Abstract
Objective
To provide updated evidence-based recommendations for migraine prevention using pharmacologic treatment with or without cognitive behavioral therapy in the pediatric population.
Methods
The authors systematically reviewed literature from January 2003 to August 2017 and developed practice recommendations using the American Academy of Neurology 2011 process, as amended.
Results
Fifteen Class I–III studies on migraine prevention in children and adolescents met inclusion criteria. There is insufficient evidence to determine if children and adolescents receiving divalproex, onabotulinumtoxinA, amitriptyline, nimodipine, or flunarizine are more or less likely than those receiving placebo to have a reduction in headache frequency. Children with migraine receiving propranolol are possibly more likely than those receiving placebo to have an at least 50% reduction in headache frequency. Children and adolescents receiving topiramate and cinnarizine are probably more likely than those receiving placebo to have a decrease in headache frequency. Children with migraine receiving amitriptyline plus cognitive behavioral therapy are more likely than those receiving amitriptyline plus headache education to have a reduction in headache frequency.
Recommendations
The majority of randomized controlled trials studying the efficacy of preventive medications for pediatric migraine fail to demonstrate superiority to placebo. Recommendations for the prevention of migraine in children include counseling on lifestyle and behavioral factors that influence headache frequency and assessment and management of comorbid disorders associated with headache persistence. Clinicians should engage in shared decision-making with patients and caregivers regarding the use of preventive treatments for migraine, including discussion of the limitations in the evidence to support pharmacologic treatments.
This article summarizes the findings of a systematic review and practice guideline on the pharmacologic treatment of migraine prevention in children and adolescents. The unabridged practice guideline is available at https://www.aan.com/Guidelines/home/GetGuidelineContent/978 and includes full details of the methodology used, including the risk of bias assessment for each study, meta-analysis, and confidence in evidence determinations.
This guideline systematically evaluates new evidence to answer the following clinical question: In children and adolescents with migraines, do preventive pharmacologic treatments, with or without cognitive behavioral therapy (CBT), compared with placebo, reduce headache frequency?
Migraine is common in children and adolescents, with a prevalence of 1%–3% in 3- to 7-year-olds, 4%–11% in 7- to 11-year-olds, and 8%–23% by age 15 years.1 Diagnosis of primary headache disorders is based on clinical criteria by the International Classification of Headache Disorders, 3rd edition, by the International Headache Society.2 Most children benefit from acute migraine treatments along with behavioral and lifestyle changes for headache prevention and do not require additional pharmacologic or biobehavioral preventive treatment.3 Additional migraine prevention should be considered when headaches occur with sufficient frequency and severity and result in migraine-related disability. The Pediatric Migraine Disability Assessment (PedMIDAS) is a 6-question, self-administered scale developed and validated in children and adolescents to measure functional impact of pediatric migraine during a 3-month period.4
Description of analytic process
This guideline was developed according to the process described in the 2011 American Academy of Neurology (AAN) guideline development process manual as amended5 and is in compliance with the National Academy of Medicine (formerly Institute of Medicine) Standards for Systematic Reviews.6 A multidisciplinary author panel, consisting of headache experts, child neurologists, clinical psychologists, methodologists, and patients, was assembled by the Guideline Development, Dissemination, and Implementation Subcommittee of the AAN to write this guideline. This author panel was solely responsible for the final decisions about the design, analysis, and reporting of the guideline. The study protocol was posted for public comment according to the 2011 process manual as amended.
The authors included randomized clinical trials of migraine prevention in children aged 3–18 years and considered studies published in English and in other languages. The headache disorders in these studies were classified according to either the International Classification of Headache Disorders, 2nd edition,7 or the International Classification of Headache Disorders, 3rd edition (beta version).8 Special populations included sexually active adolescents who were of childbearing age. Patients with episodic syndromes that may be associated with migraine, including cyclic vomiting, abdominal migraine, benign paroxysmal vertigo, and benign paroxysmal torticollis, were excluded. The systematic review included all pharmacologic interventions for the preventive treatment of migraine as well as the use of CBT in combination with pharmacologic therapy, with placebo used as the comparator. The outcome measures included change in headache frequency (defined as the reduction in number of migraine days per month, reduction of number of headache days per month, or 50% reduction in these frequencies), headache severity (defined by visual analog scale or numerical rating scale), and associated disability (PedMIDAS).
This guideline is an update of the previous guideline published in 2004 on the treatment of migraine in children and adolescents. The authors performed an initial English language literature search from December 1, 2003, to February 15, 2015, of the following databases: MEDLINE, Cochran, CINAHL, EMBASE, CDSR, DARE, CENTRAL, and an updated literature search of the same databases from January 1, 2015, to August 25, 2017. The search was conducted to find articles on both acute and preventive treatment of migraine in children and adolescents, although only trials evaluating preventive therapies were included in this systematic review. Two authors independently reviewed all abstracts and full-text articles for relevance. Articles were included if (1) 90% of participants were aged 3–18 years, (2) participants had a diagnosis of migraine, (3) the article included at least 20 participants, and (4) comparison was with placebo. The initial literature search included both pharmacologic and nonpharmacologic interventions, but due to a large number of included studies, the inclusion criteria were narrowed to only prescription pharmacologic intervention alone or in combination with CBT. Nonpharmacologic interventions, such as behavioral interventions alone or nutraceuticals, are not addressed by this guideline. Differences were reconciled by discussion; where disagreements arose, a methodologist on the panel (D.G.) adjudicated. In addition, all Class I and II studies9–12 included in the 2004 guideline were also included. Following full-text screening, all included articles were reviewed independently by 2 authors who extracted key data from each article and determined the article's class using a standardized data extraction form that was developed for each clinical question by the AAN methodologists (T.P., D.G.) with input from the author panel.
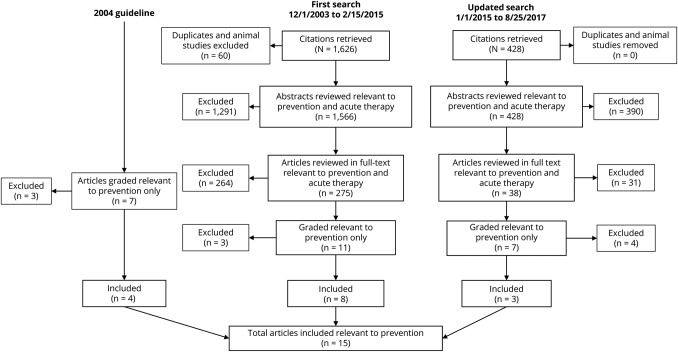
The author panel reviewed the results of a comprehensive literature search (1,994 total abstracts) and identified published studies relevant to the clinical questions (the full texts of 313 articles were reviewed), which were then classified according to the AAN's 2011 evidence-based methodology, as amended. From this search and classification strategy, 11 articles ranked as Class I, II, or III were included. In addition, the 7 prevention studies from the 2004 guideline that were previously rated as Class I or II were reclassified using the 2011 process manual, as amended, and 4 rated as Class III or higher were included in the current review (figure). All 4 articles were downgraded to Class II or III when graded according to the 2011 process as amended, typically because of failure to specify concealed allocation and to state a primary outcome.13–17 The author panel based the strength of the recommendations on the grading of evidence, with consideration of costs, risks, and feasibility as well as the AAN's modifications to the Grade of Recommendations, Assessment, Development, and Evaluation. Risk ratios (RR) and standardized mean differences (SMD) and the 95% confidence interval (CI) for the outcomes of interest were calculated. For the headache responder rate outcome (proportion of participants with a 50% reduction or greater in headache frequency from baseline), we calculated the RR. We prespecified a minimal clinically important difference of 1.25 between treatment and placebo; an RR less than 1.10 was determined to be clinically unimportant. For continuous headache frequency outcomes, including the number of headache days, the number of migraine days, and migraine-related disability at endpoint, we examined the SMD. We prespecified a minimal clinically important difference in the SMD of 0.20; an SMD less than 0.1 was determined to be clinically unimportant.18
Figure. Prevention studies from the 2004 guideline.
The panel formulated practice recommendations based on the strength of evidence and other factors, including axiomatic principles of care, the magnitude of anticipated health benefits relative to harms, financial burden, availability of interventions, and patient preferences. The panel assigned levels of obligation (A, B, C, U, R) to the recommendations using a modified Delphi process.
Analysis of evidence
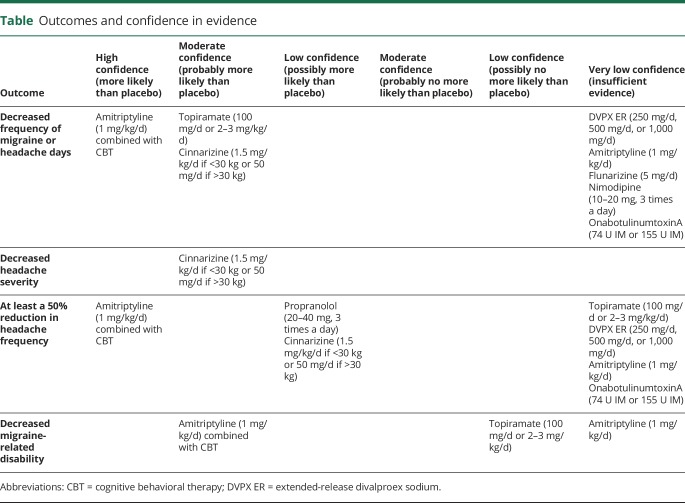
Conclusions to the analysis of evidence are listed as follows. These conclusions are also summarized in the table.
Table.
Outcomes and confidence in evidence
In children and adolescents with migraine, do preventive pharmacologic treatments, compared with placebo, reduce headache frequency?
Antiepileptic drugs
Topiramate
Children and adolescents with migraine receiving topiramate are probably more likely than those receiving placebo to have a decrease in the frequency of migraine or headache days (moderate confidence in the evidence, 4 Class I studies19–22; random effect model SMD 0.391; 95% CI 0.127–0.655; confidence in the evidence downgraded due to imprecision). There is insufficient evidence to determine whether children with migraine receiving topiramate are more or less likely than those receiving placebo to have at least a 50% reduction in headache frequency (very low confidence in the evidence; RR 1.330 [95% CI 0.933–1.894]; confidence in the evidence downgraded due to imprecision). Children with migraine receiving topiramate are possibly no more likely than those receiving placebo to have a decrease in migraine-related disability (low confidence in the evidence, 2 Class I studies20,22; SMD 0.538 [95% CI −0.097 to 1.174]; confidence in the evidence downgraded due to imprecision).
Extended-release divalproex sodium (DVPX ER)
There is insufficient evidence to determine whether children with migraine who are receiving DVPX ER (250, 500, or 1,000 mg/d) are more or less likely than those receiving placebo to have a reduction in headache frequency (very low confidence in evidence, 1 Class II study23 downgraded for imprecision). There is insufficient evidence to determine whether children with migraine who are receiving DVPX ER are more or less likely than those receiving placebo to have at least a 50% reduction in headache frequency (very low confidence in the evidence, 1 Class II study downgraded for imprecision; RR 0.92 [95% CI 0.70–1.24]).
Antidepressant drugs
Amitriptyline
There is insufficient evidence to determine whether children with migraine receiving amitriptyline are more or less likely than those receiving placebo to have a decrease in migraine attacks (SMD 0.11 [95% CI −0.18 to 0.41]), to have at least a 50% reduction in headache frequency (RR 0.86 [95% CI 0.68–1.13]), or to have a reduction in migraine-related disability (SMD 0.03 [95% CI −0.27 to 0.32]) (very low confidence in the evidence, 1 Class I study,20 confidence in the evidence downgraded for imprecision).
β-Blockers
Propranolol
Children with migraine receiving propranolol are possibly more likely than those receiving placebo to have at least a 50% reduction in headache attacks (low confidence in the evidence, 1 Class III study24; RR 5.20 [95% CI 1.59–17.00]; confidence in evidence upgraded due to magnitude of effect).
Calcium channel blockers
Flunarizine
There is insufficient evidence to determine whether children with migraine receiving flunarizine are more or less likely than those receiving placebo to have a decrease in migraine attacks (very low confidence in evidence, 1 Class III study25). Flunarizine is not available in the United States but is available in Canada.
Cinnarizine
Children with migraine receiving cinnarizine are probably more likely than those receiving placebo to have a reduction in headache frequency (moderate confidence in the evidence, 1 Class II study26; SMD 0.83 [95% CI 0.31–1.35]; confidence in the evidence upgraded due to magnitude of effect). Children with migraine receiving cinnarizine are probably more likely than those receiving placebo to have a reduction in headache severity (moderate confidence in the evidence, 1 Class II study; SMD 0.97 [95% CI 0.45–1.50]; confidence in the evidence upgraded due to magnitude of effect). Children with migraine receiving cinnarizine are possibly more likely than those receiving placebo to have at least a 50% reduction in headache frequency (low confidence in the evidence; 1 Class II study; RR 1.92 [95% CI 1.09–3.48]). Cinnarizine is not available in the United States or Canada.
Nimodipine
There is insufficient evidence to determine if children with migraine receiving nimodipine are more or less likely than those receiving placebo to have a decrease in migraine attacks (very low confidence in the evidence, 1 Class III study27).
Neurotoxins
OnabotulinumtoxinA
There is insufficient evidence to determine whether adolescents with chronic migraine receiving onabotulinumtoxinA, 74 units IM, are more or less likely than those receiving placebo to have a reduction in headache frequency (SMD 0.05 [95% CI −0.39 to 0.49]) or to have at least a 50% reduction in frequency of headache days (RR 1.10 [95% CI 0.58–2.09]) (very low confidence in the evidence, 1 Class II study28 downgraded for imprecision). There is insufficient evidence to determine whether adolescents with chronic migraine receiving onabotulinumtoxinA, 155 units IM, are more or less likely than those receiving placebo to have a reduction in headache frequency (SMD 0.75 [95% CI −0.37 to 0.51]) or to have at least a 50% reduction in frequency of headache days (RR 0.97 [95% CI 0.51–1.89]) (very low confidence in the evidence, 1 Class II study28 downgraded for imprecision).
In children and adolescents with migraine, do pharmacologic treatments combined with CBT, compared with the same pharmacologic treatments combined with a control intervention, reduce headache frequency?
Children and adolescents aged 10–17 years with chronic migraine who receive amitriptyline and CBT are more likely than those who receive amitriptyline and headache education to have a reduction in headache frequency (SMD 0.48 [95% CI 0.14–0.82]; high confidence in the evidence; 1 Class I study,29 confidence in the evidence upgraded due to magnitude of effect) and to have at least a 50% reduction in headache frequency (RR 1.79 [95% CI 1.27–2.56]; high confidence in evidence, 1 Class I study, confidence in evidence upgraded due to magnitude of effect). Children and adolescents aged 10–17 years with migraine who receive amitriptyline and CBT are probably more likely than those who receive amitriptyline and headache education to have a reduction in headache-related disability (PedMIDAS SMD 0.43 [95% CI 0.09–0.77]; moderate confidence in the evidence, 1 Class I study29).
Practice recommendations
Counseling and education for children and adolescents with migraine and their families
Recommendation 1 rationale
Individuals with a family history of migraine are at higher risk of developing migraine, and female sex is a risk factor of migraine that persists into adulthood.30 Disease prevention is the cornerstone of medical care. Migraine has multiple behavioral factors that influence headache frequency. Recurrent headache in adolescents is associated with being overweight, caffeine and alcohol use, lack of physical activity, poor sleep habits, and tobacco exposure.31 Depression is associated with higher headache disability in adolescents.32 Weight loss can contribute to headache reduction in children who are overweight.33 Identification and avoidance of factors that contribute to headache risk can reduce migraine frequency.
Statement 1a
Clinicians should counsel patients and families that lifestyle and behavioral factors may influence headache frequency (Level B).
Statement 1b
Clinicians should educate patients and families to identify and modify migraine contributors that are potentially modifiable (Level B).
Recommendation 2 rationale
In adults with migraine, headache on more than 6 days in a month is a risk factor for progression to chronic migraine, with medication overuse contributing to this progression.34 Taking triptans, ergotamines, opioids, and combination analgesics on more than 9 days in a month or taking over-the-counter simple analgesics on more than 14 days in a month can lead to medication overuse headache. (There is no evidence to support the use of opioids in children with migraine. Opioids are included in this rationale to be consistent with the International Classification of Headache Disorders35 regarding medication overuse.) It has been suggested that clinicians consider preventive treatments in these populations.36 Although there are no data on this topic in pediatric populations, it is hypothesized that similar relationships between frequent headache, medication overuse, and progression to chronic migraine may occur in children. In clinical trials of pediatric migraine prevention, inclusion criteria for headache frequency were variable and included a minimum of 4 headache days per month with no maximum and 3–4 migraine attacks per month for at least 3 months. In teenagers with migraine, those with a PedMIDAS score over 30, indicating moderate to severe migraine-related disability, had a higher risk of mood and anxiety disorders and increased severity and frequency of headache.37
Statement 2a
Clinicians should discuss the potential role of preventive treatments in children and adolescents with frequent headache or migraine-related disability or both (Level B).
Statement 2b
Clinicians should discuss the potential role of preventive treatments in children and adolescents with medication overuse (Level B).
Starting preventive treatment
Recommendation 3 rationale
The majority of randomized controlled trials that studied the efficacy of preventive medications for pediatric migraine fail to demonstrate superiority to placebo. Pediatric migraine trial results demonstrated a high response to placebo, with 30%–61% of children who received placebo having had a 50% or greater reduction in headache frequency. Children and adolescents with migraine receiving topiramate are probably more likely than those receiving placebo to have a decrease in headache days and migraine attacks; however, there is insufficient evidence to determine whether children with migraine who are receiving topiramate are more or less likely than those receiving placebo to have at least a 50% reduction in migraine frequency or headache days, and this is also the case for reduction in migraine-related disability.19–22 Children who receive propranolol are possibly more likely than those who receive placebo to have more than a 50% reduction in headache frequency.24,38 Patients receiving amitriptyline combined with CBT as compared with those treated with amitriptyline who receive headache education are more likely to experience a decreased headache frequency and have more than a 50% reduction in headache frequency and are probably more likely to have decreased migraine-associated disability.29 There is insufficient evidence to judge the independent effectiveness of amitriptyline on migraine prevention in children and adolescents.20 A Food and Drug Administration (FDA) black box warning regarding risk of suicidal thoughts and behavior with amitriptyline use especially in children, adolescents, and young adults is in effect at the time of this guideline. It is possible that CBT alone is effective in migraine prevention,10 and individual barriers to access may exist.12 There is insufficient evidence to evaluate the effects of flunarizine,25 nimodipine,27 valproate,23 and onabotulinumtoxinA28 for use in migraine prevention in children and adolescents. Although there is evidence that cinnarizine26 is probably more effective than placebo for migraine prevention, this medication is not available in the United States or Canada.
Statement 3a
Clinicians should inform patients and caregivers that in clinical trials of preventive treatments for pediatric migraine, many children and adolescents who received placebo improved and the majority of preventive medications were not superior to placebo (Level B).
Statement 3b
Acknowledging the limitations of currently available evidence, clinicians should engage in shared decision-making regarding the use of short-term treatment trials (a minimum of 2 months) for those who could benefit from preventive treatment (Level B).
Statement 3c
Clinicians should discuss the evidence for amitriptyline combined with CBT for migraine prevention, inform patients of the potential side effects of amitriptyline including risk of suicide, and work with families to identify providers who can offer this type of treatment12 (Level B).
Statement 3d
Clinicians should discuss the evidence for topiramate for migraine prevention in children and adolescents and its side effects in this population (Level B).
Statement 3e
Clinicians should discuss the evidence for propranolol for migraine prevention and its side effects in children and adolescents (Level B).
Counseling for patients of childbearing potential
Recommendation 4 rationale
Balancing benefit and risk is important when deciding among available medical treatments. Topiramate and valproate have well-demonstrated teratogenic effects, especially when used in polytherapy.39–42 Valproate use during pregnancy is also associated with developmental disorders in offspring.43,44 An FDA black box warning regarding fetal risk from valproate use exists as of the time of this guideline. Topiramate at a daily dose of 200 mg or less does not interact with oral combined hormonal contraceptives; however, at higher doses it can have drug interactions that decrease their effectiveness.45 The risk of major congenital malformation in offspring of women with epilepsy taking anticonvulsants is possibly decreased by folic acid supplementation.46
Statement 4a
Clinicians must consider the teratogenic effect of topiramate and valproate in their choice of migraine prevention therapy recommendations to patients of childbearing potential (Level A).
Statement 4b
Clinicians who offer topiramate or valproate for migraine prevention to patients of childbearing potential must counsel these patients about potential effects on fetal/childhood development (Level A).
Statement 4c
Clinicians who prescribe topiramate for migraine prevention to patients of childbearing potential must counsel these patients about the potential of this medication to decrease the efficacy of oral combined hormonal contraceptives, particularly at doses over 200 mg daily (Level A).
Statement 4d
Clinicians who prescribe topiramate or valproate for migraine prevention to patients of childbearing potential should counsel patients to discuss optimal contraception methods with their health care provider during treatment (Level B).
Statement 4e
Clinicians must recommend daily folic acid supplementation to patients of childbearing potential who take topiramate or valproate (Level A).
Monitoring and stopping medication
Recommendation 5 rationale
Migraine is a chronic disorder with spontaneous remissions and relapses. Clinical trials follow patients for limited periods of time. Patients and families often inquire about the duration of treatment. There is little information about when preventive treatment should be stopped, and the risk of relapse after discontinuation varies.
Statement 5a
Clinicians must periodically monitor medication effectiveness and adverse events when prescribing migraine preventive treatments (Level A).
Statement 5b
Clinicians should counsel patients and families about risks and benefits of stopping preventive medication once good migraine control is established (Level B).
Mental illness in children and adolescents with migraine
Recommendation 6 rationale
Several studies have been performed, with inconsistent results, that evaluated the relationship between mental illness and migraine in children. A recent systematic review of prospective or retrospective longitudinal cohort studies in children examined factors associated with the onset and course of recurrent headache in children and adolescents, with recurrent headache defined as headaches occurring at least once per month. This review found high-quality evidence suggesting that children with negative emotional states, manifesting through anxiety, depression, or mental distress, are not at greater risk of developing recurrent headache; however, it found moderate-quality evidence that suggested the presence of comorbid negative emotional states in children with headache is associated with an increased risk of headache persistence in those who already experience recurrent headaches.30
Statement 6a
Children and adolescents with migraine should be screened for mood and anxiety disorders because of the increased risk of headache persistence (Level B).
Statement 6b
In children and adolescents with migraine who have comorbid mood and anxiety disorders, clinicians should discuss management options for these disorders (Level B).
Putting the evidence into a clinical context
The goal of preventive treatment is to reduce headache frequency and headache-related disability. Achieving clinically meaningful improvements should be the standard for assessing the effect of a given treatment. Involving patients and parents helps ensure that providers understand what clinically meaningful outcomes are as well as assists with treatment adherence and respects patient preferences. The choice of treatment can be guided by the presence of comorbidities (e.g., topiramate use in patients with epilepsy or the use of drugs that either decrease or increase appetite in patients with weight-related morbidity). Although topiramate is the only FDA-approved medication for migraine prevention (in children and adolescents aged 12–17 years), the current evidence base raises some doubts about whether this treatment achieves clinically meaningful outcomes beyond those obtained by placebo. There is insufficient evidence to confidently recommend this as a known efficacious preventive intervention. Some treatments with proven efficacy in adults, such as valproate for episodic migraine prevention and onabotulinumtoxinA for chronic migraine, have not shown the same efficacy in children and adolescents, and a higher pediatric placebo response rate is observed.47,48 Analysis of placebo response rates across pediatric migraine trials show that trial designs associated with a lower placebo response rate included crossover design trials, single-center studies, and small sample size, with age and sex not predictive of placebo response rates.49 The more rigorous trials have demonstrated a robust placebo response, and this response likely has a biological basis that can be potentially explored in clinical practice.50
Suggestions for future research
Improved classification of pediatric migraine and reliable measures of outcome and disability have improved our recognition and understanding of childhood migraine and enabled more robust clinical studies. However, variation in endpoints used in trials complicates assessment and comparison of potential benefit. The presence of high placebo response rates in pediatric migraine demonstrates that children respond to treatment of their headache but makes identifying a therapeutic response from pharmaceutical treatments more challenging. To account for this effect, unique study designs should be taken into consideration when planning trials. New therapeutics (drugs, devices, behavioral treatments) and further well-designed studies are needed. Specifically, the efficacy of and access to the use of CBT alone needs to be informed by future well-designed randomized controlled trials. Mechanistic studies that examine mediators of improvement when a patient with migraine receives a preventive intervention or placebo could be critical in understanding how and why children with headaches get better. This type of science might also suggest innovations related to new approaches to preventive therapies.
More evidence about the benefits of behavioral changes on reducing migraine burden, in particular compared with pharmacologic prevention, would help guide treatment recommendation. Factors that contribute to headache occurrence and persistence such as biologic and psychologic factors, including mood disorders, need to be investigated to identify pathophysiologic pathways and biomarkers. This identification can then be used to guide the development of new treatments and inform patients and families of their effect on outcome.
Disclaimer
Practice guidelines, practice advisories, comprehensive systematic reviews, focused systematic reviews, and other guidance published by the AAN and its affiliates are assessments of current scientific and clinical information provided as an educational service. The information (1) should not be considered inclusive of all proper treatments, methods of care, or as a statement of the standard of care; (2) is not continually updated and may not reflect the most recent evidence (new evidence may emerge between the time information is developed and when it is published or read); (3) addresses only the question(s) specifically identified; (4) does not mandate any particular course of medical care; and (5) is not intended to substitute for the independent professional judgment of the treating provider, as the information does not account for individual variation among patients. In all cases, the selected course of action should be considered by the treating provider in the context of treating the individual patient. Use of the information is voluntary. AAN provides this information on an “as is” basis and makes no warranty, expressed or implied, regarding the information. AAN specifically disclaims any warranties of merchantability or fitness for a particular use or purpose. AAN assumes no responsibility for any injury or damage to persons or property arising out of or related to any use of this information or for any errors or omissions.
Conflict of interest
The AAN is committed to producing independent, critical, and truthful clinical practice guidelines (CPGs). Significant efforts are made to minimize the potential for conflicts of interest to influence the recommendations of this CPG. To the extent possible, the AAN keeps separate those who have a financial stake in the success or failure of the products appraised in the CPGs and the developers of the guidelines. Conflict of interest forms were obtained from all authors and reviewed by an oversight committee prior to project initiation. AAN limits the participation of authors with substantial conflicts of interest. The AAN forbids commercial participation in, or funding of, guideline projects. Drafts of the guideline have been reviewed by at least 3 AAN committees, a network of neurologists, Neurology peer reviewers, and representatives from related fields. The AAN Guideline Author Conflict of Interest Policy can be viewed at aan.com. For complete information on this process, access the 2011 AAN process manual, as amended.5
Glossary
- AAN
American Academy of Neurology
- CBT
cognitive behavioral therapy
- CI
confidence interval
- DVPX ER
extended-release divalproex sodium
- FDA
Food and Drug Administration
- PedMIDAS
Pediatric Migraine Disability Assessment
- RR
risk ratio
- SMD
standardized mean differences
Author contributions
Dr. Oskoui: study concept and design, acquisition of data, analysis or interpretation of data, drafting/revising the manuscript, critical revision of the manuscript for important intellectual content, study supervision. Dr. Pringsheim: study concept and design, acquisition of data, analysis or interpretation of data, drafting/revising the manuscript, critical revision of the manuscript for important intellectual content, study supervision. Dr. Billinghurst: drafting/revising the manuscript, critical revision of the manuscript for important intellectual content. Dr. Potrebic: analysis or interpretation of data, drafting/revising the manuscript, critical revision of the manuscript for important intellectual content, study supervision. E.M. Gersz: critical revision of the manuscript for important intellectual content. Dr. Gloss: study concept and design, acquisition of data, analysis or interpretation of data, drafting/revising the manuscript. Dr. Holler-Managan: study concept and design, acquisition of data, drafting/revising the manuscript, critical revision of the manuscript for important intellectual content. E. Leininger: critical revision of the manuscript for important intellectual content. Dr. Licking: acquisition of data, analysis or interpretation of data, critical revision of the manuscript for important intellectual content. Dr. Mack: study concept and design, drafting/revising the manuscript, critical revision of the manuscript for important intellectual content. Dr. Powers: drafting/revising the manuscript, critical revision of the manuscript for important intellectual content. Dr. Sowell: critical revision of the manuscript for important intellectual content. Dr. Victorio: critical revision of the manuscript for important intellectual content. Dr. Yonker: critical revision of the manuscript for important intellectual content. H. Zanitsch: critical revision of the manuscript for important intellectual content. Dr. Hershey: study concept and design, drafting/revising the manuscript, critical revision of the manuscript for important intellectual content.
Study funding
This practice guideline was developed with financial support from the AAN. Authors who serve or have served as AAN subcommittee members or as methodologists (M.O., T.P., L.B., S.P., D.G., Y.H.M., and N.L. were reimbursed for expenses related to travel to subcommittee meetings where drafts of manuscripts were reviewed. All authors on the panel were reimbursed by the AAN for expenses related to travel to in-person meetings.
Disclosure
M. Oskoui has received research as a site principal investigator for studies in spinal muscular atrophy from Biogen, Cytokinetics, and Roche Pharmaceuticals and research support from Fonds de Recherche du Québec-Santé (FRQS), Canadian Institutes of Health Research, Cerebral Palsy Alliance Foundation, McGill University Research Institute, and SickKids Foundation; served as a consultant for Biogen, Avexis, and Roche Pharmaceuticals; and received funding for travel to quarterly meetings of the Guideline Development, Dissemination, and Implementation Subcommittee by the American Academy of Neurology (AAN). Y. Holler-Managan serves on the editorial advisory board for Neurology Now. T. Pringsheim reports no disclosures relevant to the manuscript. S. Potrebic has received funding for travel to biennial Guidelines International Network meetings by the AAN; received an honorarium and funding for travel to serve as an expert from the Center for Diagnostic Imaging and Insight Imaging (CDI) Quality Institute for work on Appropriate Use Criteria for headache imaging; and has received an honorarium from the California Technology Assessment Forum for participation as expert reviewer of the Institute for Clinical and Economic Review Calcitonin Gene-Related Peptide (CGRP) Inhibitors as Preventive Treatments for Patients with Episodic or Chronic Migraine: Effectiveness and Value Final Evidence Report. L. Billinghurst and D. Gloss report no disclosures relevant to the manuscript. A. Hershey has served on a scientific advisory board for Allergan, XOC Pharma, and Amgen; served as an editor for Headache, Cephalalgia, and the Journal of Headache and Pain; has received compensation from Allergan and MAP Pharma and currently receives compensation from Alder, Amgen, Avanir, Curelator, Depomed, Impax, Lilly, Supernus, and Upsher-Smith for serving on speakers' bureaus and as a medical consultant; has received research support from GlaxoSmithKline for serving as a Local Site PI on a study on pediatric migraine treatment, from the Migraine Research Foundation and Curelator, Inc. for serving as a principal investigator on studies on migraine genomics and diagnosis, and from the National Headache Foundation for serving as a coinvestigator on a study on migraine prognosis; has received grants from the NIH/National Institute of Neurologic Disorders and Stroke (NINDS) for serving as a coinvestigator on a study on migraine management, studies on treatment, prognosis, and diagnosis of pediatric chronic migraine and headache, and for serving as a dual principal investigator on a study on amitriptyline and topiramate in the prevention of childhood migraine; and serves as a board member of the American Headache Society. N. Licking reports no disclosures relevant to the manuscript. M. Sowell has received compensation for serving on a speakers' bureau for Amgen/Novartis Pharmaceuticals; has served as manuscript editor for Headache and the Journal of Child Neurology, on a speakers' bureau for Allergan, and as an interviewer for Neurology podcasts; served as site principal investigator for the CHAMP (Childhood and Adolescent Migraine Prevention) study, for which he received research support from NINDS; and receives research support from Impax Pharmaceuticals. M.C. Victorio is the site primary investigator for a childhood and adolescent migraine prevention study funded by the NIH and site investigator for a pediatric migraine treatment study funded by Impax Laboratories (both studies were contracted through Akron Children's Hospital); has received funding for travel to meetings of the Registry Committee and Quality and Safety Subcommittee by the AAN; has received honoraria for authoring and coauthoring chapters in the Merck Manual and for authoring an article in Pediatric Annals; and performs the following clinical procedures in her practice: onabotulinumtoxinA injection for chronic migraine (2%) and peripheral nerve block injections (2%). E. Gersz and E. Leininger report no disclosures relevant to the manuscript. H. Zanitsch has received financial compensation from the Patient-Centered Outcomes Research Institute and Peer Reviewed Medical Research Program and serves as a volunteer advocate for the National Headache Foundation. M. Yonker has served on a scientific advisory board for AMGEN and for Upsher-Smith Pharmaceuticals; has served as a reviewer for Cephalalgia, Headache, Pediatrics, and the Journal of the Child Neurology Society; has received research support as a primary investigator from AstraZeneca, Allergan, Avanir, and NINDS; has received funding for travel from the American Headache Society for serving as a presenter at the Scottsdale Headache Symposium; and serves as a consultant to Impax. Dr. Kenneth Mack has served as an advisor for AMGEN; receives publishing royalties from UpToDate; performs botulinum toxin injections for headache treatment as 5% of his clinical effort; and serves as a member of the Neurology® editorial board. S. Powers has received funding from the NIH, Migraine Research Foundation, and National Headache Foundation; and has served as a manuscript reviewer for Headache, Cephalalgia, Pain, Journal of Pain, JAMA Pediatrics, and Pediatrics. Go to Neurology.org/N for full disclosures.
References
- 1.Victor TW, Hu X, Campbell JC, Buse DC, Lipton RB. Migraine prevalence by age and sex in the United States: a life-span study. Cephalalgia 2010;30:1065–1072. [DOI] [PubMed] [Google Scholar]
- 2.Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2018;38:1–211. [DOI] [PubMed] [Google Scholar]
- 3.Lopez J, Bechtel KA, Rothrock JF. Pediatric headache [online]. Available at: emedicine.medscape.com/article/2110861-overview. Accessed February 28, 2015.
- 4.Hershey AD, Powers SW, Vockell AL, LeCates S, Kabbouche MA, Maynard MK. PedMIDAS: development of a questionnaire to assess disability of migraines in children. Neurology 2001;57:2034–2039. [DOI] [PubMed] [Google Scholar]
- 5.American Academy of Neurology. Clinical Practice Guideline Process Manual, 2011 ed. St. Paul: American Academy of Neurology; 2011. [Google Scholar]
- 6.Institute of Medicine. Finding what Works in Health Care: Standards for Systematic Reviews. Washington, DC:Institute of Medicine; 2011. [PubMed] [Google Scholar]
- 7.Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia 2004;24(suppl 1):9–160. [DOI] [PubMed] [Google Scholar]
- 8.Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013;33:629–808. [DOI] [PubMed] [Google Scholar]
- 9.Ng QX, Venkatanarayanan N, Kumar L. A systematic review and meta-analysis of the efficacy of cognitive behavioral therapy for the management of pediatric migraine. Headache 2017;57:349–362. [DOI] [PubMed] [Google Scholar]
- 10.Eccleston C, Palermo TM, Williams ACdC, et al. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst Rev 2014;5:CD003968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kroon Van Diest AM, Ernst MM, Vaughn L, Slater S, Powers SW. CBT for pediatric migraine: a qualitative study of patient and parent experience. Headache 2018;58:661–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ernst MM, O'Brien HL, Powers SW. Cognitive-behavioral therapy: how medical providers can increase patient and family openness and access to evidence-based multimodal therapy for pediatric migraine. Headache 2015;55:1382–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olness K, MacDonald JT, Uden DL. Comparison of self-hypnosis and propranolol in the treatment of juvenile classic migraine. Pediatrics 1987;79:593–597. [PubMed] [Google Scholar]
- 14.Ueberall MA, Wenzel D. Intranasal sumatriptan for the acute treatment of migraine in children. Neurology 1999;52:1507–1510. [DOI] [PubMed] [Google Scholar]
- 15.Sills M, Congdon P, Forsythe I. Clonidine and childhood migraine: a pilot and double-blind study. Dev Med Child Neurol 1982;24:837–841. [DOI] [PubMed] [Google Scholar]
- 16.Sillanpaa M. Clonidine prophylaxis of childhood migraine and other vascular headache: a double blind study of 57 children. Headache 1977;17:28–31. [DOI] [PubMed] [Google Scholar]
- 17.Gillies D, Sills M, Forsythe I. Pizotifen (Sanomigran) in childhood migraine: a double-blind controlled trial. Eur Neurol 1986;25:32–35. [DOI] [PubMed] [Google Scholar]
- 18.Cohen J. Statistical Power for the Behavioural Sciences. 2nd ed. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- 19.Lewis D, Winner P, Saper J, et al. Randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of topiramate for migraine prevention in pediatric subjects 12 to 17 years of age. Pediatrics 2009;123:924–934. [DOI] [PubMed] [Google Scholar]
- 20.Powers SW, Coffey CS, Chamberlin LA, et al. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med 2017;376:115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winner P, Pearlman EM, Linder SL, et al. Topiramate for migraine prevention in children: a randomized, double-blind, placebo-controlled trial. Headache 2005;45:1304–1312. [DOI] [PubMed] [Google Scholar]
- 22.Lakshmi CV, Singhi P, Malhi P, Ray M. Topiramate in the prophylaxis of pediatric migraine: a double-blind placebo-controlled trial. J child Neurol 2007;22:829–835. [DOI] [PubMed] [Google Scholar]
- 23.Apostol G, Cady RK, Laforet GA, et al. Divalproex extended-release in adolescent migraine prophylaxis: results of a randomized, double-blind, placebo-controlled study. Headache 2008;48:1012–1025. [DOI] [PubMed] [Google Scholar]
- 24.Ludvigsson J. Propranolol used in prophylaxis of migraine in children. Acta Neurol Scand 1974;50:109–115. [DOI] [PubMed] [Google Scholar]
- 25.Sorge F, De Simone R, Marano E, Nolano M, Orefice G, Carrieri P. Flunarizine in prophylaxis of childhood migraine: a double-blind, placebo-controlled, crossover study. Cephalalgia 1988;8:1–6. [DOI] [PubMed] [Google Scholar]
- 26.Ashrafi MR, Salehi S, Malamiri RA, et al. Efficacy and safety of cinnarizine in the prophylaxis of migraine in children: a double-blind placebo-controlled randomized trial. Pediatr Neurol 2014;51:503–508. [DOI] [PubMed] [Google Scholar]
- 27.Battistella PA, Ruffilli R, Moro R, et al. A placebo-controlled crossover trial of nimodipine in pediatric migraine. Headache 1990;30:264–268. [DOI] [PubMed] [Google Scholar]
- 28.Allergan. 191622-103 BOTOX® (Botulinum Toxin Type A) Purified Neurotoxin Complex as Headache Prophylaxis in Adolescents (Children 12 to < 18 Years of Age) With Chronic Migraine. Available at https://clinicaltrials.gov/ct2/show/results/NCT01662492?view=results. Published September 5, 2017. Accessed September 6, 2017. [Google Scholar]
- 29.Powers SW, Kashikar-Zuck SM, Allen JR, et al. Cognitive behavioral therapy plus amitriptyline for chronic migraine in children and adolescents: a randomized clinical trial. JAMA 2013;310:2622–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huguet A, Tougas ME, Hayden J, et al. Systematic review of childhood and adolescent risk and prognostic factors for recurrent headaches. J Pain 2016;17:855–873 e858. [DOI] [PubMed] [Google Scholar]
- 31.Robberstad L, Dyb G, Hagen K, Stovner LJ, Holmen TL, Zwart JA. An unfavorable lifestyle and recurrent headaches among adolescents: the HUNT study. Neurology 2010;75:712–717. [DOI] [PubMed] [Google Scholar]
- 32.Amouroux R, Rousseau-Salvador C, Pillant M, Antonietti JP, Tourniaire B, Annequin D. Longitudinal study shows that depression in childhood is associated with a worse evolution of headaches in adolescence. Acta Paediatr 2017;106:1961–1965. [DOI] [PubMed] [Google Scholar]
- 33.Hershey AD, Powers SW, Nelson TD, et al. Obesity in the pediatric headache population: a multicenter study. Headache 2009;49:170–177. [DOI] [PubMed] [Google Scholar]
- 34.Katsarava Z, Schneeweiss S, Kurth T, et al. Incidence and predictors for chronicity of headache in patients with episodic migraine. Neurology 2004;62:788–790. [DOI] [PubMed] [Google Scholar]
- 35.Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013;33:629–808. [DOI] [PubMed] [Google Scholar]
- 36.Alstadhaug KB, Ofte HK, Kristoffersen ES. Preventing and treating medication overuse headache. Pain Rep 2017;2:e612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fuh JL, Wang SJ, Lu SR, Liao YC, Chen SP, Yang CY. Headache disability among adolescents: a student population-based study. J Head Face Pain 2010;50:210–218. [DOI] [PubMed] [Google Scholar]
- 38.Forsythe WI, Gillies D, Sills MA. Propanolol (Inderal) in the treatment of childhood migraine. Dev Med Child Neurol 1984;26:737–741. [DOI] [PubMed] [Google Scholar]
- 39.Vajda FJ, O'Brien TJ, Lander CM, Graham J, Eadie MJ. Antiepileptic drug combinations not involving valproate and the risk of fetal malformations. Epilepsia 2016;57:1048–1052. [DOI] [PubMed] [Google Scholar]
- 40.Tomson T, Battino D, Bonizzoni E, et al. Dose-dependent teratogenicity of valproate in mono- and polytherapy: an observational study. Neurology 2015;85:866–872. [DOI] [PubMed] [Google Scholar]
- 41.Holmes LB, Mittendorf R, Shen A, Smith CR, Hernandez-Diaz S. Fetal effects of anticonvulsant polytherapies: different risks from different drug combinations. Arch Neurol 2011;68:1275–1281. [DOI] [PubMed] [Google Scholar]
- 42.Hunt S, Russell A, Smithson WH, et al. Topiramate in pregnancy: preliminary experience from the UK epilepsy and pregnancy register. Neurology 2008;71:272–276. [DOI] [PubMed] [Google Scholar]
- 43.Christensen J, Gronborg TK, Sorensen MJ, et al. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA 2013;309:1696–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bromley RL, Calderbank R, Cheyne CP, et al. Cognition in school-age children exposed to levetiracetam, topiramate, or sodium valproate. Neurology 2016;87:1943–1953. [DOI] [PubMed] [Google Scholar]
- 45.Doose DR, Wang SS, Padmanabhan M, Schwabe S, Jacobs D, Bialer M. Effect of topiramate or carbamazepine on the pharmacokinetics of an oral contraceptive containing norethindrone and ethinyl estradiol in healthy obese and nonobese female subjects. Epilepsia 2003;44:540–549. [DOI] [PubMed] [Google Scholar]
- 46.Harden CL, Pennell PB, Koppel BS, et al. Practice parameter update: management issues for women with epilepsy: focus on pregnancy (an evidence-based review): vitamin K, folic acid, blood levels, and breastfeeding: report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology 2009;73:142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Freitag FG, Collins SD, Carlson HA, et al. A randomized trial of divalproex sodium extended-release tablets in migraine prophylaxis. Neurology 2002;58:1652–1659. [DOI] [PubMed] [Google Scholar]
- 48.Dodick DW, Turkel CC, DeGryse RE, et al. OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program. Headache 2010;50:921–936. [DOI] [PubMed] [Google Scholar]
- 49.Evers S, Marziniak M, Frese A, Gralow I. Placebo efficacy in childhood and adolescence migraine: an analysis of double-blind and placebo-controlled studies. Cephalalgia 2009;29:436–444. [DOI] [PubMed] [Google Scholar]
- 50.Faria V, Linnman C, Lebel A, Borsook D. Harnessing the placebo effect in pediatric migraine clinic. J Pediatr 2014;165:659–665. [DOI] [PMC free article] [PubMed] [Google Scholar]