He et al., 2016). Interestingly, although ZO-1 (TJP1) is a major component of the adult corneal endothelium (Petroll et al., 1999; Ramachandran and Srinivas, 2010), it was not upregulated in our data sets (Supplementary Table 8). Suggesting that its expression and function may arise at a later time point than the stages of corneal development used for this study. Our data indicate that THY1, which is involved in cell polarity, is upregulated (Table 2). THY1 is predominantly expressed in neurons, where it is involved in cell attachment, migration and polarization (Tiveron et al., 1994; Leyton et al., 2001; Kong et al., 2013). THY1 is also localized on apical surfaces of epithelial cells (Powell et al., 1991), but it has not been studied in the corneal endothelium.
Genes involved in cell junctions include CLDN4, CLDN3, AND CDH2 (N-CADHERIN). Of these genes, CLDN3 is involved in tight junction barrier function in the submandibular glands (Mei et al., 2015; Yokoyama et al., 2017), and it is possible that it plays a similar role in the corneal endothelium. CDH2 mediates cell adhesion in neural cells and it is a well-known marker for corneal endothelial cells (Reneker et al., 2000). Its upregulation indicates the potential for formation of intercellular bonds at the onset of endothelial differentiation.
We also detected genes that are highly expressed in the adult corneal endothelium (Chng et al., 2013). These were either upregulated (MGARP, MYOC, COL8A2, LDHA) or constitutively expressed at all three time points (VIM, TSPAN6, TPJ2, ATP1B1, TPT1, PRDX6) (Table 2 and Supplementary Table 8).
Identification of genes involved in pNC differentiation into keratocytes
Keratocytes are characterized by their ability to synthesize the stromal ECM, which is comprised of collagens and proteoglycans that constitute more than 90% of the cornea (Knupp et al., 2009; Hassell and Birk, 2010). Our data show that genes for several ECM-related proteins are upregulated during corneal development (Table 3 and Supplementary Table 9). A majority of the upregulated corneal ECM genes belong to collagen (Supplementary Table 9). They represent fibrillar collagens (COL1/2/5/6/11/27) required for the corneal structure, and nonfibrillar collagens, which are either associated with the collagen fibrils (COL9/14/16) or expressed on cell surfaces (COL4/8/17) (Shaw and Olsen, 1991; Kadler et al., 1996; Knupp et al., 2009). Collagens form the bulk of the corneal stroma and associate with ECM proteins and regulatory enzymes that align and space the structural fibrils in configurations that elicit transparency (Rada et al., 1993; Michelacci, 2003; Hassell and Birk, 2010). Our data also show that genes for several proteins expressed in the adult cornea are upregulated in En and KEn. They include glycoproteins (NTN3, VIT, SPON2, OLFML2B, and THSB2), proteoglycans (OGN[Mimecan], KERA, and DCN), other ECM proteins (EGFL6, NPNT, PTN, and LTBP1/2), and regulatory enzymes (MMP27, ADAMTS2/5/8, and COLGALT2). We also identified some ECM genes that are significantly downregulated in the En and KEn (VWF, VTN, HAPLN1, LEPREL1, HPSE2 and CSGALNACT1). Given the functional differences between the corneal endothelium and keratocytes (Waring et al., 1982; Linsenmayer et al., 1984; Quantock and Young, 2008), we were surprised that many of the ECM related genes were expressed by both cell-types. One possibility is that some of these genes are transiently expressed in the corneal endothelium, and this could be resolved by comparing their localization at later stages of development. It is also possible that most of the ECM proteins are intracellularly degraded upon synthesis in the corneal endothelium (Ko and Kay, 2001; Lee et al., 2012), and do not contribute to the corneal matrix.
Table 3.
Differential expression of ECM related genes in En/pNC and KEn/pNC
| Gene ID | Symbol | Log2FC (En/pNC) |
q-value | Log2FC (KEn/pNC) |
q-value |
|---|---|---|---|---|---|
| Collagens | |||||
| 428448 | COL6A6 | 2.72 | 2E-17 | 11.03 | 0 |
| 396292 | COL6A2 | 6.41 | 0 | 10.60 | 0 |
| 396000 | COL6A1 | 4.51 | 8E-137 | 7.71 | 1E-306 |
| 416696 | LOC416696 | 8.85 | 9E-29 | 7.14 | 4E-21 |
| 101747382 | LOC101747382 | 4.86 | 6E-120 | 7.04 | 2E-218 |
| 107056318 | LOC107056318 | 0.19 | 0.763 | -4.18 | 3E-11 |
| 421873 | COL19A1 | 0.16 | 0.7209 | −3.16 | 9E-14 |
| 101751793 | LOC101751793 | −1.79 | 5E-13 | −2.93 | 4E-29 |
| 422350 | COL4A6 | −0.11 | 0.5729 | −2.03 | 2E-32 |
| 422348 | COL4A5 | 0.00 | 0.9913 | −1.60 | 2E-21 |
| Glycoproteins | |||||
| 396387 | NTN3 | 1.75 | 4E-07 | 6.03 | 7E-127 |
| 421471 | VIT | 10.87 | 5E-30 | 5.67 | 2E-11 |
| 422905 | SPON2 | 3.62 | 4E-44 | 5.61 | 5E-96 |
| 424366 | OLFML2B | 5.59 | 0 | 5.41 | 0 |
| 414837 | THBS2 | 2.97 | 7E-99 | 4.69 | 3E-215 |
| 419031 | VWF | −2.75 | 8E-25 | −10.87 | 6E-102 |
| 395935 | VTN | −5.57 | 3E-35 | −5.70 | 4E-39 |
| 417180 | LAMC3 | −3.01 | 4E-67 | −5.70 | 2E-161 |
| 427850 | RELN | −2.02 | 5E-14 | −5.26 | 4E-53 |
| 395531 | NID1 | −1.49 | 1E-48 | −3.49 | 2E-212 |
| Proteoglycans | |||||
| 374039 | OGN | 6.04 | 2E-295 | 8.13 | 0 |
| 373995 | KERA | 4.86 | 1E-160 | 6.42 | 1E-242 |
| 107056545 | LOC107056545 | 4.55 | 1E-05 | 6.20 | 1E-08 |
| 417892 | DCN | 2.58 | 3E-33 | 4.25 | 4E-77 |
| 395798 | ACAN | 0.23 | 0.6479 | 3.46 | 5E-20 |
| 414143 | LEPREL1 | −3.90 | 7E-71 | −6.99 | 1E-128 |
| 396475 | HAPLN1 | −2.51 | 8E-18 | −6.31 | 1E-70 |
| 770863 | GPC3 | −1.29 | 3E-08 | −4.51 | 8E-64 |
| 419184 | SDC4 | 0.01 | 0.9846 | −2.37 | 2E-16 |
| Other ECM proteins | |||||
| 418637 | EGFL6 | 4.31 | 8E-96 | 8.18 | 3E-307 |
| 422535 | NPNT | 0.65 | 0.0021 | 6.25 | 1E-151 |
| 418125 | PTN | 4.32 | 2E-290 | 5.52 | 0 |
| 421461 | LTBP1 | 2.17 | 2E-76 | 3.21 | 3E-153 |
| 428888 | LTBP2 | 2.23 | 1E-66 | 2.82 | 7E-104 |
| 395912 | MGP | 0.69 | 0.0939 | −3.23 | 4E-10 |
| Enzymes and Regulators | |||||
| 395850 | MMP27 | 8.15 | 3E-158 | 9.07 | 8E-209 |
| 416291 | ADAMTS2 | 0.82 | 0.0511 | 7.57 | 1E-120 |
| 769222 | ADAMTS8 | 5.08 | 8E-271 | 4.54 | 8E-219 |
| 424447 | COLGALT2 | 2.36 | 3E-44 | 4.28 | 3E-131 |
| 427971 | ADAMTS5 | 5.14 | 1E-174 | 4.09 | 3E-112 |
| 422488 | CSGALNACT1 | −1.49 | 2E-08 | −3.95 | 1E-34 |
| 423834 | HPSE2 | −3.12 | 2E-19 | −1.42 | 2E-05 |
Data is arranged to show the top 5 upregulated and downregulated genes in each category. Bold values indicate differentially expressed genes between the two groups (abs(log2)≥1 and q < 0.05). Minus signs indicate downregulation. The individual TPM and standard deviations for the RNAseq replicates are listed in Table 12.
CONCLUSIONS
We have used RNA-Seq analysis to provide an unbiased depiction of gene expression profile of NCC in the periocular region and during their differentiation into corneal endothelium and stromal keratocytes. We confirmed the expression patterns of candidate genes at in the periocular region and during corneal development by in situ hybridization. We highlighted minor deviations in gene expression following aggregation of cranial NCC in the periocular region, suggesting that pNC maintain a level of multipotency that enables their contribution to various ocular structures later in development. We focused on three major pathways (RA, Wnt and TGFβ) involved in corneal development and identified for the first time that several modulators are upregulated during pNC differentiation into corneal cells, which could be critical in directing the expression of downstream transcription factors with potential roles in establishing endothelial and keratocyte identities. Previous studies have shown that there is crosstalk between these pathways during corneal development. The RNA-Seq data provides a useful tool for generating gene regulatory networks for pNC migration, proliferation, and differentiation. These data not only provide a foundation for identifying genes with novel function during corneal development, they also provide a valuable resource for future studies of corneal diseases, stem cell biology, and bioengineering of corneal tissues.
EXPERIMENTAL PROCEDURES
Animals
Fertilized White Leghorn chicken (Gallus gallus domesticus) and Japanese quail eggs (Coturnix coturnix japonica) were obtained from commercial sources and incubated at 38°C under humidified conditions. Quail-chick chimeras were generated to track cells of neural crest origin in ocular tissues by grafting dorsal neural tube explants from HH stage 9 (Hamburger and Hamilton, 1951) quail donors to stage-matched chick embryos as previously described (Lwigale et al., 2005). Chick and chimeric embryos were incubated until E3, E5, or E7. Embryos were manipulated according to protocols approved by the Institutional Animal Care and Use Committee (IACUC) at Rice University.
Tissue collection from chick embryos
Periocular neural crest (pNC) mesenchyme was obtained by dissecting the anterior half of the eyes from E3 embryos. Corneal endothelium (En) tissues were obtained by trimming E5 corneas at boundary with the periocular region. Combined keratocytes and corneal endothelium (KEn) tissues were obtained by trimming E7 corneas at the boundary with the limbus region. All tissues were incubated in dispase (1.5 mg/ml; Worthington) at 38°C for 5 minutes, then rinsed twice in Ringer’s solution. For the pNC, the ectoderm, lens vesicles, and optic cups were removed and discarded, and the periocular mesenchyme from 26 eyes was pooled into each sample. For the En, the corneal epithelium was removed and discarded, and the endothelial layers from 120 eyes were pooled into each sample. For the KEn, the corneal epithelium was removed and discarded, and the stroma and endothelium from 10 corneas were pooled into each sample. The KEn samples included the corneal endothelium to capture any changes in gene expression at this time point. Three biological repeats were made for each group for a total of 9 samples. All samples were immediately immersed in Trizol (Life Technologies), flash frozen in liquid nitrogen, and shipped in dry ice to BGI.US® for library preparation and sequencing.
RNA Sequencing and Mapping
Sequencing library of each sample was prepared according to Illumina Standard Protocols. High-throughput sequencing was performed by Illumina HiSeq™4000 (Single Read, 50bp) and generated an average of 47,074,369 raw sequencing reads from each sample. Raw reads were filtered by removing reads with adaptors, removing reads with more than 10% unknown bases, and removing low quality reads (if more than 50% of base has Phred quality score (Q score) <5). An average of 46,928,426 clean reads with Q30 >95% (Richterich, 1998) were generated for all nine samples for further analysis. Clean reads were mapped to Gallus gallus reference assembly Galgal5 and annotated gene model (GenBank: GCA_000002315.3) using Bowtie 2 (Langmead and Salzberg, 2012) and HISAT (Kim et al., 2015). The average mapping ratio with reference gene was 76.26% and average genome mapping ratio was 93.10%. The mapped reads were then counted by RSEM (Li and Dewey, 2011). Transcripts Per Kilobase Million (TPM) values were generated by normalization of counts with library size and gene length (Wagner et al., 2012).
Data access
RNA-Seq data sets have been deposited in the in the NCBI’s GEO database (https://www.ncbi.nlm.nih.gov/geo/browse/). Accession number (GSE120434).
DEGs and Pathway Analyses
Differentially expressed genes (DEGs) were screened out by EdgeR (Robinson et al., 2010) and NoiseqBio (Tarazona et al., 2011; Tarazona et al., 2015). Each pair (En/pNC, KEn/pNC, or KEn/En) were compared and DEGs chosen based on the following standards: (1) CPM (count per million) greater than 1 in at least three samples; (2) Log2 fold change greater than 1 in both EdgeR and NoiseqBIO; (3) FDR (corrected p-value, q-value) less than 0.05 in EdgeR and Probability (equal to 1-FDR) more than 95% in NoiseqBio. To reduce false positive rate, overlapped (common) genes from EdgeR and NoiseqBio were defined as DEGs for further analysis (Supplementary Table 1). KEGG pathway analysis (Kanehisa et al., 2012) was performed by DAVID Bioinformatics Resources (Huang da et al., 2009). Top significant pathways were ordered by q-value, which is a corrected p-value by multiple hypothesis testing using the Benjamini-Hochberg, with 0.01 as a cutoff.
Neural crest genes with TPM greater than 1 in three pNC samples are considered to be expressed. Genes associated with RA, Wnt and TGF-beta pathways were analyzed with KEGG pathway, Map Database, and published data from Pubmed. Transcription factors, corneal endothelium, and ECM genes were analyzed with various tools including KEGG Orthology database, KEGG pathway database, InterPro database, and published data from Pubmed.
In situ hybridization
Freshly isolated E3 chick heads and either E5 or E7 eyes were fixed overnight at 4°C in modified Carnoy’s fixative (60% ethanol, 30% formaldehyde, and 10% glacial acetic acid). Samples were embedded in paraffin and sectioned between 10–12 μm. Probes were designed by Primer-blast (https://www.ncbi.nlm.nih.gov/tools/primer-blast/) or adapted from Gallus Expression in Situ Hybridization Analysis (Geisha; http://geisha.arizona.edu/geisha/) and cloned into pCR®II-TOPO® vector or pCR®4-TOPO® with dual promoters (Invitrogen). A list of primer sequences used for generating in situ probes is provided in Table 4. Digoxigenin-labeled riboprobes were generated by in vitro transcription with Superscript III®. Section in situ hybridization was performed as previously described (Etchevers et al., 2001). Brightfield images were captured using a Zeiss Axiocam mounted on AxioImager2 microscope (Zeiss).
Table 4.
List of primers used for in situ hybridization.
| Gene ID | Symbol | Forward primer | Reverse primer |
|---|---|---|---|
| NCC genes | |||
| 395245 | MSX21 | 5’-CGAGGAGCACCACAAAGTCAAG-3’ | 5’-GACAGGAGTAGCATAGAGTCCAACG-3’ |
| 432368 | SNAI2 | 5’-CTGTGTGGACTACAACCGGG-3’ | 5’-CTTCACATCCGAGTGGGTCT-3’ |
| 395734 | RHOB | 5’-TCGTCTTCAGCAAGGACGAG-3’ | 5’-GCAATTGATGCAGCCGTTCT-3’ |
| RA genes | |||
| 416880 | ALDH2 | 5’-ATCCCTTGGAACTTCCCCCT-3’ | 5’-ACTGATGCCCAACAGCAACT-3’ |
| 419480 | DHRS3 | 5’-CCTTCCCTTCCTTCGCTTTAT-3’ | 5’-GTGCTTCCAAACTCCACATTTC-3’ |
| 386585 | NR2F2 | 5’-CAAAGTTGGCATGAGACGGG-3’ | 5’-AGCTTCCCGAATCGTGTTGG-3’ |
| TGF-β | |||
| 421461 | LTBP12 | 5’-TGCATCAAACCTAACTGTGCA-3’ | 5’-TCGGAAGTTAGTGGCTGTCA-3’ |
| 428413 | BAMBI3 | 5’-GATCGCCATTCCAGCTACAT-3’ | 5’-TTTGCTGTCGTTGATCTTGC-3’ |
| 395897 | TGFBI | 5’-CACACAGCTCTACTCCGACC-3’ | 5’-GGCCAACTCAAACAGGGTCT-3’ |
| Wnt genes | |||
| 100858542 | AES | 5’-TGTTTCCACAAAGCCGACAC-3’ | 5’-TTCTCCCCATCGTCGTCTTG-3’ |
| 424707 | WLS | 5’-AGTGATCGCCTTTCTGGTGG-3’ | 5’-GCTATGGGTCCAACCTGCTT-3’ |
| 395862 | PITX2 | 5’-AGCGGACGCACTTCACCAGC-3’ | 5’-CGCAGCTCAGTCCGTGGCAA-3’ |
Adapted from Geisha (http://geisha.arizona.edu/geisha/search.jsp?entrez_gene=461423)
Adapted from Geisha (http://geisha.arizona.edu/geisha/search.jsp?entrez_gene=461645)
Immunostaining
Paraffin sections of E3, E5, and E7 quail-chick chimeras were rehydrated and prepared for immunostaining following standard protocols. Mouse anti-QCPN monoclonal antibody (1:1, IgG1, DHSB) was used to identify quail neural crest-derived cells during corneal development. Secondary antibody (Alexa 594 Goat anti-mouse IgG1, Invitrogen) was used at a concentration of 1:200. Imaging was performed with AxioImager2 fluorescent microscope with ApoTome (Zeiss).
Supplementary Material
Key findings:
RNA-Seq profiling revealed enrichment of genes between periocular neural crest corneal endothelium, and keratocytes.
The top KEGG pathways of the differentially express genes include focal adhesion, ECM-receptor interaction, cell adhesion, melanogenesis, and MAPK signaling.
Candidate neural crest genes are expressed in the periocular neural crest, but they are either differentially expressed or maintained during cornea development.
Modulators of major signaling pathways associated with ocular development (retinoic acid, TGFβ, and Wnt), transcription factors, and differentiation genes were differentially expressed.
ACKNOWLEDGMENTS
This work was supported by NIH grant EY027048 to P.Y.L. The authors would like to thank Dr. Luay Nakhleh, Dr. Huw Ogilvie, and Ms. Yanwan Dai for the helpful discussions and assistance with Bioinformatics. We also thank members of the Lwigale laboratory for the critical reading and helpful comments on the manuscript.
REFERENCES
- 1.Al-Hussain H, Zeisberger SM, Huber PR, Giunta C, Steinmann B. 2004. Brittle cornea syndrome and its delineation from the kyphoscoliotic type of Ehlers-Danlos syndrome (EDS VI): report on 23 patients and review of the literature. Am J Med Genet A 124A:28–34. [DOI] [PubMed] [Google Scholar]
- 2.Balmer JE, Blomhoff R. 2002. Gene expression regulation by retinoic acid. J Lipid Res 43:1773–1808. [DOI] [PubMed] [Google Scholar]
- 3.Bankhead EJ, Colasanto MP, Dyorich KM, Jamrich M, Murtaugh LC, Fuhrmann S. 2015. Multiple requirements of the focal dermal hypoplasia gene porcupine during ocular morphogenesis. Am J Pathol 185:197–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baulmann DC, Ohlmann A, Flugel-Koch C, Goswami S, Cvekl A, Tamm ER. 2002. Pax6 heterozygous eyes show defects in chamber angle differentiation that are associated with a wide spectrum of other anterior eye segment abnormalities. Mech Dev 118:3–17. [DOI] [PubMed] [Google Scholar]
- 5.Beebe DC, Coats JM. 2000. The lens organizes the anterior segment: specification of neural crest cell differentiation in the avian eye. Dev Biol 220:424–431. [DOI] [PubMed] [Google Scholar]
- 6.Blanco-Mezquita JT, Hutcheon AE, Zieske JD. 2013. Role of thrombospondin-1 in repair of penetrating corneal wounds. Invest Ophthalmol Vis Sci 54:6262–6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Border WA, Noble NA, Yamamoto T, Harper JR, Yamaguchi Y, Pierschbacher MD, Ruoslahti E. 1992. Natural inhibitor of transforming growth factor-beta protects against scarring in experimental kidney disease. Nature 360:361–364. [DOI] [PubMed] [Google Scholar]
- 8.Briata P, Ilengo C, Corte G, Moroni C, Rosenfeld MG, Chen CY, Gherzi R. 2003. The Wnt/beta-catenin-->Pitx2 pathway controls the turnover of Pitx2 and other unstable mRNAs. Mol Cell 12:1201–1211. [DOI] [PubMed] [Google Scholar]
- 9.Brugmann SA, Tapadia MD, Helms JA. 2006. The molecular origins of species-specific facial pattern. Curr Top Dev Biol 73:1–42. [DOI] [PubMed] [Google Scholar]
- 10.Chai Y, Ito Y, Han J. 2003. TGF-beta signaling and its functional significance in regulating the fate of cranial neural crest cells. Crit Rev Oral Biol Med 14:78–88. [DOI] [PubMed] [Google Scholar]
- 11.Chao-Shern C, Me R, DeDionisio LA, Ke BL, Nesbit MA, Marshall J, Moore CBT. 2018. Post-LASIK exacerbation of granular corneal dystrophy type 2 in members of a chinese family. Eye (Lond) 32:39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen KG, Szakacs G, Annereau JP, Rouzaud F, Liang XJ, Valencia JC, Nagineni CN, Hooks JJ, Hearing VJ, Gottesman MM. 2005. Principal expression of two mRNA isoforms (ABCB 5alpha and ABCB 5beta ) of the ATP-binding cassette transporter gene ABCB 5 in melanoma cells and melanocytes. Pigment Cell Res 18:102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Carlson EC, Chen ZY, Hamik A, Jain MK, Dunwoodie SL, Yang YC. 2009. Conditional deletion of Cited2 results in defective corneal epithelial morphogenesis and maintenance. Dev Biol 334:243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chng Z, Peh GS, Herath WB, Cheng TY, Ang HP, Toh KP, Robson P, Mehta JS, Colman A. 2013. High throughput gene expression analysis identifies reliable expression markers of human corneal endothelial cells. PLoS One 8:e67546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Churchill A, Booth A. 1996. Genetics of aniridia and anterior segment dysgenesis. Br J Ophthalmol 80:669–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cook CS. 1989. Experimental models of anterior segment dysgenesis. Ophthalmic Paediatr Genet 10:33–46. [DOI] [PubMed] [Google Scholar]
- 17.Creuzet S, Couly G, Le Douarin NM. 2005a. Patterning the neural crest derivatives during development of the vertebrate head: insights from avian studies. J Anat 207:447–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Creuzet S, Vincent C, Couly G. 2005b. Neural crest derivatives in ocular and periocular structures. Int J Dev Biol 49:161–171. [DOI] [PubMed] [Google Scholar]
- 19.Dong D, Ruuska SE, Levinthal DJ, Noy N. 1999. Distinct roles for cellular retinoic acid-binding proteins I and II in regulating signaling by retinoic acid. J Biol Chem 274:23695–23698. [DOI] [PubMed] [Google Scholar]
- 20.Dottori M, Gross MK, Labosky P, Goulding M. 2001. The winged-helix transcription factor Foxd3 suppresses interneuron differentiation and promotes neural crest cell fate. Development 128:4127–4138. [DOI] [PubMed] [Google Scholar]
- 21.Dupe V, Matt N, Garnier JM, Chambon P, Mark M, Ghyselinck NB. 2003. A newborn lethal defect due to inactivation of retinaldehyde dehydrogenase type 3 is prevented by maternal retinoic acid treatment. Proc Natl Acad Sci U S A 100:14036–14041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dutton KA, Pauliny A, Lopes SS, Elworthy S, Carney TJ, Rauch J, Geisler R, Haffter P, Kelsh RN. 2001. Zebrafish colourless encodes sox10 and specifies non-ectomesenchymal neural crest fates. Development 128:4113–4125. [DOI] [PubMed] [Google Scholar]
- 23.Etchevers HC, Vincent C, Le Douarin M, Couly GF. 2001. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development 128:1059–1068. [DOI] [PubMed] [Google Scholar]
- 24.Evans AL, Gage PJ. 2005. Expression of the homeobox gene Pitx2 in neural crest is required for optic stalk and ocular anterior segment development. Hum Mol Gen 14:3347–3359. [DOI] [PubMed] [Google Scholar]
- 25.Feng L, Hernandez RE, Waxman JS, Yelon D, Moens CB. 2010. Dhrs3a regulates retinoic acid biosynthesis through a feedback inhibition mechanism. Dev Biol 338:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fischer A, Schumacher N, Maier M, Sendtner M, Gessler M. 2004. The Notch target genes Hey1 and Hey2 are required for embryonic vascular development. Genes Dev 18:901–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fokina VM, Frolova EI. 2006. Expression patterns of Wnt genes during development of an anterior part of the chicken eye. Dev Dyn 235:496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujiwara M, Uchida T, Osumi-Yamashita N, Eto K. 1994. Uchida rat (rSey): a new mutant rat with craniofacial abnormalities resembling those of the mouse Sey mutant. Differentiation 57:31–38. [DOI] [PubMed] [Google Scholar]
- 29.Gage PJ, Kuang C, Zacharias AL. 2014. The homeodomain transcription factor PITX2 is required for specifying correct cell fates and establishing angiogenic privilege in the developing cornea. Dev Dyn 243:1391–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gage PJ, Qian M, Wu DQ, Rosenberg KI. 2008. The canonical Wnt signaling antagonist DKK2 is an essential effector of PITX2 function during normal eye development. Dev Biol 317:310–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gage PJ, Suh HY, Camper SA. 1999. Dosage requirement of Pitx2 for development of multiple organs. Development 126:4643–4651. [DOI] [PubMed] [Google Scholar]
- 32.Good DJ, Polverini PJ, Rastinejad F, Le Beau MM, Lemons RS, Frazier WA, Bouck NP. 1990. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci U S A 87:6624–6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamburger V, Hamilton HL. 1951. A series of normal stages in the development of the chick embryo. J Morphol 88:49–92. [PubMed] [Google Scholar]
- 34.Hashimoto K, Noshiro M, Ohno S, Kawamoto T, Satakeda H, Akagawa Y, Nakashima K, Okimura A, Ishida H, Okamoto T, Pan H, Shen M, Yan W, Kato Y. 1997. Characterization of a cartilage-derived 66-kDa protein (RGD-CAP/beta ig-h3) that binds to collagen. Biochim Biophys Acta 1355:303–314. [DOI] [PubMed] [Google Scholar]
- 35.Hassell JR, Birk DE. 2010. The molecular basis of corneal transparency. Exp Eye Res 91:326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hay ED, Revel JP. 1969. Fine structure of the developing avian cornea. Monogr Dev Biol 1:1–144. [PubMed] [Google Scholar]
- 37.He Z, Forest F, Gain P, Rageade D, Bernard A, Acquart S, Peoc’h M, Defoe DM, Thuret G. 2016. 3D map of the human corneal endothelial cell. Sci Rep 6:29047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heanue TA, Reshef R, Davis RJ, Mardon G, Oliver G, Tomarev S, Lassar AB, Tabin CJ. 1999. Synergistic regulation of vertebrate muscle development by Dach2, Eya2, and Six1, homologs of genes required for Drosophila eye formation. Genes Dev 13:3231–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hill RE, Favor J, Hogan BL, Ton CC, Saunders GF, Hanson IM, Prosser J, Jordan T, Hastie ND, van Heyningen V. 1991. Mouse small eye results from mutations in a paired-like homeobox-containing gene. Nature 354:522–525. [DOI] [PubMed] [Google Scholar]
- 40.Hiscott P, Armstrong D, Batterbury M, Kaye S. 1999. Repair in avascular tissues: fibrosis in the transparent structures of the eye and thrombospondin 1. Histology and Histopathology 14:1309–1320. [DOI] [PubMed] [Google Scholar]
- 41.Hiscott P, Paraoan L, Choudhary A, Ordonez JL, Al-Khaier A, Armstrong DJ. 2006. Thrombospondin 1, thrombospondin 2 and the eye. Prog Retin Eye Res 25:1–18. [DOI] [PubMed] [Google Scholar]
- 42.Huang da W, Sherman BT, Lempicki RA. 2009. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4:44–57. [DOI] [PubMed] [Google Scholar]
- 43.Ishii M, Han J, Yen HY, Sucov HM, Chai Y, Maxson RE Jr., 2005. Combined deficiencies of Msx1 and Msx2 cause impaired patterning and survival of the cranial neural crest. Development 132:4937–4950. [DOI] [PubMed] [Google Scholar]
- 44.Ittner LM, Wurdak H, Schwerdtfeger K, Kunz T, Ille F, Leveen P, Hjalt TA, Suter U, Karlsson S, Hafezi F, Born W, Sommer L. 2005. Compound developmental eye disorders following inactivation of TGFbeta signaling in neural-crest stem cells. J Biol 4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jin EJ, Burrus LW, Erickson CA. 2002. The expression patterns of Wnts and their antagonists during avian eye development. Mech Dev 116:173–176. [DOI] [PubMed] [Google Scholar]
- 46.Johnston MC, Noden DM, Hazelton RD, Coulombre JL, Coulombre AJ. 1979. Origins of avian ocular and periocular tissues. Exp Eye Res 29:27–43. [DOI] [PubMed] [Google Scholar]
- 47.Kadler KE, Holmes DF, Trotter JA, Chapman JA. 1996. Collagen fibril formation. Biochem J 316 ( Pt 1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kam RK, Shi W, Chan SO, Chen Y, Xu G, Lau CB, Fung KP, Chan WY, Zhao H. 2013. Dhrs3 protein attenuates retinoic acid signaling and is required for early embryonic patterning. J Biol Chem 288:31477–31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kanakubo S, Nomura T, Yamamura K, Miyazaki J, Tamai M, Osumi N. 2006. Abnormal migration and distribution of neural crest cells in Pax6 heterozygous mutant eye, a model for human eye diseases. Genes Cells 11:919–933. [DOI] [PubMed] [Google Scholar]
- 50.Kanehisa M, Goto S, Sato Y, Furumichi M, Tanabe M. 2012. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res 40:D109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kattan JM, Serna-Ojeda JC, Sharma A, Kim EK, Ramirez-Miranda A, Cruz-Aguilar M, Cervantes AE, Frausto RF, Zenteno JC, Graue-Hernandez EO, Aldave AJ. 2017. Vortex Pattern of Corneal Deposits in Granular Corneal Dystrophy Associated With the p.(Arg555Trp) Mutation in TGFBI . Cornea 36:210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim D, Langmead B, Salzberg SL. 2015. HISAT: a fast spliced aligner with low memory requirements. Nat Methods 12:357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kioussi C, Briata P, Baek SH, Rose DW, Hamblet NS, Herman T, Ohgi KA, Lin C, Gleiberman A, Wang J, Brault V, Ruiz-Lozano P, Nguyen HD, Kemler R, Glass CK, Wynshaw-Boris A, Rosenfeld MG 2002. Identification of a Wnt/Dvl/beta-Catenin –>Pitx2 pathway mediating cell-type-specific proliferation during development. CELL 111: 673–685. [DOI] [PubMed] [Google Scholar]
- 54.Kitamura K, Miura H, Miyagawa-Tomita S, Yanazawa M, Katoh-Fukui Y, Suzuki R, Ohuchi H, Suehiro A, Motegi Y, Nakahara Y, Kondo S, Yokoyama M. 1999. Mouse Pitx2 deficiency leads to anomalies of the ventral body wall, heart, extra- and periocular mesoderm and right pulmonary isomerism. Development 126:5749–5758. [DOI] [PubMed] [Google Scholar]
- 55.Kliewer SA, Umesono K, Heyman RA, Mangelsdorf DJ, Dyck JA, Evans RM. 1992. Retinoid X receptor-COUP-TF interactions modulate retinoic acid signaling. Proc Natl Acad Sci U S A 89:1448–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Knupp C, Pinali C, Lewis PN, Parfitt GJ, Young RD, Meek KM, Quantock AJ. 2009. The architecture of the cornea and structural basis of its transparency. Adv Protein Chem Struct Biol 78:25–49. [DOI] [PubMed] [Google Scholar]
- 57.Ko MK, Kay EP. 2001. Subcellular localization of procollagen I and prolyl 4-hydroxylase in corneal endothelial cells. Exp Cell Res 264:363–371. [DOI] [PubMed] [Google Scholar]
- 58.Kong M, Munoz N, Valdivia A, Alvarez A, Herrera-Molina R, Cardenas A, Schneider P, Burridge K, Quest AF, Leyton L. 2013. Thy-1-mediated cell-cell contact induces astrocyte migration through the engagement of alphaVbeta3 integrin and syndecan-4. Biochim Biophys Acta 1833:1409–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kruse SW, Suino-Powell K, Zhou XE, Kretschman JE, Reynolds R, Vonrhein C, Xu Y, Wang L, Tsai SY, Tsai MJ, Xu HE. 2008. Identification of COUP-TFII orphan nuclear receptor as a retinoic acid-activated receptor. PLoS Biol 6:e227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ksander BR, Kolovou PE, Wilson BJ, Saab KR, Guo Q, Ma J, McGuire SP, Gregory MS, Vincent WJ, Perez VL, Cruz-Guilloty F, Kao WW, Call MK, Tucker BA, Zhan Q, Murphy GF, Lathrop KL, Alt C, Mortensen LJ, Lin CP, Zieske JD, Frank MH, Frank NY. 2014. ABCB5 is a limbal stem cell gene required for corneal development and repair. Nature 511:353–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kume T, Seo S. 2010. The Forkhead Transcription Factor Foxc1 is Required for Corneal Avascularity. Invest Ophthalmol Vis Sci 51. [Google Scholar]
- 62.Labosky PA, Kaestner KH. 1998. The winged helix transcription factor Hfh2 is expressed in neural crest and spinal cord during mouse development. Mech Dev 76:185–190. [DOI] [PubMed] [Google Scholar]
- 63.Langmead B, Salzberg SL. 2012. Fast gapped-read alignment with Bowtie 2. Nat Methods 9:357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee JG, Ko MK, Kay EP. 2012. Endothelial mesenchymal transformation mediated by IL-1beta-induced FGF-2 in corneal endothelial cells. Exp Eye Res 95:35–39. [DOI] [PubMed] [Google Scholar]
- 65.Lehmann OJ, Sowden JC, Carlsson P, Jordan T, Bhattacharya SS. 2003. Fox’s in development and disease. Trends in Genetics 19:339–344. [DOI] [PubMed] [Google Scholar]
- 66.Leyton L, Schneider P, Labra CV, Ruegg C, Hetz CA, Quest AF, Bron C. 2001. Thy-1 binds to integrin beta(3) on astrocytes and triggers formation of focal contact sites. Curr Biol 11:1028–1038. [DOI] [PubMed] [Google Scholar]
- 67.Li B, Dewey CN. 2011. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li H, Wagner E, McCaffery P, Smith D, Andreadis A, Drager UC. 2000. A retinoic acid synthesizing enzyme in ventral retina and telencephalon of the embryonic mouse. Mech Dev 95:283–289. [DOI] [PubMed] [Google Scholar]
- 69.Liem KF Jr., Tremml G, Roelink H, Jessell TM 1995. Dorsal differentiation of neural plate cells induced by BMP-mediated signals from epidermal ectoderm. Cell 82:969–979. [DOI] [PubMed] [Google Scholar]
- 70.Lin Z, Gao C, Ning Y, He X, Wu W, Chen YG. 2008. The pseudoreceptor BMP and activin membrane-bound inhibitor positively modulates Wnt/beta-catenin signaling. J Biol Chem 283:33053–33058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Linsenmayer TF, Fitch JM, Mayne R. 1984. Extracellular matrices in the developing avian eye: type V collagen in corneal and noncorneal tissues. Invest Ophthalmol Vis Sci 25:41–47. [PubMed] [Google Scholar]
- 72.Liu JP, Jessell TM. 1998. A role for rhoB in the delamination of neural crest cells from the dorsal neural tube. Development 125:5055–5067. [DOI] [PubMed] [Google Scholar]
- 73.Liu YH, Tang Z, Kundu RK, Wu L, Luo W, Zhu D, Sangiorgi F, Snead ML, Maxson RE. 1999. Msx2 gene dosage influences the number of proliferative osteogenic cells in growth centers of the developing murine skull: a possible mechanism for MSX2-mediated craniosynostosis in humans. Dev Biol 205:260–274. [DOI] [PubMed] [Google Scholar]
- 74.Lorda-Diez CI, Montero JA, Garcia-Porrero JA, Hurle JM. 2010. Tgfbeta2 and 3 are coexpressed with their extracellular regulator Ltbp1 in the early limb bud and modulate mesodermal outgrowth and BMP signaling in chicken embryos. BMC Dev Biol 10:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lwigale PY, Bronner-Fraser M. 2009. Semaphorin3A/neuropilin-1 signaling acts as a molecular switch regulating neural crest migration during cornea development. Dev Biol 336:257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lwigale PY, Conrad GW, Bronner-Fraser M. 2004. Graded potential of neural crest to form cornea, sensory neurons and cartilage along the rostrocaudal axis. Development 131:1979–1991. [DOI] [PubMed] [Google Scholar]
- 77.Lwigale PY, Cressy PA, Bronner-Fraser M. 2005. Corneal keratocytes retain neural crest progenitor cell properties. Dev Biol 288:284–293. [DOI] [PubMed] [Google Scholar]
- 78.Maden M 2007. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat Rev Neurosci 8:755–765. [DOI] [PubMed] [Google Scholar]
- 79.Maden M, Sonneveld E, van der Saag PT, Gale E. 1998. The distribution of endogenous retinoic acid in the chick embryo: implications for developmental mechanisms. Development 125:4133–4144. [DOI] [PubMed] [Google Scholar]
- 80.Marcos-Ramiro B, Garcia-Weber D, Barroso S, Feito J, Ortega MC, Cernuda-Morollon E, Reglero-Real N, Fernandez-Martin L, Duran MC, Alonso MA, Correas I, Cox S, Ridley AJ, Millan J. 2016. RhoB controls endothelial barrier recovery by inhibiting Rac1 trafficking to the cell border. J Cell Biol 213:385–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mardon G, Solomon NM, Rubin GM. 1994. dachshund encodes a nuclear protein required for normal eye and leg development in Drosophila. Development 120:3473–3486. [DOI] [PubMed] [Google Scholar]
- 82.Martinez-Morales PL, Diez del Corral R, Olivera-Martinez I, Quiroga AC, Das RM, Barbas JA, Storey KG, Morales AV. 2011. FGF and retinoic acid activity gradients control the timing of neural crest cell emigration in the trunk. J Cell Biol 194:489–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Martino VB, Sabljic T, Deschamps P, Green RM, Akula M, Peacock E, Ball A, Williams T, West-Mays JA. 2016. Conditional deletion of AP-2beta in mouse cranial neural crest results in anterior segment dysgenesis and early-onset glaucoma. Dis Model Mech 9:849–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Matsuba M, Hutcheon AE, Zieske JD. 2011. Localization of thrombospondin-1 and myofibroblasts during corneal wound repair. Exp Eye Res 93:534–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Matt N, Dupe V, Garnier JM, Dennefeld C, Chambon P, Mark M, Ghyselinck NB. 2005. Retinoic acid-dependent eye morphogenesis is orchestrated by neural crest cells. Development 132:4789–4800. [DOI] [PubMed] [Google Scholar]
- 86.McKeown SJ, Lee VM, Bronner-Fraser M, Newgreen DF, Farlie PG. 2005. Sox10 overexpression induces neural crest-like cells from all dorsoventral levels of the neural tube but inhibits differentiation. Dev Dyn 233:430–444. [DOI] [PubMed] [Google Scholar]
- 87.Mei M, Xiang RL, Cong X, Zhang Y, Li J, Yi X, Park K, Han JY, Wu LL, Yu GY. 2015. Claudin-3 is required for modulation of paracellular permeability by TNF-alpha through ERK1/2/slug signaling axis in submandibular gland. Cell Signal 27:1915–1927. [DOI] [PubMed] [Google Scholar]
- 88.Michalik L, Wahli W. 2007. Guiding ligands to nuclear receptors. Cell 129:649–651. [DOI] [PubMed] [Google Scholar]
- 89.Michelacci YM. 2003. Collagens and proteoglycans of the corneal extracellular matrix. Braz J Med Biol Res 36:1037–1046. [DOI] [PubMed] [Google Scholar]
- 90.Mohan RR, Gupta R, Mehan MK, Cowden JW, Sinha S. 2010. Decorin transfection suppresses profibrogenic genes and myofibroblast formation in human corneal fibroblasts. Exp Eye Res 91:238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Molotkov A, Molotkova N, Duester G. 2006. Retinoic acid guides eye morphogenetic movements via paracrine signaling but is unnecessary for retinal dorsoventral patterning. Development 133:1901–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Monsoro-Burq AH, Fletcher RB, Harland RM. 2003. Neural crest induction by paraxial mesoderm in Xenopus embryos requires FGF signals. Development 130:3111–3124. [DOI] [PubMed] [Google Scholar]
- 93.Mundell NA, Labosky PA. 2011. Neural crest stem cell multipotency requires Foxd3 to maintain neural potential and repress mesenchymal fates. Development 138:641–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nielsen NS, Juhl DW, Poulsen ET, Lukassen MV, Poulsen EC, Risor MW, Scavenius C, Enghild JJ. 2017. Mutation-Induced Deamidation of Corneal Dystrophy-Related Transforming Growth Factor beta-Induced Protein. Biochemistry 56:6470–6480. [DOI] [PubMed] [Google Scholar]
- 95.Noden DM. 1975. An analysis of migratory behavior of avian cephalic neural crest cells. Dev Biol 42:106–130. [DOI] [PubMed] [Google Scholar]
- 96.Ochoa SD, Salvador S, LaBonne C. 2012. The LIM adaptor protein LMO4 is an essential regulator of neural crest development. Dev Biol 361:313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Onichtchouk D, Chen YG, Dosch R, Gawantka V, Delius H, Massague J, Niehrs C. 1999. Silencing of TGF-beta signalling by the pseudoreceptor BAMBI. Nature 401:480–485. [DOI] [PubMed] [Google Scholar]
- 98.Petroll WM, Hsu JK, Bean J, Cavanagh HD, Jester JV. 1999. The spatial organization of apical junctional complex-associated proteins in feline and human corneal endothelium. Curr Eye Res 18:10–19. [DOI] [PubMed] [Google Scholar]
- 99.Pickens BS, Teets BW, Soprano KJ, Soprano DR. 2013. Role of COUP-TFI during retinoic acid-induced differentiation of P19 cells to endodermal cells. J Cell Physiol 228:791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Powell SK, Lisanti MP, Rodriguez-Boulan EJ. 1991. Thy-1 expresses two signals for apical localization in epithelial cells. Am J Physiol 260:C715–720. [DOI] [PubMed] [Google Scholar]
- 101.Quantock AJ, Young RD. 2008. Development of the corneal stroma, and the collagen-proteoglycan associations that help define its structure and function. Dev Dyn 237:2607–2621. [DOI] [PubMed] [Google Scholar]
- 102.Rada JA, Cornuet PK, Hassell JR. 1993. Regulation of corneal collagen fibrillogenesis in vitro by corneal proteoglycan (lumican and decorin) core proteins. Exp Eye Res 56:635–648. [DOI] [PubMed] [Google Scholar]
- 103.Ramachandran C, Srinivas SP. 2010. Formation and disassembly of adherens and tight junctions in the corneal endothelium: regulation by actomyosin contraction. Invest Ophthalmol Vis Sci 51:2139–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Reis LM, Semina EV. 2011. Genetics of anterior segment dysgenesis disorders. Curr Opin Ophthalmol 22:314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Reneker LW, Silversides DW, Xu L, Overbeek PA. 2000. Formation of corneal endothelium is essential for anterior segment development - a transgenic mouse model of anterior segment dysgenesis. Development 127:533–542. [DOI] [PubMed] [Google Scholar]
- 106.Richterich P 1998. Estimation of errors in “raw” DNA sequences: a validation study. Genome Res 8:251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Robinson MD, McCarthy DJ, Smyth GK. 2010. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26:139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sahar DE, Longaker MT, Quarto N. 2005. Sox9 neural crest determinant gene controls patterning and closure of the posterior frontal cranial suture. Dev Biol 280:344–361. [DOI] [PubMed] [Google Scholar]
- 109.Saika S, Miyamoto T, Ishida I, Shirai K, Ohnishi Y, Ooshima A, McAvoy JW. 2002. TGFbeta-Smad signalling in postoperative human lens epithelial cells. Br J Ophthalmol 86:1428–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Saika S, Saika S, Liu CY, Azhar M, Sanford LP, Doetschman T, Gendron RL, Kao CW, Kao WW. 2001. TGFbeta2 in corneal morphogenesis during mouse embryonic development. Dev Biol 240:419–432. [DOI] [PubMed] [Google Scholar]
- 111.Saint-Jeannet JP, He X, Varmus HE, Dawid IB. 1997. Regulation of dorsal fate in the neuraxis by Wnt-1 and Wnt-3a. Proc Natl Acad Sci U S A 94:13713–13718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sakai Y, Luo T, McCaffery P, Hamada H, Drager UC. 2004. CYP26A1 and CYP26C1 cooperate in degrading retinoic acid within the equatorial retina during later eye development. Dev Biol 276:143–157. [DOI] [PubMed] [Google Scholar]
- 113.Sakai Y, Meno C, Fujii H, Nishino J, Shiratori H, Saijoh Y, Rossant J, Hamada H. 2001. The retinoic acid-inactivating enzyme CYP26 is essential for establishing an uneven distribution of retinoic acid along the anterio-posterior axis within the mouse embryo. Genes Dev 15:213–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Salie R, Kneissel M, Vukevic M, Zamurovic N, Kramer I, Evans G, Gerwin N, Mueller M, Kinzel B, Susa M. 2010. Ubiquitous overexpression of Hey1 transcription factor leads to osteopenia and chondrocyte hypertrophy in bone. Bone 46:680–694. [DOI] [PubMed] [Google Scholar]
- 115.Sato A, Yamamoto H, Sakane H, Koyama H, Kikuchi A. 2010. Wnt5a regulates distinct signalling pathways by binding to Frizzled2. EMBO J 29:41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schultz-Cherry S, Ribeiro S, Gentry L, Murphy-Ullrich JE. 1994. Thrombospondin binds and activates the small and large forms of latent transforming growth factor-beta in a chemically defined system. J Biol Chem 269:26775–26782. [PubMed] [Google Scholar]
- 117.Serbedzija GN, Bronner-Fraser M, Fraser SE. 1992. Vital dye analysis of cranial neural crest cell migration in the mouse embryo. Development 116:297–307. [DOI] [PubMed] [Google Scholar]
- 118.Shah NM, Groves AK, Anderson DJ. 1996. Alternative neural crest cell fates are instructively promoted by TGFbeta superfamily members. Cell 85:331–343. [DOI] [PubMed] [Google Scholar]
- 119.Shaw LM, Olsen BR. 1991. FACIT collagens: diverse molecular bridges in extracellular matrices. Trends Biochem Sci 16:191–194. [DOI] [PubMed] [Google Scholar]
- 120.Simoes-Costa M, Bronner ME. 2015. Establishing neural crest identity: a gene regulatory recipe. Development 142:242–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Simoes-Costa M, Tan-Cabugao J, Antoshechkin I, Sauka-Spengler T, Bronner ME. 2014. Transcriptome analysis reveals novel players in the cranial neural crest gene regulatory network. Genome Res 24:281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Skonier J, Neubauer M, Madisen L, Bennett K, Plowman GD, Purchio AF. 1992. cDNA cloning and sequence analysis of beta ig-h3, a novel gene induced in a human adenocarcinoma cell line after treatment with transforming growth factor-beta. DNA Cell Biol 11:511–522. [DOI] [PubMed] [Google Scholar]
- 123.Smith RS, Zabaleta A, Kume T, Savinova OV, Kidson SH, Martin JE, Nishimura DY, Alward WLM, Hogan BLM, John SWM. 2000. Haploinsufficiency of the transcription factors FOXC1 and FOXC2 results in aberrant ocular development. Hum Mol Gen 9:1021–1032. [DOI] [PubMed] [Google Scholar]
- 124.Southard-Smith EM, Kos L, Pavan WJ. 1998. Sox10 mutation disrupts neural crest development in Dom Hirschsprung mouse model. Nat Genet 18:60–64. [DOI] [PubMed] [Google Scholar]
- 125.Sowden JC. 2007. Molecular and developmental mechanisms of anterior segment dysgenesis. Eye (Lond) 21:1310–1318. [DOI] [PubMed] [Google Scholar]
- 126.Steinfeld J, Steinfeld I, Bausch A, Coronato N, Hampel ML, Depner H, Layer PG, Vogel-Hopker A. 2017. BMP-induced reprogramming of the neural retina into retinal pigment epithelium requires Wnt signalling. Biol Open 6:979–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sui H, Han BG, Lee JK, Walian P, Jap BK. 2001. Structural basis of water-specific transport through the AQP1 water channel. Nature 414:872–878. [DOI] [PubMed] [Google Scholar]
- 128.Taneyhill LA, Coles EG, Bronner-Fraser M. 2007. Snail2 directly represses cadherin6B during epithelial-to-mesenchymal transitions of the neural crest. Development 134:1481–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tarazona S, Furio-Tari P, Turra D, Di Pietro A, Nueda MJ, Ferrer A, Conesa A. 2015. Data quality aware analysis of differential expression in RNA-seq with NOISeq R/Bioc package. Nucleic Acids Res 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tarazona S, Garcia-Alcalde F, Dopazo J, Ferrer A, Conesa A. 2011. Differential expression in RNA-seq: a matter of depth. Genome Res 21:2213–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tiveron MC, Nosten-Bertrand M, Jani H, Garnett D, Hirst EM, Grosveld F, Morris RJ. 1994. The mode of anchorage to the cell surface determines both the function and the membrane location of Thy-1 glycoprotein. J Cell Sci 107 ( Pt 7):1783–1796. [DOI] [PubMed] [Google Scholar]
- 132.Tolsma SS, Stack MS, Bouck N. 1997. Lumen formation and other angiogenic activities of cultured capillary endothelial cells are inhibited by thrombospondin-1. Microvasc Res 54:13–26. [DOI] [PubMed] [Google Scholar]
- 133.Tran P, Zhang XK, Salbert G, Hermann T, Lehmann JM, Pfahl M. 1992. COUP orphan receptors are negative regulators of retinoic acid response pathways. Mol Cell Biol 12:4666–4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tritschler I, Gramatzki D, Capper D, Mittelbronn M, Meyermann R, Saharinen J, Wick W, Keski-Oja J, Weller M. 2009. Modulation of TGF-beta activity by latent TGF-beta-binding protein 1 in human malignant glioma cells. Int J Cancer 125:530–540. [DOI] [PubMed] [Google Scholar]
- 135.Trousse F, Esteve P, Bovolenta P. 2001. Bmp4 mediates apoptotic cell death in the developing chick eye. J Neurosci 21:1292–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Villanueva S, Glavic A, Ruiz P, Mayor R. 2002. Posteriorization by FGF, Wnt, and retinoic acid is required for neural crest induction. Dev Biol 241:289–301. [DOI] [PubMed] [Google Scholar]
- 137.Vincent AL, Jordan CA, Cadzow MJ, Merriman TR, McGhee CN. 2014. Mutations in the zinc finger protein gene, ZNF469, contribute to the pathogenesis of keratoconus. Invest Ophthalmol Vis Sci 55:5629–5635. [DOI] [PubMed] [Google Scholar]
- 138.Wagner GP, Kin K, Lynch VJ. 2012. Measurement of mRNA abundance using RNA-seq data: RPKM measure is inconsistent among samples. Theory Biosci 131:281–285. [DOI] [PubMed] [Google Scholar]
- 139.Waring GO 3rd, Bourne WM, Edelhauser HF, Kenyon KR 1982. The corneal endothelium. Normal and pathologic structure and function. Ophthalmology 89:531–590. [PubMed] [Google Scholar]
- 140.Wurdak H, Ittner LM, Lang KS, Leveen P, Suter U, Fischer JA, Karlsson S, Born W, Sommer L. 2005. Inactivation of TGFbeta signaling in neural crest stem cells leads to multiple defects reminiscent of DiGeorge syndrome. Genes Dev 19:530–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yamaguchi Y, Mann DM, Ruoslahti E. 1990. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature 346:281–284. [DOI] [PubMed] [Google Scholar]
- 142.Yamanaka H, Moriguchi T, Masuyama N, Kusakabe M, Hanafusa H, Takada R, Takada S, Nishida E. 2002. JNK functions in the non-canonical Wnt pathway to regulate convergent extension movements in vertebrates. EMBO Rep 3:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yokoyama M, Narita T, Sakurai H, Katsumata-Kato O, Sugiya H, Fujita-Yoshigaki J. 2017. Maintenance of claudin-3 expression and the barrier functions of intercellular junctions in parotid acinar cells via the inhibition of Src signaling. Arch Oral Biol 81:141–150. [DOI] [PubMed] [Google Scholar]
- 144.Zhao Q, Eberspaecher H, Lefebvre V, De Crombrugghe B 1997. Parallel expression of Sox9 and Col2a1 in cells undergoing chondrogenesis. Dev Dyn: 209, 377–386. [DOI] [PubMed] [Google Scholar]
- 145.Zlotogora J, BenEzra D, Cohen T, Cohen E. 1990. Syndrome of brittle cornea, blue sclera, and joint hyperextensibility. Am J Med Genet 36:269–272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


