Abstract
Background
Approximately 2.5% of all hospitalisations in people with cirrhosis are for spontaneous bacterial peritonitis (SBP). Antibiotics, in addition to supportive treatment (fluid and electrolyte balance, treatment of shock), form the mainstay treatments of SBP. Various antibiotics are available for the treatment of SBP, but there is uncertainty regarding the best antibiotic for SBP.
Objectives
To compare the benefits and harms of different antibiotic treatments for spontaneous bacterial peritonitis (SBP) in people with decompensated liver cirrhosis.
Search methods
We searched CENTRAL, MEDLINE, Embase, Science Citation Index Expanded, World Health Organization International Clinical Trials Registry Platform, and trials registers until November 2018 to identify randomised clinical trials on people with cirrhosis and SBP.
Selection criteria
We included only randomised clinical trials (irrespective of language, blinding, or publication status) in adults with cirrhosis and SBP. We excluded randomised clinical trials in which participants had previously undergone liver transplantation.
Data collection and analysis
Two review authors independently identified eligible trials and collected data. The outcomes for this review included mortality, serious adverse events, any adverse events, resolution of SBP, liver transplantation, and other decompensation events. We performed a network meta‐analysis with OpenBUGS using Bayesian methods and calculated the odds ratio, rate ratio, and hazard ratio with 95% credible intervals (CrIs) based on an available‐case analysis, according to the National Institute of Health and Care Excellence (NICE) Decision Support Unit guidance.
Main results
We included a total of 12 trials (1278 participants; 13 antibiotics) in the review. Ten trials (893 participants) were included in one or more outcomes in the review. The trials that provided the information included patients having cirrhosis with or without other features of decompensation of varied aetiologies. The follow‐up in the trials ranged from one week to three months. All the trials were at high risk of bias. Only one trial was included under each comparison for most of the outcomes. Because of these reasons, there is very low certainty in all the results. The majority of the randomised clinical trials used third‐generation cephalosporins, such as intravenous ceftriaxone, cefotaxime, or ciprofloxacin as one of the interventions.
Overall, approximately 75% of trial participants recovered from SBP and 25% of people died within three months. There was no evidence of difference in any of the outcomes for which network meta‐analysis was possible: mortality (9 trials; 653 participants), proportion of people with any adverse events (5 trials; 297 participants), resolution of SBP (as per standard definition, 9 trials; 873 participants), or other features of decompensation (6 trials; 535 participants). The effect estimates in the direct comparisons (when available) were very similar to those of network meta‐analysis. For the comparisons where network meta‐analysis was not possible, there was no evidence of difference in any of the outcomes (proportion of participants with serious adverse events, number of adverse events, and proportion of participants requiring liver transplantation). Due to the wide CrIs and the very low‐certainty evidence for all the outcomes, significant benefits or harms of antibiotics are possible.
None of the trials reported health‐related quality of life, number of serious adverse events, or symptomatic recovery from SBP.
Funding: the source of funding for two trials were industrial organisations who would benefit from the results of the trial; the source of funding for the remaining 10 trials was unclear.
Authors' conclusions
Short‐term mortality after SBP is about 25%. There is significant uncertainty about which antibiotic therapy is better in people with SBP.
We need adequately powered randomised clinical trials, with adequate blinding, avoiding post‐randomisation dropouts (or performing intention‐to‐treat analysis), and using clinically important outcomes, such as mortality, health‐related quality of life, and adverse events.
Plain language summary
Antibiotic treatment for spontaneous bacterial peritonitis in people with advanced liver disease
What is the aim of this Cochrane Review?
To find out the best available antibiotic for spontaneous bacterial peritonitis (fluid collection in the tummy (abdomen), infected with bacteria) in people with advanced liver disease (liver cirrhosis, or late stage scarring of the liver with complications). The abnormal buildup of fluid in people with liver cirrhosis is called ascites. Sometimes, this fluid may get infected with bacteria, with no obvious source of infection. This is called 'spontaneous bacterial peritonitis'. The main treatment of spontaneous bacterial peritonitis is antibiotics, but it is unclear which antibiotic is best for treating it. The authors collected and analysed all relevant studies to answer this question and found 12 randomised clinical trials (participants receive the treatment based on methods similar to a coin toss; this is to ensure that the people who receive the different treatments are similar in all aspects except the treatment, so that any differences in the results between the treatments can be attributed to the treatment rather than differences in the type of people who received the treatment). During the analysis of data, authors used standard Cochrane techniques, which allows comparison of two treatments at a time. Authors also used advanced techniques, that allows comparison of many treatments at the same time (usually referred as 'network meta‐analysis' or 'multiple treatment comparisons'). The aim is to gather reliable evidence on the relative benefits and harms of the different antibiotics.
Date of literature search November 2018.
Key messages None of the studies were conducted without flaws, and because of the very low certainty in the results, the authors cannot suggest which antibiotic, given alone or in combination to remove the bacteria from one's tummy, is better or worse than other antibiotics in the treatment of spontaneous bacterial peritonitis.
The funding source was unclear in 10 studies; industrial organisations funded two studies.
What was studied in the review?
This review studied people, of any sex, age, and origin, with advanced liver disease due to various causes, and who had developed spontaneous bacterial peritonitis. People were administered different antibiotics for the treatment of spontaneous bacterial peritonitis. The authors excluded studies with liver transplanted participants and bacterial peritonitis due to other causes. The participants' age, when reported, ranged from 42 to 60 years. The number of females, when reported, ranged from 18 to 42 out of 100. The administered antibiotic groups were cephalosporins, penicillins, and quinolones. The review authors wanted to gather and analyse data on death, quality of life, serious and non‐serious complications, time to liver transplantation (replacement of a diseased liver with a healthy one), time until disappearance of spontaneous bacterial peritonitis, and disappearance of symptoms.
What were the main results of the review?
The 12 studies included a small number of participants (1278 participants). The study data were sparse; 10 studies with 893 participants provided data for analyses. Follow‐up in the trials ranged from one week to three months. The review shows the following.
‐ Out of the 13 different antibiotics compared in the trials, ceftriaxone and cefotaxime administered into the vein, were most commonly used.
‐ The type of antibiotic provided may make no difference to the number or percentage of people with serious complications or with any complications; number of (any) complications per person; percentage of people undergoing liver transplantation; or who recovered from spontaneous bacterial peritonitis as per laboratory tests, or other complications of liver cirrhosis.
‐ Twenty‐five out of every 100 people died within three months, and 75 out of every 100 people recovered from spontaneous bacterial peritonitis.
‐ None of the trials reported health‐related quality of life, number of serious adverse events, or symptomatic recovery from spontaneous bacterial peritonitis.
‐ We have very low confidence in the overall results. Whether some antibiotics may cause important or less important benefits or harms compared to others when given to people with advanced liver disease and spontaneous bacterial peritonitis is questionable.
‐ We need data from trials of proper design and quality in order to be able to clarify the best antibiotic for spontaneous bacterial peritonitis.
Summary of findings
Background
Description of the condition
Liver cirrhosis
The liver is a complex organ with multiple functions, including metabolism of carbohydrates, fats, proteins, and drugs; it also has synthetic, storage, digestive, excretory, and immunological functions (Read 1972). Liver cirrhosis is a liver disease in which the normal microcirculation, the gross vascular anatomy, and the hepatic architecture have been variably destroyed and altered, with fibrous septa surrounding regenerated or regenerating parenchymal nodules (Tsochatzis 2014; NCBI 2018a). The major causes of liver cirrhosis include excessive alcohol consumption, viral hepatitis, non‐alcohol‐related fatty liver disease, autoimmune liver disease, and metabolic liver disease (Williams 2014; Ratib 2015; Setiawan 2016). The global prevalence of liver cirrhosis is difficult to estimate as most estimates correspond to chronic liver disease (which includes liver fibrosis and liver cirrhosis). In studies from the USA, the prevalence of chronic liver disease varies between 0.3% and 2.1% (Scaglione 2015; Setiawan 2016); in the UK, the prevalence was 0.1% in one study (Fleming 2008). In 2010, liver cirrhosis caused an estimated 2% of all global deaths, equivalent to one million deaths (Mokdad 2014). There is an increasing trend of cirrhosis‐related deaths in some countries, like the UK, while there is a decreasing trend in other countries, for example France (Mokdad 2014; Williams 2014). The major cause of complications and deaths in people with liver cirrhosis is due to the development of clinically significant portal hypertension ‐ hepatic venous pressure gradient at least 10 mmHg (de Franchis 2015). Some of the clinical features of decompensation include jaundice, coagulopathy, ascites, variceal bleeding, hepatic encephalopathy, and renal failure (de Franchis 2015; McPherson 2016; EASL 2018). Decompensated cirrhosis is the most common indication for liver transplantation (Merion 2010; Adam 2012).
Spontaneous bacterial peritonitis (SBP)
Ascites is accumulation of free fluid in the abdomen (peritoneal cavity) (NCBI 2018b), and is a feature of liver decompensation (Tsochatzis 2017; EASL 2018). Approximately 20% of people with cirrhosis have ascites (D'Amico 2014). Approximately 1% to 4% of people with cirrhosis develop ascites each year (D'Amico 2006; D'Amico 2014). Ascites is the first sign of liver decompensation in about one‐third of people with compensated liver cirrhosis (D'Amico 2014). When the ascitic fluid is infected with bacteria, it is called 'spontaneous bacterial peritonitis' (SBP). Due to the poor sensitivity of ascitic fluid culture, SBP is diagnosed by a polymorphonuclear (PMN) leukocyte count of more than 250 per mm3 in the ascitic fluid (Rimola 2000; EASL 2018). In the presence of haemorrhagic ascites (ascites with red blood cell count of more than 10,000 per mm3), one PMN leukocyte count should be subtracted for every 250 red blood cells to account for the presence of blood in the ascitic fluid (Rimola 2000). People with SBP may or may not display symptoms of peritonitis, such as abdominal pain fever,chills, and hypotension (Rimola 2000; Nousbaum 2007; EASL 2010).
The overall incidence and prevalence of SBP in people with cirrhosis is difficult to estimate. Approximately 2.5% of all hospitalisations in people with cirrhosis are for SBP (Devani 2017). The prevalence of SBP in patients with cirrhosis and ascites undergoing paracentesis varies from 0.5% to 8.7% (Nousbaum 2007; Castellote 2008; Khan 2009; Cadranel 2013). The incidence of SBP in people with decompensated liver cirrhosis is about 20% over a period of one to 12 months (Saab 2009).
The short‐term mortality (that is, death within 30 days of diagnosis or death in hospital) after SBP is about 15% to 40% (Khan 2009; Tandon 2011; Devani 2017). In addition, SBP is associated with significant resource utilisation; a study conducted in the USA showed that the average length of hospital stay was approximately six days and the average hospital costs per patient were approximately USD 17,000 (Devani 2017).
Pathophysiology of SBP
Increased bacterial translocation (gut bacteria or bacterial products migrating outside the intestinal lumen) and decreased local and systemic immune responses in patients having cirrhosis are believed to be the cause of SBP (Bernardi 2010).
Description of the intervention
Antibiotics, in addition to supportive treatment (fluid and electrolyte balance, treatment of shock) form the mainstay treatment of SBP. There are various classes of antibiotics available for the treatment of SBP. If bacteria can be cultured from the ascitic fluid, antibiotic therapy can be based on the susceptibility of the bacteria to different antibiotics (EASL 2010; Runyon 2013; EASL 2018). However, bacteria can be cultured only in 40% to 60% of people with SBP (Rimola 2000; EASL 2010). Therefore, empirical antibiotic treatment is used in the majority of people with SBP (EASL 2010; Runyon 2013; EASL 2018). The major classes of empirical antibiotics used in the treatment of SBP include third‐generation cephalosporins, such as ceftriaxone, cefotaxime, and ‐ less commonly ‐ penicillins, such as amoxicillin/clavulanic acid, and fluoroquinolones, such as ciprofloxacin (in people who have not taken fluoroquinolones for prophylaxis of SBP) (EASL 2010; Runyon 2013; EASL 2018).
How the intervention might work
Different antibiotic classes have different mechanisms of action to kill bacteria (bactericidal effect) or reduce their growth (bacteriostatic effect). Penicillins and cephalosporins inhibit bacterial cell wall synthesis (Yocum 1980; Yotsuji 1988). Fluoroquinolones are type II topoisomerase inhibitors; type II topoisomerases at appropriate levels are required for normal cellular processes, and altering their levels leads to bacterial cell death (Aldred 2014). Other antibiotics act via bacteriostatic effects.
Why it is important to do this review
SBP is associated with significant short‐term mortality (Khan 2009; Tandon 2011; Devani 2017). It is important to provide optimal empirical treatment to people with SBP while waiting for the results of ascitic fluid culture and sensitivity (susceptibility of bacteria to the specific antibiotic) to improve their survival. Bacteria can be cultured only in 40% to 60% of people with SBP (Rimola 2000; EASL 2010). Several different antibiotic treatments are available, but their relative efficacy and the optimal combination are not known. There has been one Cochrane Review on the role of antibiotics in patients with cirrhosis and SBP (Chavez‐Tapia 2009); however, there have been no previous network meta‐analyses on the topic. Network meta‐analysis allows for a combination of direct and indirect evidence, and the ranking of different interventions for different outcomes (Salanti 2011; Salanti 2012). With this systematic review and network meta‐analysis, we aim to provide the best level of evidence for the benefits and harms of different antibiotic treatments for SBP in people with decompensated liver cirrhosis.
Objectives
To compare the benefits and harms of different antibiotic treatments for spontaneous bacterial peritonitis (SBP) in people with decompensated liver cirrhosis.
Methods
Criteria for considering studies for this review
Types of studies
We considered only randomised clinical trials for this network meta‐analysis, irrespective of language, publication status, or date of publication. We excluded studies with a quasi‐randomised design or non‐randomised design because of the risk of bias in such studies. Inclusion of indirect observational evidence could weaken our network meta‐analysis, but this could also be viewed as a strength for assessing rare adverse events. It is well established that exclusion of non‐randomised studies increases the focus on potential benefits and reduces the focus on the risks of serious adverse events and those of any adverse events. However, due to the exponentially increased amount of work required for non‐randomised studies, we planned to register and perform a new systematic review and meta‐analysis of non‐randomised studies for adverse events, if there was uncertainty in the balance of benefits and harms of effective treatment(s). We did not perform this because of the findings of the review.
Types of participants
We included randomised clinical trials with adult participants with decompensated liver cirrhosis, who are undergoing treatment for spontaneous bacterial peritonitis (SBP). We excluded randomised clinical trials in which participants have previously undergone liver transplantation, have SBP due to other causes, or have secondary peritonitis (i.e. peritonitis due to hollow viscus perforation or inflammation of other intra‐abdominal organs, such as appendicitis or pancreatitis).
Types of interventions
We included any of the following different antibiotic interventions for comparison with one another, either alone or in combination.
Cephalosporins
Penicillins
Quinolones
Other classes of antibiotics
We did not include trials evaluating interventions targeted at fluid and electrolyte balance, or the treatment of shock. However, we included trials in which such cointerventions are administered equally in all the intervention arms.
We evaluated the plausibility of the transitivity assumption (the assumption that participants included in the different trials with different treatments can be considered to be a part of a multiarm randomised clinical trial and could potentially have been randomised to any of the interventions) (Salanti 2012), by looking at the inclusion and exclusion criteria in the studies. In other words, any participant that meets the inclusion criteria is, in principle, equally likely to be randomised to any of the above eligible interventions. This necessitates that information on potential effect modifiers, such as the presence of other features of decompensation (hepatorenal syndrome, hepatic encephalopathy, or variceal bleeding) are the same across trials. Since, there was no concern about the transitivity assumption, we did not perform a separate meta‐analysis for people with cirrhosis and SBP versus without other features of decompensation.
Types of outcome measures
Primary outcomes
All‐cause mortality at maximal follow‐up (time to death)
Health‐related quality of life using a validated scale, such as the EQ‐5D or 36‐Item Short Form Health Survey (SF‐36) (EuroQol 2018; Optum 2018), at maximal follow‐up
-
Serious adverse events (during or within 6 months after cessation of the intervention). We defined a serious adverse event as any event that would increase mortality; is life‐threatening; requires hospitalisation; results in persistent or significant disability; is a congenital anomaly/birth defect; or any important medical event that might jeopardise the person or require intervention to prevent it (ICH‐GCP 1997). However, none of the authors defined serious adverse events. Therefore, we used the definitions provided by trial authors for serious adverse events (as indicated in the protocol).
Proportion of participants with one or more serious adverse event(s)
Number of serious adverse events per participant
Secondary outcomes
-
Any adverse event (during or within 6 months after cessation of the intervention): we defined an adverse event as any untoward medical occurrence, not necessarily having a causal relationship with the intervention, but resulting in a dose reduction or discontinuation of intervention (any time after commencement of the intervention) (ICH‐GCP 1997). However, none of the authors defined 'adverse event'. Therefore, we used the definitions provided by trial authors for adverse events (as indicated in the protocol).
Proportion of participants with one or more adverse event
Number of any adverse events per participant
Time to liver transplantation (maximal follow‐up)
-
Time to resolution of spontaneous bacterial peritonitis (SBP) (however defined by study authors at maximal follow‐up)
Symptomatic recovery
Recovery according to definitions used for SBP
Number of other decompensation episodes (maximal follow‐up)
Exploratory outcomes
Length of hospital stay (all hospital admissions until maximal follow‐up)
Number of days of lost work (in people who work) (maximal follow‐up)
Treatment costs (including the cost of the treatment and any resulting complications)
We have chosen outcomes based on their importance to patients in a survey related to research priorities for people with liver diseases (Gurusamy 2019), based on feedback of the patient and public representative of this project, and based on an online survey about the outcomes promoted through the Cochrane Consumer Network.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library, MEDLINE Ovid, Embase Ovid, and Science Citation Index Expanded (Web of Science) from inception to 10 November 2018, without applying any language restrictions (Royle 2003). We searched for all possible comparisons formed by the interventions of interest. To identify further ongoing or completed trials, we also searched clinicaltrials.gov, and the World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch/) which included various trial registers, including ISRCTN and ClinicalTrials.gov on 10 November 2018. We also searched the European Medical Agency (EMA) (www.ema.europa.eu/ema/), and US Food and Drug Administration (FDA) registries (www.fda.gov), for randomised clinical trials on 10 November 2018. The search strategies are provided in Appendix 1.
Searching other resources
We searched the references of the identified trials and the existing Cochrane Review on antibiotic treatments in liver cirrhosis to identify additional trials for inclusion (Chavez‐Tapia 2009).
Data collection and analysis
Selection of studies
Two review authors (KG and LP) independently identified trials for inclusion by screening the titles and abstracts, and sought full‐text articles for any references identified by at least one of the review authors for potential inclusion. We selected trials for inclusion based on the full‐text articles. We provided the list of references that we excluded and the reasons for their exclusion in the 'Characteristics of excluded studies' table. We also planned to list any ongoing trials identified primarily through the search of the clinical trial registers for further follow‐up. We resolved any discrepancies through discussion.
Data extraction and management
Two review authors (KG and LP) independently extracted the following data in a piloted Microsoft Excel‐based data extraction form (after translation of non‐English articles).
-
Outcome data (for each outcome and for each intervention group whenever applicable)
number of participants randomised
number of participants included for the analysis
number of participants with events for binary outcomes, mean and standard deviation for continuous outcomes, number of events and the mean follow‐up period for count outcomes, and number of participants with events and the mean follow‐up period for time‐to‐event outcomes
natural logarithm of hazard ratio and its standard error, if this was reported, rather than the number of participants with events and the mean follow‐up period for time‐to‐event outcomes
definition of outcomes or scale used, if appropriate
-
Data on potential effect modifiers
participant characteristics, such as age, sex, presence of other features of decompensation (hepatorenal syndrome, hepatic encephalopathy, and variceal bleeding), the aetiology for cirrhosis, and the interval between diagnosis of SBP and treatment
details of the intervention and control (including dose, frequency, and duration)
length of follow‐up
information related to 'Risk of bias' assessment (please see below)
-
Other data
year and language of publication
country in which the participants were recruited
year(s) in which the trial was conducted
inclusion and exclusion criteria
We collected outcomes at maximum follow‐up, but also at short‐term (up to 3 months) and medium‐term (from 3 months to 5 years), if applicable.
We attempted to contact the trial authors in the case of unclear or missing information. If there was any doubt as to whether trials shared the same participants, completely or partially (by identifying common authors and centres), we planned to contact the trial authors to clarify whether the trial report was duplicated. We resolved any differences in opinion through discussion.
Assessment of risk of bias in included studies
We followed the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) to assess the risk of bias in included trials. Specifically, we assessed sources of bias as defined below (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008; Savović 2012a; Savović 2012b; Savović 2018).
Allocation sequence generation
Low risk of bias: the study authors performed sequence generation using computer random number generation or a random number table. Drawing lots, tossing a coin, shuffling cards, and throwing dice are adequate if performed by an independent person not otherwise involved in the study. In general, we classified the risk of bias as low if the method used for allocation concealment suggested that it was extremely likely that the sequence was generated randomly (for example, use of interactive voice response system).
Unclear risk of bias: the study authors did not specify the method of sequence generation.
High risk of bias: the sequence generation method was not random. We excluded such quasi‐randomised studies.
Allocation concealment
Low risk of bias: the participant allocations could not have been foreseen in advance of, or during, enrolment. A central and independent randomisation unit controlled allocation. The investigators were unaware of the allocation sequence (e.g. if the allocation sequence was hidden in sequentially numbered, opaque, and sealed envelopes).
Unclear risk of bias: the study authors did not describe the method used to conceal the allocation so that the intervention allocations may have been foreseen before, or during, enrolment.
High risk of bias: it is likely that the investigators who assigned the participants knew the allocation sequence. We excluded such quasi‐randomised studies.
Blinding of participants and personnel
Low risk of bias: blinding of participants and key study personnel was ensured, and it was unlikely that the blinding could have been broken; or there was rarely no blinding or incomplete blinding, but the review authors judged that the outcome was not likely to be influenced by lack of blinding.
Unclear risk of bias: insufficient information to permit a judgement of 'low risk' or 'high risk'; or the trial did not address this outcome.
High risk of bias: no blinding or incomplete blinding, and the outcome was likely to be influenced by lack of blinding; or blinding of key study participants and personnel was attempted, but it was likely that the blinding could have been broken, and the outcome was likely to be influenced by lack of blinding.
Blinded outcome assessment
Low risk of bias: blinding of outcome assessment was ensured, and it was unlikely that the blinding could have been broken; or rarely no blinding of outcome assessment, but the review authors judged that the outcome measurement was not likely to be influenced by lack of blinding.
Unclear risk of bias: insufficient information to permit a judgement of 'low risk' or 'high risk'; or the trial did not address this outcome.
High risk of bias: any of the following ‐ no blinding of outcome assessment, and the outcome measurement was likely to be influenced by lack of blinding; or blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement was likely to be influenced by lack of blinding.
Incomplete outcome data
Low risk of bias: missing data were unlikely to make treatment effects depart from plausible values. The study used sufficient methods, such as multiple imputation, to handle missing data.
Unclear risk of bias: there was insufficient information to assess whether missing data in combination with the method used to handle missing data were likely to induce bias on the results.
High risk of bias: the results were likely to be biased due to missing data.
Selective outcome reporting
Low risk of bias: the trial reported the following predefined outcomes: at least one of the outcomes related to the main reason for treatment of people with SBP, namely, all‐cause mortality, resolution of SBP along with adverse events. If the original trial protocol was available, the outcomes should have been those called for in that protocol. If the trial protocol was obtained from a trial registry (e.g. ClinicalTrials.gov), the outcomes sought should have been those enumerated in the original protocol if the trial protocol was registered before or at the time that the trial was begun. If the trial protocol was registered after the trial was begun, those outcomes will not be considered to be reliable.
Unclear risk of bias: not all predefined, or clinically relevant and reasonably expected, outcomes were reported fully; or it was unclear whether data on these outcomes were recorded or not.
High risk of bias: one or more predefined or clinically relevant and reasonably expected outcomes were not reported, despite the fact that data on these outcomes should have been available and recorded.
Other bias
Low risk of bias: the trial appeared to be free of other components that could put it at risk of bias (e.g. inappropriate control or dose or administration of control, baseline differences, early stopping).
Unclear risk of bias: the trial may or may not have been free of other components that could put it at risk of bias.
High risk of bias: there were other factors in the trial that could put it at risk of bias (e.g. baseline differences, early stopping).
We considered a trial to be at low risk of bias if we assessed the trial to be at low risk of bias across all listed bias risk domains. Otherwise, we considered trials to be at high risk of bias. At the outcome level, we classified an outcome to be at low risk of bias if the allocation sequence generation, allocation concealment, blinding of participants, healthcare professionals, and outcome assessors, incomplete outcome data, and selective outcome reporting (at the outcome level) were at low risk of bias for objective and subjective outcomes (Savović 2018).
Measures of treatment effect
Relative treatment effects
For dichotomous variables (e.g. proportion of participants with serious adverse events or any adverse events), we calculated the odds ratio (OR) with 95% credible interval (CrI) (or Bayesian confidence interval) (Severini 1993). For continuous variables (e.g. length of hospital stay), we calculated the mean difference (MD) with 95% Crl. We planned to use standardised mean difference (SMD) values with 95% Crl for health‐related quality of life if included trials used different scales. We planned to obtain the final scores whenever possible. For count outcomes (e.g. number of serious adverse events or number of any adverse events), we calculated the rate ratio with 95% Crl. This assumes that the events are independent of each other, i.e. if a person has had an event they are not at an increased risk of further outcomes, which is the assumption in Poisson likelihood. For time‐to‐event data (e.g. all‐cause mortality at maximal follow‐up), we calculated hazard ratio (HR) with 95% Crl.
Relative ranking
We estimated the ranking probabilities with 95% CrI for all interventions of being at each possible rank for each intervention. We obtained the surface under the cumulative ranking curve (SUCRA) (cumulative probability), rankogram, and relative ranking table with CrI for the ranking probabilities (Salanti 2011; Chaimani 2013).
Unit of analysis issues
The unit of analysis was the participant undergoing treatment for SBP according to the intervention group to which the participant was randomly assigned.
Cluster‐randomised clinical trials
If we identified any cluster‐randomised clinical trials, we planned to include cluster‐randomised clinical trials, provided that the effect estimate adjusted for cluster correlation was available or if there was sufficient information available to calculate the design effect (which would allow us to take clustering into account). We also planned to assess additional domains of risk of bias for cluster‐randomised trials according to guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Cross‐over randomised clinical trials
If we identified any cross‐over randomised clinical trials, we planned to include only the outcomes after the period of first intervention because the included treatments could have residual effects.
Trials with multiple intervention groups
We collected data for all trial intervention groups that met the inclusion criteria. The codes, we used for analysis, accounted for the correlation between the effect sizes from trials with more than two groups.
Dealing with missing data
We performed an intention‐to‐treat analysis whenever possible (Newell 1992); otherwise, we used the data available to us. When intention‐to‐treat analysis is not used and the data are not missing at random (for example, treatment was withdrawn due to adverse events or duration of treatment was shortened because of lack of response and such participants were excluded from analysis), this can lead to biased results; therefore, we conducted best‐worst case scenario analysis (assuming a good outcome in the intervention group and bad outcome in the control group) and worst‐best case scenario analysis (assuming a bad outcome in the intervention group and good outcome in the control group) as sensitivity analyses whenever possible for binary and time‐to‐event outcomes, where binomial likelihood was used.
For continuous outcomes, we planned to impute the standard deviation from P values, according to guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If the data were likely to be normally distributed, we planned to use the median for meta‐analysis when the mean was not available; otherwise, we planned to simply provide a median and interquartile range of the difference in medians. If it was not possible to calculate the standard deviation from the P value or the confidence intervals, we planned to impute the standard deviation using the largest standard deviation in other trials for that outcome. This form of imputation can decrease the weight of the study for calculation of mean differences and may bias the effect estimate to no effect for calculation of standardised mean differences (Higgins 2011).
Assessment of heterogeneity
We assessed clinical and methodological heterogeneity by carefully examining the characteristics and design of included trials. We also planned to assess the presence of clinical heterogeneity by comparing effect estimates (please see Subgroup analysis and investigation of heterogeneity) in trial reports of different drug dosages, presence of other features of decompensation (hepatorenal syndrome, hepatic encephalopathy, or variceal bleeding), different aetiologies for cirrhosis (for example, alcohol‐related liver disease, viral liver diseases, autoimmune liver disease), and based on the cointerventions (for example, both groups receive albumin). Different study designs and risk of bias can contribute to methodological heterogeneity.
We assessed statistical heterogeneity by comparing the results of the fixed‐effect model meta‐analysis and the random‐effects model meta‐analysis, between‐study variance (Tau2, and comparing this with values reported in the study of the distribution of between‐study heterogeneity) (Turner 2012), and by calculating I2 (Jackson 2014), using Stata/SE 15.1 (if applicable). If we identified substantial clinical, methodological, or statistical heterogeneity, we planned to explore and address the heterogeneity in subgroup analysis (see 'Subgroup analysis and investigation of heterogeneity').
Assessment of transitivity across treatment comparisons
We assessed the transitivity assumption by comparing the distribution of the potential effect modifiers (clinical: presence of other features of decompensation, i.e. hepatorenal syndrome, hepatic encephalopathy, or variceal bleeding; methodological: risk of bias, year of randomisation, duration of follow‐up) across the different pairwise comparisons.
Assessment of reporting biases
For the network meta‐analysis, we planned to perform a comparison‐adjusted funnel plot. However, to interpret a comparison‐adjusted funnel plot, it is necessary to rank the studies in a meaningful way as asymmetry may be due to small sample sizes in newer studies (comparing newer treatments with older treatments) or higher risk of bias in older studies (comparing older treatments with placebo) (Chaimani 2012). As there was no meaningful way in which to rank these studies (i.e. there was no specific change in the risk of bias in the studies, sample size, or the control group used over time), we judged the reporting bias by the completeness of the search (Chaimani 2012).
Data synthesis
Methods for indirect and mixed comparisons
We conducted network meta‐analyses to compare multiple interventions simultaneously for each of the primary and secondary outcomes. Network meta‐analysis combines direct evidence within trials and indirect evidence across trials (Mills 2012). We obtained a network plot to ensure that the trials were connected by interventions using Stata/SE 15.1 (Chaimani 2013). We excluded any trials that were not connected to the network from the network meta‐analysis, and we reported only the direct pairwise meta‐analysis for such comparisons. We summarised the population and methodological characteristics of the trials included in the network meta‐analysis in a table based on pairwise comparisons. We conducted a Bayesian network meta‐analysis using the Markov chain Monte Carlo method in OpenBUGS 3.2.3, according to guidance from the National Institute for Health and Care Excellence (NICE) Decision Support Unit (DSU) documents (Dias 2016). We modelled the treatment contrast (i.e. log odds ratio for binary outcomes, mean difference or standardised mean difference for continuous outcomes, log rate ratio for count outcomes, and log hazard ratio for time‐to‐event outcomes) for any two interventions ('functional parameters') as a function of comparisons between each individual intervention and the reference group ('basic parameters'), using appropriate likelihood functions and links (Lu 2006). We used binomial likelihood and logit link for binary outcomes, Poisson likelihood and log link for count outcomes, binomial likelihood and complementary log‐log link (a semiparametric model which excludes censored individuals from the denominator of 'at risk' individuals at the point when they are censored), and normal likelihood and identity link for continuous outcomes. We used 'ceftriaxone' as the reference group as this was the commonest intervention in the trials included in this review. We used a fixed‐effect model and a random‐effects model for the network meta‐analysis. We planned to report both models for comparison with the reference group in a forest plot, when applicable. For each pairwise comparison in a table, we reported the fixed‐effect model if the two models reported similar results; otherwise, we planned to report the more conservative model.
We used a hierarchical Bayesian model using three different sets of initial values to start the simulation‐based parameter estimation, employing codes provided by NICE DSU (Dias 2016). We used a normal distribution with large variance (10,000) for treatment effect priors (vague or flat priors) centred at no effect. For the random‐effects model, we used a prior distributed uniformly (limits: 0 to 5) for the between‐trial standard deviation and assumed this variability would be the same across treatment comparisons (Dias 2016). We used a 'burn‐in' of 30,000 iterations, checked for convergence (of effect estimates and between‐study heterogeneity) visually (i.e. checked whether the values in different chains mix very well by visualisation), and ran the models for another 10,000 simulations to obtain effect estimates. If we did not obtain convergence, we increased the number of simulations for the 'burn‐in' and used the 'thin' and 'over relax' functions to decrease the autocorrelation. If we still did not obtain convergence, we planned to use alternate initial values and priors employing methods suggested by van Valkenhoef 2012. We estimated the probability that each intervention ranks at each of the possible positions using the NICE DSU codes (Dias 2016).
Assessment of inconsistency
We assessed inconsistency (statistical evidence of the violation of transitivity assumption) by fitting both an inconsistency model and a consistency model. We used inconsistency models employed in the NICE DSU manual, as we used a common between‐study standard deviation (Dias 2014). In addition, we planned to use design‐by‐treatment full interaction model and inconsistency factor plots to assess inconsistency when applicable (Higgins 2012; Chaimani 2013). We planned to report inconsistency factor plots when possible using Stata/SE 15.1. In the presence of inconsistency, we planned to assess whether the inconsistency was due to clinical or methodological heterogeneity by performing separate analyses for each of the different subgroups mentioned in the 'Subgroup analysis and investigation of heterogeneity' section.
If there was evidence of inconsistency, we planned to identify areas in the network where substantial inconsistency might be present in terms of clinical and methodological diversities between trials and, when appropriate, limit network meta‐analysis to a more compatible subset of trials.
Direct comparison
We performed the direct comparisons using the same codes and the same technical details.
Subgroup analysis and investigation of heterogeneity
We planned to assess the differences in the effect estimates between the following subgroups and investigate heterogeneity and inconsistency using meta‐regression with the help of the codes provided in the NICE DSU guidance (Dias 2012a), if we included a sufficient number of trials (when there were at least two trials in at least two of the subgroups) and when the interaction term could be calculated. We planned to use the following trial‐level covariates for meta‐regression.
Trials at low risk of bias compared to trials at high risk of bias
The presence of other features of decompensation (hepatorenal syndrome, hepatic encephalopathy, or variceal bleeding)
The aetiology for cirrhosis (for example, alcohol‐related liver disease, viral liver diseases, autoimmune liver disease)
Community acquired or nosocomial SBP
The interval between the diagnosis of SBP and the start of treatment
Different types of cointerventions (for example, both groups receive albumin as the cointervention)
The period of follow‐up (short‐term: up to 3 months; medium‐term: more than 3 months to 5 years; long‐term: more than 5 years)
The definition used by authors for serious adverse events and any adverse events compared to other definitions (ICH‐GCP 1997)
We planned to calculate a single common interaction term (which assumes that each relative treatment effect versus a common comparator treatment is impacted in the same way by the covariate in question) when applicable (Dias 2012a). If the 95% Crl of the interaction term did not overlap zero, we would have considered this statistically significant heterogeneity or inconsistency (depending upon the factor being used as covariate).
Sensitivity analysis
If there were post‐randomisation dropouts, we reanalysed the results using the best‐worst case scenario and worst‐best case scenario analyses as sensitivity analyses whenever possible. We also planned to perform a sensitivity analysis excluding the trials in which mean or standard deviation, or both were imputed, and use the median standard deviation in the trials to impute missing standard deviations.
Presentation of results
We followed the PRISMA‐NMA statement while reporting the results (Hutton 2015). We presented the effect estimates with 95% CrI for each pairwise comparison calculated from the direct comparisons and the network meta‐analysis. We originally planned to present the cumulative probability of the treatment ranks (i.e. the probability that the intervention is within the top two, the probability that the intervention is within the top three, etc.) in graphs (SUCRA) (Salanti 2011). We plotted the probability that each intervention was best, second best, third best, etc. for each of the different outcomes (rankograms), which are generally considered more informative (Salanti 2011; Dias 2012b), but we did not present these because of the sparse data which can lead to misinterpretation of results due to large uncertainty in the rankings (the CrI was 0 to 1 for all the ranks). We uploaded all the raw data and the codes used for analysis in The European Organization for Nuclear Research open source database (Zenodo) (the link is doi.org/10.5281/zenodo.3256132).
Grading of evidence
We presented 'Summary of findings' tables for all the primary and secondary outcomes (see Primary outcomes; Secondary outcomes). We followed the approach suggested by Yepes‐Nunez and colleagues (Yepes‐Nunez 2019). First, we calculated the direct and indirect effect estimates (when possible) and 95% Crl using the node‐splitting approach (Dias 2010), that is, calculating the direct estimate for each comparison by including only trials in which there was direct comparison of interventions and the indirect estimate for each comparison by excluding the trials in which there was direct comparison of interventions (and ensuring a connected network). Next, we rated the quality of direct and indirect effect estimates using GRADE methodology which takes into account the risk of bias, inconsistency (heterogeneity), directness of evidence (including incoherence, the term used in GRADE methodology for inconsistency in network meta‐analysis), imprecision, and publication bias (Guyatt 2011). We then presented the relative and absolute estimates of the meta‐analysis with the best certainty of evidence (Yepes‐Nunez 2019). We also presented the 'Summary of findings' tables in a second format presenting all the outcomes for selected interventions (Yepes‐Nunez 2019): we selected the three interventions (cetriaxone, cefotaxime, and ciprofloxacin) which were compared in most trials.
Recommendations for future research
We provided recommendations for future research regarding the population, intervention, control, outcomes, period of follow‐up, and study design, based on the uncertainties that we identified from the existing research.
Results
Description of studies
Results of the search
We identified 1322 references through electronic searches of CENTRAL (n = 183), MEDLINE Ovid (n = 501), Embase Ovid (n = 238), Science Citation Index Expanded (n = 316), ClinicalTrials.gov (n = 35) and WHO Trials register (n = 49). After removing duplicate references, there were 1050 references. We excluded 1022 clearly irrelevant references through reading titles and abstracts. We did not identify any additional eligible trial by reference searching or by searching the European Medicines Agency (EMA) or Food and Drug Administration (FDA). We retrieved a total of 28 full‐text references for further assessment in detail. We excluded 11 references for the reasons stated in the Characteristics of excluded studies. Thus, we included a total of 12 trials described in 17 references (Characteristics of included studies). The reference flow is shown in Figure 2.
2.
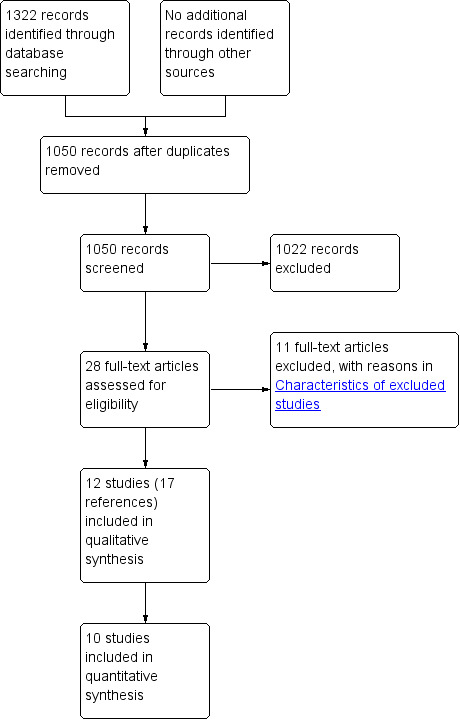
Study flow diagram.
Included studies
We included 12 trials (Gomez‐Jimenez 1993; Navasa 1996; Figueiredo 1997; Rastegar 1998; Tuncer 2003; Chen 2005; Angeli 2006; Ahmed Ather Ch 2014; Abd‐Elsalam 2016; Jindal 2016; Piano 2016; Yim 2017). A total of 1272 participants were randomised to different interventions. The number of participants ranged from 20 to 261. A total of 893 participants from 10 trials provided data for one or more outcomes (Gomez‐Jimenez 1993; Navasa 1996; Figueiredo 1997; Rastegar 1998; Tuncer 2003; Chen 2005; Angeli 2006; Ahmed Ather Ch 2014; Jindal 2016; Piano 2016). The mean or median age in the trials ranged from 42 to 60 years in the trials that reported this information (Gomez‐Jimenez 1993; Navasa 1996; Figueiredo 1997; Rastegar 1998; Tuncer 2003; Chen 2005; Angeli 2006; Ahmed Ather Ch 2014; Jindal 2016; Piano 2016). The proportion of females ranged from 18.3% to 42.1% in the trials that reported this information (Gomez‐Jimenez 1993; Navasa 1996; Figueiredo 1997; Rastegar 1998; Tuncer 2003; Chen 2005; Angeli 2006; Ahmed Ather Ch 2014; Jindal 2016; Piano 2016). The most common organism isolated was Eschericia coli (E coli) (18.6% to 56.7%) in the trials that reported the isolated micro‐organisms (Gomez‐Jimenez 1993; Navasa 1996; Chen 2005; Angeli 2006). The next most common was the Klebsiella species (5.0% to 5.7%) and Streptococcus pneumoniae (S pneumoniae) (1.7% to 9.8%) (Gomez‐Jimenez 1993; Navasa 1996); the remaining organisms were less frequent.
All trials had short‐term follow‐up (Gomez‐Jimenez 1993; Navasa 1996; Figueiredo 1997; Rastegar 1998; Tuncer 2003; Chen 2005; Angeli 2006; Ahmed Ather Ch 2014; Abd‐Elsalam 2016; Jindal 2016; Piano 2016; Yim 2017), ranging from one week to three months.
A total of 13 interventions were compared in the trials. Ceftriaxone, cefotaxime, and ciprofloxacin were the commonest antibiotics compared in the trials. The important characteristics, antibiotics compared, potential effect modifiers, and follow‐up in each trial are reported in Table 3. None of the trials compared antibiotics with no treatment or placebo. Overall, no systematic differences between any of the comparisons seemed to exist.
1. Characteristics and potential effect modifiers of included studies ordered by comparison.
| Study name | Intervention 1 | Intervention 2 | Intervention 1: number of participants | Intervention 2: number of participants | Presence of other features of decompensation (hepatorenal syndrome, hepatic encephalopathy, or variceal bleeding) | Alcohol‐related cirrhosis | Viral‐related cirrhosis | Other causes for cirrhosis | Treated for ascites in addition to antibiotics (e.g. albumin or diuretics) | Follow‐up in months | Year of recruitment |
| Abd‐Elsalam 2016 | Cefotaxime | Ceftriaxone | Not stated | Not stated | Not stated | Not stated | Not stated | Not stated | Not stated | 0.25 | 2014 |
| Tuncer 2003 | Cefotaxime | Ceftriaxone | 18 | 19 | Includes people with and without other features of decompensation | Not stated | Includes people with and without viral‐related cirrhosis | Includes people with and without other causes of cirrhosis | Not stated | 0.25 | Not stated |
| Yim 2017 | Cefotaxime | Ceftriaxone | Not stated | Not stated | Not stated | Not stated | Not stated | Not stated | Not stated | 0.25 | 2007‐2016 |
| Ahmed Ather Ch 2014 | Ciprofloxacin | Ceftriaxone | 120 | 120 | Not stated | Not stated | Not stated | Not stated | Not stated | 0.25 | 2011 |
| Tuncer 2003 | Ciprofloxacin | Ceftriaxone | 16 | 19 | Includes people with and without other features of decompensation | Not stated | Includes people with and without viral‐related cirrhosis | Includes people with and without other causes of cirrhosis | Not stated | 0.25 | Not stated |
| Yim 2017 | Ciprofloxacin | Ceftriaxone | Not stated | Not stated | Not stated | Not stated | Not stated | Not stated | Not stated | 0.25 | 2007‐2016 |
| Figueiredo 1997 | Cefixime | Ceftriaxone | 20 | 18 | Includes people with and without other features of decompensation | Includes people with and without alcohol‐related cirrhosis | Not stated | Not stated | Not stated | 0.25 | Not stated |
| Gomez‐Jimenez 1993 | Cefonicid | Ceftriaxone | 30 | 30 | Not stated | Includes people with and without alcohol‐related cirrhosis | Not stated | Includes people with and without other causes of cirrhosis | Not stated | 0.5 | 1987‐1990 |
| Tuncer 2003 | Ciprofloxacin | Cefotaxime | 16 | 18 | Includes people with and without other features of decompensation | Not stated | Includes people with and without viral‐related cirrhosis | Includes people with and without other causes of cirrhosis | Not stated | 0.25 | Not stated |
| Yim 2017 | Ciprofloxacin | Cefotaxime | Not stated | Not stated | Not stated | Not stated | Not stated | Not stated | Not stated | 0.25 | 2007‐2016 |
| Chen 2005 | Amikacin | Cefotaxime | 18 | 19 | Not stated | Includes people with and without alcohol‐related cirrhosis | Includes people with and without viral‐related cirrhosis | Includes people with and without other causes of cirrhosis | Not stated | 1 | 2000‐2002 |
| Navasa 1996 | Ofloxacin | Cefotaxime | 64 | 59 | Includes people with and without other features of decompensation | Includes people with and without alcohol‐related cirrhosis | Not stated | Includes people with and without other causes of cirrhosis | Not stated | 0.5 | 1992‐1994 |
| Angeli 2006 | Ceftazidime | Ciprofloxacin | 55 | 61 | Not stated | Includes people with and without alcohol‐related cirrhosis | Includes people with and without viral‐related cirrhosis | Includes people with and without other causes of cirrhosis | Includes people receiving and not receiving other treatments for ascites | 3 | Not stated |
| Piano 2016 | Meropenem plus daptomycin | Ceftazidime | 15 | 16 | Not stated | Includes people with and without alcohol‐related cirrhosis | Includes people with and without viral‐related cirrhosis | Includes people with and without other causes of cirrhosis | Not stated | 3 | 2011 to 2014 |
| Rastegar 1998 | Pefloxacin | Ampicillin plus gentamycin | 11 | 9 | Not stated | Not stated | Includes people with and without viral‐related cirrhosis | Includes people with and without other causes of cirrhosis | Not stated | 0.25 | Not stated |
| Jindal 2016 | Imipenem | Cefepime | 87 | 88 | Includes people with and without other features of decompensation | Includes people with and without alcohol‐related cirrhosis | Includes people with and without viral‐related cirrhosis | Includes people with and without other causes of cirrhosis | Not stated | 3 | 2012‐2014 |
None of the trials reported the proportion of people with other features of decompensation, such as hepatorenal syndrome and active variceal bleeding. The proportion of participants with alcohol‐related cirrhosis ranged between 13.5% to 64.5% in the trials that reported this information (Gomez‐Jimenez 1993; Navasa 1996; Chen 2005; Angeli 2006; Jindal 2016; Piano 2016). The proportion of participants with viral‐related cirrhosis ranged between 20.6% to 81.1% in the trials that reported this information (Chen 2005; Angeli 2006; Jindal 2016; Piano 2016). None of the trials reported the proportion of people with autoimmune disease‐related cirrhosis. The proportion of participants with other causes for cirrhosis ranged between 5.4% to 56.9% in the trials that reported this information (Gomez‐Jimenez 1993; Navasa 1996; Chen 2005; Angeli 2006; Jindal 2016; Piano 2016). None of the trials reported the proportion of people treated for ascites, in addition to antibiotics (for example, albumin or diuretics).
Funding: the source of funding for two trials was industrial organisations who would benefit from the results of the study (Navasa 1996; Piano 2016); the source of funding for the remaining 10 trials was unclear (Gomez‐Jimenez 1993; Figueiredo 1997; Rastegar 1998; Tuncer 2003; Chen 2005; Angeli 2006; Ahmed Ather Ch 2014; Abd‐Elsalam 2016; Jindal 2016; Yim 2017).
Excluded studies
The reasons for exclusion are provided in the 'Characteristics of excluded studies' tables.
Risk of bias in included studies
The risk of bias is summarised in Figure 3, Figure 4, and in Table 4. As none of the trials were at low risk of bias in all domains, we considered all trials to be at high risk of bias.
3.
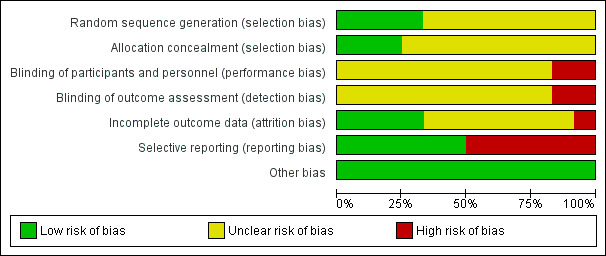
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
4.
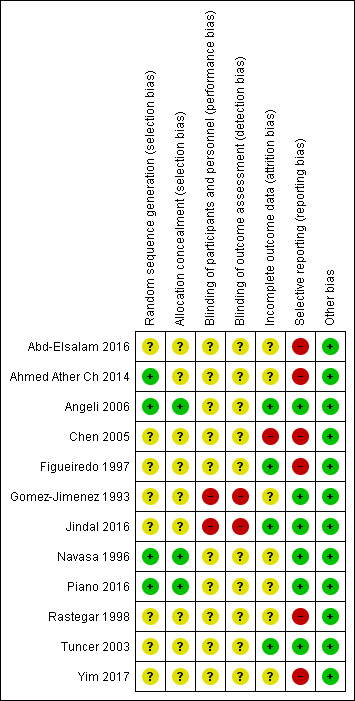
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
2. Risk of bias ordered by comparison.
| Study name | Intervention 1 | Intervention 2 | Sequence generation | Allocation concealment | Blinding of patients and healthcare providers | Blinding of outcome assessors | Missing outcome bias | Selective outcome reporting | Source of funding |
| Abd‐Elsalam 2016 | Cefotaxime | Ceftriaxone | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear |
| Tuncer 2003 | Cefotaxime | Ceftriaxone | Unclear | Unclear | Unclear | Unclear | Low | Low | Unclear |
| Yim 2017 | Cefotaxime | Ceftriaxone | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear |
| Ahmed Ather Ch 2014 | Ciprofloxacin | Ceftriaxone | Low | Unclear | Unclear | Unclear | Unclear | High | Unclear |
| Tuncer 2003 | Ciprofloxacin | Ceftriaxone | Unclear | Unclear | Unclear | Unclear | Low | Low | Unclear |
| Yim 2017 | Ciprofloxacin | Ceftriaxone | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear |
| Figueiredo 1997 | Cefixime | Ceftriaxone | Unclear | Unclear | Unclear | Unclear | Low | High | Unclear |
| Gomez‐Jimenez 1993 | Cefonicid | Ceftriaxone | Unclear | Unclear | High | High | Unclear | Low | Unclear |
| Tuncer 2003 | Ciprofloxacin | Cefotaxime | Unclear | Unclear | Unclear | Unclear | Low | Low | Unclear |
| Yim 2017 | Ciprofloxacin | Cefotaxime | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear |
| Chen 2005 | Amikacin | Cefotaxime | Unclear | Unclear | Unclear | Unclear | High | High | Unclear |
| Navasa 1996 | Ofloxacin | Cefotaxime | Low | Low | Unclear | Unclear | Unclear | Low | High |
| Angeli 2006 | Ceftazidime | Ciprofloxacin | Low | Low | Unclear | Unclear | Low | Low | Unclear |
| Piano 2016 | Meropenem plus daptomycin | Ceftazidime | Low | Low | Unclear | Unclear | Unclear | Low | High |
| Rastegar 1998 | Pefloxacin | Ampicillin plus gentamycin | Unclear | Unclear | Unclear | Unclear | Unclear | High | Unclear |
| Jindal 2016 | Imipenem | Cefepime | Unclear | Unclear | High | High | Low | Low | Unclear |
Allocation
Four trials were at low risk of sequence generation bias (Navasa 1996; Angeli 2006; Ahmed Ather Ch 2014; Piano 2016); the remaining eight trials, which did not provide sufficient details, were at unclear risk of sequence generation bias (Gomez‐Jimenez 1993; Figueiredo 1997; Rastegar 1998; Tuncer 2003; Chen 2005; Abd‐Elsalam 2016; Jindal 2016; Yim 2017). Three trials were at low risk of allocation concealment bias (Navasa 1996; Angeli 2006; Piano 2016); the remaining nine trials, which did not provide sufficient details, were at unclear risk of allocation concealment bias (Gomez‐Jimenez 1993; Figueiredo 1997; Rastegar 1998; Tuncer 2003; Chen 2005; Ahmed Ather Ch 2014; Abd‐Elsalam 2016; Jindal 2016; Yim 2017).
Blinding
None of the trials were at low risk of blinding of patients and healthcare providers' bias; 10 trials were at unclear risk of blinding of patients and healthcare providers' bias (Navasa 1996; Figueiredo 1997; Rastegar 1998; Tuncer 2003; Chen 2005; Angeli 2006; Ahmed Ather Ch 2014; Abd‐Elsalam 2016; Piano 2016; Yim 2017); the remaining two trials were at high risk of blinding of patients and healthcare providers' bias (Gomez‐Jimenez 1993; Jindal 2016).
None of the trials were at low risk of blinding of outcome assessors' bias; 10 trials were at unclear risk of blinding of outcome assessors' bias (Navasa 1996; Figueiredo 1997; Rastegar 1998; Tuncer 2003; Chen 2005; Angeli 2006; Ahmed Ather Ch 2014; Abd‐Elsalam 2016; Piano 2016; Yim 2017); the remaining two trials were at high risk of blinding of outcome assessors' bias (Gomez‐Jimenez 1993; Jindal 2016).
Incomplete outcome data
Four trials were at low risk of missing outcome bias (Figueiredo 1997; Tuncer 2003; Angeli 2006; Jindal 2016); seven trials were at unclear risk of missing outcome bias (Gomez‐Jimenez 1993; Navasa 1996; Rastegar 1998; Ahmed Ather Ch 2014; Abd‐Elsalam 2016; Piano 2016; Yim 2017), because they either did not state the number of post‐randomisation dropouts or we could not assess whether the post‐randomisation dropouts were related to the intervention and outcome; the remaining one trial was at high risk of missing outcome bias, because the post‐randomisation dropouts were likely to be related to the outcome (Chen 2005).
Selective reporting
Six trials were at low risk of selective outcome reporting bias (Gomez‐Jimenez 1993; Navasa 1996; Tuncer 2003; Angeli 2006; Jindal 2016; Piano 2016): although a protocol published prior to recruitment was not available for these trials, these trials reported all‐cause mortality or resolution of SBP along with adverse events; the remaining six trials were at high risk of selective outcome reporting bias (Figueiredo 1997; Rastegar 1998; Chen 2005; Ahmed Ather Ch 2014; Abd‐Elsalam 2016; Yim 2017): a protocol published prior to recruitment was not available for these trials, and these trials did not report reasonably expected clinical outcomes which would have been measured in a trial of this nature.
Other potential sources of bias
There were no other biases in the trials.
Effects of interventions
for the main comparison.
| Antibiotic treatment for spontaneous bacterial peritonitis in people with decompensated liver cirrhosis | ||||||
|
Patient or population: people with cirrhosis and spontaneous bacterial peritonitis Settings: secondary or tertiary care Intervention: various interventions Comparison: ceftriaxone Follow‐up period: 1 week to 3 months Network geometry plots: Figure 1 | ||||||
| Interventions | Relative effect (95% CrI) | Anticipated absolute effect* (95% CrI) | Certainty of evidence | Ranking** | ||
| Ceftriaxone | Various interventions | Difference | ||||
| All‐cause mortality Total studies: 7 Total participants: 458 | ||||||
| Cefotaxime (1 RCT; 37 participants) | HR 0.56 (0.11 to 2.28) Network estimate | 263 per 1000 | 146 per 1000 (28 to 599) | 117 fewer per 1000 (235 fewer to 336 more) | Very lowa,b,c | ‐ |
| Ciprofloxacin (1 RCT; 35 participants) | HR 0.65 (0.12 to 2.72) Network estimate | 263 per 1000 | 171 per 1000 (31 to 717) | 92 fewer per 1000 (232 fewer to 454 more) | Very lowa,b,c | ‐ |
| Ceftazidime (No direct RCT) | HR 1.15 (0.17 to 6.37) Network estimate | 263 per 1000 | 301 per 1000 (45 to 1000) | 38 more per 1000 (218 fewer to 737 more) | Very lowa,b,c | ‐ |
| Amikacin (No direct RCT) | HR 0.76 (0.09 to 5.90) Network estimate | 263 per 1000 | 201 per 1000 (24 to 1000) | 62 fewer per 1000 (239 fewer to 737 more) | Very lowa,b,c | ‐ |
| Cefixime (1 RCT; 38 participants) | HR 1.26 (0.26 to 6.90) Network estimate | 263 per 1000 | 331 per 1000 (68 to 1000) | 68 more per 1000 (195 fewer to 737 more) | Very lowa,b,c | ‐ |
| Cefonicid (1 RCT; 60 participants) | HR 1.30 (0.52 to 3.24) Network estimate | 263 per 1000 | 341 per 1000 (138 to 853) | 78 more per 1000 (126 fewer to 590 more) | Very lowa,b,c | ‐ |
| Meropenem plus daptomycin (No direct RCT) | HR 0.64 (0.05 to 6.13) Network estimate | 263 per 1000 | 169 per 1000 (14 to 1000) | 94 fewer per 1000 (249 fewer to 737 more) | Very lowa,b,c | ‐ |
| Ofloxacin (No direct RCT) | HR 0.56 (0.09 to 2.93) Network estimate | 263 per 1000 | 147 per 1000 (23 to 770) | 116 fewer per 1000 (240 fewer to 507 more) | Very lowa,b,c | ‐ |
| Health‐related quality of life | ||||||
| None of the trials reported this outcome | ||||||
| Serious adverse events (proportion of participants) | ||||||
| None of the trials with ceftriaxone as control group reported this outcome | ||||||
| Serious adverse events (number of events per participant) | ||||||
| None of the trials reported this outcome | ||||||
| Adverse events (proportion of participants) Total studies: 5 Total participants: 297 | ||||||
| Cefotaxime (1 RCT; 37 participants) | OR 0.64 (0.15 to 2.62) Network estimate | 67 per 1000 | 44 per 1000 (10 to 157) | 23 fewer per 1000 (56 fewer to 91 more) | Very lowa,b,c | ‐ |
| Ciprofloxacin (1 RCT; 35 participants) | OR 1.02 (0.24 to 4.16) Network estimate | 67 per 1000 | 68 per 1000 (17 to 229) | 1 more per 1000 (50 fewer to 162 more) | Very lowa,b,c | ‐ |
| Ceftazidime (No direct RCT) | OR 1.97 (0.38 to 10.18) Network estimate | 67 per 1000 | 123 per 1000 (27 to 421) | 57 more per 1000 (40 fewer to 354 more) | Very lowa,b,c | ‐ |
| Amikacin (No direct RCT) | OR 0.69 (0.04 to 10.94) Network estimate | 67 per 1000 | 47 per 1000 (3 to 439) | 20 fewer per 1000 (64 fewer to 372 more) | Very lowa,b,c | ‐ |
| Cefonicid (1 RCT; 60 participants) | OR 1.00 (0.10 to 10.16) Network estimate | 67 per 1000 | 67 per 1000 (7 to 420) | 0 fewer per 1000 (60 fewer to 354 more) | Very lowa,b,c | ‐ |
| Meropenem plus daptomycin (No direct RCT) | OR 1.20 (0.10 to 13.53) Network estimate | 67 per 1000 | 79 per 1000 (7 to 491) | 12 more per 1000 (60 fewer to 425 more) | Very lowa,b,c | ‐ |
| Adverse events (number of events per participant) | ||||||
| None of the trials with ceftriaxone as control group reported this outcome | ||||||
| Liver transplantation | ||||||
| None of the trials with ceftriaxone as control group reported this outcome | ||||||
| Spontaneous bacterial peritonitis (symptomatic) | ||||||
| None of the trials reported this outcome | ||||||
| Spontaneous bacterial peritonitis (as per definition used for spontaneous bacterial peritonitis) Total studies: 7 Total participants: 638 | ||||||
| Cefotaxime (1 RCT; 37 participants) | HR 0.90 (0.42 to 1.86) Network estimate | 733 per 1000 | 661 per 1000 (308 to 1000) | 73 fewer per 1000 (425 fewer to 267 more) | Very lowa,b,c | ‐ |
| Ciprofloxacin (2 RCT; 275 participants) | HR 0.93 (0.69 to 1.25) Network estimate | 733 per 1000 | 679 per 1000 (504 to 916) | 54 fewer per 1000 (230 fewer to 183 more) | Very lowa,b,c | ‐ |
| Ceftazidime (No direct RCT) | HR 0.97 (0.55 to 1.72) Network estimate | 733 per 1000 | 713 per 1000 (403 to 1000) | 21 fewer per 1000 (330 fewer to 267 more) | Very lowa,b,c | ‐ |
| Amikacin (No direct RCT) | HR 0.54 (0.17 to 1.62) Network estimate | 733 per 1000 | 393 per 1000 (126 to 1000) | 340 fewer per 1000 (608 fewer to 267 more) | Very lowa,b,c | ‐ |
| Cefixime (1 RCT; 38 participants) | HR 0.78 (0.32 to 1.90) Network estimate | 733 per 1000 | 575 per 1000 (232 to 1000) | 159 fewer per 1000 (501 fewer to 267 more) | Very lowa,b,c | ‐ |
| Meropenem plus daptomycin (No direct RCT) | HR 1.29 (0.43 to 3.92) Network estimate | 733 per 1000 | 944 per 1000 (315 to 1000) | 211 more per 1000 (419 fewer to 267 more) | Very lowa,b,c | ‐ |
| Ofloxacin (No direct RCT) | HR 1.12 (0.45 to 2.70) Network estimate | 733 per 1000 | 825 per 1000 (330 to 1000) | 91 more per 1000 (404 fewer to 267 more) | Very lowa,b,c | ‐ |
| Other features of decompensation (per participant) Total studies: 5 Total participants: 360 | ||||||
| Cefotaxime (1 RCT; 37 participants) | Rate ratio 1.22 (0.43 to 3.53) Network estimate | 368 per 1000 | 449 per 1000 (160 to 1301) | 80 more per 1000 (209 fewer to 933 more) | Very lowa,b,c | ‐ |
| Ciprofloxacin (1 RCT; 35 participants) | Rate ratio 1.01 (0.32 to 3.16) Network estimate | 368 per 1000 | 373 per 1000 (119 to 1164) | 4 more per 1000 (249 fewer to 795 more) | Very lowa,b,c | ‐ |
| Ceftazidime (No direct RCT) | Rate ratio 1.43 (0.40 to 5.19) Network estimate | 368 per 1000 | 527 per 1000 (147 to 1911) | 159 more per 1000 (222 fewer to 1542 more) | Very lowa,b,c | ‐ |
| Amikacin (No direct RCT) | Rate ratio 1.28 (0.11 to 15.66) Network estimate | 368 per 1000 | 471 per 1000 (40 to 5769) | 103 more per 1000 (329 fewer to 5400 more) | Very lowa,b,c | ‐ |
| Meropenem plus daptomycin (No direct RCT) | Rate ratio 2.19 (0.49 to 9.49) Network estimate | 368 per 1000 | 807 per 1000 (179 to 3495) | 438 more per 1000 (189 fewer to 3127 more) | Very lowa,b,c | ‐ |
| Ofloxacin (No direct RCT) | Rate ratio 1.12 (0.31 to 4.09) Network estimate | 368 per 1000 | 412 per 1000 (113 to 1508) | 44 more per 1000 (255 fewer to 1139 more) | Very lowa,b,c | ‐ |
| *Anticipated absolute effect. Anticipated absolute effect compares two risks by calculating the difference between the risks of the intervention group with the weighted median risk of the control group. **Ranking is not provided as the median rank was not 1 for at least one of the ranking positions for each intervention for the outcome. CrI: credible interval; HR: hazard ratio; OR: odds ratio; RCT: randomised clinical trial | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aThe trial(s) included in the analysis was/were at high risk of bias (downgraded 1 level). bThe sample size was small (downgraded 1 level). cThe credible intervals were wide (includes clinical benefit and harms) (downgraded 1 level).
2.
| Antibiotic treatment for spontaneous bacterial peritonitis in people with decompensated liver cirrhosis | ||||
|
Patient or population: people with cirrhosis and spontaneous bacterial peritonitis Settings: secondary or tertiary care Intervention: various interventions Comparison: ceftriaxone Follow‐up period: 1 week to 3 months Network geometry plots: Figure 1 | ||||
| Outcomes | Cefotaxime | Ciprofloxacin | ||
| All‐cause mortality | ||||
| Ceftriaxone 263 per 1000 (26.3%) | HR 0.56 (0.11 to 2.28) Network estimate | 117 fewer per 1000 (235 fewer to 336 more) | HR 0.65 (0.12 to 2.72) Network estimate | 92 fewer per 1000 (232 fewer to 454 more) |
| Very low1,2,3 | Very low1,2,3 | |||
| Rank*: ‐ | Rank: ‐ | Rank: ‐ | ||
| Based on 37 participants (1 RCT) | Based on 35 participants (1 RCT) | |||
| Adverse events (proportion of participants) | ||||
| Ceftriaxone 67 per 1000 (6.7%) | OR 0.64 (0.15 to 2.62) Network estimate | 23 fewer per 1000 (56 fewer to 91 more) | OR 1.02 (0.24 to 4.16) Network estimate | 1 more per 1000 (50 fewer to 162 more) |
| Very low1,2,3 | Very low1,2,3 | |||
| Rank: ‐ | Rank: ‐ | Rank: ‐ | ||
| Based on 37 participants (1 RCT) | Based on 35 participants (1 RCT) | |||
| Spontaneous bacterial peritonitis (as per definition used for spontaneous bacterial peritonitis) | ||||
| Ceftriaxone 733 per 1000 (73.3%) | HR 0.90 (0.42 to 1.86) Network estimate | 73 fewer per 1000 (425 fewer to 267 more) | HR 0.93 (0.69 to 1.25) Network estimate | 54 fewer per 1000 (230 fewer to 183 more) |
| Very low1,2,3 | Very low1,2,3 | |||
| Rank: ‐ | Rank: ‐ | Rank: ‐ | ||
| Based on 37 participants (1 RCT) | Based on 275 participants (2 RCT) | |||
| Other features of decompensation (per participant) | ||||
| Ceftriaxone 368 per 1000 (36.8 per 100 participants) | Rate ratio 1.22 (0.43 to 3.53) Network estimate | 80 more per 1000 (209 fewer to 933 more) | Rate ratio 1.01 (0.32 to 3.16) Network estimate | 4 more per 1000 (249 fewer to 795 more) |
| Very low1,2,3 | Very low1,2,3 | |||
| Rank: ‐ | Rank: ‐ | Rank: ‐ | ||
| Based on 37 participants (1 RCT) | Based on 35 participants (1 RCT) | |||
| *Ranking is not provided as the median rank was not 1 for at least one of the ranking positions for each intervention for the outcome. CrI: credible interval; HR: hazard ratio; OR: odds ratio; RCT: randomised clinical trial | ||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||
aThe trial(s) included in the analysis was/were at high risk of bias (downgraded one level). bThe sample size was small (downgraded one level). cThe credible intervals were wide (includes clinical benefit and harms) (downgraded one level).
The network plot for all outcomes for which network meta‐analysis was performed is shown in Figure 1. If a network meta‐analysis was not performed, the reason for not performing the network meta‐analysis is reported under the outcome. Only one trial was included for each comparison in all outcomes other than resolution of spontaneous bacterial peritonitis (SBP). Even for resolution of SBP, where one of the comparisons had two trials, the between‐study standard deviation was the same as the mean of the prior distribution: the 95% credible intervals (CrIs) of the random‐effects model were not representative. Therefore, we used the fixed‐effect model for all the network meta‐analyses and did not present the forest plots comparing the fixed‐effect and random‐effects models. In addition, the deviance information criteria statistics showed that model fit was not improved with the random‐effects model (Table 5), and the random‐effects model did not alter the interpretation on the effectiveness of treatments. These findings support the use of the fixed‐effect model.
1.
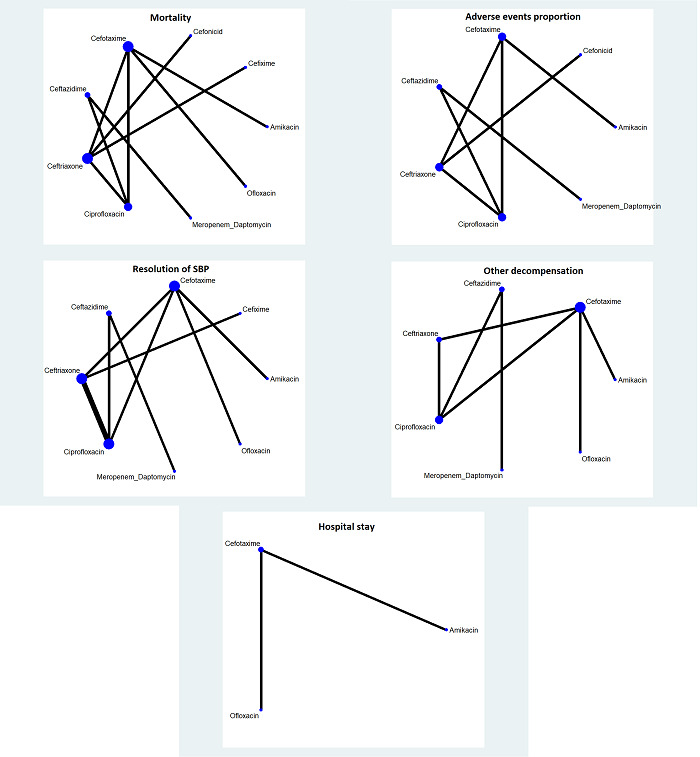
The network plots showing the outcomes for which network meta‐analysis was performed. The size of the node (circle) provides a measure of the number of trials in which the particular intervention was included as one of the intervention groups. The thickness of the line provides a measure of the number of direct comparisons between two nodes (interventions).
3. Model fit.
| All‐cause mortality at maximal follow‐up | Fixed‐effect model | Random‐effects model | Inconsistency model |
| Dbar | 65.76 | 65.69 | 65.73 |
| DIC | 80.48 | 80.35 | 80.43 |
| pD | 14.72 | 14.66 | 14.7 |
| Adverse events proportion | Fixed‐effect model | Random‐effects model | Inconsistency model |
| Dbar | 45.75 | 45.77 | 45.73 |
| DIC | 56.81 | 56.86 | 56.79 |
| pD | 11.06 | 11.09 | 11.06 |
| SBP resolution | Fixed‐effect model | Random‐effects model | Inconsistency model |
| Dbar | 61.72 | 62.34 | 62.42 |
| DIC | 75.77 | 77.07 | 77.24 |
| pD | 14.05 | 14.73 | 14.82 |
| Other decompensation | Fixed‐effect model | Random‐effects model | Inconsistency model |
| Dbar | 56.05 | 56.01 | 56.05 |
| DIC | 66.88 | 66.78 | 66.87 |
| pD | 10.83 | 10.77 | 10.83 |
| Length of hospital stay | Fixed‐effect model | Random‐effects model | Inconsistency model |
| Dbar | 14.39 | 14.38 | 14.39 |
| DIC | 18.39 | 18.36 | 18.39 |
| pD | 3.999 | 3.986 | 3.999 |
Abbreviations
Dbar: posterior mean of deviance; pD: effective number of parameters or leverage; DIC: deviance information criteria
There was no evidence of inconsistency, as indicated by deviance information criteria. As a consequence of the sparse data (only 1 trial was included for each comparison for most outcomes), we did not consider the results from the design‐by‐treatment interaction model to assess inconsistency. We were unable to obtain inconsistency factor plots in Stata/SE 15.1. This was either because there was only one closed loop resulting from a single three‐arm trial or because heterogeneity could not be calculated due to the presence of a single trial for the comparison.
The 95% CrI of the probability ranks were wide and included 0 and 1 in all the comparisons for all the outcomes. This was probably because of the sparse data from small trials. Therefore, we did not present the ranking probabilities (in a table), rankograms, and SUCRA plots as we considered that presenting this information would be misleading due to large uncertainty in the rankings.
Certainty of evidence was very low for all the comparisons. This was because all the trials were at unclear or high risk of bias for two or more risk of bias domains at the outcome level (downgraded 1 level), the sample size was small (downgraded 1 level), and the wide CrIs overlapping significant clinical effect and no effect (downgraded 1 level). There was no evidence of indirectness or publication bias. We could not assess inconsistency in the GRADE context (i.e. heterogeneity) as there was only one trial for each comparison for most outcomes.
All‐cause mortality
Nine trials (653 participants) reported all‐cause mortality (Gomez‐Jimenez 1993; Navasa 1996; Figueiredo 1997; Rastegar 1998; Tuncer 2003; Chen 2005; Angeli 2006; Jindal 2016; Piano 2016). A total of 13 interventions were compared with each other. There were nine interventions connected to the network (Figure 1), and four unconnected interventions. Only one trial was included in each pairwise comparison. There were no significant differences in the all‐cause mortality between any of the interventions included in the network meta‐analysis (Table 6). There were also no significant differences in the all‐cause mortality between the following comparisons which could not be included in the network meta‐analysis because they were not connected.
4. Effect estimates (network meta‐analysis).
| All‐cause mortality | Ceftriaxone | Cefotaxime | Ciprofloxacin | Ceftazidime | Amikacin | Cefixime | Cefonicid | Meropenem + daptomycin | Ofloxacin |
| Ceftriaxone | ‐ | 0.58[0.11,2.54] | 0.63[0.12,2.88] | ‐ | ‐ | 1.26[0.27,7.01] | 1.29[0.53,3.23] | ‐ | ‐ |
| Cefotaxime | 0.56[0.11,2.28] | ‐ | 1.13[0.20,6.67] | ‐ | 1.40[0.36,5.85] | ‐ | ‐ | ‐ | 1.01[0.44,2.36] |
| Ciprofloxacin | 0.65[0.12,2.72] | 1.16[0.20,6.90] | ‐ | 1.76[0.72,4.66] | ‐ | ‐ | ‐ | ‐ | ‐ |
| Ceftazidime | 1.15[0.17,6.37] | 2.08[0.28,15.24] | 1.77[0.73,4.58] | ‐ | ‐ | ‐ | ‐ | 0.56[0.10,2.47] | ‐ |
| Amikacin | 0.76[0.09,5.90] | 1.39[0.35,6.00] | 1.20[0.13,11.68] | 0.67[0.06,7.62] | ‐ | ‐ | ‐ | ‐ | ‐ |
| Cefixime | 1.26[0.26,6.90] | 2.32[0.27,23.93] | 1.99[0.23,21.07] | 1.11[0.11,13.68] | 1.66[0.12,25.20] | ‐ | ‐ | ‐ | ‐ |
| Cefonicid | 1.30[0.52,3.24] | 2.34[0.43,15.52] | 2.02[0.36,13.61] | 1.14[0.16,9.30] | 1.68[0.18,17.44] | 1.02[0.15,6.39] | ‐ | ‐ | ‐ |
| Meropenem + daptomycin | 0.64[0.05,6.13] | 1.16[0.09,13.48] | 1.00[0.15,5.60] | 0.57[0.11,2.41] | 0.83[0.04,13.94] | 0.50[0.03,7.76] | 0.49[0.03,5.81] | ‐ | ‐ |
| Ofloxacin | 0.56[0.09,2.93] | 1.01[0.44,2.35] | 0.87[0.12,6.19] | 0.49[0.06,4.25] | 0.73[0.14,3.63] | 0.43[0.04,4.34] | 0.43[0.05,2.87] | 0.87[0.06,12.52] | ‐ |
| Proportion of people with any adverse event | Ceftriaxone | Cefotaxime | Ciprofloxacin | Ceftazidime | Amikacin | Cefonicid | Meropenem + daptomycin | ‐ | |
| Ceftriaxone | ‐ | 0.65[0.14,2.69] | 1.03[0.25,4.33] | ‐ | ‐ | 1.00[0.10,10.23] | ‐ | ||
| Cefotaxime | 0.64[0.15,2.62] | ‐ | 1.60[0.36,7.38] | ‐ | 1.07[0.10,11.19] | ‐ | ‐ | ||
| Ciprofloxacin | 1.02[0.24,4.16] | 1.59[0.36,7.46] | ‐ | 1.95[0.86,4.49] | ‐ | ‐ | ‐ | ||
| Ceftazidime | 1.97[0.38,10.18] | 3.06[0.56,17.74] | 1.93[0.87,4.47] | ‐ | ‐ | ‐ | 0.61[0.10,3.56] | ||
| Amikacin | 0.69[0.04,10.94] | 1.07[0.10,11.31] | 0.66[0.04,10.95] | 0.34[0.02,6.58] | ‐ | ‐ | ‐ | ||
| Cefonicid | 1.00[0.10,10.16] | 1.58[0.10,24.34] | 0.98[0.06,14.86] | 0.51[0.03,8.72] | 1.47[0.04,54.82] | ‐ | ‐ | ||
| Meropenem + daptomycin | 1.20[0.10,13.53] | 1.88[0.15,22.83] | 1.17[0.16,8.47] | 0.61[0.09,3.62] | 1.72[0.06,52.67] | 1.18[0.04,34.61] | ‐ | ||
| Resolution of SBP | Ceftriaxone | Cefotaxime | Ciprofloxacin | Ceftazidime | Amikacin | Cefixime | Meropenem + daptomycin | Ofloxacin | ‐ |
| Ceftriaxone | ‐ | 0.95[0.41,2.15] | 0.93[0.69,1.25] | ‐ | ‐ | 0.78[0.32,1.88] | ‐ | ‐ | |
| Cefotaxime | 0.90[0.42,1.86] | ‐ | 1.08[0.45,2.53] | ‐ | 0.59[0.25,1.34] | ‐ | ‐ | 1.23[0.75,2.06] | |
| Ciprofloxacin | 0.93[0.69,1.25] | 1.03[0.50,2.24] | ‐ | 1.05[0.64,1.72] | ‐ | ‐ | ‐ | ‐ | |
| Ceftazidime | 0.97[0.55,1.72] | 1.08[0.44,2.71] | 1.05[0.64,1.72] | ‐ | ‐ | ‐ | 1.32[0.51,3.46] | ‐ | |
| Amikacin | 0.54[0.17,1.62] | 0.60[0.25,1.38] | 0.58[0.18,1.78] | 0.55[0.16,1.86] | ‐ | ‐ | ‐ | ‐ | |
| Cefixime | 0.78[0.32,1.90] | 0.87[0.28,2.76] | 0.85[0.33,2.14] | 0.80[0.28,2.31] | 1.47[0.35,6.11] | ‐ | ‐ | ‐ | |
| Meropenem + daptomycin | 1.29[0.43,3.92] | 1.43[0.39,5.38] | 1.38[0.48,4.08] | 1.32[0.52,3.51] | 2.40[0.51,11.53] | 1.64[0.40,6.83] | ‐ | ‐ | |
| Ofloxacin | 1.12[0.45,2.70] | 1.24[0.76,2.06] | 1.21[0.48,2.92] | 1.15[0.41,3.23] | 2.08[0.79,5.66] | 1.43[0.40,5.03] | 0.87[0.21,3.53] | ‐ | |
| Other decompensation | Ceftriaxone | Cefotaxime | Ciprofloxacin | Ceftazidime | Amikacin | Meropenem + daptomycin | Ofloxacin | ‐ | |
| Ceftriaxone | ‐ | 1.21[0.43,3.48] | 1.01[0.31,3.13] | ‐ | ‐ | ‐ | ‐ | ||
| Cefotaxime | 1.22[0.43,3.53] | ‐ | 0.82[0.26,2.40] | ‐ | 1.05[0.11,10.05] | ‐ | 0.92[0.43,1.95] | ||
| Ciprofloxacin | 1.01[0.32,3.16] | 0.83[0.27,2.46] | ‐ | 1.40[0.78,2.51] | ‐ | ‐ | ‐ | ||
| Ceftazidime | 1.43[0.40,5.19] | 1.18[0.33,3.97] | 1.42[0.81,2.50] | ‐ | ‐ | 1.52[0.72,3.32] | ‐ | ||
| Amikacin | 1.28[0.11,15.66] | 1.05[0.11,10.27] | 1.27[0.10,15.24] | 0.89[0.07,11.72] | ‐ | ‐ | ‐ | ||
| Meropenem + daptomycin | 2.19[0.49,9.49] | 1.81[0.41,7.57] | 2.17[0.85,5.64] | 1.53[0.73,3.31] | 1.72[0.12,24.31] | ‐ | ‐ | ||
| Ofloxacin | 1.12[0.31,4.09] | 0.92[0.43,1.96] | 1.10[0.30,4.24] | 0.78[0.19,3.35] | 0.88[0.08,9.51] | 0.51[0.10,2.63] | ‐ | ||
| Length of hospital stay | Cefotaxime | Amikacin | Ofloxacin | ‐ | |||||
| Cefotaxime | ‐ | 1.01[‐4.47,6.41] | ‐0.99[‐4.07,2.09] | ||||||
| Amikacin | 1.01[‐4.43,6.47] | ‐ | ‐ | ||||||
| Ofloxacin | ‐0.99[‐4.02,2.02] | ‐2.03[‐8.30,4.25] | ‐ | ||||||
Abbreviations: SBP: spontaneous bacterial peritonitis
The table provides the effect estimates (proportion of people with any adverse events; hazard ratio for all‐cause mortality and spontaneous resolution for SBP; rate ratio for other decompensation; and mean difference in days for length of hospital stay) of each pairwise comparison for the different outcomes. The top half of the table indicates the effect estimates from the direct comparisons. The bottom half of the table indicates the effect estimates from the network meta‐analysis. For network meta‐analysis, to identify the effect estimate of a comparison, say A versus B, look at the cell that occupies the row corresponding to intervention A and the column corresponding to intervention B for the direct effect estimate. If that cell is empty (indicated by a '‐'), look at the row corresponding to intervention B and the column corresponding to intervention A. Take the inverse of this number (i.e. 1/number) to arrive at the treatment effect of A versus B. For direct comparisons, this is exactly the opposite; look at the cell that occupies the column corresponding to intervention A and the row corresponding to intervention B for the direct effect estimate. If that cell is empty, look at the column corresponding to intervention B and the row corresponding to intervention A. Take the inverse of this number to arrive at the treatment effect of A versus B. If the cell corresponding to B versus A is also missing in direct comparisons, this means that there was no direct comparison.
There were no significant differences in the effect estimates for any of the comparisons in any of the outcomes for which network meta‐analysis could be performed.
Pefloxacin versus ampicillin plus gentamycin: HR 0.80 (95% CrI 0.02 to 30.66).
Imipenem versus cefepime: HR 0.98 (95% CrI 0.60 to 1.57).
Health‐related quality of life
None of the trials reported health‐related quality of life.
Serious adverse events
One trial (31 participants) reported the proportion of participants with serious adverse events (Piano 2016). It was not clear whether the authors used the ICH‐GCP definition of serious adverse events (ICH‐GCP 1997). There was no significant difference in the proportion of people with serious adverse events between meropenem plus daptomycin versus ceftazidime: odds ratio (OR) 1.51 (95% credible interval (CrI) 0.35 to 6.71). None of the trials reported the number of serious adverse events per participant.
Any adverse event
Five trials (297 participants) reported the proportion of participants with any adverse event (Gomez‐Jimenez 1993; Tuncer 2003; Chen 2005; Angeli 2006; Piano 2016). It was not clear whether the authors used the ICH‐GCP definition of adverse events (ICH‐GCP 1997). A total of seven interventions were compared with each other. All the interventions were connected (Figure 1). Only one trial compared each pairwise comparison. The fixed‐effect model was used. There were no significant differences in the proportion of people who developed any adverse event between any of the interventions (Table 6).
Two trials (291 participants) reported the number of any adverse events (Angeli 2006; Jindal 2016). It was not clear whether the authors used the ICH‐GCP definition of adverse events (ICH‐GCP 1997). The two trials compared two different pairs of interventions. Therefore, network meta‐analysis was not appropriate. There were no significant differences in the number of adverse events per participant in the following comparisons.
Ceftazdime versus ciprofloxacin: rate ratio 1.39 (95% CrI 0.79 to 2.48).
Imipenem versus cefepime: rate ratio 1.00 (95% CrI 0.83 to 1.20).
Liver transplantation
One trial (31 participants) reported the proportion of participants requiring liver transplantation (Piano 2016). There was no significant difference in the proportion of people who underwent liver transplantation between meropenem plus daptomycin versus ceftazidime: hazard ratio (HR) 1.77 (95% CrI 0.26 to 15.21).
Resolution of SBP
None of the trials reported resolution of SBP, defined as symptomatic recovery from SBP. Nine trials (873 participants) reported recovery from SBP, as per definitions used for SBP (Gomez‐Jimenez 1993; Navasa 1996; Figueiredo 1997; Tuncer 2003; Chen 2005; Angeli 2006; Ahmed Ather Ch 2014; Jindal 2016; Piano 2016). We compared a total of 11 interventions with each other. There were eight connected interventions (Figure 1), and 3 unconnected interventions. Of these, we excluded one comparison because both interventions in the comparison were unconnected. We excluded one intervention as it was connected to the network solely by one trial in which all participants had resolution of SBP. We included only one trial for each pairwise comparison, except for one pairwise comparison. There were no significant differences in the proportion of people in whom resolution of SBP could be achieved (Table 6).
There was no significant difference in the resolution of SBP between imipenem versus cefepime: HR 0.72 (95% CrI 0.45 to 1.12). We could not obtain convergence for the other comparison involving cefonicid versus ceftriaxone, in which all 30 participants in the ceftriaxone group had resolution of SBP and 28/30 (93.3%) of the cefonicid group had resolution of SBP.
Other features of decompensation
Six trials (535 participants) reported other features of decompensation (Navasa 1996; Tuncer 2003; Chen 2005; Angeli 2006; Jindal 2016; Piano 2016). None of the trials reported the number of people who developed decompensation; only the number of decompensation events in each group was reported. A total of nine interventions were compared with each other. There were seven connected interventions (Figure 1), and two unconnected interventions. Only one trial was included for each pairwise comparison. There were no significant differences in the number of other decompensation events between any of the interventions included in the network meta‐analysis (Table 6). There was also no significant difference in the number of other decompensation events between the unconnected interventions (imipenem versus cefepime): rate ratio 0.97 (95% CrI 0.75 to 1.25).
Length of hospital stay
Two trials (160 participants) reported length of hospital stay (Navasa 1996; Chen 2005). A total of three interventions were compared with each other. All the interventions were connected (Figure 1). Only one trial was included in each pairwise comparison. There were no significant differences in the length of hospital stay between any of the interventions (Table 6).
Number of work days lost
None of the trials reported this outcome.
Treatment costs
None of the trials reported total treatment costs. Two trials (91 participants) reported antibiotic costs for the regimen used (Figueiredo 1997; Tuncer 2003). The standard deviation of the costs were not reported and could not be calculated from other data available in either trial.
In one trial, oral cefixime was compared with intravenous ceftriaxone (Figueiredo 1997). The non‐inflated costs of antibiotic treatment were BRL 62 for the oral cefixime group compared to BRL 2160 for the intravenous ceftriaxone group (Figueiredo 1997). In the second trial, oral ciprofloxacin was compared with intravenous cefotaxime and intravenous ceftriaxone. The non‐inflated costs of antibiotic treatment were USD 6.61 for oral ciprofloxacin, USD 127 for intravenous cefotaxime, and USD 118 for intravenous ceftriaxone groups (Tuncer 2003).
Subgroup analysis
We did not perform any of the planned subgroup analysis because of sparse data in the trials, short follow‐up in all trials, and the unclear or high risk of bias for each of the outcomes in all trials.
Sensitivity analysis
The scenario analysis we performed for post‐randomisation dropouts for binary outcomes and time‐to‐event outcomes (where binomial likelihood was used). None of these revealed any alterations in the results. We did not impute the standard deviation for any continuous outcome.
Assessment of reporting biases
We were unable to perform the comparison‐adjusted funnel plot because there was no meaningful way in which to rank the studies (i.e. there was no specific change in the risk of bias in the studies, sample size, or the control group used over time).
Discussion
Summary of main results
We performed a systematic review and network meta‐analysis of all the antibiotic treatments for spontaneous bacterial peritonitis (SBP) in people with decompensated liver cirrhosis. We included a total of 12 trials, with a total of 1272 participants in this review. In the four trials that reported the isolated organisms, the most common organism isolated was Eschericia coli (E coli) (18.6% to 56.7%) (Gomez‐Jimenez 1993; Navasa 1996; Chen 2005; Angeli 2006). We compared a total of 13 interventions in these trials. A total of 10 trials, including 893 participants were included for one or more outcomes of this review (Gomez‐Jimenez 1993; Navasa 1996; Figueiredo 1997; Rastegar 1998; Tuncer 2003; Chen 2005; Angeli 2006; Ahmed Ather Ch 2014; Jindal 2016; Piano 2016). Only one outcome, resolution of SBP, featured more than one trial for the same comparison.
Approximately 75% of participants with SBP had resolution of SBP, and 25% of participants died from SBP. Overall, there was no evidence of differences for any of the primary or secondary outcomes of this review. However, it should be noted that the data were sparse and most of the comparisons involved a single trial. Therefore, clinically important differences in the outcomes between the interventions are possible.
Future trials can and should be powered on short‐term all‐cause mortality. Based on the probability ranks, it is not clear which interventions should be compared in future trials. Intravenous ceftriaxone and cefotaxime were the commonest interventions used in the trials. The sample size required in such trials based on a control group proportion of 25% (weighted median control group proportion in ceftriaxone in the trials), a relative risk reduction of all‐cause mortality of 20% in the experimental group, type I error of 5%, and type II error of 20% is 2188 participants. In such a trial, health‐related quality of life, and adverse events (due to any cause: disease‐related, treatment‐related, or comorbidity‐related) should be assessed as outcomes. A short period of follow‐up of 90 days may be sufficient to determine the effectiveness of an intervention.
Overall completeness and applicability of evidence
The trials included a wide variety of patients with SBP. There did not seem to be any restriction based on the aetiology or the presence of other features of decompensation in the trials that provided this information. Therefore, the results of the study are applicable in all people with cirrhosis and SBP. We excluded trials in which participants had undergone liver transplantation. Therefore, the findings of this review are not applicable in people with liver decompensation after liver transplantation.
Certainty of the evidence
The overall certainty of evidence was very low. One of the main reasons for the very low‐certainty evidence was the unclear or high risk of bias in many of the trials. It is possible to perform trials at low risk of bias in the field. To perform a trial at low risk of bias, randomisation can be performed using standard methods, for example, web‐based central randomisation; blinding can be achieved by using a double placebo design (i.e. a placebo for intervention and a placebo for control); an intention‐to‐treat analysis can be performed; and a protocol can be published prior to recruitment. None of these have any major ethical considerations; therefore, a low risk of bias trial is very much feasible.
Another major reason for very low‐certainty evidence is imprecision: the trials had small sample sizes and the credible intervals (CrIs) overlapped clinically significant benefits and clinically significant harms for all comparisons. Therefore, future trials should be adequately powered with sample sizes, as described in the previous section.
Heterogeneity could not be measured because of the presence of a single trial for most comparisons. We used clinical outcomes; therefore, there is no issue of indirectness due to outcomes. The direct comparisons and network meta‐analysis results did not result in altered conclusions and the indirect evidence was applicable only in very few comparisons because of the nature of the network. In the comparisons for which indirect evidence was available, the effect estimates were similar to that of direct comparisons. There was no evidence that the patient selection or methodological differences were systematically different across comparisons (i.e. there was no concern regarding the transitivity assumption). Therefore, there is no concern about indirectness of evidence. There was no meaningful way to rank these studies (i.e. there was no specific change in the risk of bias in the studies, sample size, or the control group used over time); we have completed a thorough search for studies on effectiveness. We found no evidence of publication bias.
Potential biases in the review process
We selected a range of databases to search without using any language restrictions and conducted the network meta‐analysis according to NICE DSU guidance (Dias 2016). In addition, we have analysed data using the fixed‐effect and random‐effects model, although we used the fixed‐effect model for all the outcomes for the reasons described above. These are the strengths of the review process.
We have excluded studies that compared variations in duration or dose in the different interventions. Hence, this review does not provide information on whether one variation is better than another. Another major limitation of this review was the paucity of data. Few trials were included for each comparison; in many comparisons, only one trial was included. This makes it difficult to assess whether the effect estimates are reproducible. This paucity of data decreases the confidence in the results.
All of the network meta‐analyses included only sparse data from trials at high risk of bias. We were able to compare the direct and indirect estimates for very few comparisons. However, the potential effect modifiers in the trials that reported them were broadly similar across comparisons. The results of direct comparisons and indirect comparisons were also similar, when applicable. However, one cannot rule out violation of the transitivity assumption because of the sparse data; potential differences in the cointerventions, and potential differences in the definitions used by trial authors for adverse events and serious adverse events.
We included only randomised clinical trials which are known to focus mostly on benefits and do not collect and report harms in a detailed manner. Therefore, it is possible that we missed a large number of non‐randomised studies addressing the reporting of harms. A significant effort is required to identify the non‐randomised studies and assess the risk of bias in those studies. Since it is possible to conduct future studies powered on mortality, a systematic review on adverse events appears to be unnecessary in a superiority trial (as a treatment that reduces short‐term mortality will be used even if it increases the adverse events).
Agreements and disagreements with other studies or reviews
This is the first network meta‐analysis on the topic. The only systematic review on this topic compared third‐generation cephalosporins with other empirical broad‐spectrum antibiotics in patients with nosocomial SBP (Fiore 2018). This study included eight non‐randomised studies and concluded that there was higher prevalence of antibiotic resistance to third‐generation cephalosporins than empirical broad‐spectrum antibiotics in patients with nosocomial SBP (Fiore 2018). Only one of the included studies in this review included solely people with nosocomial SBP (Piano 2016). There was no evidence of a difference in any of the outcomes between the meropenem plus daptomycin versus ceftazidime group in this trial. However, this was a small trial and included only 31 participants, indicating that clinically important benefits or harms are possible in this comparison. Therefore, our conclusions are that there is significant uncertainty about whether broad‐spectrum antibiotics (meropenem plus daptomycin) are better than third‐generation cephalosporins (ceftazidime) in the treatment of nosocomial SBP.
Authors' conclusions
Implications for practice.
Short‐term mortality after spontaneous bacterial peritonitis (SBP) is high (around 25%). There is significant uncertainty about which antibiotic therapy is better in people with SBP. The majority of the randomised clinical trials used third‐generation cephalosporins, such as intravenous ceftriaxone or cefotaxime as one of the interventions.
Implications for research.
Further well‐designed randomised clinical trials are necessary. Some aspects of the design of the randomised clinical trials should be as follows.
-
Study design
Placebo‐controlled, parallel, randomised clinical trial
-
Participants
People with cirrhosis and SBP
-
Intervention
Not identified from this network meta‐analysis. This could be one of the newer broad‐spectrum antibiotics (or combination of antibiotics) or oral third‐generation cephalosporins or oral ciprofloxacin.
-
Control
Intravenous ceftriaxone or cefotaxime until at least resolution of SBP or availability of culture results.
-
Outcomes
Primary outcome: short‐term mortality (90‐day all‐cause mortality)
Secondary outcomes: health‐related quality of life, adverse events, resolution of SBP, and resource utilisation measures including length of hospital stay
Minimum length of follow‐up: 90 days
Sample size: please see Discussion.
Trials need to be conducted and reported according to the SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) statement (Chan 2013), and CONSORT statement (Schulz 2010).
Notes
The methods section of this protocol is based on a standard Cochrane Hepato‐Biliary Group template incorporating advice by the Complex Reviews Support Unit for a network meta‐analysis protocol (Best 2018).
Acknowledgements
We acknowledge the help and support of the Cochrane Hepato‐Biliary Group, Cochrane Editorial Unit, and the copy editors. The authors would also like to thank the people listed below who provided comments to improve the protocol and the review.
Peer reviewers of protocol: Anastasios Koulaouzidis, UK; Rohit Sinha, UK Peer reviewers of review: Kannan Sridharan, Bahrain; Jose Lopez‐Lopez, UK Contact Editor of protocol and review: Christian Gluud, Denmark Sign‐off Editor of protocol and review: Christian Gluud, Denmark
Cochrane Abdomen and Endocrine Network Editor of the review: Liz Bickerdike, UK.
Cochrane Review Group funding acknowledgement: the Danish State is the largest single funder of the Cochrane Hepato‐Biliary Group through its investment in The Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Copenhagen University Hospital, Denmark.
This project was funded by the National Institute for Health Research Systematic Reviews Programme (project number 16/114/17) and was supported by the Complex Reviews Support Unit, also funded by the National Institute for Health Research (project number 14/178/29).
Department of Health disclaimer
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the 16/114/17 or 14/178/29 Programme, NIHR, NHS, or the Department of Health.
Danish State and The Copenhagen Trial Unit disclaimer
The views and opinions expressed in this review are those of the authors and do not necessarily reflect those of the Danish State or The Copenhagen Trial Unit.
Appendices
Appendix 1. Search strategies
| Database | Time span | Search strategy |
| Central Register of Controlled Trials (CENTRAL) in the Cochrane Library | Issue 11, 2018 | #1 (spontaneous near/3 bacterial near/3 peritonitis) #2 MeSH descriptor: [Liver Cirrhosis] explode all trees #3 ((hepatic or liver) and (fibrosis or cirrhosis or cirrhotic)) #4 #2 or #3 #5 #1 and #4 |
| MEDLINE Ovid | January 1947 to November 2018 | 1. (spontaneous adj3 bacterial adj3 peritonitis).ti,ab. 2. exp Liver Cirrhosis/ 3. ((hepatic or liver) and (fibrosis or cirrhosis or cirrhotic)).ti,ab. 4. 2 or 3 5. 1 and 4 6. randomized controlled trial.pt. 7. controlled clinical trial.pt. 8. randomized.ab. 9. placebo.ab. 10. drug therapy.fs. 11. randomly.ab. 12. trial.ab. 13. groups.ab. 14. 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 15. exp animals/ not humans.sh. 16. 14 not 15 17. 5 and 16 |
| Embase Ovid | January 1974 to November 2018 | 1. exp bacterial peritonitis/ 2. (spontaneous adj3 bacterial adj3 peritonitis).ti,ab. 3. 1 or 2 4. exp liver cirrhosis/ 5. ((hepatic or liver) and (fibrosis or cirrhosis or cirrhotic)).ti,ab. 6. 4 or 5 7. 3 and 6 8. exp crossover‐procedure/ or exp double‐blind procedure/ or exp randomized controlled trial/ or single‐blind procedure/ 9. (((((random* or factorial* or crossover* or cross over* or cross‐over* or placebo* or double*) adj blind*) or single*) adj blind*) or assign* or allocat* or volunteer*).af. 10. 8 or 9 11. 7 and 10 |
| Science Citation Index Expanded (Web of Science) | January 1945 to November 2018 | #1 TS=(spontaneous near/3 bacterial near/3 peritonitis)
#2 TS=((hepatic or liver) and (fibrosis or cirrhosis or cirrhotic)) #3 TS=(random* OR rct* OR crossover OR masked OR blind* OR placebo* OR meta‐analysis OR systematic review* OR meta‐analys*) #4 #1 AND #2 AND #3 |
| World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch/Default.aspx) | November 2018 | spontaneous bacterial peritonitis |
| ClinicalTrials.gov | November 2018 | cirrhosis | Interventional Studies | Spontaneous Bacterial Peritonitis | Phase 2, 3, 4 |
| European Medical Agency (www.ema.europa.eu/ema/) and US Food and Drug Administration (www.fda.gov) | November 2018 | spontaneous bacterial peritonitis |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abd‐Elsalam 2016.
| Methods | Randomised clinical trial | |
| Participants | Country: Egypt Number randomised: not stated Post‐randomisation dropouts: not stated Revised sample size: not stated Average age: not stated Females: not stated Presence of other features of decompensation (hepatorenal syndrome, hepatic encephalopathy, or variceal bleeding): not stated Alcohol‐related cirrhosis: not stated Viral‐related cirrhosis: not stated Autoimmune disease‐related cirrhosis (e.g. PSC, PBC, AIH): not stated Other causes for cirrhosis: not stated Treated for ascites in addition to antibiotics (e.g. albumin or diuretics): not stated Follow‐up in months: 0.25 Years of recruitment: 2014 Inclusion criteria
|
|
| Interventions | Participants were randomly assigned to two groups Group 1: cefotaxime (n = not stated) Further details: cefotaxime 2 gm twice/day (route and duration not stated clearly, but appears to be 5 days or 1 week) Group 2: ceftriaxone (n = not stated) Further details: ceftriaxone 2 gm once/day (route duration not stated clearly, but appears to be 5 days or 1 week) Total number of participants and number of participants in each group was not reported | |
| Outcomes | None of the outcomes of interest were reported. | |
| Notes | Attempts were made to contact the authors in December 2018; there were no replies. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: a prepublished protocol was not available, but the authors do not report routinely measured clinical outcomes adequately. |
| Other bias | Low risk | Comment: no other bias was noted. |
Ahmed Ather Ch 2014.
| Methods | Randomised clinical trial | |
| Participants | Country: Pakistan Number randomised: 240 Post‐randomisation dropouts: not stated Revised sample size: 240 Average age: 44 years Females: 67 (27.9%). Presence of other features of decompensation (hepatorenal syndrome, hepatic encephalopathy, or variceal bleeding): not stated Alcohol‐related cirrhosis: not stated Viral‐related cirrhosis: not stated Autoimmune disease‐related cirrhosis (e.g. PSC, PBC, AIH): not stated Other causes for cirrhosis: not stated Treated for ascites in addition to antibiotics (e.g. albumin or diuretics): not stated Follow‐up in months: 0.25 Years of recruitment: 2011 Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to two groups Group 1: ciprofloxacin (n = 120) Further details: ciprofloxacin 200 mg IV twice/day for 5 days Group 2: ceftriaxone (n = 120) Further details: ceftriaxone 1 gm IV twice/day for 5 days | |
| Outcomes | Outcomes reported
|
|
| Notes | Attempts were made to contact the authors in December 2018; there were no replies. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly allocated in two groups using random number table". |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: a prepublished protocol was not available, but the authors do not report routinely measured clinical outcomes adequately. |
| Other bias | Low risk | Comment: no other bias was noted. |
Angeli 2006.
| Methods | Randomised clinical trial | |
| Participants | Country: Italy Number randomised: 116 Post‐randomisation dropouts: 0 Revised sample size: 116 Average age: 60 years Females: 48 (41.4%) Presence of other features of decompensation (hepatorenal syndrome, hepatic encephalopathy, or variceal bleeding): not stated Alcohol‐related cirrhosis: both Viral‐related cirrhosis: both Autoimmune disease‐related cirrhosis (e.g. PSC, PBC, AIH): not stated Other causes for cirrhosis: both Treated for ascites in addition to antibiotics (e.g. albumin or diuretics): both Follow‐up in months: 3 Years of recruitment: not stated Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to two groups. Group 1: ceftazidime (n = 55) Further details: ceftazidime 1 g to 2 g once/day or twice/day depending on creatinine levels for 8 days Group 2: ciprofloxacin (n = 61) Further details: ciprofloxacin 200 mg IV once/day or twice/day depending on serum creatinine converted to oral ciprofloxacin 250 mg twice/day to 500 mg twice/day depending on serum creatinine when possible for a total of 8 days | |
| Outcomes | Outcomes reported
|
|
| Notes | Attempts were made to contact the authors in December 2018; there were no replies. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was performed with sealed envelopes containing treatment options prepared with random numbers generated by the STATISTICA 6.1 software." |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was performed with sealed envelopes containing treatment options prepared with random numbers generated by the STATISTICA 6.1 software." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: a prepublished protocol was not available but the authors report routinely measured clinical outcomes adequately. |
| Other bias | Low risk | Comment: no other bias was noted. |
Chen 2005.
| Methods | Randomised clinical trial | |
| Participants | Country: China Number randomised: 45 Post‐randomisation dropouts: 8 Revised sample size: 37 Average age: 56 years Females: 9 (24.3%) Presence of other features of decompensation (hepatorenal syndrome, hepatic encephalopathy, or variceal bleeding): not stated Alcohol‐related cirrhosis: both Viral‐related cirrhosis: both Autoimmune disease‐related cirrhosis (e.g. PSC, PBC, AIH): not stated Other causes for cirrhosis: both Treated for ascites in addition to antibiotics (e.g. albumin or diuretics): not stated Follow‐up in months: 1 Years of recruitment: 2000‐2002 Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to two groups. Group 1: cefotaxime (n = 19) Further details: cefotaxime 1 g IV three times/day for minimum 5 day Group 2: amikacin (n = 18) Further details: amikacin with plasma level maintained at <= 30 mg/dL after a loading dose of 500 mg or 8 mg/Kg depending on weight for a total duration of minimum 5 days | |
| Outcomes | Outcomes reported
|
|
| Notes | Attempts were made to contact the authors in December 2018; there were no replies. Reason for post‐randomisation dropouts: other causes of peritonitis, death, discharged against medical advice before starting treatment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were post‐randomisation dropouts which were related to the outcomes. |
| Selective reporting (reporting bias) | High risk | Comment: a prepublished protocol was not available but the authors do not report routinely measured clinical outcomes adequately |
| Other bias | Low risk | Comment: no other bias was noted. |
Figueiredo 1997.
| Methods | Randomised clinical trial | |
| Participants | Country: Brazil Number randomised: 38 Post‐randomisation dropouts: 0 Revised sample size: 38 Average age: 54 years Females: 16 (42.1%) Presence of other features of decompensation (hepatorenal syndrome, hepatic encephalopathy, or variceal bleeding): not stated Alcohol‐related cirrhosis: not stated Viral‐related cirrhosis: not stated Autoimmune disease‐related cirrhosis (e.g. PSC, PBC, AIH): not stated Other causes for cirrhosis: not stated Treated for ascites in addition to antibiotics (e.g. albumin or diuretics): yes Follow‐up in months: 0.25 Years of recruitment: not stated Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to two groups. Group 1: cefixime (n = 20) Further details: cefixime 400 mg/day oral until 2 days after resolution of signs/symptoms/polymorphonuclear count < 250/mm3 Group 2: ceftriaxone (n = 18) Further details: ceftriaxone 1g IV twice/day until 2 days after resolution of signs/symptoms/polymorphonuclear count < 250/mm3 | |
| Outcomes | Outcomes reported
|
|
| Notes | We were unable to locate the current contact details of the author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Sealed envelope method" Comment: further details were not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | High risk | Comment: prepublished protocol was not available but the authors do not report routinely measured clinical outcomes adequately. |
| Other bias | Low risk | Comment: no other bias was noted. |
Gomez‐Jimenez 1993.
| Methods | Open randomised clinical trial | |
| Participants | Country: Spain Number randomised: 60 Post‐randomisation dropouts: not stated Revised sample size: 60 Average age: 59 years Females: 13 (21.7%) Presence of other features of decompensation (hepatorenal syndrome, hepatic encephalopathy, or variceal bleeding): not stated Alcohol‐related cirrhosis: both Viral‐related cirrhosis: not stated Autoimmune disease‐related cirrhosis (e.g. PSC, PBC, AIH): not stated Other causes for cirrhosis: both Treated for ascites in addition to antibiotics (for example albumin or diuretics): not stated Follow‐up in months: 0.5 Years of recruitment: 1987‐1990 Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to two groups. Group 1: cefonicid (n = 30) Further details: cefonicid 2g IV twice/day for 10 or 4 days after becoming afebrile, whichever was shortest. Group 2: ceftriaxone (n = 30) Further details: ceftriaxone 2g IV once/day for 10 or 4 days after becoming afebrile, whichever was shortest. | |
| Outcomes | Outcomes reported
|
|
| Notes | We were unable to locate the current contact details of the author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: open clinical trial |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: open clinical trial |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | Low risk | Comment: a prepublished protocol was not available but the authors report routinely measured clinical outcomes adequately. |
| Other bias | Low risk | Comment: no other bias was noted. |
Jindal 2016.
| Methods | Open randomised clinical trial | |
| Participants | Country: India Number randomised: 175 Post‐randomisation dropouts: 0 Revised sample size: 175 Average age: 49 years Females: 32 (18.3%) Presence of other features of decompensation (hepatorenal syndrome, hepatic encephalopathy, or variceal bleeding): both Alcohol‐related cirrhosis: both Viral‐related cirrhosis: both Autoimmune disease‐related cirrhosis (e.g. PSC, PBC, AIH): not stated Other causes for cirrhosis: both Treated for ascites in addition to antibiotics (e.g. albumin or diuretics): not stated Follow‐up in months: 3 Years of recruitment: 2012‐2014 Inclusion criteria (if at least one of the following conditions is met)
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to two groups. Group 1: cefepime (n = 88) Further details: cefepime 2 g IV twice/day for 5 days Group 2: imipenem (n = 87) Further details: imipenem 1 g IV three times/day for 5 days | |
| Outcomes | Outcomes reported
|
|
| Notes | Attempts were made to contact the authors in December 2018; there were no replies. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: open clinical trial |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: open clinical trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: a prepublished protocol was not available but the authors report routinely measured clinical outcomes adequately. |
| Other bias | Low risk | Comment: no other bias was noted. |
Navasa 1996.
| Methods | Randomised clinical trial | |
| Participants | Country: Spain Number randomised: 132 Post‐randomisation dropouts: 9 Revised sample size: 123 Average age: 59 years Females: 44 (35.8%) Presence of other features of decompensation (hepatorenal syndrome, hepatic encephalopathy, or variceal bleeding): both Alcohol‐related cirrhosis: both Viral‐related cirrhosis: not stated Autoimmune disease‐related cirrhosis (e.g. PSC, PBC, AIH): not stated Other causes for cirrhosis: both Treated for ascites in addition to antibiotics (e.g. albumin or diuretics): not stated Follow‐up in months: 0.5 Years of recruitment: 1992‐1994 Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to two groups. Group 1: ofloxacin (n = 64) Further details: ofloxacin 100 mg/day to 800 mg/day depending upon creatinine levels (4 days to 2 weeks) Group 2: cefotaxime (n = 59) Further details: cefotaxime 1 g/day to 8 g/day depending upon creatinine levels (4 days to 2 weeks) | |
| Outcomes | Outcomes reported
|
|
| Notes | We were unable to locate the current contact details of the author. Reason for post‐randomisation dropouts: secondary peritonitis, voluntary dropout |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed with sealed envelopes containing the treatment options prepared with random numbers generated by the SAS statistical package (SAS Institute Inc., Cary, NC)". |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed with sealed envelopes containing the treatment options prepared with random numbers generated by the SAS statistical package (SAS Institute Inc., Cary, NC)". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: there were post‐randomisation dropouts; it is not clear whether this was related to treatment and/or outcomes. |
| Selective reporting (reporting bias) | Low risk | Comment: a prepublished protocol was not available but the authors report routinely measured clinical outcomes adequately. |
| Other bias | Low risk | Comment: no other bias was noted. |
Piano 2016.
| Methods | Randomised clinical trial | |
| Participants | Country: Italy Number randomised: 32 Post‐randomisation dropouts: 1 Revised sample size: 31 Average age: 60 years Females: 12 (38.7%) Presence of other features of decompensation (hepatorenal syndrome, hepatic encephalopathy, or variceal bleeding): not stated Alcohol‐related cirrhosis: both Viral‐related cirrhosis: both Autoimmune disease‐related cirrhosis (e.g. PSC, PBC, AIH): not stated Other causes for cirrhosis: both Treated for ascites in addition to antibiotics (e.g. albumin or diuretics): not stated Follow‐up in months: 3 Years of recruitment: 2011‐2014 Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to two groups. Group 1: ceftazidime (n = 16) Further details: ceftazidime 2 g/day to 6 g/day depending on glomerular filtration rate for at least 7 days Group 2: meropenem + daptomycin (n = 15) Further details: meropenem 0.5 g to 3 g/day plus daptomycin 3 mg/Kg/day to 6 mg/Kg/day depending on glomerular filtration rate for at least 7 days | |
| Outcomes | Outcomes reported
|
|
| Notes | Attempts were made to contact the authors in December 2018; there were no replies. Reason for post‐randomisation dropout: secondary peritonitis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed using consecutively numbered, computer‐generated, sealed, opaque envelopes containing the treatment assigned." |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed using consecutively numbered, computer‐generated, sealed, opaque envelopes containing the treatment assigned." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Independent laboratory examiners, blinded to assigned treatment, manually assessed the ascitic fluid PMN count". Comment: it is not clear whether patients and healthcare providers were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Independent laboratory examiners, blinded to assigned treatment, manually assessed the ascitic fluid PMN count". Comment: it is not clear whether patients and healthcare providers were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: there were post‐randomisation dropouts; it is not clear whether this was related to treatment and/or outcomes. |
| Selective reporting (reporting bias) | Low risk | Comment: a prepublished protocol was not available but the authors report routinely measured clinical outcomes adequately. |
| Other bias | Low risk | Comment: no other bias was noted. |
Rastegar 1998.
| Methods | Randomised clinical trial | |
| Participants | Country: Iran Number randomised: 20 Post‐randomisation dropouts: not stated Revised sample size: 20 Average age: 42 years Females: 5 (25%) Presence of other features of decompensation (hepatorenal syndrome, hepatic encephalopathy, or variceal bleeding): not stated Alcohol‐related cirrhosis: not stated Viral‐related cirrhosis: both Autoimmune disease‐related cirrhosis (e.g. PSC, PBC, AIH): not stated Other causes for cirrhosis: both Treated for ascites in addition to antibiotics (e.g. albumin or diuretics): not stated Follow‐up in months: 0.25 Years of recruitment: not stated Inclusion and exclusion criteria: not stated |
|
| Interventions | Participants were randomly assigned to two groups. Group 1: ampicillin + gentamycin (n = 9) Further details: ampicillin IV 4 g/day plus gentamycin 40 to 60 mg IV three times/day for 10 to 14 days Group 2: pefloxacin (n = 11) Further details: pefloxacin 400 mg/36 hours for 7 to 10 days | |
| Outcomes | Outcomes reported
|
|
| Notes | We were unable to locate the current contact details of the author. Follow‐up information was not available, but the follow‐up was probably until the end of hospitalisation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: a prepublished protocol was not available but the authors do not report routinely measured clinical outcomes adequately. |
| Other bias | Low risk | Comment: no other bias was noted. |
Tuncer 2003.
| Methods | Randomised clinical trial | |
| Participants | Country: Turkey Number randomised: 53 Post‐randomisation dropouts: 0 Revised sample size: 53 Average age: 46 years Females: 19 (35.8%) Presence of other features of decompensation (hepatorenal syndrome, hepatic encephalopathy, or variceal bleeding): both Alcohol‐related cirrhosis: not stated Viral‐related cirrhosis: both Autoimmune disease‐related cirrhosis (e.g. PSC, PBC, AIH): not stated Other causes for cirrhosis: both Treated for ascites in addition to antibiotics (e.g. albumin or diuretics): not stated Follow‐up in months: 0.25 Years of recruitment: not stated Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to three groups.
Group 1: cirpofloxacin (n = 16)
Further details: ciprofloxacin 500 mg oral twice/day for 5 days
Group 2: cefotaxime (n = 18)
Further details: cefotaxime 2 g IV three times/day for 5 days Group 3: ceftriaxone (n = 19) Further details: ceftriaxone 2 g/day IV for 5 days |
|
| Outcomes | Outcome reported
|
|
| Notes | Attempts were made to contact the authors in December 2018; there were no replies. Follow‐up information was not available but the follow‐up was probably until the end of hospitalisation. Although the authors excluded 3 patients from the analysis, these have been included for all outcomes other than complications and decompensated cirrhosis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all patients were included in the analysis of mortality and SBP resolution. There were post‐randomisation dropouts related to the treatment and outcome for remaining outcomes; therefore risk of bias is high for these outcomes. |
| Selective reporting (reporting bias) | Low risk | Comment: a prepublished protocol was not available but the authors report routinely measured clinical outcomes adequately. |
| Other bias | Low risk | Comment: no other bias was noted. |
Yim 2017.
| Methods | Randomised clinical trial | |
| Participants | Country: South Korea Number randomised: 261 Post‐randomisation dropouts: not stated Revised sample size: 261 Average age: not stated Females: not stated Presence of other features of decompensation (hepatorenal syndrome, hepatic encephalopathy, or variceal bleeding): not stated Alcohol‐related cirrhosis: not stated Viral‐related cirrhosis: not stated Autoimmune disease‐related cirrhosis (e.g. PSC, PBC, AIH): not stated Other causes for cirrhosis: not stated Treated for ascites in addition to antibiotics (e.g. albumin or diuretics): not stated Follow‐up in months: 0.25 Years of recruitment: 2007‐2016 Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to three groups.
Group 1: ciprofloxacin (n = not stated)
Further details: (route and duration not stated)
Group 2: cefotaxime (n = not stated)
Further details: (route and duration not stated) Group 3: ceftriaxone (n = not stated) Further details: (route and duration not stated) Number of participants in each group not stated |
|
| Outcomes | None of the outcomes of interest were reported. | |
| Notes | Attempts were made to contact the authors in December 2018; there were no replies. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: this information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: this information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: this information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: a prepublished protocol was not available but the authors do not report routinely measured clinical outcomes adequately. |
| Other bias | Low risk | Comment: no other bias was noted. |
AIH: autoimmune hepatitis IV: intravenous PBC: primary biliary cholangitis PSC: primary sclerosing cholangitis SBP: spontaneous bacterial peritonitis
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ariza 1991 | Only one group was allowed to receive other antibiotics in addition to the randomised treatment. |
| Badawy 2013 | Randomisation was performed after matching to similar patients; the allocation of the second person can be predicted with 100% accuracy. |
| Felisart 1985 | It includes infections other than SBP and no separate data are available. |
| Grange 2004 | It includes infections other than SBP and no separate data are available. |
| Lim 2011 | This is a review. |
| Liu 2000 | This is not a randomised clinical trial. |
| McCue 1981 | This is a review. |
| Piano 2011 | This is a review. |
| Ricart 2000 | It includes infections other than SBP and no separate data are available. |
| Rimola 1984 | This is not a randomised clinical trial. |
| Taskiran 2004 | This is not a randomised clinical trial. |
SBP: spontaneous bacterial peritonitis
Characteristics of studies awaiting assessment [ordered by study ID]
Zafar 2018.
| Methods | Randomised clinical trial |
| Participants | People with cirrhosis and spontaneous bacterial peritonitis |
| Interventions | Oral antibiotics versus intravenous antibiotics |
| Outcomes | Mortality |
| Notes | It was not clear whether a single oral antibiotic was compared with intravenous antibiotic or a group of oral antibiotics was compared with a group of intravenous antibiotics or whether the same antibiotic was compared by oral and intravenous antibiotics. We made attempts to clarify this information from the authors in December 2018, but we did not receive any replies. |
Differences between protocol and review
We did not perform Trial Sequential Analysis (TSA), as the risk of false positive results with Bayesian meta‐analysis is probably less or at least equivalent to TSA.
We used the latest guidance from the GRADE Working group (Yepes‐Nunez 2019), rather than the previous guidance (Puhan 2014), for presenting the 'Summary of findings' table.
The trials did not report the proportion of people with other episodes of decompensation but reported the number of episodes of decompensation. Therefore, we treated this as a count outcome and used the Poisson likelihood to calculate the rate ratio.
We used ceftriaxone rather than cefotaxime as the control group, since ceftriaxone was the commonest control group in the trials.
In the absence of a protocol published prior to the start of the study, we have classified the risk of bias as low for selective reporting bias only when mortality, adverse events, and spontaneous bacterial peritonitis (SBP) were reported, as we anticipated these outcomes to be routinely measured in clinical trials of this nature.
We used 30,000 iterations as a minimum for burn‐in.
We did not present some information because of the concern about the misinterpretation of the results. We have highlighted this clearly within the text of the review along with the reasons for not presenting them.
Contributions of authors
Protocol
Conceiving the protocol: KG Designing the protocol: KG Co‐ordinating the protocol: KG Designing search strategies: KG Writing the protocol: KG Providing general advice on the protocol: ET, PW Securing funding for the protocol: KG Performing previous work that was the foundation of the current study: not applicable
Review
Co‐ordinating the review: KG Study selection: KG, LP, AB, MP, DR Data extraction: KG, LP Writing the review: KG with contribution of LP for characteristics and 'Risk of bias' tables Providing advice on the review: EJM, PW, AJS, NJC, NH, SF, DT, CSP, BRD, ET Securing funding for the review: KG
Sources of support
Internal sources
-
University College London, UK.
Writing equipment, software, etc.
External sources
-
National Institute for Health Research, UK.
Payment for writing reviews, writing equipment, software
Declarations of interest
None known for any of the authors.
New
References
References to studies included in this review
Abd‐Elsalam 2016 {published data only}
- Abd‐Elsalam SM, Soliman HH, Elkhalawany WA, Soliman SM, Awny SA, Khalil HS, et al. Is spontaneous bacterial peritonitis still responding to third generation cephalosporins?. Hepatology International 2016;10(1 Suppl):s489. [Google Scholar]
Ahmed Ather Ch 2014 {published data only}
- Ahmed Ather Ch A, Chaudhary S, Khan IM. Comparison of intravenous ciprofloxacin and ceftriaxone in the management of spontaneous bacterial peritonitis in cirrhosis of liver at Mayo hospital, Lahore. Pakistan Journal of Medical and Health Sciences 2014;8(1):82‐4. [Google Scholar]
Angeli 2006 {published data only}
- Angeli P, Guarda S, Fasolato S, Miola E, Craighero R, Piccolo F, et al. Switch therapy with ciprofloxacin vs. Intravenous ceftazidime in the treatment of spontaneous bacterial peritonitis in patients with cirrhosis: similar efficacy at lower cost. Alimentary Pharmacology & Therapeutics 2006;23(1):75‐84. [DOI] [PubMed] [Google Scholar]
Chen 2005 {published data only}
- Chen TA, Lo GH, Lai KH, Lin WJ. Single daily amikacin versus cefotaxime in the short‐course treatment of spontaneous bacterial peritonitis in cirrhotics. World Journal of Gastroenterology 2005;11(43):6823‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Figueiredo 1997 {published data only}
- Figueiredo FA, Coelho HS, Alvariz RG, Silva FD, Godinho F, Salgueiro E. Randomized trial comparing intravenous ceftriaxone with oral cefixime for treatment of spontaneous bacterial peritonitis (SBP) in cirrhotic patients: pilot study. United European Gastroenterology Week 1996:4 0103.
- Figueiredo FA, Coelho HS, Soares JA, Salgueiro E, Pinto CA. Oral cephalosporin for the treatment of non‐severe spontaneous bacterial peritonitis in liver disease: a prospective study of 38 cases. Gastrenterologia Endoscopia Digestiva 1997;16(6):231‐6. [Google Scholar]
Gomez‐Jimenez 1993 {published data only}
- Gomez‐Jimenez J, Ribera E, Gasser I, Artaza MA, Valle O, Pahissa A, et al. Randomized trial comparing ceftriaxone with cefonicid for treatment of spontaneous bacterial peritonitis in cirrhotic patients. Antimicrobial Agents and Chemotherapy 1993;37(8):1587‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jindal 2016 {published data only}
- Jindal A, Kumar M, Bhadoria AS, Maiwall R, Sarin SK. A randomized open label study of 'imipenem vs. cefepime' in spontaneous bacterial peritonitis. Liver International 2016;36(5):677‐87. [DOI] [PubMed] [Google Scholar]
- Jindal A, Maiwal R, Vashishta C, Kumar M, Kumar Chandan KN, Bhatia V, et al. A randomized, comparative open label study of imipenem versus cefepime in difficult to treat spontaneous bacterial peritonitis. Indian Journal of Gastroenterology 2014;33(1 Suppl 1):a22. [Google Scholar]
Navasa 1996 {published data only}
- Navasa M, Follo A, Llovet JM, Clemente G, Vargas V, Rimola A, et al. Randomized, comparative study of oral ofloxacin versus intravenous cefotaxime in spontaneous bacterial peritonitis. Gastroenterology 1996;111(4):1011‐7. [DOI] [PubMed] [Google Scholar]
- Navasa M, Planas R, Clemente G, Vargas V, Guarner C, Follo A, et al. Oral ofloxacin versus intravenous cefotaxime in the treatment of non‐complicated spontaneous bacterial peritonitis (SBP) in cirrhosis results of a multicenter, prospective, randomized trial. Journal of Hepatology 1994;21(Suppl 1):s11. [Google Scholar]
Piano 2016 {published data only}
- Piano S, Fasolato S, Salinas F, Romano A, Tonon M, Morando F, et al. The empirical antibiotic treatment of nosocomial spontaneous bacterial peritonitis: results of a randomized, controlled clinical trial. Hepatology (Baltimore, Md.) 2016;63(4):1299‐309. [DOI] [PubMed] [Google Scholar]
- Piano S, Salinas F, Morando F, Cavallin M, Romano A, Rosi S, et al. The empirical antibiotic treatment of nosocomial spontaneous bacterial peritonitis in patients with decompensated liver cirrhosis: Results of a randomized controlled clinical trial. Journal of Hepatology 2015;62:s241. [DOI] [PubMed] [Google Scholar]
- Piano S, Salinas F, Morando F, Cavallin M, Romano A, Rosi S, et al. The empirical antibiotic treatment of nosocomial spontaneous bacterial peritonitis in patients with decompensated liver cirrhosis: results of a randomized controlled clinical trial. Hepatology (Baltimore, Md.) 2014;60:479a. [DOI] [PubMed] [Google Scholar]
Rastegar 1998 {published data only}
- Rastegar LA, Omrani G, Dehbashi N, Malec F. Evaluation of the therapeutic effect of pefloxacin in comparison with ampicillin and gentamicin in cirrhotic patients with spontaneous bacterial peritonitis. Hepato‐gastroenterology 1998;45(21):783‐5. [PubMed] [Google Scholar]
Tuncer 2003 {published data only}
- Tuncer I, Topcu N, Durmus A, Turkdogan MK. Oral ciprofloxacin versus intravenous cefotaxime and ceftriaxone in the treatment of spontaneous bacterial peritonitis. Hepato‐gastroenterology 2003;50(53):1426‐30. [PubMed] [Google Scholar]
Yim 2017 {published data only}
- Yim HJ, Suh SJ, Jung YK, Kim MY, Baik SK, Kim HS, et al. Comparison of efficacy of cefotaxime, ceftriaxone, and ciprofloxacin for the treatment of spontaneous bacterial peritonitis in patients with liver cirrhosis: a randomized controlled trial. Journal of Hepatology 2017;66(1):s374‐s5. [Google Scholar]
References to studies excluded from this review
Ariza 1991 {published data only}
- Ariza J, Xiol X, Esteve M, Fernandez Baneres F, Linares J, Alonso T, et al. Aztreonam vs. cefotaxime in the treatment of gram‐negative spontaneous peritonitis in cirrhotic patients. Hepatology (Baltimore, Md.) 1991;14(1):91‐8. [DOI] [PubMed] [Google Scholar]
Badawy 2013 {published data only}
- Badawy AA, Zaher TI, Sharaf SM, Emara MH, Shaheen NE, Aly TF. Effect of alternative antibiotics in treatment of cefotaxime resistant spontaneous bacterial peritonitis. World Journal of Gastroenterology 2013;19(8):1271‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Felisart 1985 {published data only}
- Felisart J, Rimola A, Arroyo V, Perez‐Ayuso RM, Quintero E, Gines P, et al. Cefotaxime is more effective than is ampicillin‐tobramycin in cirrhotics with severe infections. Hepatology (Baltimore, Md.) 1985;5(3):457‐62. [DOI] [PubMed] [Google Scholar]
Grange 2004 {published data only}
- Grange JD, Thabut D, Lucidarme D, Adler M, Navasa M, Petit P, et al. Randomized, comparative study of moxifloxacin versus amoxicillin‐clavulanate in the treatment of bacterial infections in cirrhotic patients. Hepatology (Baltimore, Md.) 2004;40(4 Suppl 1):631a. [Google Scholar]
Lim 2011 {published data only}
- Lim JK, Tandon P, Garcia‐Tsao G. Antibiotic prophylaxis and management of bacterial infections. Chronic Liver Failure: Mechanisms and Management. Ginès P, Kamath PS, Arroyo V, 2011:395‐410. [Google Scholar]
Liu 2000 {published data only}
- Liu XM, Wu Y. The value of therapeutic paracentesis, peritoneal lavage and abdominal antibiotic administration in cirrhotic patients with spontaneous bacterial peritonitis. Chinese Journal of Clinical Hepatology 2000;16(3):175‐7. [Google Scholar]
McCue 1981 {published data only}
- McCue JD. Spontaneous bacterial peritonitis. Hospital Practice 1981;16(8):117, 20, 22‐3 passim. [DOI] [PubMed] [Google Scholar]
Piano 2011 {published data only}
- Piano S, Morando F, Angeli P. Spontaneous bacterial peritonitis in patients with cirrhosis and ascites. link.springer.com/chapter/10.1007/978‐3‐642‐18081‐1_51 2011 (accessed 16 August 2019).
Ricart 2000 {published data only}
- Ricart E, Soriano G, Novella MT, Ortiz J, Sabat M, Kolle L, et al. Amoxicillin‐clavulanic acid versus cefotaxime in the therapy of bacterial infections in cirrhotic patients. Journal of Hepatology 2000;32(4):596‐602. [DOI] [PubMed] [Google Scholar]
Rimola 1984 {published data only}
- Rimola A, Felisart J, Terés J, Gatell J, Rodés J. Controlled study of the therapeutic efficacy of two antibiotic regimens in spontaneous bacterial peritonitis in cirrhosis prognostic value of bacteriologic data. Gastroenterologia y Hepatologia 1984;7(5):235‐41. [Google Scholar]
Taskiran 2004 {published data only}
- Taskiran B, Colakoglu O, Sozmen B, Unsal B, Aslan SL, Buyrac Z. Comparison of cefotaxime and ofloxacin in treatment of spontaneous bacterial peritonitis. Turkish Journal of Gastroenterology 2004;15(1):34‐8. [PubMed] [Google Scholar]
References to studies awaiting assessment
Zafar 2018 {published data only}
- Zafar MH, Asghar A, Ather U. Role of oral verses intravenous antibiotic in patients with spontaneous bacterial peritonitis. Medical Forum Monthly 2018;29(7):32‐4. [Google Scholar]
Additional references
Adam 2012
- Adam R, Karam V, Delvart V, O'Grady J, Mirza D, Klempnauer J, et al. Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR). Journal of Hepatology 2012;57(3):675‐88. [DOI] [PubMed] [Google Scholar]
Aldred 2014
- Aldred KJ, Kerns RJ, Osheroff N. Mechanism of quinolone action and resistance. Biochemistry 2014;53(10):1565‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bernardi 2010
- Bernardi M. Spontaneous bacterial peritonitis: from pathophysiology to prevention. Internal and Emergency Medicine 2010;5(Suppl 1):S37‐44. [DOI] [PubMed] [Google Scholar]
Best 2018
- Best LM, Freeman S, Sutton AJ, Hawkins N, Tsochatzis E, Gurusamy KS. Treatment for hepatorenal syndrome in people with decompensated liver cirrhosis: a network meta‐analysis. Cochrane Database of Systematic Reviews 2018, Issue 9. [DOI: 10.1002/14651858.CD013103] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cadranel 2013
- Cadranel JF, Nousbaum JB, Bessaguet C, Nahon P, Nguyen‐Khac E, Moreau R, et al. Low incidence of spontaneous bacterial peritonitis in asymptomatic cirrhotic outpatients. World Journal of Hepatology 2013;5(3):104‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Castellote 2008
- Castellote J, Girbau A, Maisterra S, Charhi N, Ballester R, Xiol X. Spontaneous bacterial peritonitis and bacterascites prevalence in asymptomatic cirrhotic outpatients undergoing large‐volume paracentesis. Journal of Gastroenterology and Hepatology 2008;23(2):256‐9. [DOI] [PubMed] [Google Scholar]
Chaimani 2012
- Chaimani A, Salanti G. Using network meta‐analysis to evaluate the existence of small‐study effects in a network of interventions. Research Synthesis Methods 2012;3(2):161‐76. [DOI] [PubMed] [Google Scholar]
Chaimani 2013
- Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta‐analysis in STATA. PloS One 2013;8(10):e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2013
- Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gotzsche PC, Krleza‐Jeric K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Annals of Internal Medicine 2013;158(3):200‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chavez‐Tapia 2009
- Chavez‐Tapia NC, Soares‐Weiser K, Brezis M, Leibovici L. Antibiotics for spontaneous bacterial peritonitis in cirrhotic patients. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD002232.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
D'Amico 2006
- D'Amico G, Garcia‐Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. Journal of Hepatology 2006;44(1):217‐31. [DOI] [PubMed] [Google Scholar]
D'Amico 2014
- D'Amico G, Pasta L, Morabito A, D'Amico M, Caltagirone M, Malizia G, et al. Competing risks and prognostic stages of cirrhosis: a 25‐year inception cohort study of 494 patients. Alimentary Pharmacology & Therapeutics 2014;39(10):1180‐93. [DOI] [PubMed] [Google Scholar]
de Franchis 2015
- Franchis R, Baveno VIF. Expanding consensus in portal hypertension: report of the Baveno VI consensus workshop: stratifying risk and individualizing care for portal hypertension. Journal of Hepatology 2015;63(3):743‐52. [DOI] [PubMed] [Google Scholar]
Devani 2017
- Devani K, Charilaou P, Jaiswal P, Patil N, Radadiya D, Patel P, et al. Trends in hospitalization, acute kidney injury, and mortality in patients with spontaneous bacterial peritonitis. Journal of Clinical Gastroenterology 2019; Vol. 53, issue 2:e68‐e74. [DOI: 10.1097/MCG.0000000000000973] [DOI] [PubMed]
Dias 2010
- Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta‐analysis. Statistics in Medicine 2010;29(7‐8):932‐44. [DOI] [PubMed] [Google Scholar]
Dias 2012a
- Dias S, Sutton AJ, Welton NJ, Ades AE. NICE DSU technical support document 3: heterogeneity: subgroups, meta‐regression, bias and bias‐adjustment, September 2011 (last updated April 2012). nicedsu.org.uk/wp‐content/uploads/2016/03/TSD3‐Heterogeneity.final‐report.08.05.12.pdf 2012 (accessed 17 July 2018).
Dias 2012b
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU technical support document 1: introduction to evidence synthesis for decision making, April 2011 (last updated April 2012). scharr.dept.shef.ac.uk/nicedsu/wp‐content/uploads/sites/7/2016/03/TSD1‐Introduction.final_.08.05.12.pdf 2012 (accessed 17 July 2018).
Dias 2014
- Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. NICE DSU technical support document 4: inconsistency in networks of evidence based on randomised controlled trials, May 2011 (last updated April 2014). nicedsu.org.uk/wp‐content/uploads/2016/03/TSD4‐Inconsistency.final_.15April2014.pdf 2014 (accessed 17 July 2018).
Dias 2016
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU technical support document 2: a generalised linear modelling framework for pairwise and network meta‐analysis of randomised controlled trials, August 2011 (last updated September 2016). www.ncbi.nlm.nih.gov/pubmedhealth/PMH0088912/pdf/PubMedHealth_PMH0088912.pdf 2016 (accessed 17 July 2018). [PubMed]
EASL 2010
- European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. Journal of Hepatology 2010;53(3):397‐417. [DOI] [PubMed] [Google Scholar]
EASL 2018
EuroQol 2018
- EuroQol. EQ‐5D Instruments | About EQ‐5D, 2018. euroqol.org/eq‐5d‐instruments/ (accessed 17 July 2018).
Fiore 2018
- Fiore M, Gentile I, Maraolo AE, Leone S, Simeon V, Chiodini P, et al. Are third‐generation cephalosporins still the empirical antibiotic treatment of community‐acquired spontaneous bacterial peritonitis? A systematic review and meta‐analysis. European Journal of Gastroenterology & Hepatology 2018;30(3):329‐36. [DOI] [PubMed] [Google Scholar]
Fleming 2008
- Fleming KM, Aithal GP, Solaymani‐Dodaran M, Card TR, West J. Incidence and prevalence of cirrhosis in the United Kingdom, 1992‐2001: a general population‐based study. Journal of Hepatology 2008;49(5):732‐8. [DOI] [PubMed] [Google Scholar]
Gurusamy 2019
- Gurusamy K, Walmsley M, Davidson BR, Frier C, Fuller B, Madden A, et al. Top research priorities in liver and gallbladder disorders in the UK. BMJ Open 2019;9(3):e025045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2012
- Higgins JP, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta‐analysis: concepts and models for multi‐arm studies. Research Synthesis Methods 2012;3(2):98‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hutton 2015
- Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta‐analyses of health care interventions: checklist and explanations. Annals of Internal Medicine 2015;162(11):777‐84. [DOI] [PubMed] [Google Scholar]
ICH‐GCP 1997
- International Conference on Harmonisation Expert Working Group. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline. Guideline for Good Clinical Practice CFR & ICH Guidelines. Vol. 1, Philadelphia (PA): Barnett International/PAREXEL, 1997. [Google Scholar]
Jackson 2014
- Jackson D, Barrett JK, Rice S, White IR, Higgins JP. A design‐by‐treatment interaction model for network meta‐analysis with random inconsistency effects. Statistics in Medicine 2014;33(21):3639‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 2009
- Khan J, Pikkarainen P, Karvonen AL, Makela T, Peraaho M, Pehkonen E, et al. Ascites: aetiology, mortality and the prevalence of spontaneous bacterial peritonitis. Scandinavian Journal of Gastroenterology 2009;44(8):970‐4. [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Lu 2006
- Lu G, Ades AE. Assessing evidence inconsistency in mixed treatment comparisons. Journal of the American Statistical Association 2006;101(474):447‐59. [Google Scholar]
McPherson 2016
- McPherson S, Lucey MR, Moriarty KJ. Decompensated alcohol related liver disease: acute management. BMJ (Clinical Research Ed.) 2016;352:i124. [DOI] [PubMed] [Google Scholar]
Merion 2010
- Merion RM. Current status and future of liver transplantation. Seminars in Liver Disease 2010;30(4):411‐21. [DOI] [PubMed] [Google Scholar]
Mills 2012
- Mills EJ, Ioannidis JP, Thorlund K, Schunemann HJ, Puhan MA, Guyatt GH. How to use an article reporting a multiple treatment comparison meta‐analysis. JAMA 2012;308(12):1246‐53. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
Mokdad 2014
- Mokdad AA, Lopez AD, Shahraz S, Lozano R, Mokdad AH, Stanaway J, et al. Liver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysis. BMC Medicine 2014;12:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCBI 2018a
- NCBI. Liver cirrhosis. www.ncbi.nlm.nih.gov/mesh/68008103 (accessed 17 July 2018).
NCBI 2018b
- National Center for Biotechnology Information. Ascites. www.ncbi.nlm.nih.gov/mesh/68001201 (accessed 17 July 2018).
Newell 1992
- Newell DJ. Intention‐to‐treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837‐41. [DOI] [PubMed] [Google Scholar]
Nousbaum 2007
- Nousbaum JB, Cadranel JF, Nahon P, Khac EN, Moreau R, Thevenot T, et al. Diagnostic accuracy of the multistix 8 sg reagent strip in diagnosis of spontaneous bacterial peritonitis. Hepatology (Baltimore, Md.) 2007;45(5):1275‐81. [DOI] [PubMed] [Google Scholar]
Optum 2018
- Optum. Patient‐reported Outcomes | What We Do | SF Health Surveys | SF‐36v2 Health Survey, 2018. campaign.optum.com/optum‐outcomes/what‐we‐do/health‐surveys/sf‐36v2‐health‐survey.html (accessed 14 April 2018).
Puhan 2014
- Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello‐Petersen R, Singh JA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta‐analysis. BMJ (Clinical Research Ed.) 2014;349:g5630. [DOI] [PubMed] [Google Scholar]
Ratib 2015
- Ratib S, Fleming KM, Crooks CJ, Walker AJ, West J. Causes of death in people with liver cirrhosis in England compared with the general population: a population‐based cohort study. American Journal of Gastroenterology 2015;110(8):1149‐58. [DOI] [PubMed] [Google Scholar]
Read 1972
- Read AE. Clinical physiology of the liver. British Journal of Anaesthesia 1972;44(9):910‐7. [DOI] [PubMed] [Google Scholar]
Rimola 2000
- Rimola A, Garcia‐Tsao G, Navasa M, Piddock LJ, Planas R, Bernard B, et al. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. International Ascites Club. Journal of Hepatology 2000;32(1):142‐53. [DOI] [PubMed] [Google Scholar]
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
Runyon 2013
- Runyon BA, Committee APG. Management of adult patients with ascites due to cirrhosis: an update. Hepatology (Baltimore, Md.) 2013;57(4):1651‐3. [DOI] [PubMed] [Google Scholar]
Saab 2009
- Saab S, Hernandez JC, Chi AC, Tong MJ. Oral antibiotic prophylaxis reduces spontaneous bacterial peritonitis occurrence and improves short‐term survival in cirrhosis: a meta‐analysis. American Journal of Gastroenterology 2009;104(4):993‐1001. [DOI] [PubMed] [Google Scholar]
Salanti 2011
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple‐treatment meta‐analysis: an overview and tutorial. Journal of Clinical Epidemiology 2011;64(2):163‐71. [DOI] [PubMed] [Google Scholar]
Salanti 2012
- Salanti G. Indirect and mixed‐treatment comparison, network, or multiple‐treatments meta‐analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Research Synthesis Methods 2012;3(2):80‐97. [DOI] [PubMed] [Google Scholar]
Savović 2012a
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized controlled trials: combined analysis of meta‐epidemiological studies. Health Technology Assessment 2012;16(35):1‐82. [DOI] [PubMed] [Google Scholar]
Savović 2012b
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. [DOI] [PubMed] [Google Scholar]
Savović 2018
- Savović J, Turner RM, Mawdsley D, Jones HE, Beynon R, Higgins JP, et al. Association between risk‐of‐bias assessments and results of randomized trials in Cochrane Reviews: The ROBES Meta‐Epidemiologic Study. American Journal of Epidemiology 2018;187(5):1113‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scaglione 2015
- Scaglione S, Kliethermes S, Cao G, Shoham D, Durazo R, Luke A, et al. The epidemiology of cirrhosis in the united states: a population‐based study. Journal of Clinical Gastroenterology 2015;49(8):690‐6. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ (Clinical Research Ed.) 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Setiawan 2016
- Setiawan VW, Stram DO, Porcel J, Lu SC, Marchand L, Noureddin M. Prevalence of chronic liver disease and cirrhosis by underlying cause in understudied ethnic groups: the multiethnic cohort. Hepatology (Baltimore, Md.) 2016;64(6):1969‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Severini 1993
- Severini Thomas A. Bayesian interval estimates which are also confidence intervals. Journal of the Royal Statistical Society. Series B (Methodological) 1993;55(2):533‐40. [Google Scholar]
Stata/SE 15.1 [Computer program]
- StataCorp. Stata. Version 15. College Station, TX, USA: StataCorp, 2017.
Tandon 2011
- Tandon P, Garcia‐Tsao G. Renal dysfunction is the most important independent predictor of mortality in cirrhotic patients with spontaneous bacterial peritonitis. Clinical Gastroenterology and Hepatology 2011;9(3):260‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsochatzis 2014
- Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet 2014;383(9930):1749‐61. [DOI] [PubMed] [Google Scholar]
Tsochatzis 2017
- Tsochatzis EA, Gerbes AL. Diagnosis and treatment of ascites. Journal of Hepatology 2017;67(1):184‐5. [DOI] [PubMed] [Google Scholar]
Turner 2012
- Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP. Predicting the extent of heterogeneity in meta‐analysis, using empirical data from the Cochrane Database of Systematic Reviews. International Journal of Epidemiology 2012;41(3):818‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Valkenhoef 2012
- Valkenhoef G, Lu G, Brock B, Hillege H, Ades AE, Welton NJ. Automating network meta‐analysis. Research Synthesis Methods 2012;3(4):285‐99. [DOI] [PubMed] [Google Scholar]
Williams 2014
- Williams R, Aspinall R, Bellis M, Camps‐Walsh G, Cramp M, Dhawan A, et al. Addressing liver disease in the UK: a blueprint for attaining excellence in health care and reducing premature mortality from lifestyle issues of excess consumption of alcohol, obesity, and viral hepatitis. Lancet 2014;384(9958):1953‐97. [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical Research Ed.) 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yepes‐Nunez 2019
Yocum 1980
- Yocum RR, Rasmussen JR, Strominger JL. The mechanism of action of penicillin. Penicillin acylates the active site of Bacillus stearothermophilus d‐alanine carboxypeptidase. Journal of Biological Chemistry 1980;255(9):3977‐86. [PubMed] [Google Scholar]
Yotsuji 1988
- Yotsuji A, Mitsuyama J, Hori R, Yasuda T, Saikawa I, Inoue M, et al. Mechanism of action of cephalosporins and resistance caused by decreased affinity for penicillin‐binding proteins in Bacteroides fragilis. Antimicrobial Agents and Chemotherapy 1988;32(12):1848‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Gurusamy 2018
- Gurusamy KS, Wilson P, Tsochatzis E. Antibiotic treatment for spontaneous bacterial peritonitis in people with decompensated liver cirrhosis: a network meta‐analysis. Cochrane Database of Systematic Reviews 2018, Issue 9. [DOI: 10.1002/14651858.CD013120] [DOI] [PMC free article] [PubMed] [Google Scholar]


